Abstract
Several factors that modulate migraine, a common primary headache disorder, also affect susceptibility to cortical spreading depolarization (CSD). CSD is a wave of neuronal and glial depolarization and thought to underlie the migraine aura and possibly headache. Here, we tested whether caffeine, known to alleviate or trigger headache after acute exposure or chronic use/withdrawal, respectively, modulates CSD. We injected C57BL/6J mice with caffeine (30, 60, or 120 mg/kg; i.p.) once (acute) or twice per day for one or two weeks (chronic). Susceptibility to CSD was evaluated by measuring the electrical CSD threshold and by assessing KCl-induced CSD. Simultaneous laser Doppler flowmetry was used to assess CSD-induced cortical blood flow changes. Recordings were performed 15 min after caffeine/vehicle administration, or 24 h after the last dose of chronic caffeine in the withdrawal group. The latter paradigm was also tested in mice carrying the familial hemiplegic migraine type 1 R192Q missense mutation, considered a valid migraine model. Neither acute/chronic administration nor withdrawal of caffeine affected CSD susceptibility or related cortical blood flow changes, either in WT or R192Q mice. Hence, adverse or beneficial effects of caffeine on headache seem unrelated to CSD pathophysiology, consistent with the non-migrainous clinical presentation of caffeine-related headache.
Keywords: Caffeine, cortical spreading depolarization, migraine, withdrawal, headache
Introduction
Migraine is a severe common episodic primary headache disorder characterized by recurrent attacks of throbbing unilateral head pain that is accompanied by vomiting, nausea, photophobia and phonophobia and typically lasts between 4 and 72 h.1 In one-third of patients, headaches are preceded by focal neurological symptoms, the so-called migraine aura. Human imaging studies and animal experiments suggest that cortical spreading depolarization (CSD) is the electrophysiologic event underlying migraine aura.2,3 CSD is an intense wave of neuronal and glial depolarization, slowly propagating through cortical gray matter. Various factors known to modulate migraine also affect the susceptibility to CSD in experimental animal models. For instance, migraine prophylactic drugs suppress CSD susceptibility,4 whereas female gonadal hormones6,7 and human gene mutations causing migraine-associated syndromes5 increase CSD susceptibility. The importance of CSD in migraine pathophysiology is underscored by accumulating evidence that CSD can activate headache mechanisms,8–10 at least in experimental animal models.
Caffeine is the most widely used psychostimulant drug; over 87% of the US population consumes caffeine every day, with about 30% ingesting over 500 mg/day.11 Average daily caffeine consumption is around 70 mg, which corresponds to one large cup of coffee.12 The relation between caffeine and headache is not straightforward. Notably, acute administration of caffeine can resolve headaches13 and increase the efficacy of analgesics to treat tension-type headache or migraine,14 whereas chronic caffeine exposure is known to cause headache, in particular when the subject is female and young.15 In a population-based study, patients with chronic daily headache were more likely to have high caffeine consumption before onset of chronic daily headache in comparison with episodic headache controls.15 In a large cross-sectional study with 50,483 participants high caffeine consumption was associated with an increased prevalence of infrequent headache.16 Similarly, withdrawal from caffeine has been shown to produce headache under placebo-controlled double-blind conditions.17
Here, we used electrocortical recording with simultaneous laser Doppler flow measurement to investigate whether acute or chronic administration of or withdrawal from caffeine in wild-type (WT) C57BL/6 J and familial hemiplegic migraine 1 (FHM1) R192Q mice affects susceptibility to CSD, which may shed light on how caffeine exerts its clinical effect on headache.
Materials and methods
Experimental animals
All experimental procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996) and were approved by the institutional review board (MGH Subcommittee on Research Animal Care, SRAC). Experiments have been reported in compliance with the ARRIVE guidelines. In total, 124 female mice were used, 112 adult (2 and 6 months of age) WT C57BL/6 J mice (Charles River Laboratories) for acute and chronic caffeine administration as well as caffeine withdrawal and 12 adult (same age range as for WT) FHM1 R192Q mutant mice for caffeine withdrawal. In addition, three WT mice and one R192Q mutant mouse were used but excluded from further investigation because of failed surgery. As CSD susceptibility is higher in young adult female compared to male R192Q mutant mice,6 we decided to study only female mice.
Experimental timeline and treatment
Caffeine was administered via intraperitoneal (i.p.) injection according to the following paradigms. (a) Acute administration: single dose of 30, 60 or 120 mg/kg, equivalent to 2, 5 and 10 cups of coffee in humans.18 CSD recording was performed 15 min after caffeine administration; (b) Chronic administration: twice a day for one or two weeks,19 with a dose of 60, 120, or 240 mg/kg/day. CSD recording was performed 15 min after the last dose of caffeine in the chronic group, or (c) 24 h after the last dose of the one-week chronic treatment in the withdrawal group. Protocols build on the notion that caffeine reaches its maximal plasma level, in humans, about 30 min after administration; caffeine is eliminated by the liver with an average plasma half-life of approximately 3–6 h.20 In mice, the half-life is 40–60 min with complete elimination after 4–5 h.21 Saline was used as vehicle for all groups. No gross weight loss or mortality occurred during the treatment period. All experiments were carried out with the investigator blinded to genotype and treatment.
