Abstract
Resting-state functional magnetic resonance imaging (rs-fMRI) is used to interrogate the functional connectivity and network organization amongst brain regions. Functional connectivity is determined by measuring the extent of synchronization in the spontaneous fluctuations of blood oxygenation level dependent (BOLD) signal. Here, we review current rs-fMRI studies in headache disorders including migraine, trigeminal autonomic cephalalgias, and medication overuse headache. We discuss (1) brain network alterations that are shared amongst the different headache disorders and (2) network abnormalities distinct to each headache disorder. In order to focus the section on migraine, the headache disorder that has been most extensively studied, we chose to include articles that interrogated functional connectivity: (i) during the attack phase; (ii) in migraine patients with aura compared to migraine patients without aura; and (iii) of regions within limbic, sensory, motor, executive and default mode networks and those which participate in multisensory integration. The results of this review show that headache disorders are associated with atypical functional connectivity of regions associated with pain processing as well as atypical functional connectivity of multiple core resting state networks such as the salience, sensorimotor, executive, attention, limbic, visual, and default mode networks.
Keywords: Functional connectivity, magnetic resonance imaging, medication-overuse headache, migraine, trigeminal autonomic cephalalgias
Background on resting-state fMRI and the rationale for application in headache
Magnetic resonance imaging (MRI) allows for the non-invasive interrogation of (1) brain structure and (2) brain function. Functional magnetic resonance imaging (fMRI) is based on the blood-oxygenation-level-dependent-signal (BOLD signal) to investigate regional changes in blood oxygenation patterns using task-based or stimulus-driven designs. For example, as migraine is associated with hypersensitivities during and between attacks, a number of migraine fMRI studies have used pain stimulation paradigms to better understand how migraine-specific hypersensitivities are manifested in the brain and to interrogate whether patients with migraine respond differently to pain compared to healthy controls (HCs). Task-based fMRI techniques and structural T1-weighted imaging have yielded evidence of wide-spread abnormalities of brain structure and function, but are not ideally suited for interrogating whether abnormalities within these wide-spread regions might underlie a brain network defect. Resting-state functional connectivity magnetic resonance imaging (rs-fMRI) measures synchronicity of spontaneous fluctuations in the BOLD signal, i.e. in the absence of a task or stimulation, and provides an indirect measurement of brain connectivity.
This review aims to discuss recent results of rs-fMRI studies of headache disorders and to discuss common and unique functional connectivity (fc) patterns in migraine, trigeminal autonomic cephalalgias, and medication overuse headache (MOH).
Search criteria
We searched PubMed for English language articles that were published between 1966 and 10 July 2017. The reference lists of included articles and the authors' own files were searched for additional articles. Articles that investigated brain functional connectivity in migraine, trigeminal autonomic cephalalgias (TACs) or MOH were considered for inclusion in this review. To provide a more focused review, we only included migraine studies that investigated functional connectivity: (1) during the migraine attack; (2) differences between migraine with aura and migraine without aura; and (3) analyses of regions within limbic, sensory, motor, executive and default mode networks, and those participating in multisensory integration. Consistent with many narrative reviews, we did not document specific reasons for excluding individual papers. Publications were carefully reviewed and were selected for inclusion based on author discussion and on the basis of their relevance to the topic, originality, and the extent to which the study results were deemed to contribute to the description of brain functional connectivity within the specified areas of focus.
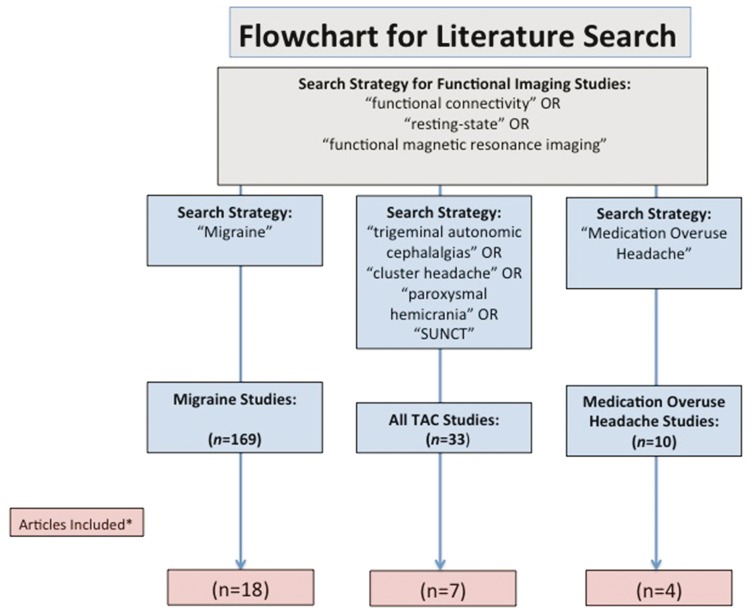
The following search terms were used for migraine: “functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “migraine”. This produced 169 articles.
Search terms for all TACs included the following:
“Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “cluster headache”. This produced 23 papers.
The search “Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “hemicrania continua” produced 2 papers.
The search “Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “paroxysmal hemicrania” produced 0 papers.
The search “Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “SUNCT” produced 4 papers.
The search “Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “trigeminal autonomic cephalalgias” produced 4 papers.
Search terms for MOH included the following:
“Functional connectivity” OR “resting state” OR “functional magnetic resonance imaging” AND “medication overuse headache”. This search produced 10 papers.
An illustrative flowchart of the search criteria is shown in Figure 1.
Figure 1.
Flowchart for literature search.
*Articles were selected for inclusion based on author discussion, originality and contribution to the topic of “brain functional connectivity.”
Migraine
Migraine is a common neurological disorder consisting of headache, sensitivities to light, sound, touch and odor, nausea and vomiting.1–3 As expected with any chronic pain disorder, functional and structural neuroimaging studies indicate that migraine is associated with changes in brain pain processing regions, regions that are often referred to as components of the ‘pain-matrix’.4–7 Herein, we discuss studies that have investigated connectivity of these pain matrix regions, but also focus on summarizing studies that yield insights that might be specific for migraine: (1) functional connectivity during migraine attacks; (2) functional connectivity in those who have migraine with aura compared to those who have migraine without aura; (3) studies investigating the functional connectivity of regions within limbic, sensory, motor, executive and default mode networks and regions that participate in multisensory integration.
Functional connectivity during migraine attacks
Four studies have used functional connectivity (rs-fc) to interrogate brain changes in migraine during the attack (ictal phase) (see Table 1). Coppola et al.8 have studied fc changes in 13 migraineurs without aura (MwoA) during migraine attacks compared to 19 HCs using a whole-brain independent component analysis (ICA). During naturally occurring migraines (not medication induced), migraineurs had less fc between the executive and dorso-ventral attention network. Furthermore, weaker executive network connectivity related to higher monthly headache frequency in patients with migraine. Less fc between cognitive and attentional networks during the attack phase are intriguing results since they might relate to the difficulties with memory and attention that are often experienced by migraineurs during attacks.9,10 Furthermore, the authors described a negative correlation between executive network functional connectivity and headache frequency indicating that less network functional connectivity might relate to higher disease burden. Whereas in HC subjects, there was a relationship between stronger fc in areas of the dorso-ventral attention system and lower bilateral thalamic fractional anisotropy measures, this relationship was absent in migraine patients potentially indicating a functional decoupling of thalamo-cortical control networks during the attack. In a recent follow-up study by Coppola et al.11 (using the same subject cohort), authors also found stronger fc between the medial prefrontal cortex and the posterior cingulate cortex and stronger fc between the medial prefrontal cortex and the insula. In migraineurs during the attack, fc strength between the medial prefrontal cortex and insula regions negatively related to perceived pain intensity. Abnormal connectivity between these large-scale networks and regions important for emotional processing of pain including the insula could be considered an adaptive response in the setting of acute pain if it allows for greater focus on the acute pain, even at the expense of impaired higher order functions. However, in the setting of a chronic pain condition like migraine, this response might be maladaptive if it leads to excessive focus on the pain experience. Of note, since the authors compared ictal migraine patients to HCs only, and not to interictal patients, it is not possible to determine if these changes are specific to the attack phase of migraine or if they are general features of migraine pathophysiology.
Table 1.
Brain functional connectivity in ictal migraine patients.
| Study | Subject cohorts | Analysis | Main finding |
|---|---|---|---|
| Ictal migraine | |||
| Coppola et al.11 | MwoA, ictal (n = 13) HC (n = 19) | ROI-based analysis of the insula and of seed regions in the default mode network | Ictal MwoA: Stronger fc between the medial prefrontal cortex and the posterior cingulate cortex. Stronger fc between the medial prefrontal cortex and the bilateral insula. Stronger fc between medial prefrontal cortex and insula related to less pain intensity during the attack. |
| Coppola et al.8 | MwoA, ictal (n = 13) HC (n = 19) | ICA executive and dorso-ventral attention network | Ictal MwoA: Weaker fc between the executive and dorso-ventral attention networks during untreated migraine attacks. Weaker executive network connectivity (z-score) related to higher headache frequency. |
| Amin et al.12 | MwoA, ictal PACAP38 induced (n = 16) MWoA, interictal (n = 16) | ROI-based analysis of seed regions in the salience network, default mode network and sensorimotor network. | Resting-state connectivity before and after PACAP38 induced migraine: Altered fc connectivity in the salience network (stronger fc to bilateral inferior frontal regions), the default mode network (less fc with right cerebellum and left frontal lobe) and the sensorimotor network (stronger fc in right premotor cortex and weaker fc in left visual cortex). |
| Hougaard et al.13 | MwA, ictal (n = 16) | ROI-based analysis of visual areas, pons, hypothalamus, and periaqueductal grey Exploratory ICA | Ictal vs. interictal fc changes in MwA: Increased fc between pons and somatosensory cortex. Increased fc between visual area V5 of symptomatic hemispheres (contralateral to aura symptoms) and middle frontal gyrus. |
HC: healthy controls; MwA: migraine with aura; MwoA: migraineurs without aura; fc: functional connectivity; ICA: independent component analysis, ROI: region of interest.
