Abstract
Familial hemiplegic migraine type 1 (FHM1) is a rare migraine subtype. Whereas transgenic knock-in mice with the human pathogenic FHM1 R192Q missense mutation in the Cacna1a gene reveal overall neuronal hyperexcitability, the effects on the trigeminovascular system and calcitonin gene-related peptide (CGRP) receptor are largely unknown. This gains relevance as blockade of CGRP and its receptor are therapeutic targets under development. Hence, we set out to test these effects in FHM1 mice. We characterized the trigeminovascular system of wild-type and FHM1 mice through: (i) in vivo capsaicin- and CGRP-induced dural vasodilation in a closed-cranial window; (ii) ex vivo KCl-induced CGRP release from isolated dura mater, trigeminal ganglion and trigeminal nucleus caudalis; and (iii) peripheral vascular function in vitro. In mutant mice, dural vasodilatory responses were significantly decreased compared to controls. The ex vivo release of CGRP was not different in the components of the trigeminovascular system between genotypes; however, sumatriptan diminished the release in the trigeminal ganglion, trigeminal nucleus caudalis and dura mater but only in wild-type mice. Peripheral vascular function was similar between genotypes. These data suggest that the R192Q mutation might be associated with trigeminovascular CGRP receptor desensitization. Novel antimigraine drugs should be able to revert this complex phenomenon.
Keywords: Calcitonin gene-related peptide, migraine, R192Q, trigeminovascular, vasodilation
Introduction
Migraine is a disabling neurovascular brain disorder that is characterized by severe attacks of throbbing headache, which can be accompanied by nausea, vomiting, osmo-, photo-, and phonophobia.1 In one-third of patients, attacks may be preceded by neurological symptoms (migraine with aura), the likely consequence of a wave of neuronal and glial depolarization with subsequent temporary inactivity, called cortical spreading depression.2 Migraine headache is associated with activation of the trigeminovascular system and calcitonin gene-related peptide (CGRP) release from sensory nerves.3 Monogenic familial hemiplegic migraine, a rare subtype of migraine with transient hemiparesis during the aura, has shown validity as a genetic model of migraine.4 Familial hemiplegic migraine type 1 (FHM1) is caused by specific missense mutations in the Cacna1a gene that encodes the α1A subunit of neuronal voltage-gated CaV2.1 (P/Q-type) calcium channels.5,6
A transgenic knock-in mouse model was generated by introducing the human FHM1 R192Q mutation into the mouse Cacna1a gene using a gene targeting approach.7 The mutation results in a gain of CaV2.1 channel function with enhanced cortical glutamatergic neurotransmission,8 an increased susceptibility to cortical spreading depression,7,9 and signs of spontaneous unilateral head pain, as evidenced by increased head grooming and eye blinking.10 Few studies have investigated CGRP expression and function in the trigeminovascular system of FHM1 R192Q mice; however, the results are inconsistent and mostly only the trigeminal ganglion was investigated,11–13 making it difficult to understand the exact role of this mutation in the different components of the trigeminal system. Furthermore, the effect the R192Q mutation may have on perivascular CGRP release and CGRP-induced (dural artery) vasodilation has not been investigated. Also, it is not known whether the R192Q mutation could affect the peripheral vascular function.
Hence, to study the effects of the R192Q mutation on the trigeminovascular system, we investigated in FHM1 R192Q mice: (i) trigeminovascular dural vasodilation induced by endogenous and exogenous CGRP in vivo, (ii) CGRP release in the trigeminovascular components including the trigeminal nucleus caudalis, trigeminal ganglion and dura mater ex vivo, and (iii) peripheral vascular CGRP receptor function in vitro. We hypothesized that the R192Q mutation will increase dural vasodilation and CGRP release in the trigeminovascular system but will not disturb peripheral vascular function.
Material and methods
Experimental animals
Experiments were performed in 13- to 14-week-old homozygous FHM1 R192Q mice (“R192Q”) and wild-type littermates (“wild-type”) of both sexes. Mice were backcrossed for at least five generations with C57BL/6J mice so that the genetic background is >97% C57BL/6J, as described before.7 All mice were bred at the Leiden University Medical Center and transported to our animal facility at least 14 days before the start of the experiment to allow for sufficient equilibration time at the Erasmus MC. Animals were housed under a 12-h dark-light cycle and given free access to food and water. All experiments were approved by the Erasmus University Medical Center’s institutional animal ethics committee and in accordance with the European directive 2010/63/EU and ARRIVE (Animal Research: Reporting in Vivo Experiments) reporting guidelines for the care and use of laboratory animals.
In vivo: Intravital microscopy and dural artery vasodilation
Animals (n = 32; 13 wild-type and 19 R192Q) were anaesthetized throughout the experiment using intraperitoneal (i.p.) sodium pentobarbital (80 mg/kg, i.p. and then 20 mg/kg/h, i.p.). The trachea was cannulated and connected to a pressure ventilator (small animal ventilator SAR-830 series; CWE, Inc., Ardmore, PA, USA). The jugular vein was cannulated for intravenous (i.v.) administration of drugs and the femoral artery for continuous monitoring of mean arterial pressure. During the experiment, the core temperature of the animal was monitored via a rectal thermometer and maintained between 36.5 and 37.5℃ using a homeothermic blanket system for rodents (Harvard Instruments, Edenbridge, UK). Subsequently, the mouse was placed in a stereotaxic frame, and the parietal bone was drilled thin until the dural artery was clearly visible. As the mouse skull is very thin, care was taken to drill with constant application of ice-cold saline. In four out of 32 mice, bleeding was observed underneath the skull, making visualization of the artery difficult; these animals were excluded from the study. The drilled area was covered with mineral oil to prevent drying of the skull and to facilitate visualization of the artery. The dural artery was captured with an intravital microscope (Leica MZ 16; Leica Microsystem Ltd, Heerbrugg, Switzerland), using a cyan filter on a cold source of light. A zoom lens (80 × magnification) and camera (DCx V3.52, Thorlabs LTD, Ely, UK) were used to capture the image of the dural artery, which was displayed and measured on a computer using a dedicated software package (IDA-Intravital Dimension Analyser; http://www.beneryx.co.uk) integrated with a ADC/DAC board (DI-158, DATAQ instruments, ‘s-Hertogenbosch, The Netherlands). Data of dural artery diameter, mean arterial pressure and exhaled CO2 were recorded using Labchart data acquisition system (AD Instruments Ltd, Oxford, UK).
