Abstract
Migraine abortives likely target both peripheral-dural and central trigeminovascular mechanisms in mediating their therapeutic effects. However, in preclinical assays, many migraine preventives have little success at inhibiting similar trigeminovascular-mediated peripheral changes within the dural microenvironment. In addition, their effects on central trigeminovascular neuronal responses are largely unknown. Using a validated preclinical model of acute dural-intracranial (migraine-like) head pain, using Sprague Dawley rats, we tested whether migraine preventives suppress ongoing firing of central trigeminocervical neurons, and evoked responses to cranial neurovascular activation. Flunarizine, sodium valproate, propranolol, and amitriptyline, all dose-dependently inhibited ongoing spontaneous firing of dural trigeminovascular neurons, and differentially affected neuronal responses to intracranial-dural and extracranial-cutaneous somatosensory stimulation. Lamotrigine, only effective in the treatment of migraine aura, did not affect responses. These data provide a mechanistic rationale for the clinical effects of migraine preventives in the treatment of migraine, via the modulation of dural-responsive central trigeminovascular neurons. Also, given their limited effect on peripheral dural vasdilatory responses, these data also suggest that migraine preventives specifically target central, rather than peripheral, components of trigeminal neurovascular mechanisms involved in migraine pathophysiology, to mediate their preventive action. Finally, these data further validate this preclinical model of central trigeminovascular activation to screen migraine preventives.
Keywords: Migraine, preventives, trigeminovascular system, peripheral, central
Introduction
Migraine is thought of as a neurovascular disorder, with migraine headache thought to involve activation and sensitization,1 and the dysfunctional processing of somatosensory information,2 of the trigeminal neurovascular system. The ‘trigeminovascular system’ includes the trigeminal primary afferents that innervate the nociceptive-specific dural vasculature, and their central projections to the medullary dorsal horn and upper cervical spinal cord. Sensitization of central trigeminovascular neurons, which also receive convergent inputs from facial cutaneous areas, mediates extracranial facial hypersensitivity within the area of referred head pain that can manifest as cutaneous allodynia and hyperalgesia in migraine patients.3,4 The importance of both the primary afferent and central second-order projections in migraine pathophysiology cannot be overstated.
Studies from the 1940s in conscious patients undergoing open cranial surgery demonstrate that noxious stimulation, including electrical and mechanical manipulation of intracranial structures, particularly the dural vasculature, results in pain referred to the head, similar to migraine.5,6 This pain is localized to specific head regions depending on the site of stimulation, similar to migraine headache localization, and other symptoms associated with migraine including nausea and photophobia are also mediated. Furthermore, stimulation of sites away from these blood vessels is much less nociceptive, with correspondingly less severe symptoms of headache. Thus, activation, or the perception of activation, of the primary afferent projection to specifically dural vascular structures is crucially important in mediating migraine-like headache. Trigeminal neurons of the medullary dorsal horn and upper cervical spinal cord, commonly known as the ‘trigeminocervical complex’ (TCC), relay all somatosensory information coming from the face and head, including the intracranial dural vasculature, before it is processed in higher pain centers. These neurons are also under the control of pain-modulatory circuits in the brainstem.7 TCC neurons are therefore considered as important integrative relay neurons between peripheral and central pain mechanisms.
This experimental approach is modeled in preclinical studies using noxious dural electrical stimulation, and it results in dural meningeal vasodilation8 and activation of primary afferent and second-order trigeminocervical neurons.9–11 Both peripheral and central trigeminal neurovascular responses are effectively inhibited by established abortive migraine treatments, including triptans,12–16 calcitonin gene-related peptide (CGRP) receptor antagonists,12,17,18 and non-steroidal anti-inflammatory drugs (NSAIDs).19,20 However, the responses of first-line migraine preventives (Table 1) to neurogenic dural vasodilation in the peripheral arm of the trigeminal neurovascular system are far less predictive of therapeutic migraine efficacy.8,21–23 Only the response of topiramate would predict clinical efficacy.8 Topiramate also effectively inhibits trigeminocervical and trigeminothalamic neuronal responses to dural vascular stimulation,24,25 yet the trigeminocervical neuronal effects of other migraine preventives to dural trigeminovascular activation are not known. In the present study, we used this validated preclinical approach to determine whether migraine preventives preferentially effect central trigeminocervical neuronal responses to dural neurovascular activation, compared to their general lack of effect on peripheral dural neurovascular mechanisms. This would highlight a clear difference in the responses of abortive and preventive migraine drugs in peripheral and central neurovascular mechanisms related to migraine pathophysiology, as well as providing a rationale for using these methods to screen therapeutic efficacy of novel drug targets.
Table 1.
Clinical and preclinical doses of established preventives of migraine and migraine aura.
| Migraine preventive | Class | Human dose range35–38a | Preclinical dose range – Intravenous |
|---|---|---|---|
| Flunarizine | Ca2+ channel blocker | 0.1–0.2 mg/kg, q.i.d, oral | 1–5 mg/kg22,59 |
| Sodium valproate | Anti-epileptic | 5–20 mg/kg b.i.d., oral | 30–100 mg/kg23,60–62 |
| Topiramate | Anti-epileptic | 1–5 mg/kg, oral | 5–50 mg/kg8,24,25,52,53 |
| Propranolol | β-blocker | 1–3 mg/kg, oral | 1–5 mg/kg21,63 |
| Amitriptyline | Tricyclic anti-depressant | 0.3–1.5 mg/kg, oral | 1–5 mg/kg52 |
| Lamotrigine | Na+ channel blocker | 3 mg/kg, oral | 1–5 mg/kg50 |
Assuming an average weight for adults of 75 kg, b.i.d., twice daily, q.i.d., once a day, HBC, 2-hydroxypropyl-β-cylcodextrin.