Amin et al.12 used pituitary adenylate cyclase-activating polypeptide-38 (PACAP38) to induce migraine attacks in 16 MwoA. Patients were scanned during the early migraine phase. Compared to the migraine-free phase, migraine attacks were associated with abnormal fc within the salience network (stronger fc in bilateral inferior frontal regions), sensorimotor network (stronger fc in right premotor cortex and weaker fc in left visual cortex), and within the default mode network (less fc in the right cerebellum and in the left frontal lobe), networks that are known to be involved with the cognitive, sensory, and emotional components of pain.
Hougaard et al.13 investigated 16 episodic migraine (EM) patients during spontaneous attacks of migraine with visual aura. Patients were scanned outside the aura phase (on average 8.2 h after aura onset), while experiencing headache and associated migraine symptoms, and again outside of attacks being migraine-free for at least 72 h. The authors applied both an ROI-based approach to investigate connectivity with visual and pain-related areas and an ICA-based approach to explore potential changes in other intrinsic networks. During attacks, relative to the attack-free state, fc increased between the dorsolateral pons and the ipsilateral primary somatosensory cortex corresponding to the head and face somatotopic areas and between visual area V5 and the ipsilateral middle frontal gyrus of the “symptomatic” hemispheres (i.e. hemispheres contralateral to the perceived visual aura symptoms). The ICA approach did not reveal other abnormal networks. These findings highlight the importance of the dorsolateral pons, an area referred to as a “migraine generator,”14 for the ictal pathophysiological mechanisms of migraine.
Results of these four important studies investigating functional connectivity during migraine attacks are difficult to compare. Whereas Coppola et al. used a whole-brain ICA to compare brain connectivity patterns in migraine patients during naturally occurring attacks to HC subjects, Amin et al. induced migraines using PACAP38 and interrogated fc in patients during the early stages of a migraine attack compared to the pain-free state using a seed-based analysis, and Hougaard et al. specifically studied migraine patients with aura during the headache phase of spontaneous attacks using both ICA and seed-based analyses. Despite these differences in study designs, the studies indicate marked abnormalities during the ictal phase in networks relevant for mediating cognitive, attentional, somatosensory and emotional components of pain (see Figure 2).
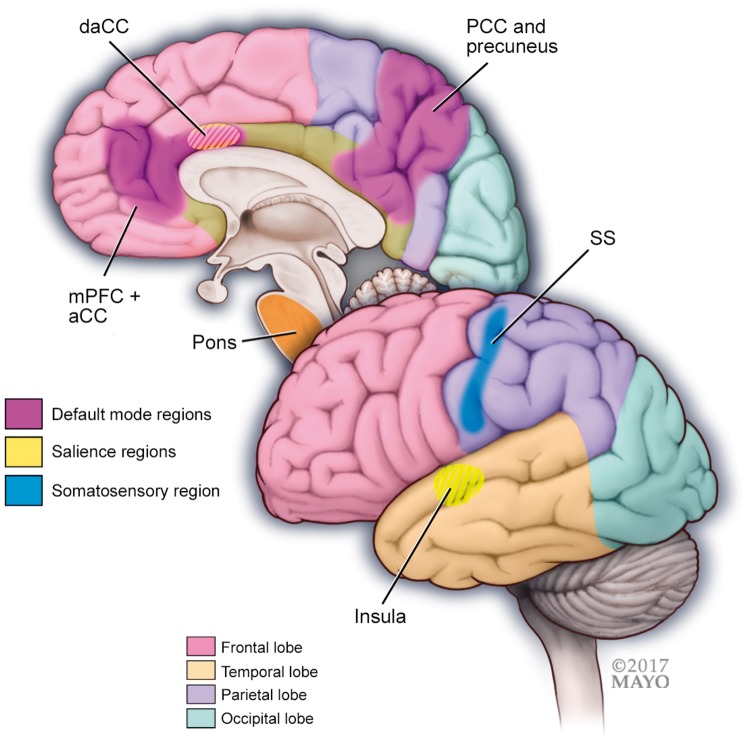
Figure 2.
A schematic illustration of regions and functional networks (default mode, salience, sensory) where studies have shown altered functional connectivity in migraine patients during attacks compared to between attacks. Important regions of functional networks that are altered in migraineurs during the attack compared to between attacks include the following:
Default mode network regions: medial prefrontal cortex (mPFC) and anterior cingulate cortex (aCC), precuneus and posterior cingulate cortex (pCC).
Salience network regions: insula and (dorsal) anterior cingulate.
Sensory network region: somatosensory cortex (SS).
Migraineurs during the attack have stronger functional connectivity between the pons and the somatosensory cortex (SS).
Interictal migraine with aura
Approximately 1/3 to 1/4 of patients with migraine experience attacks that are accompanied by aura symptoms.15,16 The majority of migraine auras consist of visual symptoms such as flashing, moving or flickering lights.17 Visual aura symptoms are attributed to cortical spreading depression in the occipital cortex.18 Electrophysiology studies using electroencephalography (EEG) and event-related fMRI have found visual cortex hyper-responsivity as well as increased activation to visual stimuli in MwA.19–21 Several functional imaging studies have compared brain connectivity differences between interictal MwA and interictal MwoA (see Table 2). Niddam et al.22 explored the fc of two regions that might assert top-down influences on information conveyed to the visual cortex23,24; the anterior insula and middle frontal gyrus. Relative to HCs, MwA and MwoA both had stronger fc between the middle frontal gyrus and the right temporal region. Their results further indicated that MwA compared to MwoA have weaker fc between the anterior insula and the V3A in the visual cortex. A correlation analysis indicated that weaker fc in MwA was inversely related to migraine severity. These results indicate that MwA and MwoA share common network abnormalities between middle frontal and the right temporal regions and that MwA (relative to MwoA) have weaker fc between the anterior insula, a key region of the limbic system, and the visual cortex, perhaps representing network abnormalities distinct to aura.
Table 2.
Brain functional connectivity in interictal migraine patients with aura.
| Study | Subject cohorts | Analysis | Main finding |
|---|---|---|---|
| Migraine with aura | |||
| Farago et al.a 19 | MwA (n = 18) MwoA (n = 35) HC (n = 32) | ICA Frequency spectrum analysis | MwA compared to HC: No differences between groups in the frequency amplitudes of the default mode, visual, and attention networks. MwA compared to MwoA: Higher frequency amplitudes in the default mode, visual and attention networks in MwA, specifically in frontal cortex, cingulate, inferior parietal lobule and the cerebellum. |
| Niddam et al.22 | EMwA (n = 26) EMwoA (n = 26) HC (n = 26) | ROI-based analysis of anterior insula and middle frontal gyrus | MwA and MwoA vs. HC: Stronger fc between middle frontal gyrus and the right temporal region in MwA and MwoA relative to HC. MwA vs. MwoA: Weaker fc between anterior insula and occipital cortex (V3A) in MwA. Weaker connectivity in MwA correlated with headache severity. |
| Tedeschi et al.25 | MwA (n = 20) MwoA (n = 20) HC (n = 20) | ICA | MwA compared to MwoA and HC: Stronger fc in visual network (centering in the lingual gyrus) in MwA. Stronger fc was not related to migraine severity. |
| Hougaard et al.28 | MwA (n = 40) HC (n = 40) | ROI-based (Visual areas, periaqueductal grey, amygdala) ICA | No differences between groups for any of the investigated networks. |
Note: A summary of published studies investigating the brain functional connectivity in interictal migraine patients with aura compared to migraineurs without aura and to healthy controls.
HC: healthy controls; MwA: migraine with aura; MwoA: migraineurs without aura; fc: functional connectivity; ICA: independent component analysis; ROI: region of interest.
Studies that interrogated multiple networks, or patient sub-types.
Tedeschi et al.25 interrogated visual cortex fc patterns of MwA compared to MwoA during the interictal phase. Compared to MwoA, MwA had stronger fc within the visual network centering around the lingual gyrus, an extrastriate region important for visual-spatial processing.26 Results did not show a correlation between clinical parameters and visual cortex connectivity patterns in MwA, which lead the authors to postulate that aberrant fc within extrastriate regions could indicate a ‘brain biomarker’ for MwA.