As described in a previous study,14 mice dural arteries were first constricted with endothelin-1 (ET-1) before other pharmacological interventions, as it is not possible to observe significant vasodilation in mouse dural arteries without artery preconstriction. ET-1 (1–6 µg/kg i.v.) was titrated to induce vasoconstriction, reducing the diameter to 30–40% of its original diameter. Capsaicin (30 µg/kg) and α-CGRP (10 µg/kg) were administrated i.v. as a bolus after the constriction induced by ET-1 was stable (around 5 minutes). The arterial diameter was recorded for another 10 min; 30 min were allowed to elapse after each of the treatments for the recovery of baseline diameter.
Ex vivo: CGRP release in trigeminal nucleus caudalis, trigeminal ganglion, and dura mater
Mice were anesthetized using sodium pentobarbital (80 mg/kg, i.p.) and decapitated at the atlanto-occipital joint. The skin and galea aponeurotica were retracted from the skull, which was divided into halves by a clear cut along the sagittal suture. The brain halves, together with the brainstem, were carefully removed while the cranial dura was left attached to the skull.15 The trigeminal nucleus caudalis (Sp5C), which runs caudally from approximately 9–13 mm from bregma, was isolated from the brainstem. The trigeminal ganglia were harvested by dissection 1 mm proximal and distal to the point where the mandibular nerve branches off and the dura mater around the trigeminal ganglion had been carefully removed.16 All other tissues, except for the dura mater, were extracted from the skull without damaging the dura. The isolated trigeminal nucleus caudalis, trigeminal ganglion and the skull with the dura mater were immersed and washed in carbogenated synthetic interstitial fluid, containing (mM): NaCl (108), KCl (3.48), MgSO4 (3.5), NaHCO3 (26), NaH2PO4 (11.7), CaCl2 (1.5), sodium gluconate (9.6), glucose (5.55) and sucrose (7.6) for 30 min at 37℃.
Isolated trigeminal nucleus caudalis, trigeminal ganglion and dura mater were placed in a 24-well plate containing 500 µL synthetic interstitial fluid. The 24-well plate was fixed in a water bath that formed a closed humid chamber of 37℃. To induce CGRP release, tissues were stimulated with 60 mM KCl, this concentration was chosen based on previous literature on CGRP release in rats and mice.17 To test reproducibility, a second stimulation with 60 mM KCl was done after two wash steps of 10 min each. In the experiments with sumatriptan (30 µM), the agonist was applied 10 min prior to the challenge with 60 mM KCl. For every sample, including basal, the solution was collected after 10 min incubation and mixed with aprotinin (500 KUI/mL; n = 6–12). For the assessment of CGRP content, samples were stored at −80℃ until processed with a commercial CGRP RIA kit according to the manual (Phoenix Pharmaceuticals, Burlingame, CA, USA). The assay has a detection level of ∼0.1 pg/mL, if the CGRP content of a sample was below the detection limit, the value for that sample was set at 0.1 pg/mL.
In vitro: Peripheral vascular function
Aortas and mesenteric arteries were dissected from the mice and placed in a carbogenated Krebs bicarbonate buffer solution containing (mM): NaCl (118), KCl (4.7), CaCl2 (2.5), MgSO4 (1.2), KH2PO4 (1.2), NaHCO3 (25) and glucose (8.3); pH 7.4. The arteries were cut in small segments of about 2–4 mm each, which were suspended in Mulvany myographs (ADinstruments, Danish Myograph Technology, Aarhus, Denmark) containing oxygenated Krebs bicarbonate solution at 37℃. After equilibration for at least 30 min, with two changes of solution at 15-min intervals, blood vessel segments were subsequently stretched to a tension normalized to 90% of l100.18 Then, the vessels were exposed to 30 mM KCl. The maximum contractile response to KCl was determined using 100 mM KCl. After washout and stabilisation, concentration-response curves to α-CGRP, acetylcholine, sodium nitroprusside and 5-hydroxytryptamine (5-HT) were constructed in a parallel design,19 U46619 (10-300 nM) was used to induce precontraction of the vessel segments, before constructing relaxation curves to acetylcholine, sodium nitroprusside and α-CGRP. After the concentration-response curves to acetylcholine, a single concentration of sodium nitroprusside (0.1 mM) was administered.
Data analysis
Dural artery diameter was calculated from the area under the curve of the intensity measured and expressed in arbitrary units (AU). Vasodilation induced by capsaicin or α-CGRP was corrected for diameter change before induction of vasodilation to compare differences between wild-type and mutant mice. Changes in mean arterial pressure were expressed in mm Hg.20 Paired and unpaired student t-tests and one-way ANOVA were conducted to compare changes in dural diameter and blood pressure after each experimental intervention, followed by the Bonferroni multiple comparisons post-test.
CGRP samples were measured in duplicate and total CGRP release was calculated as the average of the duplicates. CGRP release was expressed as relative stimulated CGRP release, which was calculated by the ratio of KCl-induced CGRP release and basal CGRP release.12 Statistical differences of the relative CGRP release between genotypes were calculated using one-tailed Mann–Whitney test for unpaired observations and Friedmann test, followed by Dunns multiple comparison post hoc test. For the repeated stimulation with KCl and the sumatriptan experiments, a one-tailed Wilcoxon matched pairs test for non-parametric analysis of paired data was used.