Materials and methods
Ethics statement
All experiments were conducted under license of the UCSF or NYU Institutional Animal Care and Use Committee, and conforming to the National Institute of Health Guide for the Care and Use of Laboratory Animals, and adhered to the guidelines of the Committee for Research and Ethical Issues of IASP,26 and adhering to ARRIVE guidelines.27
Animal preparation
The surgical preparation, recording methods and analyses have been reported in detail previously;19,28,29 107 male Sprague-Dawley rats (245–440 g, Charles River laboratories, MA) were housed in pairs in a light and temperature controlled environment for at least seven days prior to use, with access to food and water ad libitum. They were anesthetized with sodium pentobarbital (80 mg/kg, intraperitoneal, Diamondback Drugs, AZ) and maintained with propofol (PropoFlo™ 15–25 mg/kg/h intravenous infusion, Abbott Laboratories, IL). During electrophysiological recording, rats were also paralyzed with pancuronium bromide (Pavulon®, Organon, NJ) 0.4 mg initially and maintained with 0.2 mg every 35 min, or gallamine triethiodide (Sigma-Aldrich, MI) 25 mg/kg initially and maintained with 15 mg/kg every 35–40 min. All rats were prepared for physiological measurement and drug administration with cannulation of a femoral artery, and both femoral veins, and received a tracheal cannulation. They were placed in a stereotaxic frame (David Kopf Instruments, CA) and continuously monitored for blood pressure (PM-1000 transducer amplifier, CWE Inc., PA), and body temperature (Homeothermic Blanket System, Harvard Apparatus, MA) and kept within normal physiological parameters; blood pressure maintained at mean 80–100 mmHg and temperature maintained at 37.6℃. Rats were also artificially ventilated with oxygen-enriched air, 2 ml, 80–90 strokes per minute (Small Animal Ventilator, Ugo Basile, Italy) and expired CO2 (Capstar-100, CWE Inc., PA) was measured and kept between 3.5 and 4.5%. It is acknowledged that a potential adverse event of pentobarbital dosing, especially if it is increased, is respiratory suppression. Artificially ventilating the rats is an approach that will alleviate this suppression. Here we report no adverse events during this study. A sufficient depth of anesthesia was judged by the absence of paw withdrawal and corneal blink reflex, and during muscular paralysis by fluctuations of blood pressure and changes to expired CO2. At the end of each experiment, all rats were euthanized with a lethal dose of intravenous pentobarbital and phenytoin sodium (200 mg/kg, intravenous, Euthasol, Henry Schein Animal Health, OH).
Cranial surgery preparation for electrophysiological recording
To model the intracranial pain believed to be responsible for migraine-like headache, we used stimulation of the trigeminal innervation of the dural vasculature. For this, the skull was exposed and a partial craniotomy of the parietal and temporal bones was performed with saline-cooled drilling to expose the dural middle meningeal artery, and the area was covered in mineral oil (Figure 1(a)). To gain access to the trigeminocervical complex (TCC) for electrophysiological recording, the muscles of the dorsal neck were separated and a C1 laminectomy was performed and the dura mater incised to expose the brainstem at the level of the caudal medulla. A tungsten recording electrode (0.5–1 MΩ, tip diameter 0.5 µm, World Precision Instruments, Inc. FL) was lowered into the TCC region of the brainstem at 5 µm increments with a piezoelectric motor controller (Burleigh Inc., USA). The neuronal signal was amplified (Neurolog, Digitimer, UK), filtered (Humbug, Quest Scientific, Vancouver) and fed to a gated amplitude discriminator and analogue-to-digital converter (Micro 1401, Cambridge Electronic Design, UK) and to a microprocessor-based computer for analysis using Spike 2 v8. Additionally, the signal was fed to a loudspeaker for audio monitoring and displayed on an analogue oscilloscope to assist isolation of action potentials from adjacent cell activity and noise. Post and peri-stimulus time histograms of neural activity were displayed and analyzed.
Figure 1.
Experimental set-up for electrophysiological studies. (a). Neurons of the trigeminocervical complex were recorded in response to electrical stimulation of the trigeminal innervation of the dural meninges, and measurements of the cutaneous facial receptive field (shaded area). (b). Somatotopic representation of the trigeminal territories for receptive field characterization; V1, ophthalmic; V2, maxillary; V3, mandibular; 5Gn, trigeminal ganglion; C1-4, cervical regions. (c). Original tracing of a single sweep (stimulus) of a reproducible, dural-evoked multi-unit neuronal cluster classified as receiving Aδ-fiber input (<20 ms latency, ‘fast’ neuronal responses), but also with later unitary discharges that are classified as receiving C-fiber input (between 35 and 65 ms; slow neuronal responses), prior to any treatment (baseline – upper plot). As an example (lower plot), after administration of an effective migraine preventive, amitriptyline (5 mg/kg), the number of Aδ-fiber and C-fiber latency action potentials spikes is attenuated, indicative of inhibiting activation of these nociceptive nerve fibers. (d). Original example of the electrophysiological neuronal response to innocuous brush and noxious pinch of the cutaneous V1 receptive field. Top panel is original electrophysiological output, bottom panel is responses that cross the window discriminator.
Characterization of neurons
Extracellular recordings were made from multi-unit neuronal clusters in the TCC, identified as having cutaneous and deep facial receptive fields, and were assessed in all three trigeminal territories (Figure 1(b)). The receptive field was assessed for both non-noxious, with gentle brushing using a cotton tip applicator, and noxious inputs, with pinching with forceps that was painful when applied to humans. Neuronal clusters identified as being sensitive to stimulation of at least the ophthalmic facial dermatome of the trigeminal nerve were then tested for convergent nociceptive input from the dura mater. Nociceptive-responsive trigeminal afferents were activated using electrical stimulation of the dura mater adjacent to the middle meningeal artery through the open cranial window, with a stainless steel bipolar stimulating electrode (0.2 mm contact diameter, separation 0.5 mm; NE200, Clark Electromedical, Harvard Apparatus, MA) using square-wave stimuli (100–200 µs pulse, 0.25 Hz and 4–15 V: S88 Stimulator, Grass Instruments, RI). Having established neuronal clusters sensitive to stimulation of the ophthalmic dermatome of the trigeminal nerve, and inputs from the dura mater, baseline responses were characterized under test conditions. This consisted of trains of 20 stimuli delivered at 5 min intervals, stimulating the dura mater. Responses were analyzed using post-stimulus histograms with a sweep length of 100 ms and a bin width of 1 ms that separated Aδ-fiber and C-fiber activated firing for dural-evoked responses. The distinction of both trigeminal Aδ (‘fast’ responses) and C-fibers (‘slow’ responses; Figure 1(c)) that innervate the dura mater is based on the approximate conduction velocities of Aδ (2.0–30.0 m/s) and C-fiber (0.5–2.0 m/s)30 primary afferents and the distance of the trigeminal ganglion from the dural stimulation site, plus the distance from the trigeminal ganglion to the recording site in the TCC (approximately 30–40 mm).28 Facial receptive field characterization consisted of 10 brush strokes applied to the cutaneous receptive field region over 7–8 s for the innocuous response, and pinch with forceps for 4 s for the noxious response (Figure 1(d)). Spontaneous activity (spikes per second, Hz) was recorded throughout and measures for analysis taken for 300 s preceding the dural stimulation using a peri-stimulus histogram. Reliable TCC neuronal baseline responses were then tested in response to intravenous (IV) administration of first-line migraine preventives.
Drugs
Sodium valproate (valproic acid sodium salt; Sigma-Aldrich, MI), (S)-(-)-propranolol hydrochloride, amitriptyline hydrochloride, and lamotrigine isethionate (all Tocris Bioscience, MN) were dissolved in 0.9% NaCl. Flunarizine dihydrochloride was dissolved in a 45% w/v 2-hydroxypropyl-β-cylcodextrin (Sigma-Aldrich, MI) aqueous solution. All drug doses used were based on clinical doses for migraine and those used previously in preclinical studies, summarized in Table 1. All drugs and vehicles were administered intravenously (IV) in a volume of 0.3 ml, over several minutes, to avoid profound physiological changes to blood pressure and expired CO2. As a consequence, there were only transient and mild (less than 10 mmHg) changes to blood pressure in response to drug infusion, and these changes returned to baseline levels (within 5 min) prior to the subsequent measurements of neuronal responses.