Electrophysiological recordings
The femoral artery was catheterized for blood sampling and for measurement of the mean arterial pressure, and the trachea was intubated for mechanical ventilation under inhalational isoflurane (2.5% induction, 1% maintenance, in 70% N2O/30% O2) or intraperitoneal urethane anesthesia (1.3–1.5 g/kg). Arterial blood gases and pH were measured every 20 min and maintained within normal limits by adjusting ventilation during the ∼90 min experiment. Mice were placed in a stereotaxic frame and three burr holes were drilled in the skull at coordinates as described previously.22 Two glass capillary microelectrodes were placed at a cortical depth of 300 µm to record extracellular direct current (DC) potentials and an electrocorticogram. After surgical preparation, the cortex was allowed to recover for 20 min under saline irrigation. In each hemisphere, first, the electrical CSD threshold was determined using a stimulus isolator (A385, WPI, Sarasota, FL, USA) and a bipolar stimulation electrode (400 µm tip diameter; Frederick Haer Company, Bowdoin, ME, USA) placed in the occipital cortex. Single pulses of increasing duration and intensity (0.2–900 µC) were applied at 3-min intervals, until a CSD was observed.5 To investigate a possible effect of pharmacokinetic changes of caffeine on CSD along the time course after administration, electrical thresholds of the two hemispheres were studied consecutively. After confirming no difference between electrical thresholds of both hemispheres, we averaged results of the bilateral hemispheres. Following the assessment of the electrical thresholds, we moved back to the already recovered first hemisphere to assess the frequency of CSDs during continuous application of 300 mM KCl as described previously.6 The amplitude, propagation speed, and duration at half-amplitude of the first CSD in each hemisphere were measured as secondary endpoints.
Laser Doppler flowmetry to assess cortical blood flow
Regional cerebral blood flow (CBF) was recorded using laser Doppler flowmetry (LDF) (PF2, Perimed, Jarfalla, Sweden). The LDF probe (0.48-mm tip diameter) was placed immediately proximal to the proximal glass micropipette, and away from large vessels. The amplitudes of CBF changes, expressed as % of initial baseline prior to the onset of the CSD and plotted as a function of time, were measured at 5-s intervals, with time 0 indicating the inflection point of initial hypoperfusion. Data from the first and second CSD were used for analysis.
Measurement of caffeine plasma levels
Whole blood was collected via cardiac puncture 10 min after injection of 60 or 120 mg/kg caffeine in EDTA-coated microtainers (n = 4 for each dose). Blood plasma was separated by centrifugation and supernatants were collected. Plasma samples were diluted 1000-fold with phosphate-buffered saline and caffeine concentrations were determined using a commercial kit (Neogen, Lansing, MI, USA), according to manufacturer instructions. Briefly, 20 µL of caffeine standards from 200 ng/mL to 0.39 ng/mL or diluted plasma samples were added to the coated plate in duplicate. Enzyme conjugate stock solution was diluted 1 to 180 times with the provided EIA buffer, and 180 µL was added to each well. Next, 150 µL of K-Blue Substrate was added to each well. The reaction was terminated after 30-min incubation by the addition of 50 µL Red Stop solution. The absorbance was immediately measured using a plate reader (Victor, Perkin Elmer, Waltham, MA, USA) set to a wavelength of 650 nm, and caffeine concentrations were determined using the standard curve.
Statistics
The primary endpoint of the study was to investigate the effect of caffeine (acute exposure, chronic exposure, withdrawal) on CSD susceptibility (electrical threshold and frequency of CSD upon continuous topical KCl). The secondary endpoints were the effects of caffeine on propagation speed, duration of CSD, and CSD-associated cortical blood flow changes. Data were analyzed using SPSS (v11.0), and presented as mean ± standard deviation, or median (interquartile range). Using a general linear model approach, we tested for an effect of the independent variables treatment type (caffeine vs. vehicle) and paradigm (acute vs. chronic vs. withdrawal) on the dependent variables CSD frequency, CSD propagation speed, and CSD duration. CSD threshold was compared between treatment groups using the non-parametric Mann–Whitney U-test. Other electrophysiological measures of CSD and systemic physiological data were compared among groups using one-way analysis of variance (ANOVA). For the analysis of the effect of caffeine on cortical blood flow response to CSD, general linear models for repeated measures were used to compare mean values over time according to treatment groups. The number of animals used per group was based on previous studies.22 For the frequency of CSD, we considered an at least 30% reduction of CSD frequency to be meaningful. For electrical threshold, we assumed the minimum significant difference of electrical threshold to be 2-fold. With such effect sizes, an estimated sample size of four to eight per group is expected to have a statistical power of approximately 95% when using non-parametric tests. P-values are two-tailed, and a p < 0.05 was considered statistically significant.