Vasorelaxant responses to α-CGRP, acetylcholine and sodium nitroprusside were expressed relative to the contraction induced by U46619 (10 nM–100 nM). Contraction by 5-HT was expressed relative to the contraction induced by KCl (100 mM). For each agonist, the maximal effect (Emax) was calculated. The concentration-response curves of the agonists were analyzed using nonlinear regression analysis, and the potency of the agonists was expressed as pEC50 (i.e. negative logarithm of the molar concentration of agonist inducing half maximum response) using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Statistical differences of the Emax and pEC50 were calculated using the unpaired student t-test.21
While the experimenter was aware of the genotype of the animals during the experiment because of logistic reasons, all analyses were performed in a manner blinded to the genotype. All data are expressed as mean ± SEM. Statistical significance was accepted at p < 0.05
Compounds
The compounds used in the present study (obtained from the sources indicated) were: rat α-CGRP and ET-1 (NeoMPS S.A., Strasbourg, France), sumatriptan succinate, capsaicin, acetylcholine, sodium nitroprusside and 5-HT (Sigma Chemical Co., Steinheim, Germany). α-CGRP, ET-1, sumatriptan, acetylcholine, sodium nitroprusside and 5-HT were dissolved in water. Capsaicin (1 mg/mL) was dissolved in a mixture of Tween-80, ethanol 70% and water (1:1:8). All stock solutions were stored at −80℃ until required. Just before use, the stock solutions were further diluted to appropriate concentrations in isotonic saline for injection and in water for application.
Results
General
There was no difference in body weight between the wild-type (22.4 ± 1.0 g) and R192Q (24.6 ± 1.2) mice included in our study (p = 0.807). Also, in none of the experiments, there was a difference between the results obtained in male and female animals. Therefore, the results from both sexes were pooled for further analysis.
In vivo: Effect on dural artery dilation induced by capsaicin and CGRP
A trace obtained during an experiment is shown in Figure 1. Diameter changes induced by ET-1, capsaicin or CGRP are shown in Figure 2. Blood vessel baseline diameter before any pharmacological intervention was significantly higher in the wild-type (721 ± 48 AU; n = 12) than mutant (560 ± 53 AU; n = 16, p < 0.05) mice. Administration of ET-1 reduced the dural artery diameter in the capsaicin-treated group to 40 ± 3% and 31 ± 4% of its original diameter in wild-type and mutant mice, respectively. In the CGRP-treated group, ET-1 reduced the diameter to 37 ± 6% of its original diameter in wild-type mice and to 34 ± 9% of its original diameter in the mutant mice. There was no significant difference between the two genotypes in the dose of ET-1 required to reach this preconstriction (wild-type: 3.3 ± 0.6 mg/kg; n = 22 vs. R192Q: 3.7 ± 0.4 mg/kg; n = 27; p = 0.174).
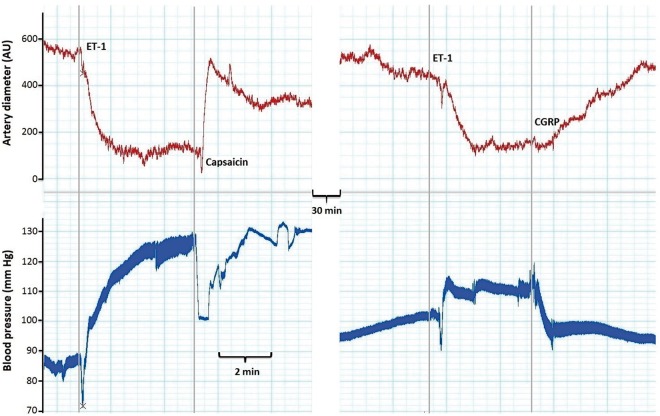
Figure 1.
A trace obtained during an experiment on the closed-cranial window. The upper red line represents the dural artery diameter (in arbitrary units, AU) and the lower blue line represents the blood pressure (in mm Hg) simultaneously measured in the femoral artery. The gray vertical lines represent the moment of intravenous administration of endothelin-1 (ET-1, 2 µg/kg), capsaicin (30 µg/kg) or CGRP (10 µg/kg).
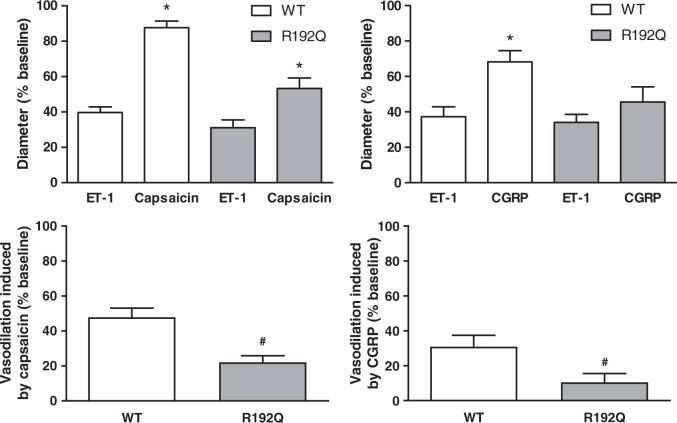
Figure 2.
Trigeminovascular dural artery vasodilation. Diameter change (%) induced by endothelin-1 (ET-1), capsaicin or CGRP in wild-type (WT, white bars) and R192Q (grey bars) mice. ET-1 induced comparable dural vasoconstriction in all groups (upper left and right panels). Capsaicin induced significant dural vasodilation in both genotypes (upper left panel), while CGRP only induced dural vasodilation in the wild-type group (upper right panel). The vasodilation induced by capsaicin and CGRP, corrected for the ET-1 baseline, is significantly lower in the R192Q groups than in the wild-type groups (lower left and right panel). Data are expressed as mean ± SEM, n = 9–16, *p < 0.05 vs. the corresponding dose to ET-1, #p < 0.05 between genotypes.
Capsaicin induced significant (p < 0.001) dural vasodilation in wild-type (88 ± 4%) and mutant (53 ± 6%) mice compared to the preconstriction. Capsaicin-induced vasodilation corrected for the diameter change before induction of vasodilation was significantly lower in the mutant mice (22 ± 4%; n = 16) than in the wild-type (48 ± 5%; n = 11; p < 0.001).
CGRP-induced dural vasodilation was significantly different compared to the preconstriction in wild-type (68 ± 6%; n = 11; p < 0.01), showing a tendency for significance in mutant mice (46 ± 9%; n = 11; p = 0.06). In accordance, CGRP-induced vasodilation corrected for the diameter change before induction of vasodilation was also significantly lower in mutant mice (11 ± 5%; n = 11) than in the wild-type (31 ± 7%; n = 11; p < 0.05).