Statistical analysis
Rats were randomly assigned to treatment or control groups. All data were tested for normality and if not normally distributed non-parametric statistics were used. Sample sizes were determined using previous experience, and published data, which typically sees differences in means = 25–30%, SD = 15–20%, two-sided alpha = 0.05 and power = 0.8, which provides a sample size of 8–10 animals. Each experimental group within a series was assigned an integer and all rats were randomly assigned to the different experimental protocols and drug groups using the Microsoft Excel random number generator with the parameters set by the number of groups. We worked through these groups in the order assigned by the random number generator.
The exact latency of multi-unit neuronal clusters for Aδ-fiber inputs and unitary discharges for C-fiber inputs was established separately in each experiment and this latency window used throughout. The data collected from post-stimulus histograms after electrical stimulation of the dura mater represent the number of action potential spikes that fired within the latency window per stimulation, averaged over 20 stimulations (sweeps; spikes/sweep, s/s). Cutaneous receptive field responses and ongoing spontaneous neuronal activity were measured in cell firings per second (Hz). Data were assessed over 1 h using the average of all data points taken every 15 min. Statistical analysis was performed using an ANOVA for repeated measures with Bonferroni post hoc correction for multiple comparisons, to measure the time course of migraine preventive action, using a 95% confidence interval. If Mauchly’s test of sphericity was violated, we made appropriate corrections to degrees of freedom according to Greenhouse-Geisser. Student’s paired two-tailed t-test for post-hoc analysis was used to test for the time points of significance, using the average of the three baselines for comparison, again using the criteria of Bonferroni correction. Kruskal–Wallis test was used for non-parametric data, where applicable. Statistical significance was set at *P < 0.05 (using IBM SPSS 22.0 throughout).
Results
Neuronal identification and properties
We tested whether migraine preventive drugs altered ongoing neuronal firing of trigeminocervical neurons in the central nervous system, and changes in the responses of these neurons to intra- and extra-cranial somatosensory stimulation, to determine if they alter central trigeminocervical responses similar to their efficacy as migraine preventives. Electrophysiological recordings were made from 107 multi-unit neuronal clusters that responded to electrical stimulation of the trigeminal afferent innervation of the dura mater, and were also characterized as having noxious and/or innocuous cutaneous facial receptor fields that included predominantly the 1st (ophthalmic) division of the trigeminal nerve, and on occasions also the 2nd (maxillary) and 3rd (mandibular) division (Figure 1(b)). Of these 107, 66 multi-unit neuronal clusters (all wide dynamic range (WDR), being responsive to both noxious and innocuous somatosensory inputs) exhibited a combination of either reproducible burst of discharges at 3–20 ms (Aδ fiber) or 4–30 ms (both Aδ and C-fiber), with also a unitary discharge at 18–92 ms (C-fiber), which were classified as receiving both Aδ and C-fiber inputs. Forty-one multi-unit neuronal clusters (all WDR) exhibited only early discharge responses at 3–20 ms (Aδ-fiber), which were classified as receiving only Aδ-fiber inputs. Neurons were located in mainly nociceptive-specific superficial (laminae I-II) and deeper layers (laminae V and VI) of the dorsal horn of the TCC at range of depth, 100–1100 µm. This distribution of neuronal properties is similar to previous studies,31 and these properties were similar across the different laminae (superficial/deeper) locations. While the assignment of neurons to drug groups was random, these different locations were evenly distributed throughout all the groups, and the proportion of neurons classified as receiving both Aδ and C-fiber inputs, or Aδ-fiber only, within each drug group was within a normal distribution. This suggests the neuronal recording location and nerve fiber properties within the TCC for each drug group could not, and did not, affect the overall results, based on having different neuronal properties. An example of an Aδ-fiber response with C-fiber unitary discharges, and how these responses change after amitriptyline treatment is highlighted in Figure 1(c).
Through all animals/groups studied, the average baseline ongoing spontaneous firing rate was 20.4 ± 2 Hz, which is a similar range to previous studies,28,32 and there was no significant difference in firing across all the different groups (F11,106 = 0.67, P = 0.76). There was also no significant difference at baseline between all groups for dural-evoked multi-units classified as within the Aδ-fiber range (3–20 ms) and with extended C-fiber latencies (4–30 ms, F11,106 = 1.7, P = 0.08), or unitary discharges within the C-fiber range (18–92 ms; H11 = 14.6, P = 0.20), with an average baseline of 8.2 ± 0.2 and 1.6 ± 0.2 action potential spikes per sweep (s/s), respectively. There was also no significant difference at baseline between groups in response to both innocuous (F11,104 = 0.81, P = 0.64) and noxious (F11,104 = 0.53, P = 0.88) somatosensory stimulation of the facial receptive field. Therefore, despite inter-animal variation in the baseline responses, these differences can be considered normal, and support the validity of the experimental model and subsequent analyses.
Effects of flunarizine on cranial-evoked somatosensory trigeminocervical neuronal responses
Flunarizine (1–5 mg/kg, IV) dose-dependently inhibited trigeminocervical neuronal responses. At 1 mg/kg it had no effect on ongoing spontaneous trigeminal neuronal firing (F1.7,12.0 = 0.45, P = 0.62, n = 8), or neuronal responses within the Aδ-fiber (F4,28 = 1.1, P = 0.35) and C-fiber range (F4,16 = 1.9, P = 0.17) to intracranial-dural electrical stimulation, or responses to innocuous (F4,28 = 0.4, P = 0.82) and noxious (F4,28 = 0.18, P = 0.95) somatosensory stimulation of the cutaneous receptive field in the ophthalmic dermatome, over 60 min. However, at 5 mg/kg, flunarizine significantly inhibited ongoing spontaneous trigeminal neuronal firing (F2.1,16.9 = 4.0, P = 0.035, n = 9), specifically after 15 min (t8 = 3.31, P = 0.011) from 13.4 ± 2 to 7.9 ± 2 Hz that returned to baseline levels after 60 min. It also inhibited trigeminocervical neuronal responses to intracranial-dural electrical stimulation within both the Aδ-fiber (F1.6,13.0 = 4.2, P = 0.046) and C-fiber range (F4,16 = 3.7, P = 0.026). Aδ-fiber multi-unit neuronal clusters were specifically inhibited after 30 min (t8 = 4.7, P = 0.002, from 6.1 ± 0.3 to 5.0 ± 0.6 s/s), which remained until at least 60 min; 5 mg/kg also significantly inhibited neuronal responses to both innocuous (F4,32 = 3.3, P = 0.024) and noxious (F4,32 = 3.6, P = 0.021) somatosensory stimulation of the cutaneous facial receptive field. These data are summarized in Figure 2.
Figure 2.