Results
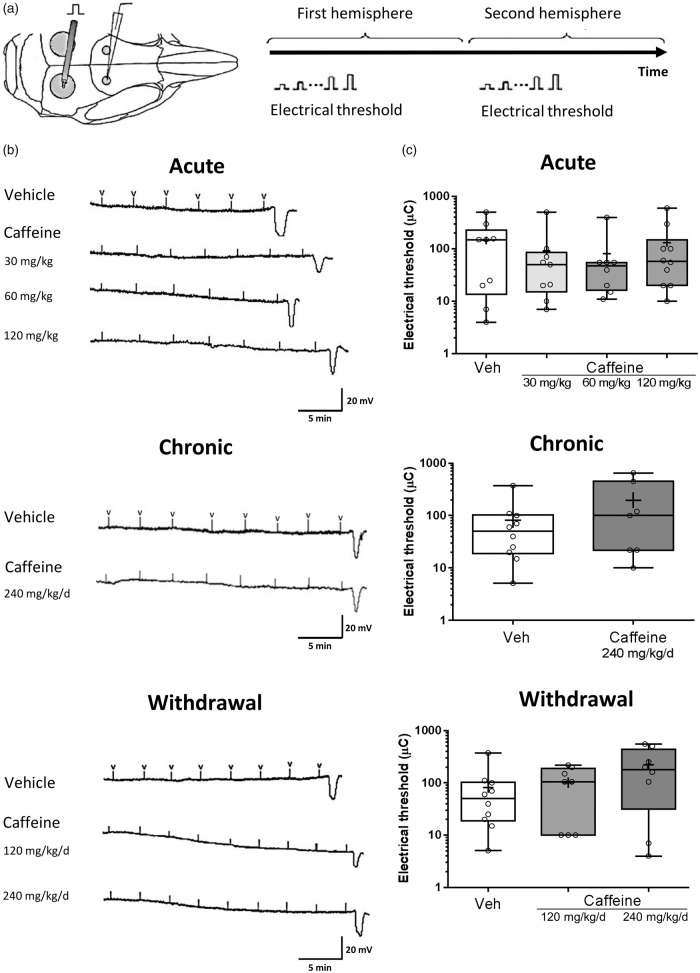
Acute administration of caffeine in mice produced plasma levels that were similar to those reported previously (Supplemental Figure 1).20,23 Acute administration of vehicle or a single dose of caffeine (0, 30, 60, or 120 mg/kg) did not affect the electrical threshold for CSD in WT C57BL/6 J mice with a median (in µC) of 150.00, 50.10, 47.50 and 57.50, respectively (Figure 1). Chronic administration of caffeine (240 mg/kg/day for one week) also did not affect the electrical threshold for CSD in WT mice with a median (in µC) of 100.00 (Figure 1). Caffeine withdrawal, 24 h after the last dose of the chronic exposure paradigm, i.e. with vehicle, 120 mg/kg/day or 240 mg/kg/day caffeine, did not affect the electrical CSD threshold with a median (in µC) of 40.00, 105.00, and 180.00, respectively (Figure 1).
Figure 1.
Caffeine does not affect the electrical threshold for cortical spreading depolarization in mice.(a) Single-squared pulses of increasing duration and intensity (2–900 µC) were applied at 3-min intervals, until a CSD was observed. The two hemispheres were studied consecutively to investigate a possible effect of pharmacokinetic changes of caffeine on CSD along the time course after administration. (b) Representative tracings of electrical threshold for CSD in each group, with “V” indicating electrical stimulus administration. (c) Whisker box plots show that acute (0 mg/kg n = 9; 30 mg/kg n = 9; 60 mg/kg n = 9; 120 mg/kg n = 10) or chronic (0 mg/kg/day n = 10 (1 week); 240 mg/kg/day n = 7 (1 week)) caffeine administration did not affect the electrical threshold for CSD. Similarly, caffeine withdrawal (0 mg/kg n = 10; 120 mg/kg/day n = 8 (1 week); 240 mg/kg/day n = 8 (1 week)) had no effect on CSD threshold in C57BL/6 J mice (p > 0.05). The ends of the whiskers represent minimal and maximal data points. The horizontal lines within the box indicate the median, and the “+” sign represents the mean. Open circles show individual data points.
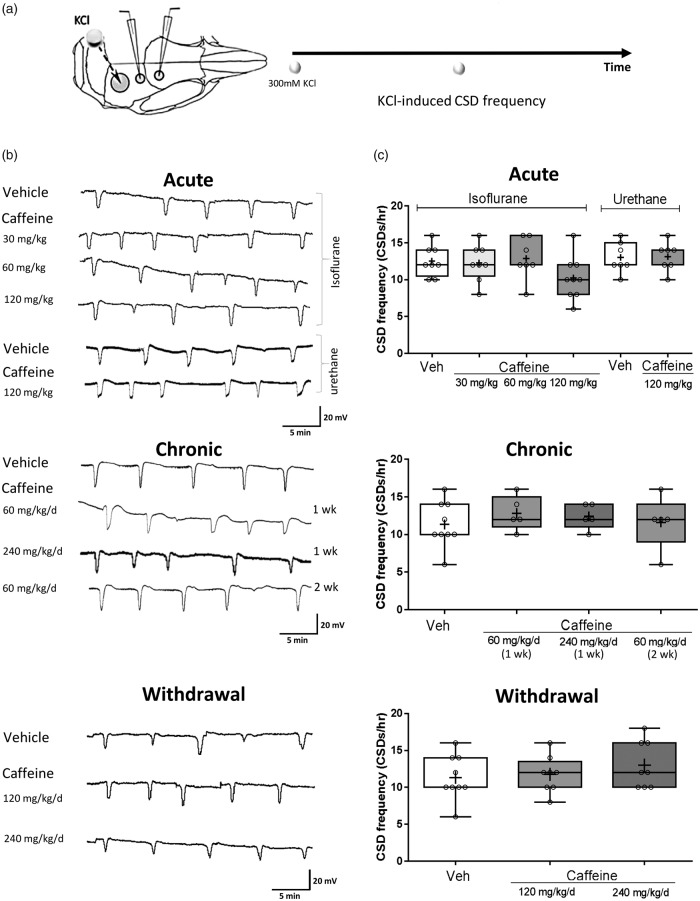
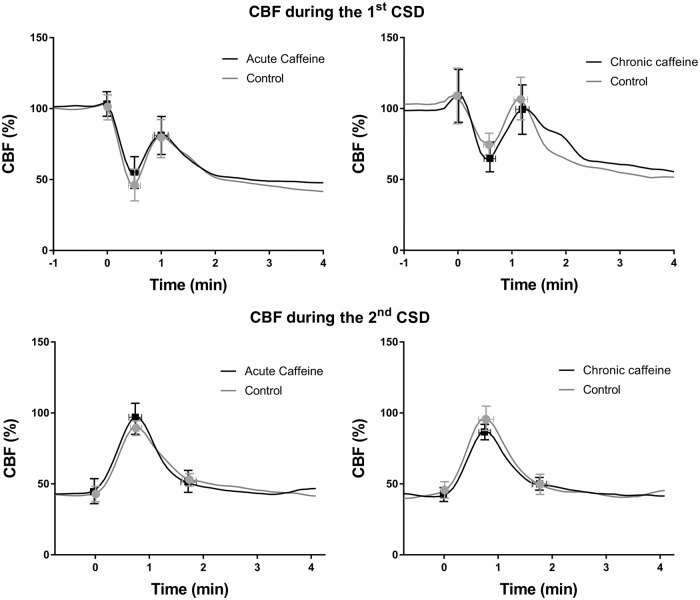
Acute administration of caffeine also did not alter KCl-induced CSD. Acute administration of caffeine (0, 30, 60, or 120 mg/kg) had no effect on CSD frequency, measured as number of CSDs per hour (mean ± SD: 12 ± 2 vs. 12 ± 2 vs. 12 ± 2 vs. 10 ± 4, respectively). Chronic administration of caffeine (0, 60, 240 mg/kg/day (one week), or 60 mg/kg/day (two weeks)) also did not alter CSD frequency (mean ± SD: 11 ± 3 vs. 12 ± 2 vs. 12 ± 3 vs. 13 ± 2, respectively). Similarly, withdrawal after chronic exposure with caffeine (0, 120 or 240 mg/kg/day) did not affect CSD frequency (mean ± SD: 12 ± 2 vs. 12 ± 2 vs. 14 ± 4, respectively) (Figure 2). The peak change of CBF during CSD was not altered by caffeine; neither was the time needed to return to post-CSD baseline blood flow level (Figure 3).