In vivo: Effect on mean arterial pressure of capsaicin and CGRP
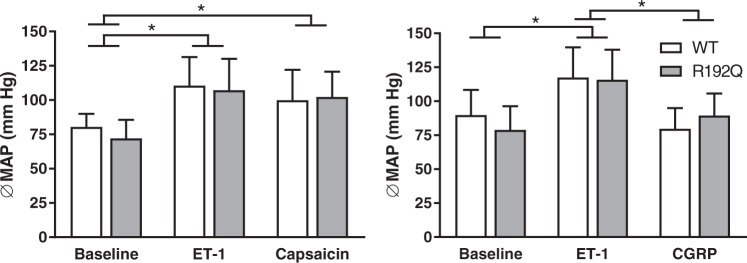
As shown in Figure 3, baseline mean arterial pressure was comparable between the two genotypes (wild-type: 80 ± 3 mm Hg; n = 10 vs. R192Q: 72 ± 4 mm Hg; n = 14; p = 0.11). ET-1 increased the mean arterial pressure similarly in both genotypes (wild-type: 111 ± 7 mm Hg; n = 10 vs. R192Q: 107 ± 6 mm Hg; n = 14; p < 0.001 vs. baselines). Capsaicin did not reverse the elevated blood pressure caused by ET-1 in either genotype (wild-type: 100 ± 7 mm Hg; n = 10 vs. R192Q: 102 ± 5 mm Hg; n = 14). Administration of CGRP caused a normalization of the elevated blood pressure in both genotypes (wild-type: 80 ± 5 mm Hg; n = 9, vs. R192Q: 89 ± 5 mm Hg; n = 10; p < 0.001 vs. baselines). Mean arterial pressure changes induced by ET-1, capsaicin or CGRP were not different between wild-type and mutant mice.
Figure 3.
Effect of pharmacological intervention on mean arterial pressure. Mean arterial pressure (MAP; mm Hg) after administration of endothelin-1 (ET-1), capsaicin or CGRP in wild-type (white bars) and R192Q (grey bars) mice. ET-1 increased the MAP compared to the baseline in both genotypes, and this increase was reverted after administration of only CGRP. Data are expressed as mean ± SEM, n = 9–14, *p < 0.05.
Ex vivo: Relative stimulated CGRP release after KCl stimulation
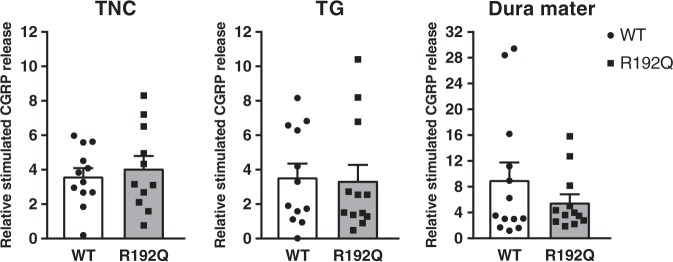
Basal CGRP release (in absolute values) in trigeminal caudal nucleus (wild-type: 4.9 ± 1.0; n = 11 vs. R192Q: 6.2 ± 1.3 n = 12; p = 0.203), trigeminal ganglion (wild-type: 1.3 ± 0.3; n = 11 vs. R192Q: 1.3 ± 0.2 n = 12; p = 0.367) and dura mater (wild-type: 0.9 ± 0.6; n = 12 vs. R192Q: 1.0 ± 0.2 n = 12; p = 0.321) was not different between wild-type and mutant mice. Relative stimulated CGRP release (measured as the ratio of KCl-induced CGRP release and CGRP release at basal) was used to compare the effect of KCl in the different trigeminal components of wild-type and mutant mice (Figure 4).Relative stimulated CGRP release induced by KCl was comparable between wild-type and mutant mice in the trigeminal nucleus caudalis (wild-type: 3.6 ± 0.5; n = 12 vs. R192Q: 4.1 ± 0.8; n = 11; p = 0.415), trigeminal ganglion (wild-type: 3.6 ± 0.8; n = 12 vs. R192Q: 3.4 ± 0.9; n = 12; p = 0.375), and dura mater (wild-type: 9.0 ± 2.7; n = 12 vs. R192Q: 5.6 ± 1.3; n = 12; p = 0.425). Moreover, there was no difference in relative stimulated CGRP release between the different trigeminovascular components of wild-type and mutant mice.
Figure 4.
CGRP release in the trigeminovascular system. Relative stimulated CGRP release in the trigeminal nucleus caudalis (TNC), trigeminal ganglion (TG) and dura mater of wild-type (WT, white bars and circles) and R192Q (grey bars and squares) mice. There is no statistical difference in CGRP release between wild-type and R192Q mice in the trigeminal nucleus caudalis, trigeminal ganglion or dura mater. Data are expressed as mean ± SEM, n = 11–12.
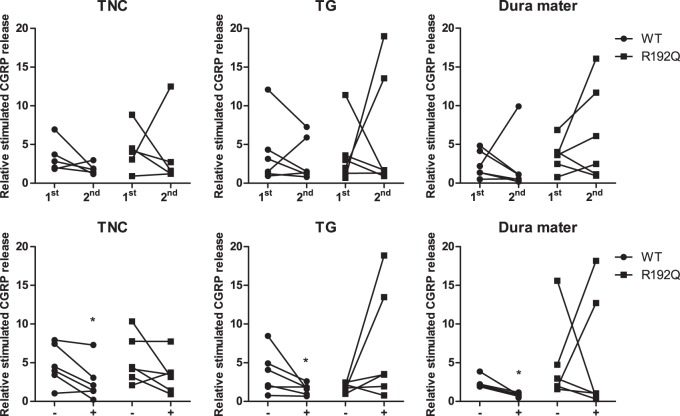
Ex vivo: Relative stimulated CGRP release after repeated stimulation with KCl
CGRP release was measured after the first and second stimulation with KCl to test the reproducibility of CGRP release. The relative stimulated CGRP release of wild-type mice was similar for the first and second stimulation for trigeminal nucleus caudalis (1st: 3.7 ± 0.8 vs. 2nd: 1.8 ± 0.3; n = 6; p = 0.156), trigeminal ganglion (1st: 3.9 ± 1.7 vs. 2nd: 3.0 ± 1.2; n = 6; p = 0.281) and dura mater (1st: 2.4 ± 0.7 vs. 2nd: 2.2 ± 1.5; n = 6; p = 0. 281) (Figure 5, top panel). Also for the mutant mice, no statistical differences were observed between the first and second stimulation for trigeminal nucleus caudalis (1st: 4.3 ± 1.1 vs. 2nd: 3.8 ± 2.2; n = 6; p = 0.406), trigeminal ganglion (1st: 3.7 ± 1.6 vs. 2nd: 6.3 ± 3.2; n = 6; p = 0.422) and dura mater (1st: 3.6 ± 0.8 vs. 2nd: 6.4 ± 2.6; n = 6; p = 0.156) (Figure 5, top panel), although there was a trend for increased release in trigeminal ganglion and dura mater, with means 77% and 78% higher with the 2nd stimulation lowered release for all other tissues in wild-type or mutant.