Effects of flunarizine on dural-evoked trigeminocervical neuronal responses. (a) Time course changes in ongoing spontaneous trigeminocervical neuronal firing in response to flunarizine (1 and 5 mg/kg). Only 5 mg/kg caused a significant inhibition of ongoing spontaneous neuronal firing. The data have been normalized to represent the percentage change from baseline, and are expressed as mean ± SEM. Histograms of the time course changes in the average number of action potential spikes per sweep (mean ± SEM) of (b) intracranial dural-evoked multi-unit trigeminocervical neuronal clusters with inputs in the Aδ-fiber range (3–20 ms; ‘fast’ neuronal responses), and (c) unitary discharges within the C-fiber latency range (‘slow’ neuronal responses). Only 5 mg/kg flunarizine inhibited dural-evoked Aδ and C-fiber neuronal responses in the trigeminocervical complex (TCC). It also inhibited TCC neuronal responses to both (d) innocuous and (e) noxious somatosensory-evoked stimulation of the cutaneous facial receptive field. In all panels, vehicle control (hydroxypropyl-β-cyclodextrin; HBC) had no significant effects on neuronal responses. *P < 0.05 compared to baseline.
Effects of sodium valproate on cranial-evoked somatosensory trigeminocervical neuronal responses
Sodium valproate (30–100 mg/kg, IV) dose-dependently inhibited trigeminocervical neuronal responses. At 30 mg/kg, it had no effect on ongoing spontaneous trigeminal neuronal firing (F2.0,15.7 = 2.4, P = 0.12, n = 9), or innocuous-evoked cutaneous facial neuronal responses (F4,32 = 0.74, P = 0.57). However, in the same neuronal population, it significantly inhibited dural-evoked multi-unit neuronal responses within the Aδ-fiber range (F4,32 = 3.4, P = 0.021), and unitary discharges within the C-fiber range (F4,32 = 3.2, P = 0.042), as well as significantly inhibiting neuronal responses mediated by noxious cutaneous facial stimulation (F4,32 = 2.8, P = 0.044). 100 mg/kg sodium valproate significantly inhibited ongoing spontaneous trigeminal neuronal firing (F4,32 = 3.4, P = 0.019, n = 9), specifically after 30 min (t8 = 3.0, P = 0.017) from 16.4 ± 7 to 11.9 ± 6 Hz. It also inhibited trigeminocervical neuronal responses to intracranial-dural electrical stimulation within both the Aδ-fiber (F1.8,14.4 = 5.2, P = 0.022) and C-fiber range (F4,16 = 8.7, P = 0.001). Both Aδ (t8 = 3.9, P = 0.005, from 8.0 ± 0.5 to 7.1 ± 0.6) and C-fiber (t4 = 3.9, P = 0.017, from 3.0 ± 1.0 to 1.2 ± 0.5) responses were specifically inhibited after 15 min but had returned to baseline levels by 60 min. It also significantly inhibited responses to innocuous (F4,32 = 4.6, P = 0.005) and noxious (F1.5,10.7 = 4.6, P = 0.04) somatosensory stimulation of the cutaneous facial receptive field. These data are summarized in Figure 3.
Figure 3.
Effects of sodium valproate on dural-evoked trigeminocervical neuronal responses. (a) Time course changes in ongoing spontaneous trigeminocervical neuronal firing in response to sodium valproate (30 and 100 mg/kg). Only 100 mg/kg caused a significant inhibition of ongoing spontaneous neuronal firing. The data have been normalized to represent the percentage change from baseline, and are expressed as mean ± SEM. Histograms of the time course changes in the average number of action potential spikes per sweep (mean ± SEM) of (b) intracranial dural-evoked multi-unit trigeminocervical neuronal clusters with inputs in the Aδ-fiber range (3–20 ms; ‘fast’ neuronal responses), and (c) unitary discharges within the C-fiber (‘slow’ neuronal responses) latency range. Both 30 and 100 mg/kg sodium valproate inhibited dural-evoked Aδ and C-fiber neuronal responses in the trigeminocervical complex (TCC). Sodium valproate also inhibited TCC neuronal responses to (d) innocuous (100 mg/kg) and (e) noxious (30 and 100 mg/kg) somatosensory-evoked stimulation of the cutaneous facial receptive field. In all panels, vehicle control (saline) had no significant effects on neuronal responses. *P < 0.05 compared to baseline.
Effects of propranolol on cranial-evoked somatosensory trigeminocervical neuronal responses
Propranolol significantly inhibited ongoing spontaneous trigeminal neuronal firing at both 1 mg/kg (F1.9,15.5 = 5.4, P = 0.018, n = 9) and 5 mg/kg (F1.9,15.1 = 10.2, P = 0.002, n = 9). It was most efficacious at 5 mg/kg where it specifically inhibited neuronal firing after 15 min (t8 = 4.2, P = 0.003) from 20.7 ± 6 to 7.5 ± 3 Hz that lasted beyond 60 min. However, in the same neuronal populations, this inhibition did not manifest as an effect on the number of action potential spikes in response to intracranial-dural electrical stimulation of neurons classified within the Aδ-fiber range (1 mg/kg, F4,32 = 1.4, P = 0.26; 5 mg/kg, F4,28 = 1.1, P = 0.39), or within the C-fiber range (1 mg/kg, F1.6,6.3 = 0.5, P = 0.58; 5 mg/kg, F1.9,11.4 = 1.2, P = 0.33). Propranolol also had no effect on neuronal responses to innocuous (1 mg/kg, F4,32 = 0.6, P = 0.68; 5 mg/kg, F1.8,14.3 = 0.8, P = 0.48) and noxious (1 mg/kg, F2.1,16.5 = 0.3, P = 0.77; 5 mg/kg, F4,32 = 1.6, P = 0.23) somatosensory stimulation of the cutaneous facial receptive field, over the 60 min. These data are summarized in Figure 4.
Figure 4.
Effects of propranolol on dural-evoked trigeminocervical neuronal responses. (a) Time course changes in ongoing spontaneous trigeminocervical neuronal firing in response to propranolol (1 and 5 mg/kg). Both 1 and 5 mg/kg caused a significant inhibition of ongoing spontaneous neuronal firing. The data have been normalized to represent the percentage change from baseline, and are expressed as mean ± SEM. Histograms of the time course changes in the average number of action potential spikes per sweep (mean ± SEM) of (b) intracranial dural-evoked multi-unit trigeminocervical neuronal clusters with inputs in the Aδ-fiber and C-fiber range (3–30 ms; ‘fast’ neuronal responses), and (c) unitary discharges within the C-fiber (‘slow’ neuronal responses) latency range. Neither dose affected dural-evoked Aδ and C-fiber neuronal responses in the trigeminocervical complex (TCC). Similarly, neither dose affected TCC neuronal responses to (d) innocuous and (e) noxious somatosensory-evoked stimulation of the cutaneous facial receptive field. In all panels, vehicle control (saline) had no significant effects on neuronal responses. *P < 0.05 compared to baseline.