Figure 2.
Caffeine does not affect frequency of KCl-induced cortical spreading depolarization in mice.(a) CSD frequency was assessed by topical cortical application of KCl and represented as the number of CSDs per hour. (b) Representative electrophysiological tracings of CSD frequency measurement for each treatment group. (c) Whisker box plots show that acute (0 mg/kg n = 9; 30 mg/kg n = 9; 60 mg/kg n = 9; 120 mg/kg n = 10) or chronic (0 mg/kg/day n = 10 (1 week); 60 mg/kg/day n = 5 (1); 240 mg/kg/day n = 7 (1 week); 60 mg/kg/day n = 5 (2 weeks)) caffeine administration did not affect CSD frequency. The results were not affected by the choice of anesthetic (isoflurane vs. urethane (n = 7 per group)). Similarly, caffeine withdrawal (0 mg/kg/day n = 10; 120 mg/kg/day n = 8 (1 week); 240 mg/kg/day n = 8 (1 week)) had no effect on CSD frequency in C57BL/6J mice (p > 0.05). The ends of the whiskers represent minimal and maximal data points. The horizontal lines within the box indicate the median, and the “+” sign represents the mean. Open circles show individual data points.
Figure 3.
Caffeine does not affect cortical spreading depolarization-induced cortical blood flow changes. CBF response during the first or second CSD was not altered by caffeine, after acute administration (120 mg/kg) (n = 7) or chronic treatment (60 mg/kg/day for 2 weeks) (n = 5) when compared to vehicle (n = 7 and 5, respectively) (p > 0.05). The curves represent averaged CBF of individual animals at 5-s intervals, with time 0 indicating the inflection point at initial hypoperfusion. Vertical error bars show standard deviation of the amplitude of CBF change at inflection points. Horizontal error bars indicate standard deviation of time differences during CBF inflection points.
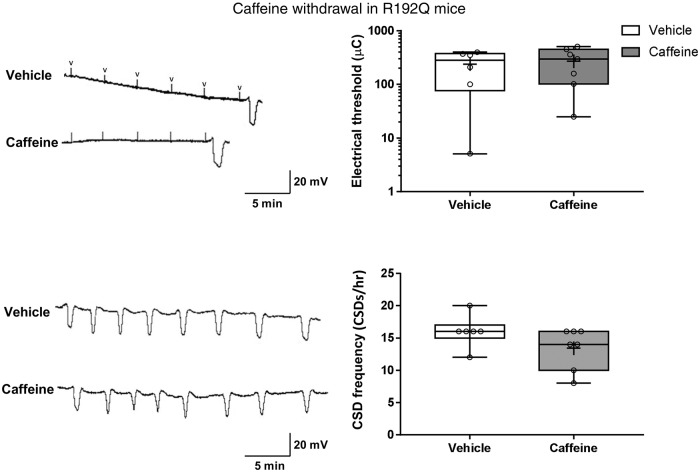
In FHM1 R192Q mutant mice, i.e. in mice with enhanced neuronal excitability and susceptibility to CSD, caffeine withdrawal also did not have an effect on the genetically low electrical threshold for CSD – the median threshold remained unchanged at 10.00 µC. Similarly, the KCl-induced CSD frequency of 16 ± 2 SD/h in the R192Q mutant mice, which is increased compared to WT mice (p = 0.01), was not affected by caffeine withdrawal (14 ± 4 SD/h; see Figure 4).