Figure 5.
Effect of repeated stimulation and sumatriptan on CGRP release. Relative stimulated CGRP release after a first (1st) and second (2nd) stimulation with KCl (panels a, b, c) or in the absence (−) or presence (+) of sumatriptan (panels d, e, f) in trigeminal nucleus caudalis (TNC), trigeminal ganglion (TG) and dura mater of wild-type (WT, circles) and R192Q (squares) mice. There were no statistically significant differences between the first and second stimulation with KCl in either genotype. Sumatriptan significantly reduced the relative stimulated CGRP release in the trigeminal nucleus caudalis, dura mater and trigeminal ganglion of wild-type, but not in all the trigeminovascular components of the R192Q mice tested. Data are expressed as mean ± SEM, n = 5–6, *p < 0.05 vs. response in the absence of sumatriptan.
Ex vivo: Relative stimulated CGRP release in the absence and presence of sumatriptan
The effect of pretreatment with sumatriptan (30 µM) on CGRP release in the trigeminovascular components is shown in Figure 5 (lower panel). In the presence of sumatriptan, relative stimulated CGRP release in wild-type mice was significantly attenuated in trigeminal nucleus caudalis (4.7 ± 1.1 vs. 2.6 ± 1.0; n = 6; p < 0.05), dura mater (2.4 ± 0.4 vs. 0.8 ± 0.1; n = 5 p < 0.05) and in the trigeminal ganglion (3.7 ± 1.1 vs. 1.6 ± 0.3; n = 6; p < 0.05). However, in mutant mice, sumatriptan did not significantly reduce the relative stimulated CGRP release in trigeminal nucleus caudalis (5.3 ± 1.3 vs. 3.4 ± 1.0; n = 6; p = 0.078), trigeminal ganglion (1.8 ± 0.2 vs. 7.0 ± 3.0; n = 6; p = 0.078) or dura mater (4.8 ± 2.2 vs. 5.6 ± 3.2; n = 6; p = 0.422), although, in contrast to our hypothesis, there was a trend for increased release in trigeminal ganglion and dura mater, with means 390% and 16% higher, whereas sumatriptan lowered release for the other tissues in wild-type or mutant.
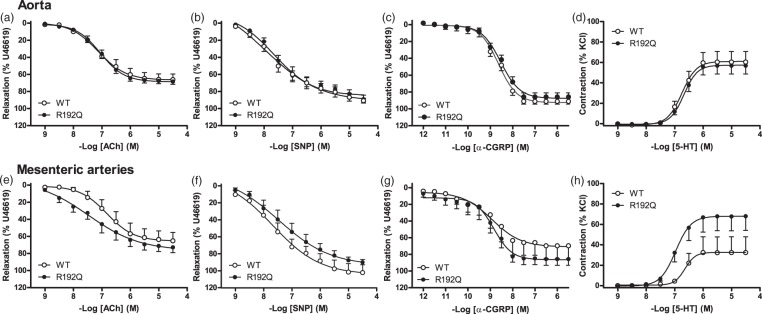
In vitro: Peripheral vascular function in aortas and mesenteric arteries
In the aortas, KCl-induced contraction was not different between both genotypes (wild-type: 8.3 ± 0.4 mN; n = 12 vs. R192Q: 7.9 ± 0.5 mN; n = 11; p = 0.560), while in mesenteric arteries, the KCl-induced contraction was significantly lower in mutant mice (wild-type: 5.7 ± 0.8 mN; n = 9 vs. R192Q: 3.4 ± 0.3 mN; n = 13; p < 0.01). There were no significant differences between full concentration-response curves to CGRP, sodium nitroprusside or acetylcholine in aortas or mesenteric arteries of both genotypes (Figure 6). To determine the endothelial function, the maximal effect of acetylcholine was corrected for the maximal effect induced by sodium nitroprusside (0.1 mM) in the same vessel segment. No differences were found in the acetylcholine/sodium nitroprusside ratio between genotypes in the aortas (wild-type: 0.76 ± 0.05; n = 11 vs. R192Q: 0.77 ± 0.03; n = 10; p = 0.812) or in the mesenteric arteries (wild-type: 0.73 ± 0.10; n = 6 vs. R192Q: 0.77 ± 0.06; n = 9; p = 0.714). 5-HT induced a concentration-dependent contraction, which was not different in aortas of both genotypes. In mesenteric arteries, the maximal effect induced by 5-HT seemed to be lower in wild-type than in mutant mice, but this was not statistically significant (p = 0.13; Figure 6). For the Emax and pEC50 values of the concentration-response curves, see Supplementary Table 1.
Figure 6.
Peripheral vascular function. Concentration-response curve to different agonists in isolated aortas and mesenteric arteries of wild-type (WT, open circles) and R192Q (closed circles) mice. There were no differences between wild-type and R192Q mice in relaxant responses to acetylcholine (a, e), sodium nitroprusside (SNP, b, f) and CGRP (c, g), which are expressed relative to the contraction induced by U46619, in the aortas (a, b, c) and mesenteric arteries (e, f, g). Contractile responses to 5-hydroxytryptamine (5-HT, d, h), which are expressed relative to 100 mM KCl, in the aortas (d) and mesenteric arteries (h) were also not different between wild-type and R192Q mice. Data are expressed as mean ± SEM, n = 10–13.