Using a frequency spectrum ICA, a post-processing method for analyzing the frequency and amplitude of the rs-BOLD signal,27 Farago et al.19 found higher amplitudes of the resting state BOLD fluctuations in the lateral visual network frequencies in MwA compared to MwoA and to HC, specifically in regions including the cingulate cortex, superior parietal lobule, cerebellum and frontal regions.
Hougaard et al.28 compared 40 MwA patients to 40 age and sex-matched HCs. The authors applied a seed-based analysis of 27 different seed locations in total, including cortical visual areas, amygdala, and periaqueductal grey. In addition, ICA was used to study intrinsic brain networks. The authors found no differences, or even trends towards differences, between migraine patients and controls for any of these networks. Specifically, previously reported abnormal fc involving the periaqueductal grey29 and the amygdala30 could not be reproduced.
In summary, these results suggest possible fc alterations in MwA compared to MwoA and HC in the visual cortex as well as in wide-spread regions involved in visual processing (including the middle frontal regions, insula, and the anterior cingulate, superior parietal lobule and the cerebellum, see Figure 3). However, the study by Hougaard et al., showing no fc abnormalities in a relatively large sample of MwA patients, questions these findings and emphasizes the general need for reproducing rs-fMRI results before firm conclusions can be drawn.
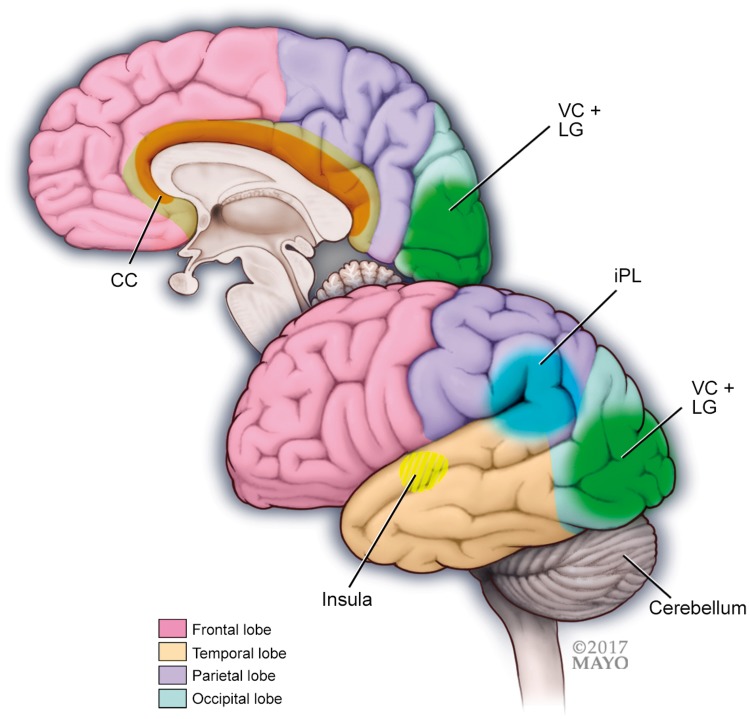
Figure 3.
Interictal migraine patients with aura compared to interictal migraine patients without aura have stronger functional connectivity within visual cortex regions (VC) including the lingual gyrus (LG) and stronger functional connectivity of occipital regions with the anterior insula.
Migraineurs with aura have higher amplitudes of resting-state fluctuations in frontal areas, cingulate cortex (CC), inferior parietal lobule (iPL) and the cerebellum.
Limbic connectivity in migraine
Hadjikhani et al.30 compared fc patterns of the amygdala in MwA and MwoA to patients with trigeminal neuralgia, to patients with carpal tunnel syndrome and to HCs. Compared to HCs and to patients with other chronic pain conditions, migraineurs had stronger fc between the amygdala and the thalamus, anterior insula and the secondary somatosensory area, potentially indicating that stronger fc patterns between the amygdala with other viscero-sensory areas might be uniquely manifested in migraine. Similar results were reported by another study that compared fc patterns of the bilateral amygdala in chronic and episodic migraineurs to HCs.31 Relative to HCs, episodic migraineurs had stronger fc of the left amygdala with the left middle cingulate and left precuneus and chronic migraineurs had stronger fc of the left and right amygdala with widespread regions in inferior temporal lobe, prefrontal gyrus, cingulate cortex, and pre-and postcentral gyri compared to episodic migraineurs. Compared to HCs, chronic migraineurs had weaker functional connectivity between the right amygdala and occipital regions. Although the roles of the left and right amygdala in migraine chronification will need to be further investigated, there is some evidence that supports the potential lateralization of right versus left amygdala function.32,33 For example, stronger fc between the left amygdala and prefrontal regions was found in healthy females with higher risk for developing anxiety disorders, whereas stronger right amygdala fc with limbic and prefrontal regions was found to be important for regulating negative affect.34
Results of studies investigating limbic region connectivity show stronger amygdala fc patterns in patients with migraine and a strengthening of fc patterns between the amygdala and regions which play important roles in the pain experience (sensory discrimination, pain modulation, multisensory integration) (see Table 3).
Table 3.
Connectivity of limbic regions, sensory and motor regions, executive networks, and default mode networks in interictal migraine patients.
| Study | Subject cohorts | Analysis | Main finding |
|---|---|---|---|
| Limbic connectivity | |||
| Chen et al.31 | CM (n = 16) EM (n = 18) HC (n = 18) | ROI-based analysis of seed regions in bilateral amygdala | EM vs. HC: Stronger left amygdala fc in EM to the left middle cingulate and the left precuneus. CM vs. EM: Stronger bilateral amygdala connectivity in CM to widespread regions including the inferior temporal, prefrontal, cingulate, and pre-and postcentral regions. CM vs. HC: Weaker right amygdala connectivity in CM to occipital regions. |
| Hadjikhani et al.30 | Migraine (n = 22) MwoA (n = 11) MwA (n = 11) compared to: HC (n = 20) trigeminal neuralgia (n = 9) compared to: HC (n = 9) carpal tunnel syndrome (n = 11) compared to: HC (n = 11) | ROI-based analysis of seed regions in bilateral amygdala | Migraineurs with and without aura compared to healthy controls and compared to patients with trigeminal neuralgia and to patients with carpal tunnel syndrome: Stronger fc between amygdala and viscero- sensory areas (thalamus, anterior insula and secondary somatosensory cortex). |
| Sensory-motor connectivity | |||
| Hodkinson et al.35 | EM (n = 40) MwoA: n = 24 MwA: n = 16 HC (n = 40) | ROI-based analysis of six regions within the primary sensory networks: vision, audition and somato-sensation | EM vs. HC: Intact fc within regions of the primary sensory areas. Impaired long-range connections of primary sensory areas to default mode and salience network. |
| Zhang et al.36 | MwoA (n = 30) HC (n = 31) | Regional homogeneity, (ReHo), amplitudes of low-frequency fluctuations, (ALFF), degree centrality (DC) Followed by ROI-based analysis of sensorimotor network | MwoA vs. HC: Decreased network spontaneous activity as measured in the right primary motor cortex and the bilateral primary sensory areas (SI) in MwoA and less fc between bilateral SI and other regions important for pain processing and pain discrimination, including; the temporal lobe, superior and inferior parietal lobes, anterior cingulate, insular cortex and brainstem regions (pons, cerebellum). Negative correlation between network spontaneous activity and measures of headache disability |
| Executive networks | |||
| Russo et al.41 | MwoA (n = 14) HC (n = 14) | ICA executive network | MwoA vs. HC: In the absence of executive function deficits, MwoA had weaker fc within the fronto-parietal network. (middle frontal gyrus and dorsal anterior cingulate cortex) Weaker fc in the middle frontal gyrus correlated with higher pain intensity in MwoA |
| Tessitore et al.42 | MwoA (n = 20) MwA (n = 20) HC (n = 20) | ICA executive network | MwA and MwoA compared to HC: Compared to HC, MwA and MwoA had weaker fc of regions within the executive network (middle frontal gyrus and dorsal anterior cingulate cortex). There were no differences in executive function between MwA, MwoA and HC. MwoA compared to MwA: No differences in executive network connectivity between MwA and MwoA |
| Default mode network | |||
| Farago et al. a 19 | MwoA (n = 35) HC (n = 32) | ICA Frequency spectrum analysis | MwoA vs HC: Lower frequency fluctuations in MwoA in the default mode network |
| Zhang et al.48 | MwoA (n = 22) HC (n = 22) | Regional homogeneity, (ReHo), amplitudes of low-frequency fluctuations, (ALFF), degree centrality (DC) Followed by ROI-based analysis of default mode network | MwoA vs. HC: More fc in left precuneus/posterior cingulate cortex within the default mode network. Increased regional homogeneity in the bilateral precuneus/ posterior cingulate cortex. Decreased fc of the precuneus/ posterior cingulate cortex with brain regions outside of the default mode network. Negative correlation between network spontaneous activity and measures of headache disability. |
| Tessitore et al.44 | Episodic MwoA (n = 20) HC (n = 20) | ICA analysis of the default mode network | EMwoA vs. HC: Less fc of in prefrontal and temporal regions of the default mode network. Less fc in regions of the default mode network was not related to structural abnormalities or to clinical parameters. |
| Xue et al.47 a | MwoA (n = 23) HC (n = 23) | ICA analysis of the default mode network | MwoA vs. HC: No alteration within the default mode network system. Greater fc between the default mode network, executive network, and the salience network (anterior insula). Greater fc amongst these networks correlated with duration of migraine. |
| Yu et al.46 | MwoA (n = 26) HC (n = 26) | ROI analysis and regional homogeneity (Re-Ho) | MwoA vs. HC: Less default mode network connectivity (and amplitude of low-frequency fluctuations) in regions including the anterior cingulate, prefrontal cortex, orbitofrontal cortex and the thalamus. Negative correlation between regional homogeneity values in the anterior cingulate and prefrontal cortex with disease duration in MwoA. |
Note: A summary of published studies investigating the brain functional connectivity of limbic regions, sensory and motor regions, executive networks, and default mode networks in interictal migraineurs compared to healthy controls.