Figure 4.
Caffeine withdrawal does not affect cortical spreading depolarization threshold and frequency in R192Q mutant mice. (a) Representative tracings of the electrical threshold for CSD in R192Q mutant mice during caffeine withdrawal, compared to vehicle-treated R192Q mutant mice. “V” indicates electrical stimulation. Whisker box plots show that withdrawal of caffeine did not affect the genetically low electrical threshold for CSD in R192Q mice (p > 0.05). (b) Representative electrophysiological tracings of KCl-induced CSD frequency in R192Q mutant mice during caffeine withdrawal compared to vehicle-treated R192Q mutant mice. (c) Whisker box plots show that caffeine withdrawal (n = 7) did not affect CSD frequency when compared to vehicle (n = 5) in R192Q mutant mice (p > 0.05).
Other characteristics, including the speed, the amplitude and the duration of CSD as well as systemic physiologic parameters, did also not differ between treatment groups (Table 1).
Table 1.
Systemic physiologic parameters, and characteristics of cortical spreading depolarization.
| Group |
Systemic physiology |
CSD |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Genotype | Caffeine | Number of Animals | Age (mo) | BW (g) | BP | pH | pCO2 | pO2 | Speed (mm/min) | Duration (s) | Amplitude (mV) |
| Acute | C57BL/6 J | Vehicle | 9 | 4 ± 1 | 22 ± 3 | 79 ± 9 | 7.37 ± 0.04 | 31 ± 4 | 135 ± 21 | 3.3 ± 0.9 | 29 ± 6 | 17 ± 3 |
| (isoflurane) | 30 mg/kg | 9 | 4 ± 1 | 23 ± 3 | 82 ± 9 | 7.34 ± 0.03 | 30 ± 5 | 134 ± 22 | 3.5 ± 0.9 | 32 ± 9 | 17 ± 4 | |
| 60 mg/kg | 9 | 3 ± 1 | 22 ± 2 | 79 ± 7 | 7.35 ± 0.04 | 32 ± 5 | 144 ± 18 | 3.7 ± 0.5 | 33 ± 10 | 19 ± 2 | ||
| 120 mg/kg | 10 | 5 ± 1 | 24 ± 3 | 79 ± 6 | 7.35 ± 0.05 | 26 ± 3 | 143 ± 17 | 3.9 ± 0.8 | 27 ± 7 | 20 ± 3 | ||
| Acute | Vehicle | 7 | 2 | 18 ± 1 | 78 ± 7 | 7.37 ± 0.05 | 38 ± 7 | 120 ± 18 | 3.9 ± 0.5 | 33 ± 8 | 19 ± 2 | |
| (urethane) | 120 mg/kg | 7 | 2 | 18 ± 1 | 77 ± 8 | 7.36 ± 0.06 | 39 ± 7 | 124 ± 19 | 3.8 ± 0.8 | 29 ± 6 | 19 ± 2 | |
| Chronic | C57BL/6 J | Vehicle (one week) | 10 | 5 ± 1 | 22 ± 2 | 85 ± 11 | 7.37 ± 0.04 | 28 ± 4 | 149 ± 13 | 4.2 ± 0.5 | 33 ± 13 | 19 ± 2 |
| 60 mg/kg/d (one week) | 5 | 2 | 18 ± 1 | 81 ± 12 | 7.39 ± 0.07 | 38 ± 8 | 119 ± 12 | 4.2 ± 0.5 | 33 ± 13 | 19 ± 2 | ||
| 240 mg/kg/d (one week) | 7 | 5 | 18 ± 2 | 76 ± 4 | 7.33 ± 0.03 | 27 ± 4 | 140 ± 12 | 4.2 ± 1.0 | 23 ± 6 | 18 ± 2 | ||
| Vehicle (two weeks) | 5 | 2 | 18 ± 2 | 77 ± 11 | 7.37 ± 0.06 | 39 ± 8 | 149 ± 13 | 3.6 ± 0.3 | 38 ± 17 | 19 ± 4 | ||
| 60 mg/kg/d (two weeks) | 5 | 2 | 18 ± 1 | 76 ± 9 | 7.39 ± 0.07 | 37 ± 7 | 140 ± 12 | 3.4 ± 0.4 | 39 ± 12 | 22 ± 2 | ||
| Withdrawal | C57BL/6 J | Vehicle | 10 | 5 ± 1 | 22 ± 2 | 85 ± 11 | 7.37 ± 0.04 | 28 ± 4 | 149 ± 13 | 4.2 ± 0.5 | 33 ± 13 | 22 ± 4 |
| 120 mg/kg/d | 8 | 5 ± 1 | 22 ± 2 | 86 ± 8 | 7.39 ± 0.04 | 28 ± 4 | 153 ± 8 | 3.8 ± 0.8 | 31 ± 11 | 22 ± 2 | ||
| 240 mg/kg/d | 8 | 4 ± 1 | 25 ± 3 | 88 ± 11 | 7.40 ± 0.05 | 27 ± 3 | 146 ± 16 | 4.4 ± 0.6 | 34 ± 8 | 22 ± 3 | ||
| R192Q | Vehicle | 6 | 5 ± 2 | 21 ± 2 | 83 ± 5 | 7.37 ± 0.03 | 29 ± 3 | 135 ± 8 | 5.8 ± 1.0 | 29 ± 6 | 25 ± 3 | |
| 240 mg/kg/d | 7 | 5 ± 2 | 21 ± 2 | 89 ± 11 | 7.38 ± 0.03 | 30 ± 3 | 143 ± 19 | 6.5 ± 1.8 | 31 ± 8 | 24 ± 5 | ||
CSD: cortical spreading depolarization.