Discussion
The mechanisms underlying migraine pathophysiology are not completely elucidated. It seems that migraine is a disorder of brain sensory processing, characterized by a generalized neuronal hyperexcitability,22 while the head pain is thought to be the consequence of activation of the trigeminovascular system, CGRP release and sensitization of trigeminal nociceptors. Transgenic mice harboring the FHM1 R192Q mutation revealed an overall hyperexcitability phenotype and increased susceptibility to CSD,4 but whether this mutation could affect the normal physiology of the trigeminovascular system is unknown. Therefore, the functionality of the trigeminovascular system was investigated in transgenic mice that express the FHM1 R192Q missense mutation in the α1A subunit of voltage-gated CaV2.1 calcium channels that leads to a gain of function of these channels by studying: (i) dural artery vasodilation induced by endogenous and exogenous CGRP in vivo, (ii) CGRP release in various components of the trigeminovascular system ex vivo, and (iii) peripheral vascular function in vitro.
Several aspects of the CGRP-related trigeminovascular function were abnormal in the mutant mice. First of all, the trigeminovascular dural artery vasodilation in vivo, either by endogenous (induced by capsaicin) or exogenous CGRP, was reduced. A priori we expected an increased vasodilation in mutant mice. After all, as CaV2.1 channels are also expressed on perivascular nerve endings,23 given the gain of function of the R192Q mutation on CaV2.1 channels,7 activation of the channels would lead to release of CGRP, a potent vasodilator, and increased vasodilation.24
One possible explanation for the reduced effects on dural vasodilation is that CGRP release may already be elevated at basal in mutant mice due to the increased activity of mutant CaV2.1 channels as proposed in previous studies.11,25 Therefore, administration or release of CGRP from an exogenous or endogenous source, may no longer exert an effect in response to a stimulus. However, more complex mechanisms seem to be involved, since we observed a lower baseline dural diameter in the mutant mice in our in vivo study; and no genotypic differences in CGRP release, at basal or after KCl stimulation, in the trigeminovascular components in our ex vivo study.
On the other hand, hyperactivity of mutant CaV2.1 channels could potentially lead to CGRP depletion – already at baseline – as may be concluded from an immunohistochemistry study that showed reduced CGRP immunoreactivity in the trigeminal ganglia and trigeminocervical complex of these mice.13 However, our data do not seem to support CGRP depletion, since no statistical differences in basal CGRP release or CGRP release after repeated stimulation between the genotypes were observed. Still, a reduction in response, in their case for intracellular Ca2+ response, was observed after somatosensory stimulation when studying the same R192Q mouse model, which was ascribed to reduced neurovascular coupling.26
An alternative explanation for the in vivo reduced dural artery vasodilation response to capsaicin may be mediated by a change in CGRP receptor function or expression, since we also observed reduced dural vasodilation induced by exogenous CGRP in the mutant mice, which might suggest a desensitization or downregulation of this receptor. Receptor desensitization, as suggested before for CGRP-mediated ATP-gated P2X3 signaling in FHM1 R192Q mice25 is more likely given that CGRP receptor expression on trigeminal ganglion neurons was shown to be similar between genotypes in culture and in situ.27
Hypothetically, the different in vivo effects on dural vasodilation between wild-type and mutant mice could have been due to changes in mean arterial pressure associated with the administration of drugs. However, this is unlikely as changes in blood pressure during intravenous injection of ET-1, capsaicin or CGRP, are below the level affecting dural vasodilation in this experimental model28 and, more importantly, they were not different between wild-type and mutant mice.
When considering the present and previous studies on FHM1 R192Q mice, the basal release of CGRP and functionality of the CGRP receptor may change with age. It is noteworthy that in 11-day-old mutant mice there is an increased basal and KCl-stimulated CGRP release from trigeminal ganglia;11 in two-week-old animals an increased basal CGRP release was identified;25 whereas in four-week-old mice an increased stimulated CGRP release but unaffected basal CGRP release was identified.12 Remarkably, in the present study of 13- to 14-week-old mutant mice, which are young adult mice, neither an increase in basal nor in KCl-stimulated CGRP release in the components of the trigeminovascular system (including trigeminal ganglia) was observed, while in our in vivo experiments, CGRP-induced dural artery vasodilation was decreased. Moreover, a reduced CGRP expression was found in the trigeminal ganglion of five to eight-month-old mutant mice in an immunohistochemistry study.13 Therefore, it is tantalizing that the age of the mutant mice may be a determining factor that could reconcile the different findings of CGRP release in these studies.
An alternative explanation for the apparent discrepancy between our FHM1 R192Q mice data and the previously studies mentioned, where an increased CGRP release was observed in the trigeminal ganglion,12 may be the concentration of KCl employed in the different experiments. While Fioretti et al.12 used 35 mM KCl to stimulate CGRP release, we used 60 mM KCl based on studies showing that this concentration activates calcium channels (including the CaV2.1 channels);29 and this induces a significant increase in CGRP release without reaching the maximal effect in the trigeminal ganglion, trigeminal nucleus caudalis and dura mater,17 which are essential characteristics for our study. However, we cannot exclude that using lower concentrations, a difference in sensitivity to KCl between wild-type and mutant mice might have been unmasked.
To assess systemic vascular CGRP receptor desensitization, we performed concentration-response curves to CGRP in isolated aortas and mesenteric arteries to understand the effect of the R192Q mutation on peripheral CGRP receptor function. Interestingly, no differences in the relaxant responses were found between genotypes, so it is unlikely that the R192Q mutation affects CGRP-induced vasodilation in these vessels. Though, if desensitization of CGRP receptors occurs due to a structurally elevated CGRP release (caused by the gain-of-function of the CaV2.1 calcium channel), it may only be occurring in dural arteries. Unfortunately, it is not possible to do functional studies with a Mulvany myograph, due to the small size of those vessels (outer diameter < 30 µm).