HC: healthy controls; CM: chronic migraineurs; EM: episodic migraineurs; MwA: migraine with aura; MwoA: migraineurs without aura; fc: functional connectivity; ROI: region of interest.
Studies that interrogated multiple networks, or patient sub-types.
Sensory and motor networks and multi-sensory integration in migraine
Hodkinson et al.35 investigated the fc of several key sensory regions associated with vision, audition, and somatosensation. Although there was not a difference between the fc amongst regions of the primary sensory areas, episodic migraineurs had altered long-range functional connections to higher order networks such as the default and the salience network. The authors postulated that weaker connectivity to long-range networks could reflect difficulties integrating multi-sensory information.
Zhang et al.36 found less sensorimotor network spontaneous activity, a technique for evaluating time-series synchronizations of neighboring voxels,27 in primary motor and primary sensory areas in MwoA compared to HC and less fc between primary sensory areas and regions relevant for pain processing and pain discrimination such as the temporal lobe, superior and inferior parietal lobes, anterior cingulate cortex, insular cortex and brainstem regions. The authors also reported a negative correlation between network spontaneous activity and measures of headache disability, indicating that weaker network activity related to more headache-specific disability.
Results of these two studies (see Table 3) indicate weaker network activity within primary sensorimotor regions as well as weaker long-range network connectivity to regions important for pain perception, pain modulation and multi-sensory integration. Results of these studies provide further evidence that might help explain some of the neural underpinnings involved with migraine-specific hypersensitivies to light, sound, touch and odor and which might underlie aberrant network processes of regions involved with multi-sensory integration.
Executive network connectivity in migraine
Although cognitive difficulties during migraine attacks are frequently reported by migraineurs, studies assessing cognitive function in migraineurs have yielded conflicting results.37–40 The executive network is known to underlie high-level cognitive processes. The interrogation of this network provides an indirect way to study cognitive integrity on a brain network level. Russo et al. 41 found that MwoA, who did not demonstrate impairments on neuropsychological tests measuring executive function, had weaker fc within regions of the executive network (middle frontal gyrus and dorsal anterior cingulate) relative to HCs. Additionally, weaker fc in the executive network negatively correlated with pain intensity in MwoA. Results of a follow-up study by the same group using larger sample sizes42 showed that weaker executive network connectivity was also present in MwA relative to HC and there was not a difference between executive function network connectivity between MwA and MwoA.
Although further studies using larger sample sizes are needed (both studies included ≤ 20 migraine patients; see Table 3), these preliminary results showing weaker connectivity of regions within the executive network in MwA and MwoA relative to HCs are intriguing and might indicate that measures of rs-fc could be more sensitive in detecting changes underlying cognition than neuropsychological measures.
Default mode network connectivity in migraine
The default mode network, the most commonly interrogated rs network, is important for interoception and self-monitoring and may also have a role in cognitive, attentional and emotional processes.43 Several studies have investigated resting state default mode network alterations in interictal migraine patients relative to HCs (see Table 3). Tessitore et al.44 investigated fc in the default mode network in 20 EM patients and 20 HCs. Authors found less fc in prefrontal and temporal regions of the default mode network. Changes in fc in default mode network regions were not related to brain structural changes and did not relate to clinical or neuropsychological measures. Similar findings were reported by two other studies; Farago et al.45 reported lower frequency fluctuations in the default mode network in 35 MwoA compared to 35 HCs and Yu et al.46 reported less fc (and amplitudes of low-frequency fluctuations) in regions of the default mode network in 26 MwoA compared to 26 HCs. Additionally, Yu et al. reported a negative correlation between regional homogeneity in the anterior cingulate and the prefrontal cortex regions of the default mode network and disease duration.
Xue et al.47 found no differences in functional network connectivity within the default mode system in 23 MwoA compared to 23 HCs. However, the authors reported that fc between the insula, a key region for pain, and regions of the default mode and executive networks correlated with duration of migraine.
Zhang et al.48 reported increased fc and regional homogeneity between the precuneus and the posterior cingulate cortex regions of the default mode network in 22 migraine patients without aura compared to 22 HCs. Furthermore, less fc was found in the precuneus and the posterior cingulate cortex with areas outside of the default mode network, which are important for pain processing, including the somatosensory and somatomotor cortex, prefrontal regions, and superior and inferior parietal regions. The precuneus and posterior cingulate are important hub areas of the default mode network but also key regions important for information transfer and multisensory integration. Increased fc in the precuneus and posterior cingulate correlated with higher headache impact test (HIT-6) scores, potentially indicating a relationship between fc in these areas and headache disability.
The majority of studies reported less functional connectivity or lower frequency fluctuations within regions of the default mode network.19,44,46 Several studies also reported altered fc between regions of the default mode network and regions of other functional networks.47,48 This between-network dysfunction could indicate a disruption of the interplay of functional networks in migraineurs between attacks. Lastly, the relationships observed between disrupted between-network activity and disease parameters, such as disease duration and headache disability could indicate that between-network dysfunctions are modulated by the migraine disease process.
TACs
The TACs are a group of primary headache disorders that manifest with cranial parasympathetic autonomic signs and symptoms ipsilateral to the headache. The TACs include cluster headache, hemicrania continua, paroxysmal hemicrania, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT), and short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA). The TACs have overlapping pathophysiology that includes activation of the posterior hypothalamus and the trigeminal autonomic reflex during headache episodes. However, the TACs differ in regards to the frequency and duration of headache attacks and their responsiveness to individual therapies.
Functional imaging studies investigating brain activation patterns during attacks of cluster headache, hemicrania continua and SUNCT have demonstrated activation in the posterior hypothalamus in addition to regions of the “pain matrix” that commonly activate during headache and non-headache pain.49–55 The posterior hypothalamus might be responsible for several key features of the TACs. For example, the circadian and circannual temporal rhythmicity by which some of the TACs occur implicates the suprachiasmatic nucleus of the hypothalamus, the so-called “autonomous circadian clock” of the brain.56 Stimulation of the posterior hypothalamus results in restlessness and a desire to move, symptoms that are common during cluster headache attacks.57 Further implicating the hypothalamus in the pathophysiology of the TACs, deep brain stimulation in the region of the posterior hypothalamus is an effective treatment for some patients with otherwise treatment refractory cluster headache, SUNCT, and paroxysmal hemicrania.58
Functional connectivity of the hypothalamus, pain-matrix regions, and core resting networks has been investigated in patients who have cluster headache using resting-state analyses. However, there is a paucity of studies investigating brain connectivity in patients with hemicrania continua, SUNCT, and SUNA. Functional connectivity studies have compared episodic cluster headache patients to HCs, episodic cluster headache patients who are in-bout vs. when they are out-of-bout (i.e. during the time period in which a person is having episodic attacks of cluster headache vs. during the period when they are in temporary remission), and episodic cluster headache patients during spontaneous cluster attacks vs. when they are in-bout but between attacks. Independent components analyses and region-of-interest analytical approaches have both been used. Table 4 summarizes these functional connectivity studies.
Table 4.
Brain functional connectivity in patients who have cluster headache.