Effects of amitriptyline on cranial-evoked somatosensory trigeminocervical neuronal responses
Amitriptyline (1–5 mg/kg, IV) significantly and dose-dependently inhibited trigeminocervical neuronal responses. At 1 mg/kg, it inhibited ongoing spontaneous trigeminal neuronal firing (F4,32 = 5.2, P = 0.003, n = 9). In the same neuronal population, it also significantly inhibited dural-evoked multi-unit neuronal clusters that received inputs classified within the Aδ-fiber range (F4,32 = 2.7, P = 0.048), and unitary discharges within the C-fiber range (F4,32 = 33.8, P = 0.018). However, it had no effect on either innocuous (F1.4,11.8 = 0.77, P = 0.45) or noxious- (F4,32 = 0.47, P = 0.76) evoked cutaneous facial trigeminocervical neuronal responses. At 5 mg/kg, it also significantly inhibited ongoing spontaneous trigeminal neuronal firing (F1.2,10.0 = 8.1, P = 0.014, n = 9), specifically after 15 min (t8 = 3.7, P = 0.006) from 19.0 ± 4 to 5.4 ± 1 Hz that returned to baseline by 60 min. It also significantly inhibited neuronal trigeminocervical neuronal responses to intracranial-dural electrical stimulation within both the Aδ-fiber (F2.2,17.5 = 10.3, P = 0.001) and C-fiber range (F4,20 = 3.3, P = 0.03). Both Aδ (t8 = 4.3, P = 0.003, from 9.0 ± 0.5 to 6.2 ± 0.5) and C-fiber (t5 = 2.7, P = 0.043, from 1.8 ± 0.5 to 1.1 ± 0.4) responses were specifically inhibited after 15 min, but Aδ-fiber responses returned to baseline by 45 min, whereas C-fiber responses returned to baseline by 30 min. Amitriptyline (5 mg/kg) also significantly inhibited responses to innocuous (F4,32 = 7.6, P = 0.001) and noxious (F4,32 = 4.1, P = 0.008) somatosensory stimulation of the cutaneous facial receptive field. These data are summarized in Figure 5.
Figure 5.
Effects of amitriptyline on dural-evoked trigeminocervical neuronal responses. (a) Time course changes in ongoing spontaneous trigeminocervical neuronal firing in response to amitriptyline (1 and 5 mg/kg). Both doses caused a significant inhibition of ongoing spontaneous neuronal firing. The data have been normalized to represent the percentage change from baseline, and are expressed as mean ± SEM. Histograms of the time course changes in the average number of action potential spikes per sweep (mean ± SEM) of (b) intracranial dural-evoked multi-unit trigeminocervical neuronal clusters with inputs in the Aδ-fiber range (3–25 ms; ‘fast’ neuronal responses), and (c) unitary discharges within the C-fiber (‘slow’ neuronal responses) latency range. Both 1 and 5 mg/kg amitriptyline significantly inhibited dural-evoked Aδ and C-fiber neuronal responses in the trigeminocervical complex (TCC). However, only 5 mg/kg inhibited TCC neuronal responses to (d) innocuous and (e) noxious somatosensory-evoked stimulation of the cutaneous facial receptive field. In all panels, vehicle control (saline) had no significant effects on neuronal responses. *P < 0.05 compared to baseline.
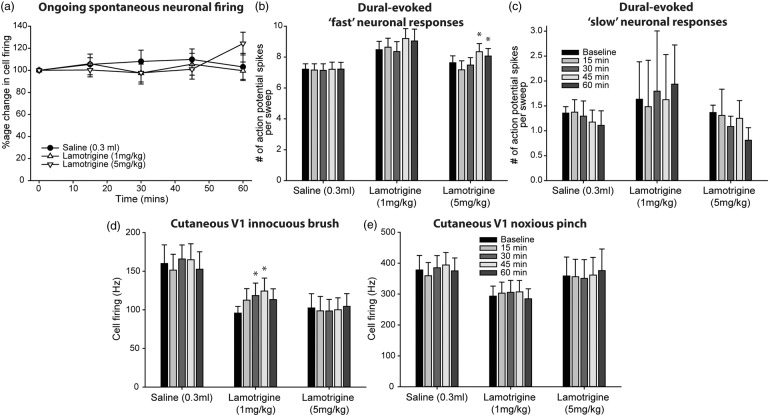
Effects of lamotrigine on cranial-evoked somatosensory trigeminocervical neuronal responses
Lamotrigine (1–5 mg/kg, IV) did not have an inhibitory effect on ongoing spontaneous trigeminocervical neuronal firing (1 mg/kg, F4,32 = 0.96, P = 0.44, n = 9; 5 mg/kg, F4,32 = 2.5, P = 0.061, n = 9). However, in these same neuronal populations, it significantly increased dural-evoked multi-unit neuronal clusters that received inputs classified within the Aδ-fiber range (1 mg/kg, F4,32 = 3.9, P = 0.01; 5 mg/kg, F2.0,16.0 = 9.4, P = 0.002), but had no effect on unitary discharges within the C-fiber range (1 mg/kg, F1.5,4.4 = 0.53, P = 0.57; 5 mg/kg, F4,32 = 0.46, P = 0.76). It also increased innocuous-evoked cutaneous facial trigeminocervical neuronal responses, only at the 1 mg/kg dose (F4,32 = 4.2, P = 0.008; 5 mg/kg, F1.8,14.4 = 0.3, P = 0.72), but had no effect on noxious-evoked neuronal responses (1 mg/kg, F1.5,12.0 = 0.6, P = 0.5; 5 mg/kg, F1.8,14.0 = 0.3, P = 0.71). These data are summarized in Figure 6.
Figure 6.
Effects of lamotrigine on dural-evoked trigeminocervical neuronal responses. (a) Time course changes in ongoing spontaneous trigeminocervical neuronal firing in response to lamotrigine (1 and 5 mg/kg). Neither dose had an effect on ongoing spontaneous neuronal firing. The data have been normalized to represent the percentage change from baseline, and are expressed as mean ± SEM. Histograms of the time course changes in the average number of action potential spikes per sweep (mean ± SEM) of (b) intracranial dural-evoked multi-unit trigeminocervical neuronal clusters with inputs in the Aδ-fiber range (3–25 ms; ‘fast’ neuronal responses), and (c) unitary discharges within the C-fiber (‘slow’ neuronal responses) latency range. Both 1 and 5 mg/kg lamotrigine significantly increased dural-evoked Aδ-fiber neuronal responses in the trigeminocervical complex (TCC), but neither dose had an effect on unitary C-fiber discharges. Only 1 mg/kg lamotrigine increased TCC neuronal responses to (d) innocuous somatosensory-evoked stimulation of the cutaneous facial receptive field. There was not effect of either dose on (e) noxious-evoked responses. In all panels, vehicle control (saline) had no significant effects on neuronal responses. *P < 0.05 compared to baseline.