The general vascular function of the aortas and mesenteric arteries was also investigated by performing concentration-response curves to acetylcholine, sodium nitroprusside, 5-HT and a single dose of KCl. Between genotypes, there were no differences in the acetylcholine and sodium nitroprusside responses, or the acetylcholine/sodium nitroprusside ratio, suggesting that the R192Q mutation does not influence peripheral nitric oxide (NO) endothelium-dependent and -independent pathways, which seems in line with a lack of effect on the baseline mean arterial pressure in vivo.
Instead, a decreased KCl-induced contraction in the mesenteric arteries of mutant mice was observed, which might suggest that the R192Q mutation (in the CaV2.1 calcium channel) indirectly influences other channels. This might also be the case with other voltage-dependent calcium channels, disturbing the levels of cytoplasmic [Ca2+], the activation of the Ca2+-calmodulin-dependent myosin light chain (MLC) kinase, MLC phosphorylation, and overall contraction of the smooth muscle cells.30 The latter seems to be supported by findings, although not confirmed by other research groups, that the CaV2.1 calcium channel may be present in smooth muscle cells of murine aortas, rat renal vessels and rabbit renal afferent arterioles;31,32 and may contribute to contraction by regulating intracellular calcium concentrations.32 The fact that this effect was only observed in mesenteric arteries and not in aortas suggests that the R192Q mutation in CaV2.1 channels might play a more prominent role in resistance arteries than in conductance arteries. This could be related to the channel expression levels in different vascular beds. Since the 5-HT-induced contraction was related to the KCl response in vitro, the trend of increased 5-HT-induced contraction observed in the mesenteric arteries of the R192Q mice, might rather be due to biological differences in the KCl responses, than differences in the function of the 5-HT1B receptors, which is responsible for the 5-HT-induced contraction.33
The ex vivo CGRP release experiments demonstrated in wild-type mice, that the presence of sumatriptan significantly reduced CGRP release upon stimulation in the trigeminal nucleus caudalis, dura mater and in the trigeminal ganglion. This seems in line with a finding that naratriptan and sumatriptan prevented the induction of sensitization in central trigeminovascular neurons, but not from peripheral terminals innervating the dura mater.34 However, there was also a trend of lower CGRP release in the trigeminal nucleus caudalis of wild-type mice after a second stimulation with KCl (Figure 5, top panel), which might suggest that the effect of sumatriptan observed is a result of lower release in response to the second stimulation, rather than inhibition of CGRP release by sumatriptan. This is unlikely since delta CGRP release in the sumatriptan experiments (CGRP release in the presence of sumatriptan – CGRP release in the absence of sumatriptan) were significantly different from the hypothetical value of zero (p = 0.05); which was not the case for the delta CGRP release in the repeated experiments (CGRP release after second stimulation – CGRP release after first stimulation), indicating that the lower CGRP release in the trigeminal nucleus caudalis of wild-type mice in the sumatriptan experiments is indeed an effect of sumatriptan.
Notably, in the mutant mice, sumatriptan did not show a statistically significant change in CGRP release upon stimulation on the different components of the trigeminovascular system. This might be explained by the gain-of-function effect on the CaV2.1 calcium channels that influence the modulation properties of sumatriptan, since activation of the 5-HT1 receptor modulates calcium channels to control CGRP release.35
In conclusion, our data do not support the a priori hypothesis that the FHM1 R192Q mutation increases dural artery vasodilation and CGRP release in the trigeminovascular system; instead, a decrease in dural vasodilation and no effect on CGRP release was observed, possibly involving trigeminovascular CGRP receptor desensitization. Thus, our data reinforce the findings of previous studies that the R192Q mutation does not only affect central aspects of migraine pathophysiology, but also the normal functioning of the trigeminovascular system. Moreover, the effects of the mutation on CGRP release in the trigeminovascular system may not just be an ‘on’ or ‘off’ phenomenon but rather of a modulatory nature, involving multiple mechanisms. Although it is still undefined whether the trigeminovascular effects of the R192Q mutation are the consequence of a direct neurovascular effect, a central neuronal dysfunction, or a combination of both; our results clearly indicate that the FHM1 R192Q mice display trigeminovascular abnormalities, underlining their relevance for migraine research.
Supplementary Material
Acknowledgements
We would like to thank technicians Sandra van Heiningen and Ludo Broos for breeding and genotyping of the mice, and Prof. Dr. H. Boersma, Erasmus MC, for the statistical advice.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants of the Netherlands Organization for Scientific Research (to A. MaassenVanDenBrink; Vidi grant nr. 917.11.349) and the Center of Medical System Biology established by the Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research (to A.M.J.M. van den Maagdenberg; EU-funded FP7 “EUROHEADPAIN” grant nr. 6026337).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KYC: performed the experiments, analyzed the data and drafted the manuscript; ALR: drafted, revised and approved the final manuscript; MRR: Mulvany experiments; SL: revised and approved the final manuscript; IMG: CGRP quantification; AHJD; revised and approved the final manuscript; AMJMvdM: revised and approved the final manuscript; AMvdB: supervised the experiments and data analysis, revised and approved the final manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Headache Classification Committee of the International Headache Society. The international classification of headache disorders, 3 rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 2.Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain 1994; 117(Pt 1): 199–210. [DOI] [PubMed] [Google Scholar]
- 3.Karsan N, Goadsby PJ. Calcitonin gene-related peptide and migraine. Curr Opin Neurol 2015; 28: 250–254. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari MD, Klever RR, Terwindt GM, et al. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015; 14: 65–80. [DOI] [PubMed] [Google Scholar]