| Study | Subject cohorts | Analysis | Main finding |
|---|---|---|---|
| Chou et al.59 | Episodic CH (n = 17) HC (n = 18) | ICA | CH in-bout vs. HC and CH out-of-bout vs. HC: Connectivity differences in temporal, frontal, salience, default mode, somatosensory, dorsal attention, and visual networks. CH in-bout vs. out-of-bout: Connectivity differences in frontal and dorsal attention networks |
| Farago et al.45 | Episodic CH (n = 17) HC (n = 26) | ICA | CH out-of-bout vs. HC: Connectivity differences in attention network and cerebellar network |
| Qiu et al.60 | Episodic CH (n = 21) HC (n = 21) | ICA | CH in-bout, inter-ictal vs. HC: Decreased resting-state hypothalamus to salience network co-activation |
| Yang et al.64 | Episodic CH (n = 18) HC (n = 19) | ROI (hypothalamus) | CH in-bout vs. out-of-bout: Decreased hypothalamic connectivity with medial frontal gyrus, middle frontal gyrus, inferior temporal gyrus, precuneus, and cerebellum. CH in-bout vs. HC and CH out-of-bout vs. HC: Altered hypothalamic connectivity with medial frontal gyrus, middle frontal gyrus, cuneus, inferior semilunar lobule, and inferior temporal gyrus. |
| Qiu et al.61 | Episodic CH (n = 12) HC (n = 12) | ROI (hypothalamus) | CH ictal vs. CH in-bout, inter-ictal: Increased connectivity hypothalamus with numerous pain matrix regions including anterior cingulate cortex, posterior cingulate cortex, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule, parahippocampal gyrus, and amygdala. CH in-bout, inter-ictal vs. HC: Stronger connectivity hypothalamus with inferior frontal gyrus, superior and middle temporal gyri, temporal pole, insula, parahippocampal gyrus, and uncus. Weaker connectivity hypothalamus with precuneus, inferior parietal lobule, and occipital lobe. |
| Qiu et al.62 | Episodic CH (n = 12) HC (n = 12) | Regional homogeneity | CH ictal vs. CH in-bout, inter-ictal: Increased regional homogeneity in posterior cingulate cortex and ventromedial prefrontal cortex. Decreased regional homogeneity in middle frontal gyrus, and parahippocampal gyrus. CH in-bout, inter-ictal vs. HC: Stronger regional homogeneity in anterior cingulate cortex, gyrus rectus, and orbital gyrus. Weaker regional homogeneity in middle prefrontal cortex, posterior cingulate cortex, insula, and dorsolateral prefrontal cortex. |
| Rocca et al.63 | Episodic CH (n = 13) HC (n = 15) | ICA ROI (hypothalamus, thalamus) | CH out-of-bout vs. HC: Connectivity differences in sensorimotor network and primary visual network. Increased connectivity of hypothalamus with anterior cingulate cortex, secondary sensorimotor cortex, primary visual cortex, middle occipital gyrus, thalamus, and insula. Increased connectivity of thalamus with primary sensorimotor cortex, supplementary motor area, and anterior cingulate cortex. |
Note: This table summarizes the methods and findings from published studies that have investigated brain functional connectivity in individuals with cluster headache.
CH: cluster headache; HC: healthy control; ICA: independent components analysis; ROI: region of interest.
Cluster vs. HC
The majority of functional connectivity studies have compared individuals with cluster headache to HCs.45,59–64 Independent components analyses including a total of 68 individuals with cluster headache and 80 HCs from four different published studies have identified atypical connectivity in numerous resting state networks amongst those with cluster headache: salience, default mode, somatosensory, attention, visual, temporal, frontal, and cerebellar.45,59,63 All of these studies were performed when individuals with cluster headache were headache free, but some were done during the in-bout period and others during the out-of-bout period. Supporting the notion that the atypical connectivity was actually associated with cluster headache, these studies demonstrated correlations between the extent of atypical connectivity with duration that an individual had cluster headache and number of cumulative headache days.45,59,63 Two studies including a total of 30 individuals with cluster headache and 31 HCs performed region-of-interest functional connectivity analyses of the hypothalamus.61,64 In these studies, compared to HCs, individuals with cluster headache were found to have atypical hypothalamic connectivity with inferior, medial and middle frontal gyri, superior, middle, and inferior temporal gyri, temporal pole, inferior parietal lobule, insula, parahippocampal gyrus, fusiform gyrus, inferior semilunar lobule, occipital lobe, uncus, precuneus, and cuneus. There were correlations between the magnitude of atypical connectivity with the frequency of bouts per year.64 Finally, a regional homogeneity study found that individuals with cluster headache have atypical regional homogeneity in anterior cingulate cortex, posterior cingulate cortex, insula, middle and dorsolateral prefrontal cortices, gyrus rectus, and orbital gyrus.62
Cluster in-bout vs. out-of-bout
Two studies, including a total of 35 individuals with episodic cluster headache, have investigated functional connectivity differences during a cluster period (i.e. in-bout) vs. between cluster periods (i.e. out-of-bout).59,64 The independent components analysis demonstrated connectivity differences in frontal and dorsal attention networks and a correlation between functional connectivity of the frontal network with disease duration.59 A hypothalamic region-of-interest analysis found differences in functional connectivity in-bout vs. out-of-bout with medial frontal gyrus, middle frontal gyrus, inferior temporal gyrus, precuneus and cerebellum.64 Frequency of cluster bouts per year was correlated with connectivity strength of the hypothalamus with the cerebellum.
Cluster during spontaneous attack (i.e. ictal) vs. in-bout but between attacks (i.e. interictal)
Two studies have compared functional connectivity during cluster attacks vs. between attacks.61,62 In a hypothalamic region-of-interest study of 12 individuals with episodic cluster headache, during a cluster attack there was increased connectivity to pain matrix regions such as the anterior cingulate cortex, posterior cingulate cortex, superior frontal gyrus, middle frontal gyrus, inferior frontal gyrus, superior temporal gyrus, inferior parietal lobule, parahippocampal gyrus, and amygdala.61 In a regional homogeneity analysis of 12 episodic cluster subjects, during a cluster attack there was increased regional homogeneity of posterior cingulate cortex and ventromedial prefrontal cortex and decreased regional homogeneity of middle frontal gyrus and parahippocampal gyrus.62
In summary, functional connectivity studies of cluster headache have further implicated the hypothalamus as a region that participates in cluster headache pathophysiology (see Figure 4). As is expected with any headache disorder, several regions of the pain-matrix have also been shown to have atypical functional connectivity. However, functional connectivity aberrations associated with cluster headache reach beyond the hypothalamus, pain-matrix regions, and expected networks such as the somatosensory and salience networks. Studies have demonstrated that cluster headache is also associated with atypical functional connectivity within networks such as the default mode, attention, and visual networks. Individuals with cluster headache have altered functional connectivity compared to HCs, and functional connectivity differs when individuals are in-bout vs. out-of-bout, and during cluster headache attacks vs. between attacks. Although it could be hypothesized that the extent of functional connectivity “abnormalities” would increase as the cluster patient moves from the out-of-bout period, to the in-bout but inter-ictal period, to the within attack phase, there are currently inadequate data to confirm or refute this hypothesis. There are correlations between the frequency of cluster attacks and disease duration with the extent of atypical connectivity, strengthening an argument that the atypical connectivity is in fact related to having cluster headache and suggesting that there is a cumulative effect of cluster attacks on altering brain functional connectivity.
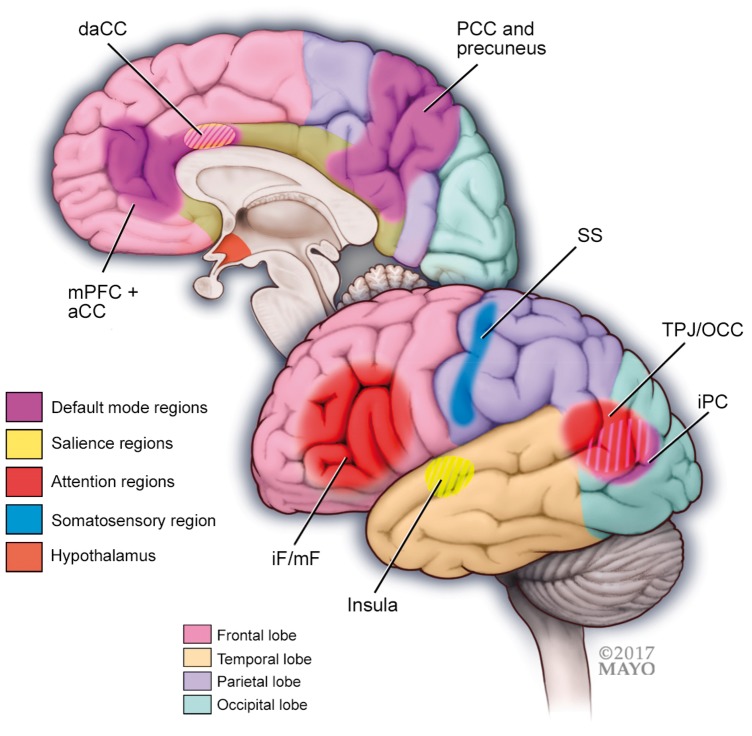
Figure 4.
A schematic illustration of regions and functional networks (default mode, salience, sensory, and attention) where studies have shown altered functional connectivity in patients with cluster headache compared to healthy controls. In cluster headache, the hypothalamus has shown abnormal functional connectivity to regions of the default mode, salience, sensory and attention networks. Important regions of functional networks that are altered in cluster headache include the following:
Default mode network regions: medial prefrontal cortex (mPFC) and anterior cingulate cortex (aCC), precuneus and posterior cingulate cortex (pCC) and the inferior parietal cortex (iPC).
Salience network regions: insula and (dorsal) anterior cingulate.
Sensory network region: somatosensory cortex (SS).
Attention network regions: inferior frontal (iF) and middle frontal (mF) cortex, and the temporo-parietal junction (TPJ) and occipital cortex (OCC).
Futures studies are needed that compare functional connectivity in cluster headache patients to individuals with other headache types to determine if certain findings are specific to cluster headache or if they are all shared by other headache types. For example, although the hypothalamus is strongly implicated in the pathophysiology of the TACs, it is also likely to be involved in migraine.65,66 It is unknown if hypothalamic connectivity in the TACs is similar or different from connectivity in migraine. Future studies should investigate connectivity in individuals with chronic cluster as well as the other TACs. Since there can be a fair amount of overlap in clinical symptoms between the TACs, it would be useful to determine if functional connectivity patterns differ between individuals with different TACs. If so, it is possible that classification models developed from functional connectivity data could be used as diagnostic aids in the circumstances when the diagnosis is especially challenging via clinical grounds.