Both vehicle controls, saline and 2-hydroxypropyl-β-cyclodextrin (HBC), had no effect on either ongoing spontaneous trigeminocervical neuronal firing (saline; F4,32 = 0.84, P = 0.51, n = 9, HBC; F4,32 = 0.50, P = 0.74, n = 9) or all somatosensory-evoked neuronal responses over the 60 min recording window. HBC data are summarized in Figure 2, and saline data presented in Figure 3, and repeated for the sake of direct comparison of each drug group in Figures 4 to 6.
Discussion
In this study, we demonstrate that established first line migraine preventives are able to inhibit ongoing and acute nociceptive activation of central trigeminal neurons, mediated by activation of the trigeminal innervation to the dural vasculature, thought to model acute intracranial migraine-like pain. These data suggest that part of the mechanism of action of these migraine preventive drugs is via modulation of intracranial trigeminovascular nociceptive neurotransmission. Overall, when compared, the efficacy of these drugs to inhibit nociceptive activation of trigeminocervical neurons differs between them. This is perhaps indicative of different mechanisms of therapeutic action, and may also explain the presence of known side-effects with some of these treatments. Ultimately, these data are suggestive that migraine preventives specifically target central components of trigeminal neurovascular mechanisms involved in migraine pathophysiology to mediate their preventive action, rather than impacting peripheral neurovasodilatory mechanisms; a significant difference to the likely mechanisms of abortive migraine drugs.
Triptans, including sumatriptan, rizatriptan and naratriptan,12–16,33 CGRP receptor antagonists,12,17,18,34 and NSAIDs19,20,22 are all able to inhibit trigeminal neurogenically mediated vasodilation of dural meningeal vessels, as well as inhibiting dural-evoked central trigeminovascular neuronal responses. These data have helped to predict their migraine abortive therapeutic efficacy. Topiramate, flunarizine, sodium valproate and propranolol, all established and effective migraine preventives,35–38 have also been tested in this assay, yet only topiramate was effective at inhibiting the neurogenically mediated peripheral dural meningeal vasodilatory response.21–23 Coupled with the previous topiramate studies,24,25 our data demonstrate that these migraine preventives all dose-dependently inhibit ongoing spontaneous firing of trigeminal neurons, which innervate the dural vasculature. In the case of propranolol it only affected spontaneous activity. Changes to ongoing spontaneous firing likely reflect changes to intrinsic activity of central trigeminovascular neurons. This might be more indicative of the necessary therapeutic action of migraine preventives, whose effectiveness is commonly determined by reduction in headache frequency, as well as headache severity. It could be hypothesized that by dampening the level of background activity of firing of trigeminocervical neurons, and its relay to other pain processing areas of the brain, it is possible these neurons are far less likely to reach the threshold that seems to predict migraine attack susceptibility, reducing the frequency of attacks being triggered. This is in line with current thinking that migraine susceptibility cycles in migraineurs, where responsiveness of spinal trigeminal neurons is lower interictally, and gradually increases nearer to an attack, raising the susceptibility of the brain to generate an attack.39
Previous studies also suggest that the ventroposteromedial (VPM) thalamus might be an important target for migraine preventives. Propranolol, sodium valproate and topiramate24,25,40,41 have all been shown to inhibit local L-glutamate-mediated activation of VPM thalamic neurons that receive inputs from the dura mater. This is likely mediated by post-synaptic mechanisms as glutamate receptors are known to be solely located post-synaptically. It therefore seems likely that within trigeminovascular neurons, the therapeutics actions of these migraine preventives are also mediated post-synaptically to alter intrinsic neuronal activity. In general, these migraine preventives have in common an ability to modulate the relay of trigeminal and thalamic neuronal responses involved in pain processing through actions on post-synaptic sites.
While modulating spontaneous firing of dural-responsive trigeminovascular neurons is common to all these migraine preventives, their direct effects on somatosensory intracranial dural-evoked and extracranial cutaneous facial-evoked trigeminal neuronal responses differ slightly. Both flunarizine and sodium valproate dose-dependently inhibited cranial-evoked responses, while propranolol had no effect on either. It seems likely the therapeutic mechanism of these preventives on spontaneous firing is not causative of the therapeutic mechanism on evoked responses. The data suggest that changes to evoked responses more likely depend upon actions on the peripheral input to block transmission of somatosensory information to the central neuron, perhaps via mechanisms mediated on trigeminal pre-synaptic terminals, or at the level of the trigeminal ganglion or its central axonal projection. This is similar to the predicted actions of triptans in the trigeminovascular system in modulating responses of sensitized dural-trigeminovasclar neurons.42,43
If we take all the data together, this demonstrates a clear difference in the way peripheral and central components of the trigeminal neurovascular system are likely to be targeted and respond, depending on their therapeutic role, and we can hypothesize and compare the likely actions of migraine abortives and preventives. When one considers migraine abortives, for them to be effective, it seems that it is equally important that they are able to inhibit the acute nociceptive signal from the dura mater mediated by changes that may occur at the dural microenvironment, and via the peripheral trigeminal projection, as it is to inhibit the relay of these inputs via the central projection to trigeminocervical neurons and higher pain processing centers. Whereas, it is crucial for migraine preventives to at least target neural components of the trigeminal neurovascular mechanisms involved in migraine pathophysiology, to modulate intrinsic activity of dural-specific trigeminovascular neurons, to mediate their preventive action. Inhibiting changes that occur at the dural vascular microenvironment, as a consequence of activation of the trigeminal innervation of the dural vasculature, seem to be far less relevant for effective migraine prevention. This does not necessarily mean that migraine preventives must enter the central nervous system to be effective. The development and growing success of monoclonal antibodies targeting CGRP and its receptors suggest peripheral neural mechanisms are still relevant.44–46 However, it does highlight the important action of migraine preventives in modulating the intrinsic activity of trigeminocervical neurons, in their role as an integrative relay between peripheral and central nociceptive mechanisms, as well as their relay in projecting to thalamocortical neurons. These actions may be mediated at the level of the trigeminal ganglion or fibers of the central trigeminal projection to be effective, but still in the periphery.
As a positive control for these studies we also used amitriptyline, a selective serotonin re-uptake inhibitor (SSRI) from the anti-depressant class of migraine preventives. Based on the above data, we predicted that amitriptyline would inhibit ongoing and dural-evoked trigeminocervical responses, and indeed this is what we found. It is likely its mechanism of action is by increasing the presence of 5-HT within the synaptic cleft, by preventing its breakdown, to act on both pre- and post-synaptic 5-HT1B/1D ‘triptan’ receptors. In addition, we used administration of lamotrigine as a negative control. Lamotrigine has been demonstrated to be largely ineffective in migraine headache prevention.47–49 However, both clinical48 and preclinical data, using models of cortical spreading depression (CSD),50,51 suggest lamotrigine may be effective in reducing the severity and frequency of aura symptoms. Here we are able to demonstrate that lamotrigine does not inhibit either ongoing or cranial somatosensory-evoked trigeminocervical neuronal responses. In fact, in some cases, it caused an increase in neuronal activity, which would be counter to a likely mechanism for migraine prevention. These data fit with the predicted response as an ineffective migraine preventive.