- 5.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 1996; 87: 543–552. [DOI] [PubMed] [Google Scholar]
- 6.van den Maagdenberg AM, Haan J, Terwindt GM, Ferrari MD. Migraine: gene mutations and functional consequences. Curr Opin Neurol 2007; 20: 299–305. [DOI] [PubMed] [Google Scholar]
- 7.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004; 41: 701–710. [DOI] [PubMed] [Google Scholar]
- 8.Tottene A, Conti R, Fabbro A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron 2009; 61: 762–773. [DOI] [PubMed] [Google Scholar]
- 9.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest 2009; 119: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chanda ML, Tuttle AH, Baran I, et al. Behavioral evidence for photophobia and stress-related ipsilateral head pain in transgenic Cacna1a mutant mice. Pain 2013; 154: 1254–1262. [DOI] [PubMed] [Google Scholar]
- 11.Ceruti S, Villa G, Fumagalli M, et al. Calcitonin gene-related peptide-mediated enhancement of purinergic neuron/glia communication by the algogenic factor bradykinin in mouse trigeminal ganglia from wild-type and R192Q Cav2.1 Knock-in mice: implications for basic mechanisms of migraine pain. J Neurosci 2011; 31: 3638–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fioretti B, Catacuzzeno L, Sforna L, et al. Trigeminal ganglion neuron subtype-specific alterations of Ca(V)2.1 calcium current and excitability in a Cacna1a mouse model of migraine. J Physiol 2011; 589: 5879–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathew R, Andreou AP, Chami L, et al. Immunohistochemical characterization of calcitonin gene-related peptide in the trigeminal system of the familial hemiplegic migraine 1 knock-in mouse. Cephalalgia 2011; 31: 1368–1380. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Akerman S, van den Maagdenberg AM, et al. Intravital microscopy on a closed cranial window in mice: a model to study trigeminovascular mechanisms involved in migraine. Cephalalgia 2006; 26: 1294–1303. [DOI] [PubMed] [Google Scholar]
- 15.Ebersberger A, Averbeck B, Messlinger K, et al. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 from rat dura mater encephali following electrical and chemical stimulation in vitro. Neuroscience 1999; 89: 901–907. [DOI] [PubMed] [Google Scholar]
- 16.Eberhardt M, Hoffmann T, Sauer SK, et al. Calcitonin gene-related peptide release from intact isolated dorsal root and trigeminal ganglia. Neuropeptides 2008; 42: 311–317. [DOI] [PubMed] [Google Scholar]
- 17.Amrutkar DV, Ploug KB, Hay-Schmidt A, et al. mRNA expression of 5-hydroxytryptamine 1B, 1D, and 1 F receptors and their role in controlling the release of calcitonin gene-related peptide in the rat trigeminovascular system. Pain 2012; 153: 830–838. [DOI] [PubMed] [Google Scholar]
- 18.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res 1977; 41: 19–26. [DOI] [PubMed] [Google Scholar]
- 19.MaassenVanDenBrink A, Reekers M, Bax WA, et al. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation 1998; 98: 25–30. [DOI] [PubMed] [Google Scholar]
- 20.Chan KY, Gupta S, de Vries R, et al. Effects of ionotropic glutamate receptor antagonists on rat dural artery diameter in an intravital microscopy model. Br J Pharmacol 2010; 160: 1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labruijere S, van Houten EL, de Vries R, et al. Analysis of the vascular responses in a murine model of polycystic ovary syndrome. J Endocrinol 2013; 218: 205–213. [DOI] [PubMed] [Google Scholar]
- 22.Akerman S, Romero-Reyes M, Holland PR. Current and novel insights into the neurophysiology of migraine and its implications for therapeutics. Pharmacol Ther 2017; 172: 151–170. [DOI] [PubMed] [Google Scholar]
- 23.Asakura K, Kanemasa T, Minagawa K, et al. α-Eudesmol, a P/Q-type Ca2+ channel blocker, inhibits neurogenic vasodilation and extravasation following electrical stimulation of trigeminal ganglion. Brain Res 2000; 873: 94–101. [DOI] [PubMed] [Google Scholar]
- 24.Akerman S, Williamson DJ, Goadsby PJ. Voltage-dependent calcium channels are involved in neurogenic dural vasodilatation via a presynaptic transmitter release mechanism. Br J Pharmacol 2003; 140: 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hullugundi SK, Ferrari MD, van den Maagdenberg AM, et al. The mechanism of functional up-regulation of P2X3 receptors of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine type 1 (FHM-1). PLoS One 2013; 8: e60677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khennouf L, Gesslein B, Lind BL, et al. Activity-dependent calcium, oxygen, and vascular responses in a mouse model of familial hemiplegic migraine type 1. Ann Neurol 2016; 80: 219–232. [DOI] [PubMed] [Google Scholar]
- 27.Vilotti S, Vana N, Van den Maagdenberg AM, et al. Expression and function of calcitonin gene-related peptide (CGRP) receptors in trigeminal ganglia of R192Q Cacna1a knock-in mice. Neurosci Lett 2016; 620: 104–110. [DOI] [PubMed] [Google Scholar]
- 28.Petersen KA, Dyrby L, Williamson D, et al. Effect of hypotension and carbon dioxide changes in an improved genuine closed cranial window rat model. Cephalalgia 2004; 25: 23–29. [DOI] [PubMed] [Google Scholar]
- 29.Amrutkar DV, Ploug KB, Olesen J, et al. Role for voltage gated calcium channels in calcitonin gene-related peptide release in the rat trigeminovascular system. Neuroscience 2011; 172: 510–517. [DOI] [PubMed] [Google Scholar]
- 30.Ratz PH, Berg KM, Urban NH, et al. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol 2005; 288: C769–C783. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen D, Friis UG, Uhrenholt TR, et al. Coexpression of voltage-dependent calcium channels Cav1.2, 2.1 a, and 2.1 b in vascular myocytes. Hypertension 2006; 47: 735–741. [DOI] [PubMed] [Google Scholar]
- 32.Hansen PB, Jensen BL, Andreasen D, et al. Vascular smooth muscle cells express the alpha(1 A) subunit of a P-/Q-type voltage-dependent Ca(2+) Channel, and it is functionally important in renal afferent arterioles. Circ Res 2000; 87: 896–902. [DOI] [PubMed] [Google Scholar]
- 33.Villalón CM, Centurión D. Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn-Schmiedebergs Arch Pharmacol 2007; 376: 45–63. [DOI] [PubMed] [Google Scholar]
- 34.Kageneck C, Nixdorf-Bergweiler BE, Messlinger K, et al. Release of CGRP from mouse brainstem slices indicates central inhibitory effect of triptans and kynurenate. J Headache Pain 2014; 15: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Y, Richter JA, Hurley JH. Release of glutamate and CGRP from trigeminal ganglion neurons: role of calcium channels and 5-HT1 receptor signaling. Mol Pain 2008; 16: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.