MOH
MOH is a worsening of a pre-existing headache disorder due to excessive intake of medication for the acute treatment of headache.1 Most MOH patients have an underlying primary headache in the form of migraine or tension-type headache (TTH). Overall, it is estimated that MOH affects between 1% and 2% of the general population and at least 50% of chronic headache patients have MOH.67
The mechanisms underlying headache aggravation as a consequence of medication overuse are largely unknown. Clinical studies of pain perception in MOH patients indicate that central sensitization to nociceptive input is a key feature of this disorder.68 In further support of a central mechanism, several neuroimaging studies have reported alterations of brain structure and function in patients with medication overuse.69
MRI studies of brain structure using voxel-based morphometry in MOH demonstrated increased gray matter volume in pain-related areas such as the peri-aqueductal gray (PAG)70 and decreased volume in orbitofrontal cortex, an area that is also involved in addictive behavior.70,71 The role of the orbitofrontal cortex is further substantiated by a PET study in patients with MOH showing decreased glucose metabolism in this area, which persisted following medication withdrawal.72 Functional MRI studies reported attenuated responses of MOH patients to painful stimulation, which normalized following discontinuation of the overused medication.73,74 So far, however, only a few rs-MRI studies have been carried out in MOH patients. These studies are summarized in Table 5.
Table 5.
Brain functional connectivity in patients who have medication-overuse headache.
| Study | Subject cohort | Analysis | Main finding |
|---|---|---|---|
| Chanraud et al.75 | MOH (n = 9) EM (n = 15) HC (n = 17) | Seed-based (left precuneus) | Compared to EM and HC: Connectivity weaker between precuneus and frontal cortical areas in MOH. Stronger connectivity with right hippocampus (positively correlated with medication use). |
| Torta et al.76 | HA w MO (n = 15) HA w/o MO (n = 15) | Seed-based (nucleus accumbens, dorsal caudal and dorsal rostral putamen). Classification analysis. | Highest discriminative power for MOH: nucleus accumbens with insula, dorsolateral prefrontal cortex, midcingulate cortex, precuneus, secondary sensorimotor cortex and thalamus; dorsal rostral putamen with sensorimotor, premotor, anterior insular and midcingulate cortices. |
| Chen et al.77 | MOH (n = 37) EM (n = 18) HC (n = 32) | Functional connectivity density followed by seed-based analysis | Weaker functional connectivity density in MOH compared to EM in the right caudate and left insula. Weaker connectivity between these areas and left frontal inferior gyrus. |
| Michels et al.78 | MOH (n = 12) MYO (n = 11) HC (n = 16) | ICA and seed-based (peri-aqueductal gray) | Stronger connectivity in salience network (MOH > HC and MOH > MYO) and in inferior temporal network (MOH > HC and MOH > MYO). Weaker connectivity in frontoparietal network (MOH < HC). Stronger functional connectivity (MOH > HC) PAG with inferior frontal gyrus, middle frontal gyrus, cerebellum and stronger functional connectivity (MOH > MYO) of PAG with postcentral gyrus, pre- central gyrus, anterior cingulate cortex, temporo-parietal junction. Weaker functional connectivity (MOH < HC) of PAG with parahippocampal gyrus, precuneus, lingual gyrus, and fusiform gyrus. |
Note: Summary of methods and findings from published studies that have investigated brain functional connectivity in individuals with medication-overuse headache. MOH: medication-overuse headache; EM: episodic migraine; HC: healthy controls; HA: headache; MO: medication overuse; MYO: myofascial pain.
One study investigated patients with MOH and migraine compared to patients with EM, and HCs.75 The authors used a seed-based approach with the left precuneus as the region of interest to specifically study alterations of the default mode network. Compared to HCs, MOH and EM patients exhibited several regions that had either stronger or weaker connectivity with the precuneus. Interestingly, connectivity with the right hippocampus was stronger in MOH patients compared to both other groups. Of notice, MOH patients had higher self-rated medication-dependence, depression, anxiety, and pain-catastrophizing levels than episodic migraineurs, adding a potential risk of confounding. Precuneus-hippocampus connectivity was positively correlated with medication intake, suggesting that the findings could reflect a pharmaceutical effect.
Another study76 compared resting state connectivity of MOH patients to other patients with similar headache frequency, but without medication overuse. While this is a rational design that is useful for uncovering mechanisms specific for MOH, the study is somewhat limited by a lack of specification of the underlying headache disorders in the MOH and non-MOH group and further that 6/15 of patients in the MOH group had less than 15 headache days/month, thus not fulfilling the ICHD criteria for MOH.1 The authors investigated connectivity with the nucleus accumbens and the dorsal caudal and dorsal rostral putamen. The resulting connectivity maps served as input for a classification analysis, which could distinguish between the two groups of patients with 75% accuracy (based on nucleus accumbens) and 66% (dorsal rostral putamen). The authors suggested that these findings represent abnormalities of brain motivational circuits including the so-called reward system of the brain, which may relate to the addictive behavior in MOH.
A study using a data-driven approach known as functional connectivity density (FCD)77 investigated MOH patients, primarily diagnosed with migraine, compared to groups of EM patients and HC. The authors found FCD differences between all three groups, but no overlapping differences between MOH vs. EM and MOH vs. HC. Most significant findings were reduced FCD in MOH compared to EM in the right caudate and left insula. Subsequent seed-based analysis based on these areas showed decreased connectivity to the left frontal inferior gyrus from both areas.
To separate specific intrinsic connectivity changes of MOH to those of other chronic pain conditions, one study included patients with chronic myofascial pain (MYO) as well as MOH patients and HCs.78 While MOH patients had a longer disease duration, pain intensity, anxiety and depression ratings did not differ between the two patient groups. The authors used ICA and a seed-based approach with the mid-PAG region as the seed region, motivated by the observation that the PAG shows reversible structural alterations in MOH.70 Stronger functional connectivity was reported in the salience network (MOH>HC and MOH>MYO) and inferior temporal network (MOH > HC, MOH>MYO). In contrast, significantly weaker connectivity (MOH<HC and MYO<HC) was seen in the bilateral frontoparietal network (including the PAG) in both patient groups. The authors reported several areas that had either stronger or weaker connectivity with the PAG when comparing the three groups, but no areas for which MOH was consistently different from both MYO patients and HC.
In summary, resting state functional MRI studies in MOH support the notion of CNS mechanisms as key to the understanding of the pathophysiology of this disorder as also demonstrated through other neuroimaging modalities69 and clinical studies of pain thresholds.68 Current studies indicate that the mechanisms could prove to be similar to those observed in other disorders of addictive behavior including abnormal function of the brain reward system, see Figure 5. Altered brain functions in MOH likely also include pain-processing networks reflecting central sensitization. So far, only a few functional connectivity studies have been carried out to investigate this disorder and present studies are relatively small, heterogeneous and limited by the risk of confounding from, e.g. the effects of headache medication per se, comorbidity such as anxiety and depression, and health and lifestyle factors. Future studies applying this technique in MOH patients should include larger sample sizes and provide detailed clinical information regarding primary headache disorders and comorbidity. To elucidate specific features of MOH, studies should ideally compare MOH patients to patients suffering from other conditions involving chronic head pain, and to subjects with overuse of analgesics but with no headache. Also, currently lacking are prospective functional connectivity studies of the effects of medication withdrawal and change of addictive behavior.
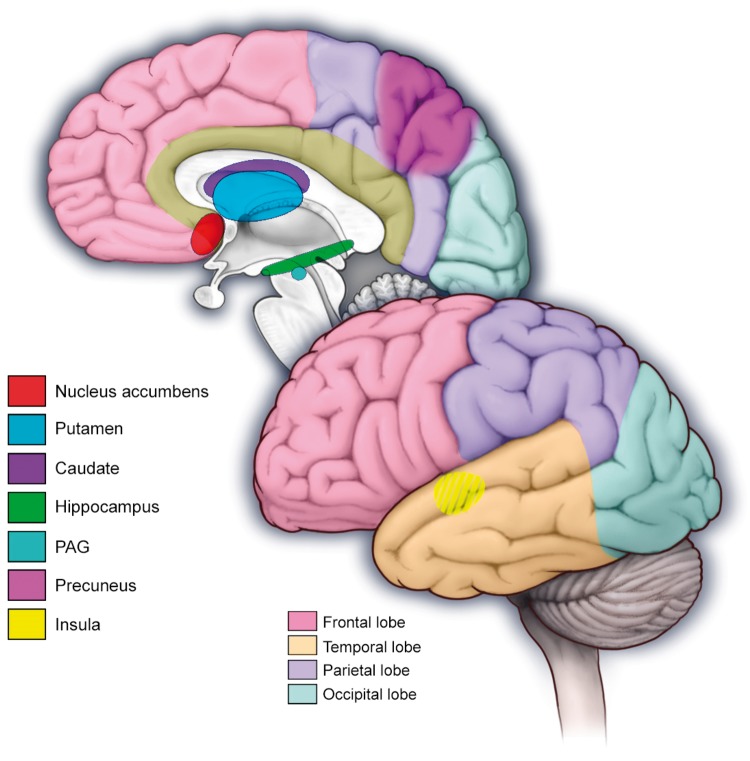
Figure 5.