Note: Data are mean ± SD. Systemic physiologic parameters and characteristics of CSD did not differ between caffeine treatment groups (p > 0.05).
Discussion
Using a multimodal approach of simultaneous electrocortical and laser Doppler recordings, we investigated the effect of caffeine on susceptibility to CSD, the electrophysiological correlate underlying migraine aura. In mice, neither acute exposure (single dose in WT), chronic treatment (one dose twice a day for one or two weeks in WT), or withdrawal of caffeine after chronic exposure (WT and FHM1 R192Q) affected susceptibility to CSD with any of the tested dosings; depending on the paradigm, 30, 60, 120 or 240 mg/kg/day of caffeine was tested. No effect of caffeine was observed on the electrical threshold to induce a CSD, the frequency of CSD events upon continuous topical application of 300 mM KCl, the amplitude or propagation speed of CSD, or the CBF response to CSD.
Not finding an effect of the non-selective competitive adenosine receptor antagonist caffeine on CSD at first glance seems unexpected. After all, adenosine has been implicated in migraine pathophysiology because adenosine levels were shown to be elevated during migraine attacks,24 an adenosine A2A receptor gene haplotype was found to be associated with migraine with aura,25 and administration of adenosine can precipitate migraine attacks.24 Given that caffeine consumption has clinical effects on headache, we hypothesized that the underlying mechanism might involve modulation of neuronal activity with an effect on CSD susceptibility through action on adenosine A1 and A2A receptors. In fact, in a brain slice study, endogenous adenosine inhibits SD while A1 receptor antagonists could reverse the effect.26 Adenosine receptor activation is responsible for the prolonged depression of synaptic transmission after SD in brain slices in vivo, while inhibition of A1 receptor antagonizes the effect.27,28
Acute caffeine administration reportedly has multiple effects on neuronal excitability, depending on the experimental paradigm. In Genetic Epilepsy Rats from Strasbourg, acute caffeine reduces spike-and-wave discharges,29 and acute caffeine was found to decrease rhythmic metrazol activity in a rat model of human absence seizures.30 In contrast, caffeine administration to the isolated cat cortex has been shown to produce convulsive discharges,31 while caffeine did not affect cortical inhibition.32 Also, no effect of caffeine was found on latency or amplitude of visual evoked potential.33 When we tested the effect of caffeine on CSD susceptibility, caffeine did not inhibit CSD in WT mice, nor did it have an effect on CBF. Our findings suggest that acute exposure to caffeine exhibits beneficial clinical effects on headache via alternative mechanisms. Perhaps the beneficial effect of acute exposure to caffeine may involve analgesia by stimulation of D2 or alpha-2 receptors, because adenosine antagonists such as caffeine increase dopamine and norepinephrine activity in the CNS, and reportedly increase D2/D3 receptor levels and their affinity in the CNS.34
Chronic caffeine consumption has been shown to be associated with chronic episodic headache (p = 0.01).15 As underlying mechanism, we postulated that chronic caffeine treatment might enhance CSD susceptibility by increasing release of excitatory neurotransmitters.35 Chronic caffeine consumption, i.e. adenosine antagonism, indeed induces compensatory mechanisms in the form of an increase in adenosine plasma levels36 and up-regulation of adenosine receptors. Chronic exposure to caffeine produces a dose-dependent increase in A2A receptor expression in the striatum of mice, and shifts low-affinity A1 receptors to a high-affinity state.37 However, we did not find an effect of chronic caffeine on CSD susceptibility or on CBF response during CSD. An alternative explanation for headache associated with chronic caffeine consumption might involve blockage of A2A receptors at the trigeminal neuron that inhibit the release of pro-nociceptive calcitonin gene-related peptide.38 During caffeine withdrawal after chronic exposure, the increased adenosine signal secondary to chronic caffeine exposure is not antagonized by caffeine anymore, thereby causing caffeine withdrawal headache that is relieved by re-exposure to caffeine. In a placebo-controlled double-blind trial of caffeine discontinuation, about 50% of the study subjects developed moderate to severe headache.17 Caffeine withdrawal headache has been reported in persons consuming the equivalent of as little as one cup of coffee per day.39 As R192Q mice exhibit a genetically enhanced CSD susceptibility and are particularly sensitive to migraine modulators6 while being less susceptible to stimulation of adenosine receptors,40 we tested whether withdrawal from caffeine has an effect on CSD in this group of mice, but no such effects were observed in this group either.
Several possible explanations may explain why caffeine did not affect CSD and related CBF changes, while exhibiting effects on headache in patients. These include pathophysiological and clinical considerations. First, the vascular and neuronal net effect of caffeine depends on the ratio of A1 and A2A receptors, receptor number and affinity in a specific brain area.41 Indeed, levels of A2A receptors responsible for the vasoconstrictive effect of caffeine are low in, for example, the frontal cortex where we performed CBF and CSD recordings.42 With respect to CSD-induced changes of CBF, mice with a predominantly vasoconstrictive response to CSD – in contrast to rats and humans – might exhibit a less pronounced vasoconstrictive effect of caffeine.33 Furthermore, caffeine normally acts as a respiratory stimulant causing a significant reduction in end-tidal carbon dioxide,43 which was not the case in our experimental setting with controlled mechanical ventilation. Another technical limitation of our study relates to our animal model of CSD. Although electrical stimulation and 300 mM topical application as CSD triggers are the most established and routinely used methods to induce CSD, these triggers are not physiological and might cause minimal injury to the cortex.44 Therefore, our study might have relevance for brain injury as well, even though the study was not designed to study this specifically. In fact, CSD is known to play an important role in brain injury, and caffeine has been found to be protective in traumatic brain injury in rodents45 and perhaps in humans.46 Our study may suggest that the protective effect of caffeine in cerebral injury might not be mediated through inhibition of injurious CSD or its associated CBF changes. Moreover, it should be mentioned that caffeine still could interfere with the true trigger condition of CSD in individuals who have migraine with aura as the current models are non-physiological and far from ideal ones.