Interestingly, in animal models, topiramate, valproate, propranolol, and amitriptyline are all known to inhibit CSD,52,53 and it is thought this action is involved in its mechanism of migraine prevention. There is also evidence that CSD mediates activation of trigeminal primary afferents that innervate the dural vasculature54,55; thus activation of the pathway that leads to headache in migraine. Although this is still a hotly debated topic.56,57 It is therefore attractive to speculate that migraine preventives in inhibiting the likelihood of CSD are also inhibiting the likely activation of trigeminal primary afferents to prevent migraine attacks. However, it seems unlikely that this is the sole mechanism of action of migraine preventives. Firstly, we clearly demonstrate new data that each of these preventive drugs inhibits intrinsic trigeminovascular activity, as well as dural-evoked responses in most cases, and there is no evidence here that blocking CSD is the driver to these responses. If blocking CSD was the mechanism, each preventive would block trigeminovascular responses similarly. Secondly, lamotrigine also inhibits CSD and can prevent migraine aura symptoms. However, evidence to suggest that it prevents migraine headache is weak, and here we clearly demonstrate that lamotrigine is ineffective at blocking trigeminovascular responses. It seems more likely that these migraine preventives affect CSD (aura) and trigeminovascular (headache) responses via independent neuronal mechanisms, directly on cortical and trigeminovascular neurons, respectively, or via an as yet unknown neural mechanism that affects both pathways to inhibit cortical and trigeminovascular responses.
These studies are also able to potentially highlight explanations for side-effects with these treatments. Flunarizine, sodium valproate and amitriptyline all inhibited extracranial facial cutaneous-evoked trigeminal neuronal responses. In contrast to other preclinical assays,9,58 which mediate hypersensitive neuronal responses to cutaneous facial stimulation by inducing pathological central sensitization, we measured only acute responses within the trigeminal neurovascular system, processing cutaneous facial nociceptive information normally. Therefore, we would not anticipate an effect on this normal somatosensory nociceptive processing, but only processing of pathological pain, such as mediated by intracranial nociception. However, these data suggest that these preventives also potentially interfere with normal somatosensory processing. It is noteworthy that each causes drowsiness, as a known side-effect, which may impair the patients’ ability to process normal somatosensory information. It is not possible to determine directly how this is achieved, it could be directly on these somatosensory-nociceptive pathways, or indirectly through mechanisms that promote drowsiness and sedation, which may also interfere with somatosensory processing. Propranolol does not cause symptoms of drowsiness, and also does not alter this normal somatosensory facial cutaneous processing, suggesting the two are linked.
In conclusion, we demonstrate that migraine preventives, ineffective at inhibiting dural meningeal vasodilatory responses to neurogenic activation of the trigeminal innervation to the dural vasculature, are able to inhibit ongoing trigeminocervical neuronal firing and their responses to dural trigeminovascular activation. These data provide a mechanistic rationale for the clinical effects of these migraine preventives in the treatment of migraine, via the modulation of dural-responsive central trigeminovascular neurons. In addition, it also highlights a clear difference of responsiveness, and likely mechanism of action, of abortive and preventive migraine drugs in peripheral and central aspects of the trigeminal neurovascular system. Finally, these data further validate this preclinical model to screen migraine preventives, but also highlights the need for the development of alternative preventive treatment strategies, given the side-effect profile of these current therapies.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been funded in part by start-up funds from NYU (MRR, SA) and by a donation from the Sandler Family Foundation to UCSF (SA).
Declaration of conflicting interests
The author(s) declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SA and MRR report an unrestricted grant, honoraria and travel reimbursements from electroCore LLC, unrelated to the submitted work.
Authors’ contributions
SA and MRR conceived and designed the study. SA performed studies, analyzed the data and wrote the first draft of the manuscript. MRR contributed to the writing and provided critical review of the manuscript. SA and MRR provided final approval of the manuscript.
References
- 1.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013; 154(Suppl 1): S44–S53. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol 2000; 47: 614–624. [PubMed] [Google Scholar]
- 4.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol 2008; 63: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penfield W, McNaughton F. Dural headache and innervation of the dura mater. Arch Neurol Psychiatry 1940; 44: 43–75. [Google Scholar]
- 6.Ray BS, Wolff HG. Experimental studies on headache. Pain sensitive structures of the head and their significance in headache. Arch Surg 1940; 41: 813–856. [Google Scholar]
- 7.Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci 2011; 12: 570–584. [DOI] [PubMed] [Google Scholar]
- 8.Akerman S, Goadsby PJ. Topiramate inhibits trigeminovascular activation: an intravital microscopy study. Br J Pharmacol 2005; 146: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 1998; 79: 964–982. [DOI] [PubMed] [Google Scholar]
- 10.Goadsby PJ, Zagami AS. Stimulation of the superior sagittal sinus increases metabolic activity and blood flow in certain regions of the brainstem and upper cervical spinal cord of the cat. Brain 1991; 114: 1001–1111. [DOI] [PubMed] [Google Scholar]
- 11.Kaube H, Keay KA, Hoskin KL, et al. Expression of c-Fos-like immunoreactivity in the caudal medulla and upper cervical spinal cord following stimulation of the superior sagittal sinus in the cat. Brain Res 1993; 629: 95–102. [DOI] [PubMed] [Google Scholar]
- 12.Williamson DJ, Hargreaves RJ, Hill RG, et al. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia 1997. b; 17: 525–531. [DOI] [PubMed] [Google Scholar]
- 13.Williamson DJ, Hill RG, Shepheard SL, et al. The anti-migraine 5-HT(1B/1D) agonist rizatriptan inhibits neurogenic dural vasodilation in anaesthetized guinea-pigs. Br J Pharmacol 2001; 133: 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goadsby PJ, Knight YE. Inhibition of trigeminal neurones after intravenous administration of naratriptan through an action at 5-hydroxy-tryptamine (5-HT(1B/1D)) receptors. Br J Pharmacol 1997; 122: 918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoskin KL, Kaube H, Goadsby PJ. Sumatriptan can inhibit trigeminal afferents by an exclusively neural mechanism. Brain 1996; 119: 1419–1428. [DOI] [PubMed] [Google Scholar]