Regions of the pain reward system where patients with medication-overuse headache show altered functional connectivity including the nucleus accumbens, putamen, caudate, hippocampus, periaqueductal gray (PAG), precuneus and the insula.
Conclusions
Results of rs-fMRI studies investigating migraine, TACs and MOH are complex and reflect functional abnormalities of multiple brain networks that relate to the multiple different pain and non-pain aspects of these headache disorders. Thus, in addition to atypical connectivity of the expected ‘pain matrix’ regions, these headache disorders are associated with functional connectivity alterations in salience, sensorimotor, executive, attention, limbic and default mode networks, and networks related to visual processing.
Although it is clear that there is much overlap between the functional connectivity findings when studying migraine, TACs, and MOH, findings that might be specific to each headache type are incompletely identified. There is strong suggestion that atypical connectivity of regions that are part of the brain reward system is uniquely involved in MOH. The hypothalamus plays an important role in cluster headache and other TACs and hypothalamic functional connectivity is clearly atypical in the presence of these headaches. However, it is likely that the hypothalamus also plays an important role in migraine and it is not clear if hypothalamic activity and functional connectivity differ between the TACs and migraine. More studies that directly compare functional connectivity across headache types are needed. The comparison of studies specific to a headache disorder and the comparison of resting-state data between headache disorders is complicated by patient heterogeneity (differences in age, depression, anxiety, demographic, psychological background) as well as by differences in data acquisition (variability due to field strength, 1.5 Tesla versus 3 Tesla; or differences in imaging protocols or scanner vendors). Comparison between studies is further complicated by differences in study design and data post-processing protocols (differences in statistical thresholding, or power calculations). Whereas some studies have used a region-of-interest approach, other studies have used an ICA. Both have inherent strengths and weaknesses. Whereas the seed-based approach allows interrogation of specific functional connections, it is also less robust than ICA, which can determine large-scale networks automatically without a priori hypothesis. Furthermore, seed-based approaches might not specifically interrogate a ‘network per se’ as certain regions can be a part of more than one network.79 Other studies have interrogated different frequency bands of BOLD fluctuations, believed to indicate yet another way of interrogating fluctuations in frequency-specific functional connections.80 Direct comparison amongst different rs-fMRI studies is challenging within any area of research due to variability amongst studies relating to the use of imaging designs, protocols, and post-processing techniques. The heterogeneity of study results is certain to relate to the relatively small sample sizes, as most of the rs-fc studies include less than 20 subjects in each group. Future studies, using replication designs as well as larger multicenter studies will be useful to validate and to solidify our current understanding of the pathophysiology underlying headache. Over the past decade, headache neuroimaging using rs-fMRI has become a rapidly growing field and recent studies have shown encouraging results for distinguishing individual patients with migraine from HCs using classification algorithms based on rs-fMRI data or rs-fMRI plus structural MRI data.81,82 Another study has shown utility of rs-fMRI data for characterizing migraine patients with MOH from patients without MOH.76 Results of these studies show the utility of rs-fMRI for querying headache pathogenesis as well and indicate future potential of rs-fMRI for identifying individual patients with headache, thus showing potential for headache classification.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary data
Over the past decade, a number of intrinsic functional resting-state brain networks have been identified.83 The major networks that showed altered fc in headache disorders are listed below:
Default mode network
Functions: Introspective thought, daydreaming, self-monitoring.
Key regions: Medial prefrontal cortex, anterior and posterior cingulate, posterior parietal lobule.43
Dorsal attention network
Functions: Selection of important sensory information (top-down system), modulation of visual processes.
Key regions: Frontal eye fields and intra-parietal regions.
Ventral attention network
Functions: Detection of salient (unattended) stimuli.
Key regions: Inferior frontal cortex and temporo-parietal junction.84
Executive network
Functions: Directed attention, complex planning, and memory.
Key regions: Prefrontal cortex, parietal cortex, and somato-motor areas.85
Salience network
Functions: Emotion, higher order cognitive function, interoceptive feedback.
Key regions: Insula and dorsal anterior cingulate cortex.85
Sensory motor network
Functions: Sensory-motor integration processing.
Key regions: Precentral and postcentral gyrus, posterior insula, middle and superior frontal gyrus.86,87
References
- 1.The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 2.Demarquay G, Royet JP, Giraud P, et al. Rating of olfactory judgements in migraine patients. Cephalalgia 2006; 26: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol 2008; 63: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maniyar FH, Sprenger T, Schankin C, et al. Photic hypersensitivity in the premonitory phase of migraine–a positron emission tomography study. Eur J Neurol 2014; 21: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 5.Maleki N, Becerra L, Brawn J, et al. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012; 32: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008; 48: 1044–1055. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Wilcke T, Ganssbauer S, Neuner T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008; 28: 1–4. [DOI] [PubMed] [Google Scholar]
- 8.Coppola G, Di Renzo A, Tinelli E, et al. Thalamo-cortical network activity during spontaneous migraine attacks. Neurology 2016; 87: 2154–2160. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Gouveia R, Oliveira AG, Martins IP. Cognitive dysfunction during migraine attacks: a study on migraine without aura. Cephalalgia 2015; 35: 662–674. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Gouveia R, Oliveira AG, Martins IP. Subjective cognitive symptoms during a migraine attack: a prospective study of a clinic-based sample. Pain Phys 2016; 19: E137–E150. [PubMed] [Google Scholar]
- 11.Coppola G, Di Renzo A, Tinelli E, et al. Resting state connectivity between default mode network and insula encodes acute migraine headache. Cephalalgia Epub ahead of print 1 January 2017. DOI: 10.1177/0333102417715230. [DOI] [PubMed] [Google Scholar]
- 12.Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology 2016; 86: 180–187. [DOI] [PubMed] [Google Scholar]
- 13.Hougaard A, Amin FM, Larsson HB, et al. Increased intrinsic brain connectivity between pons and somatosensory cortex during attacks of migraine with aura. Hum Brain Mapp 2017; 38: 2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nat Med 1995; 1: 658–660. [DOI] [PubMed] [Google Scholar]
- 15.Manzoni GC, Stovner LJ. Epidemiology of headache. Handbook Clin Neurol 2010; 97: 3–22. [DOI] [PubMed] [Google Scholar]
- 16.Foroozan R, Cutrer FM. Transient neurologic dysfunction in migraine. Neurol Clin 2009; 27: 361–378. [DOI] [PubMed] [Google Scholar]
- 17.Eriksen MK, Thomsen LL, Andersen I, et al. Clinical characteristics of 362 patients with familial migraine with aura. Cephalalgia 2004; 24: 564–575. [DOI] [PubMed] [Google Scholar]
- 18.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 2001; 98: 4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farago P, Tuka B, Toth E, et al. Interictal brain activity differs in migraine with and without aura: resting state fMRI study. J Headache Pain 2017; 18: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cucchiara B, Datta R, Aguirre GK, et al. Measurement of visual sensitivity in migraine: validation of two scales and correlation with visual cortex activation. Cephalalgia 2015; 35: 585–592. [DOI] [PubMed] [Google Scholar]
- 21.Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia 2007; 27: 1442–1453. [DOI] [PubMed] [Google Scholar]
- 22.Niddam DM, Lai KL, Fuh JL, et al. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia 2016; 36: 53–66. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert CD, Li W. Top-down influences on visual processing. Nat Rev Neurosci 2013; 14: 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petro NM, Gruss LF, Yin S, et al. Multimodal imaging evidence for a frontocortical modulation of visual cortex during the selective processing of conditioned threat. J Cogn Neurosci 2017; 29: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedeschi G, Russo A, Conte F, et al. Increased interictal visual network connectivity in patients with migraine with aura. Cephalalgia 2016; 36: 139–147. [DOI] [PubMed] [Google Scholar]
- 26.Schiltz C, Bodart JM, Dubois S, et al. Neuronal mechanisms of perceptual learning: changes in human brain activity with training in orientation discrimination. Neuroimage 1999; 9: 46–62. [DOI] [PubMed] [Google Scholar]
- 27.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010; 4: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hougaard A, Amin FM, Magon S, et al. No abnormalities of intrinsic brain connectivity in the interictal phase of migraine with aura. Eur J Neurol 2015; 22: 702–e46. [DOI] [PubMed] [Google Scholar]