Clinically, chronic caffeine and its withdrawal cause headache of non-migrainous character. Chronic caffeine consumption has been associated with daily or nearly daily headache of dull pressure-like and bilateral character.47 Similarly, caffeine withdrawal headache is “symptom-poor” headache, without typical migraine features such as throbbing or nausea, and CSD might therefore not be involved in its pathophysiology.48
In summary, caffeine does not affect susceptibility to CSD in mice with the tested exposure paradigms. Despite clinical evidence of acute caffeine improving and chronic caffeine or caffeine withdrawal worsening headache, our data suggest caffeine affects headache without modulating susceptibility to the migraine trigger CSD.
Supplemental Material
Supplemental material for Caffeine does not affect susceptibility to cortical spreading depolarization in mice by Nilufer Yalcin, Shih-Pin Chen, Esther S Yu, Tzu-Ting Liu, Jiin-Cherng Yen, Yahya B Atalay, Tao Qin, Furkan Celik, Arn MJM van den Maagdenberg, Michael A Moskowitz, Cenk Ayata and Katharina Eikermann-Haerter in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the American Heart Association (10SDG2610275 to K.E.H.); the Massachusetts General Hospital (Claflin Distinguished Award to K.E.H.); the Ministry of Science and Technology of Taiwan (MOST 104-2314-B-075 -006 - MY3 to S.P.C), Taipei Veterans General Hospital (V104C-174 to S.P.C), Taipei Veterans General Hospital-National Yang-Ming University Excellent Physician Scientists Cultivation Program (No.102-V-A-001 and No. 104-V-B-035 to S.P.C.); and the Vivian W. Yen Neurological Foundation (2013 research grant to S.P.C.) and by the Center for Medical Systems Biology (CMSB) established by the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (project No. 050060-409, A.M.J.M.v.d.M.).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
NY, SPC, MAM, CA and KEH designed the studies. NY, SPC, ESY, YBA, TTL, JCY, TQ, FC performed and analyzed the studies. NY, SPC and KEH drafted the manuscript. NY, SPC, KEH, AvdM, MAM critically revised the manuscript. All authors reviewed, edited and approved the final version of this manuscript.
References
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33: 629–808. [DOI] [PubMed]
- 2.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci U S A 2001; 98: 4687–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med 2002; 8: 136–142. [DOI] [PubMed] [Google Scholar]
- 4.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 5.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, et al. A Cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron 2004; 41: 701–710. [DOI] [PubMed] [Google Scholar]
- 6.Eikermann-Haerter K, Dilekoz E, Kudo C, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest 2009; 119: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eikermann-Haerter K, Baum MJ, Ferrari MD, et al. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Ann Neurol 2009; 66: 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of the pain and strain of migraine. J Comp Neurol 2005; 493: 9–14. [DOI] [PubMed] [Google Scholar]
- 9.Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci 1993; 13: 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013; 339: 1092–1095. [DOI] [PubMed] [Google Scholar]
- 11.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 2005; 105: 110–113. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert RM. Caffeine consumption. Prog Clin Biol Res 1984; 158: 185–213. [PubMed] [Google Scholar]
- 13.Ward N, Whitney C, Avery D, et al. The analgesic effects of caffeine in headache. Pain 1991; 44: 151–155. [DOI] [PubMed] [Google Scholar]
- 14.Diener HC, Pfaffenrath V, Pageler L, et al. The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study. Cephalalgia 2005; 25: 776–787. [DOI] [PubMed] [Google Scholar]
- 15.Scher AI, Stewart WF, Lipton RB. Caffeine as a risk factor for chronic daily headache: a population-based study. Neurology 2004; 63: 2022–2027. [DOI] [PubMed] [Google Scholar]
- 16.Hagen K, Thoresen K, Stovner LJ, et al. High dietary caffeine consumption is associated with a modest increase in headache prevalence: results from the Head-HUNT Study. J Headache Pain 2009; 10: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman K, Evans SM, Strain EC, et al. Withdrawal syndrome after the double-blind cessation of caffeine consumption. N Engl J Med 1992; 327: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 18.Arendash GW, Mori T, Cao C, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis 2009; 17: 661–680. [DOI] [PubMed] [Google Scholar]
- 19.Nikodijevic O, Jacobson KA, Daly JW. Locomotor activity in mice during chronic treatment with caffeine and withdrawal. Pharmacol Biochem Behav 1993; 44: 199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan GB, Tai NT, Greenblatt DJ, et al. Caffeine-induced behavioural stimulation is dose- and concentration-dependent. Br J Pharmacol 1990; 100: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartmann M, Czok G. [Pharmacokinetics of caffeine in mice and its modification by ethanol]. Zeitschrift fur Ernahrungswissenschaft 1980; 19: 215–227. [DOI] [PubMed] [Google Scholar]