- 16.Kaube H, Hoskin KL, Goadsby PJ. Inhibition by sumatriptan of central trigeminal neurones only after blood-brain barrier disruption. Br J Pharmacol 1993; 109: 788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer MJ, Koulchitsky S, Messlinger K. The nonpeptide calcitonin gene-related peptide receptor antagonist BIBN4096BS lowers the activity of neurons with meningeal input in the rat spinal trigeminal nucleus. J Neurosci 2005; 25: 5877–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storer RJ, Akerman S, Goadsby PJ. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br J Pharmacol 2004; 142: 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akerman S, Holland PR, Summ O, et al. A translational in vivo model of trigeminal autonomic cephalalgias: therapeutic characterization. Brain 2012; 135: 3664–3675. [DOI] [PubMed] [Google Scholar]
- 20.Summ O, Andreou AP, Akerman S, et al. A potential nitrergic mechanism of action for indomethacin, but not of other COX inhibitors: relevance to indomethacin-sensitive headaches. J Headache Pain 2010; 11: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerman S, Williamson DJ, Hill RG, et al. The effect of adrenergic compounds on neurogenic dural vasodilatation. Eur J Pharmacol 2001; 424: 53–58. [DOI] [PubMed] [Google Scholar]
- 22.Akerman S, Williamson DJ, Kaube H, et al. The effect of anti-migraine compounds on nitric oxide-induced dilation of dural meningeal vessels. Eur J Pharmacol 2002; 452: 223–228. [DOI] [PubMed] [Google Scholar]
- 23.Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microscopy Res Tech 2001; 53: 167–178. [DOI] [PubMed] [Google Scholar]
- 24.Andreou AP, Goadsby PJ. Topiramate in the treatment of migraine: a kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia 2011; 31: 1343–1358. [DOI] [PubMed] [Google Scholar]
- 25.Storer RJ, Goadsby PJ. Topiramate inhibits trigeminovascular neurons in the cat. Cephalalgia 2004; 24: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 27.Kilkenny C, Browne W, Cuthill IC, et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akerman S, Goadsby PJ. Neuronal PAC1 receptors mediate delayed activation and sensitization of trigeminocervical neurons: relevance to migraine. Sci Transl Med 2015; 7: 308ra157. [DOI] [PubMed] [Google Scholar]
- 29.Akerman S, Holland PR, Lasalandra MP, et al. Inhibition of trigeminovascular dural nociceptive afferents by Ca(2+)-activated K(+) (MaxiK/BK(Ca)) channel opening. Pain 2010; 151: 128–136. [DOI] [PubMed] [Google Scholar]
- 30.Millan MJ. The induction of pain: an integrative review. Prog Neurobiol 1999; 57: 1–164. [DOI] [PubMed] [Google Scholar]
- 31.Akerman S, Simon B, Romero-Reyes M. Vagus nerve stimulation suppresses acute noxious activation of trigeminocervical neurons in animal models of primary headache. Neurobiol Dis 2017; 102: 96–104. [DOI] [PubMed] [Google Scholar]
- 32.Akerman S, Holland PR, Lasalandra MP, et al. Endocannabinoids in the brainstem modulate dural trigeminovascular nociceptive traffic via CB1 and “triptan” receptors: implications in migraine. J Neurosci 2013; 33: 14869–14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson DJ, Shepheard SL, Hill RG, et al. The novel anti-migraine agent rizatriptan inhibits neurogenic dural vasodilation and extravasation. Eur J Pharmacol 1997; 328: 61–64. [DOI] [PubMed] [Google Scholar]
- 34.Petersen KA, Birk S, Doods H, et al. Inhibitory effect of BIBN4096BS on cephalic vasodilatation induced by CGRP or transcranial electrical stimulation in the rat. Br J Pharmacol 2004; 143: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia 2002; 22: 491–512. [DOI] [PubMed] [Google Scholar]
- 36.Silberstein SD. Preventive migraine treatment. Neurol Clin 2009; 27: 429–443. [DOI] [PubMed] [Google Scholar]
- 37.Schurks M, Diener HC, Goadsby P. Update on the prophylaxis of migraine. Curr Treat Opt Neurol 2008; 10: 20–29. [DOI] [PubMed] [Google Scholar]
- 38.Goadsby PJ. Therapeutic prospects for migraine: can paradise be regained? Ann Neurol 2013; 74: 423–434. [DOI] [PubMed] [Google Scholar]
- 39.Stankewitz A, Aderjan D, Eippert F, et al. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 2011; 31: 1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andreou AP, Shields KG, Goadsby PJ. GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol Dis 2010; 37: 314–323. [DOI] [PubMed] [Google Scholar]
- 41.Shields KG, Goadsby PJ. Propranolol modulates trigeminovascular responses in thalamic ventroposteromedial nucleus: a role in migraine? Brain 2005; 128: 86–97. [DOI] [PubMed] [Google Scholar]
- 42.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol 2004; 55: 27–36. [DOI] [PubMed] [Google Scholar]
- 43.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A 2004; 101: 4274–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodick DW, Goadsby PJ, Silberstein SD, et al. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 2014; 13: 1100–1107. [DOI] [PubMed] [Google Scholar]
- 45.Dodick DW, Goadsby PJ, Spierings EL, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014; 13: 885–892. [DOI] [PubMed] [Google Scholar]
- 46.Bigal ME, Dodick DW, Rapoport AM, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2015; 14: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 47.Lampl C, Buzath A, Klinger D, et al. Lamotrigine in the prophylactic treatment of migraine aura – a pilot study. Cephalalgia 1999; 19: 58–63. [DOI] [PubMed] [Google Scholar]
- 48.Lampl C, Katsarava Z, Diener HC, et al. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J Neurol Neurosurg Psychiatry 2005; 76: 1730–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia 1997; 17: 109–112. [DOI] [PubMed] [Google Scholar]
- 50.Bogdanov VB, Multon S, Chauvel V, et al. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol Dis 2011; 41: 430–435. [DOI] [PubMed] [Google Scholar]
- 51.Eikermann-Haerter K, Lee JH, Yalcin N, et al. Migraine prophylaxis, ischemic depolarizations, and stroke outcomes in mice. Stroke 2015; 46: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 53.Akerman S, Goadsby PJ. Topiramate inhibits cortical spreading depression in rat and cat: impact in migraine aura. Neuroreport 2005; 16: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Levy D, Kainz V, et al. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 2011; 69: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X, Levy D, Noseda R, et al. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci 2010; 30: 8807–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache 2010; 50: 725–730. [DOI] [PubMed] [Google Scholar]
- 57.Charles A. Does cortical spreading depression initiate a migraine attack? Maybe not. Headache 2010; 50: 731–733. [DOI] [PubMed] [Google Scholar]
- 58.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996; 384: 560–564. [DOI] [PubMed] [Google Scholar]
- 59.Hashimoto M, Yamamoto Y, Takagi H. Effects of KB-2796 on plasma extravasation following antidromic trigeminal stimulation in the rat. Res Commun Mol Pathol Pharmacol 1997; 97: 79–94. [PubMed] [Google Scholar]
- 60.Cutrer FM, Limmroth V, Ayata G, et al. Attenuation by valproate of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br J Pharmacol 1995; 116: 3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cutrer FM, Moskowitz MA. Wolff Award 1996. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache 1996; 36: 579–585. [DOI] [PubMed] [Google Scholar]
- 62.Lee WS, Limmroth V, Ayata C, et al. Peripheral GABAA receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance P and trigeminal stimulation. Br J Pharmacol 1995; 116: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Markowitz S, Saito K, Moskowitz MA. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia 1988; 8: 83–91. [DOI] [PubMed] [Google Scholar]