- 29.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol 2011; 70: 838–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadjikhani N, Ward N, Boshyan J, et al. The missing link: enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia 2013; 33: 1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Chen X, Liu M, et al. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J Headache Pain 2017; 18: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faria V, Erpelding N, Lebel A, et al. The migraine brain in transition: girls vs boys. Pain 2015; 156: 2212–2221. [DOI] [PubMed] [Google Scholar]
- 33.Maleki N, Linnman C, Brawn J, et al. Her versus his migraine: multiple sex differences in brain function and structure. Brain 2012; 135: 2546–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baeken C, Marinazzo D, Van Schuerbeek P, et al. Left and right amygdala – mediofrontal cortical functional connectivity is differentially modulated by harm avoidance. PloS One 2014; 9: e95740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodkinson DJ, Veggeberg R, Kucyi A. Cortico-cortical connections of primary sensory areas and associated symptoms in migraine. eNeuro 2017; 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Su J, Wang M, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol 2017; 264: 654–663. [DOI] [PubMed] [Google Scholar]
- 37.Rist PM, Kang JH, Buring JE, et al. Migraine and cognitive decline among women: prospective cohort study. BMJ 2012; 345: e5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson AJ, Chronicle EP, Maylor EA, et al. Cognitive function is not impaired in people with a long history of migraine: a blinded study. Cephalalgia 2006; 26: 74–80. [DOI] [PubMed] [Google Scholar]
- 39.Kalaydjian A, Zandi PP, Swartz KL, et al. How migraines impact cognitive function: findings from the Baltimore ECA. Neurology 2007; 68: 1417–1424. [DOI] [PubMed] [Google Scholar]
- 40.Santangelo G, Russo A, Trojano L, et al. Cognitive dysfunctions and psychological symptoms in migraine without aura: a cross-sectional study. JHeadache Pain 2016; 17: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russo A, Tessitore A, Giordano A, et al. Executive resting-state network connectivity in migraine without aura. Cephalalgia 2012; 32: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 42.Tessitore A, Russo A, Conte F, et al. Abnormal connectivity within executive resting-state network in migraine with aura. Headache 2015; 55: 794–805. [DOI] [PubMed] [Google Scholar]
- 43.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Nal Acad Sci U S A 2001; 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain 2013; 14: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farago P, Szabo N, Toth E, et al. Ipsilateral alteration of resting state activity suggests that cortical dysfunction contributes to the pathogenesis of cluster headache. Brain Topograph 2017; 30: 281–289. [DOI] [PubMed] [Google Scholar]
- 46.Yu D, Yuan K, Zhao L, et al. Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR Biomed 2012; 25: 806–812. [DOI] [PubMed] [Google Scholar]
- 47.Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PloS One 2012; 7: e52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J, Su J, Wang M, et al. Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. J Headache Pain 2016; 17: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.May A, Bahra A, Buchel C, et al. Functional magnetic resonance imaging in spontaneous attacks of SUNCT: short-lasting neuralgiform headache with conjunctival injection and tearing. Ann Neurol 1999; 46: 791–794. [DOI] [PubMed] [Google Scholar]
- 50.May A, Bahra A, Buchel C, et al. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology 2000; 55: 1328–1335. [DOI] [PubMed] [Google Scholar]
- 51.Matharu MS, Cohen AS, McGonigle DJ, et al. Posterior hypothalamic and brainstem activation in hemicrania continua. Headache 2004; 44: 747–761. [DOI] [PubMed] [Google Scholar]
- 52.Sprenger T, Valet M, Platzer S, et al. SUNCT: bilateral hypothalamic activation during headache attacks and resolving of symptoms after trigeminal decompression. Pain 2005; 113: 422–426. [DOI] [PubMed] [Google Scholar]
- 53.Sprenger T, Boecker H, Tolle TR, et al. Specific hypothalamic activation during a spontaneous cluster headache attack. Neurology 2004; 62: 516–517. [DOI] [PubMed] [Google Scholar]
- 54.May A, Bahra A, Buchel C, et al. Hypothalamic activation in cluster headache attacks. Lancet 1998; 352: 275–278. [DOI] [PubMed] [Google Scholar]
- 55.Cohen AS, Matharu MS, Kalisch R. Functional MRI in SUNCT shows differencial hypothalamic activation with increasing pain. Cephalalgia 2004; 24: 1098–1099. [Google Scholar]
- 56.Buijs FN, Leon-Mercado L, Guzman-Ruiz M, et al. The circadian system: a regulatory feedback network of periphery and brain. Physiology 2016; 31: 170–181. [DOI] [PubMed] [Google Scholar]
- 57.Bejjani BP, Houeto JL, Hariz M, et al. Aggressive behavior induced by intraoperative stimulation in the triangle of Sano. Neurology 2002; 59: 1425–1427. [DOI] [PubMed] [Google Scholar]
- 58.Leone M, Proietti Cecchini A. Deep brain stimulation in headache. Cephalalgia 2015; 36: 1143–1148. [DOI] [PubMed] [Google Scholar]
- 59.Chou KH, Yang FC, Fuh JL, et al. Bout-associated intrinsic functional network changes in cluster headache: a longitudinal resting-state functional MRI study. Cephalalgia 2016. [DOI] [PubMed]
- 60.Qiu E, Tian L, Wang Y, et al. Abnormal coactivation of the hypothalamus and salience network in patients with cluster headache. Neurology 2015; 84: 1402–1408. [DOI] [PubMed] [Google Scholar]
- 61.Qiu E, Wang Y, Ma L, et al. Abnormal brain functional connectivity of the hypothalamus in cluster headaches. PloS One 2013; 8: e57896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu EC, Yu SY, Liu RZ, et al. Altered regional homogeneity in spontaneous cluster headache attacks: a resting-state functional magnetic resonance imaging study. Chin Med J 2012; 125: 705–709. [PubMed] [Google Scholar]
- 63.Rocca MA, Valsasina P, Absinta M, et al. Central nervous system dysregulation extends beyond the pain-matrix network in cluster headache. Cephalalgia 2010; 30: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 64.Yang FC, Chou KH, Fuh JL, et al. Altered hypothalamic functional connectivity in cluster headache: a longitudinal resting-state functional MRI study. J Neurol Neurosurg Psychiatr 2015; 86: 437–445. [DOI] [PubMed] [Google Scholar]
- 65.Maniyar FH, Sprenger T, Monteith T, et al. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain 2014; 137: 232–241. [DOI] [PubMed] [Google Scholar]
- 66.Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016; 139: 1987–1993. [DOI] [PubMed] [Google Scholar]
- 67.Westergaard ML, Glumer C, Hansen EH, et al. Prevalence of chronic headache with and without medication overuse: associations with socioeconomic position and physical and mental health status. Pain 2014; 155: 2005–2013. [DOI] [PubMed] [Google Scholar]
- 68.Munksgaard SB, Jensen RH. Medication overuse headache. Headache 2014; 54: 1251–1257. [DOI] [PubMed] [Google Scholar]
- 69.Schwedt TJ, Chong CD. Medication overuse headache: pathophysiological insights from structural and functional brain MRI research. Headache 2017; 57: 1173–1178. [DOI] [PubMed] [Google Scholar]
- 70.Riederer F, Marti M, Luechinger R, et al. Grey matter changes associated with medication-overuse headache: correlations with disease related disability and anxiety. World J Biol Psychiatry 2012; 13: 517–525. [DOI] [PubMed] [Google Scholar]
- 71.Lai TH, Chou KH, Fuh JL, et al. Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 2016; 36: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 72.Fumal A, Laureys S, Di Clemente L, et al. Orbitofrontal cortex involvement in chronic analgesic-overuse headache evolving from episodic migraine. Brain 2006; 129: 543–50. [DOI] [PubMed] [Google Scholar]
- 73.Chiapparini L, Grazzi L, Ferraro S, et al. Functional-MRI evaluation of pain processing in chronic migraine with medication overuse. Neurol Sci 200930 Suppl 1: S71–S74. [DOI] [PubMed] [Google Scholar]
- 74.Grazzi L, Chiapparini L, Ferraro S, et al. Chronic migraine with medication overuse pre-post withdrawal of symptomatic medication: clinical results and FMRI correlations. Headache 2010; 50: 998–1004. [DOI] [PubMed] [Google Scholar]
- 75.Chanraud S, Di Scala G, Dilharreguy B, et al. Brain functional connectivity and morphology changes in medication-overuse headache: clue for dependence-related processes? Cephalalgia 2014; 34: 605–615. [DOI] [PubMed] [Google Scholar]
- 76.Torta DM, Costa T, Luda E, et al. Nucleus accumbens functional connectivity discriminates medication-overuse headache. Neuroimage Clin 2016; 11: 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen Z, Chen X, Liu M, et al. Altered functional connectivity architecture of the brain in medication overuse headache using resting state fMRI. J Headache Pain 2017; 18: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michels L, Christidi F, Steiger VR, et al. Pain modulation is affected differently in medication-overuse headache and chronic myofascial pain – a multimodal MRI study. Cephalalgia 2017; 37: 764–779. [DOI] [PubMed] [Google Scholar]
- 79.Joel SE, Caffo BS, van Zijl PC, et al. On the relationship between seed-based and ICA-based measures of functional connectivity. Magn Reson Med 2011; 66: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salvador R, Martinez A, Pomarol-Clotet E, et al. A simple view of the brain through a frequency-specific functional connectivity measure. Neuroimage 2008; 39: 279–289. [DOI] [PubMed] [Google Scholar]
- 81.Chong CD, Gaw N, Fu Y, et al. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia 2017; 37: 828–844. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Q, Wu Q, Zhang J, et al. Discriminative analysis of migraine without aura: using functional and structural MRI with a multi-feature classification approach. PloS One 2016; 11: e0163875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 2005; 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox MD, Corbetta M, Snyder AZ, et al. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 2006; 103: 10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 2011; 21: 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Biswal B, Yetkin FZ, Haughton VM, et al. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 1995; 34: 537–541. [DOI] [PubMed] [Google Scholar]