- 22.Chen SP, Qin T, Seidel JL, et al. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 2017; 140: 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan GB, Greenblatt DJ, Leduc BW, et al. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J Pharmacol Exp Ther 1989; 248: 1078–1083. [PubMed] [Google Scholar]
- 24.Guieu R, Devaux C, Henry H, et al. Adenosine and migraine. Can J Neurol Sci 1998; 25: 55–58. [DOI] [PubMed] [Google Scholar]
- 25.Hohoff C, Marziniak M, Lesch KP, et al. An adenosine A2A receptor gene haplotype is associated with migraine with aura. Cephalalgia 2007; 27: 177–181. [DOI] [PubMed] [Google Scholar]
- 26.Kaku T, Hada J, Hayashi Y. Endogenous adenosine exerts inhibitory effects upon the development of spreading depression and glutamate release induced by microdialysis with high K+ in rat hippocampus. Brain Res 1994; 658: 39–48. [DOI] [PubMed] [Google Scholar]
- 27.Lindquist BE, Shuttleworth CW. Adenosine receptor activation is responsible for prolonged depression of synaptic transmission after spreading depolarization in brain slices. Neuroscience 2012; 223: 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindquist BE, Shuttleworth CW. Evidence that adenosine contributes to Leao's spreading depression in vivo. J Cereb Blood Flow Metab 2017; 37: 1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germe K, Faure JB, Koning E, et al. Effect of caffeine and adenosine receptor ligands on the expression of spike-and-wave discharges in Genetic Absence Epilepsy Rats from Strasbourg (GAERS). Epilepsy Res 2015; 110: 105–114. [DOI] [PubMed] [Google Scholar]
- 30.Tchekalarova JD, Kubova H, Mares P. Different effects of postnatal caffeine treatment on two pentylenetetrazole-induced seizure models persist into adulthood. Pharmacol Rep 2013; 65: 847–853. [DOI] [PubMed] [Google Scholar]
- 31.Frank GB, Jhamandas K. Effects of general stimulant drugs on the electrical responses of isolated slabs of cat's cerebral cortex. Br J Pharmacol 1970; 39: 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krnjevic K, Randic M, Straughan DW. Pharmacology of cortical inhibition. J Physiol 1966; 184: 78–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diukova A, Ware J, Smith JE, et al. Separating neural and vascular effects of caffeine using simultaneous EEG-FMRI: differential effects of caffeine on cognitive and sensorimotor brain responses. Neuroimage 2012; 62: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Logan J, et al. Caffeine increases striatal dopamine D2/D3 receptor availability in the human brain. Transl Psychiatry 2015; 5: e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci 2001; 24: 31–55. [DOI] [PubMed] [Google Scholar]
- 36.Conlay LA, Conant JA, deBros F, et al. Caffeine alters plasma adenosine levels. Nature 1997; 389: 136. [DOI] [PubMed] [Google Scholar]
- 37.Traversa U, Rosati AM, Florio C, et al. Effects of chronic administration of adenosine antagonists on adenosine A1 and A2a receptors in mouse brain. In Vivo 1994; 8: 1073–1078. [PubMed] [Google Scholar]
- 38.Carruthers AM, Sellers LA, Jenkins DW, et al. Adenosine A(1) receptor-mediated inhibition of protein kinase A-induced calcitonin gene-related peptide release from rat trigeminal neurons. Mol Pharmacol 2001; 59: 1533–1541. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths RR, Evans SM, Heishman SJ, et al. Low-dose caffeine physical dependence in humans. J Pharmacol Exp Ther 1990; 255: 1123–1132. [PubMed] [Google Scholar]
- 40.Deboer T, van Diepen HC, Ferrari MD, et al. Reduced sleep and low adenosinergic sensitivity in cacna1a R192Q mutant mice. Sleep 2013; 36: 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, Parrish TB. Caffeine dose effect on activation-induced BOLD and CBF responses. Neuroimage 2009; 46: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer A, Holschbach MH, Meyer PT, et al. In vivo imaging of adenosine A1 receptors in the human brain with [18F]CPFPX and positron emission tomography. Neuroimage 2003; 19: 1760–1769. [DOI] [PubMed] [Google Scholar]
- 43.D'Urzo AD, Jhirad R, Jenne H, et al. Effect of caffeine on ventilatory responses to hypercapnia, hypoxia, and exercise in humans. J Appl Physiol 1990; 68: 322–328. [DOI] [PubMed] [Google Scholar]
- 44.Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron 2015; 86: 902–922. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Dai S, An J, et al. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience 2008; 151: 1198–1207. [DOI] [PubMed] [Google Scholar]
- 46.Sachse KT, Jackson EK, Wisniewski SR, et al. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab 2008; 28: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hering-Hanit R, Gadoth N. Caffeine-induced headache in children and adolescents. Cephalalgia 2003; 23: 332–335. [DOI] [PubMed] [Google Scholar]
- 48.Sjaastad O, Bakketeig LS. Caffeine-withdrawal headache. The Vaga study of headache epidemiology. Cephalalgia 2004; 24: 241–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material for Caffeine does not affect susceptibility to cortical spreading depolarization in mice by Nilufer Yalcin, Shih-Pin Chen, Esther S Yu, Tzu-Ting Liu, Jiin-Cherng Yen, Yahya B Atalay, Tao Qin, Furkan Celik, Arn MJM van den Maagdenberg, Michael A Moskowitz, Cenk Ayata and Katharina Eikermann-Haerter in Journal of Cerebral Blood Flow & Metabolism