Abstract
The cerebellum plays an important role in pain processing but its function in headache and specifically in migraine is not known. We therefore compared 54 migraineurs with pairwise matched healthy controls in a magnetic resonance imaging study on neuronal cerebellar activity in response to nociceptive trigeminal sensation and also investigated possible structural alterations. Headache frequency, disease duration, and the proximity to a migraine attack were used as co-factors. Migraine patients showed functional and structural alterations in the posterior part of the cerebellum, namely crus I and crus II. Gray matter volume changes were seen on the right side whereas functional changes were ipsilateral to the stimulation, on the left side. Neuronal activity in the crus in response to trigeminal pain was modulated by migraine severity and the migraine phase. As the crus is strongly interconnected to higher cognitive areas in the temporal, frontal, and parietal part of the cortex our results suggest an specific cerebellar involvement in migraine. This is further supported by our finding of decreased connectivity from the crus to the thalamus and higher cortical areas in the patients. We therefore suggest an abnormally decreased inhibitory involvement of the migraine cerebellum on gating and nociceptive evaluation.
Keywords: Nociception, trigeminal nervous system, functional imaging, voxel-based morphometry, co-modulation with biomarkers of migraine
Introduction
Deciphering and understanding the role of the cerebellum for other tasks than fine-tuning sensorimotor functions is a rather new subject in neuroscience. Recent studies demonstrate the involvement of the cerebellum in human nociception1–3 and even suggest a modulating role in pain perception.4 Cerebellar activity in response to trigeminal nociceptive input has been shown in quite a number of functional imaging studies in migraineurs5–8 although these findings have been usually only reported and rarely discussed. The cerebellums’ activity in response to nociceptive input seems to be higher during a migraine attack than interictally.9 This observation failed to reach significance in most aforementioned studies. This lack of difference might stem from the problem that classical whole-brain analysis of functional magnetic resonance imaging (fMRI) data fails for the cerebellum. Especially the segmentation of the structural image and the following normalization of the functional data set underrepresents cerebellar in favor of cortical areas and can be drastically enhanced using advanced methods.10
The same holds true for structural alterations of the cerebellar gray matter (GM),11 possibly even linked to the cerebellum’s functional role.12 Nevertheless, other structural13,14 and diffusivity15,16 abnormalities have been shown in the cerebellum of migraineurs compared with healthy controls. One further argument why the cerebellum may be interesting for migraine research is the finding of an increased prevalence of ischemic lesions particularly in the cerebellar posterior lobe of migraineurs.17,18 Furthermore, the cerebellum exhibits remarkably high concentrations of calcitonin gene-related peptide (GCRP),19,20 a strong vasodilator which is a target peptide for migraine treatment.21–26 Anatomically, the functional involvement of the cerebellum in migraine is further supported by direct connection with the spinal trigeminal nucleus (sTN) in cats27 and rats.28
Recently, we have shown the complex functional interaction of brainstem, cerebellum, and cortex in response to trigeminal nociceptive input in healthy controls.29 To validate our hypotheses of altered nociceptive, cerebellar processing of the cerebellum in migraine patients we performed a MRI study in a large population of migraineurs and compared them to pairwise age and gender matched healthy controls. Of special interest was whether functional abnormalities in migraineurs can be linked to the severity, here defined as the number of days with headache per month, or a specific state of migraine, i.e. whether the patient is currently in an interictal or ictal phase as current research has especially highlighted the importance of the pre-ictal state shortly before the actual migraine attack takes place.30 Additionally, structural changes of the migraine cerebellum might be linked to the functional ones and further influenced by the severity and the disease duration.
Materials and methods
Subjects and experimental design
Written informed consent was obtained from all participants and the study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee in Hamburg, Germany (PV 4522). We compared cerebellar activation in nociception of 54 migraineurs with 54 pairwise gender and age matched healthy controls (p > 0.05, comparing age distributions between groups by a Wilcoxon rank sum test). For the patient group we further acquired information about headache days per month, the duration of the disease (years), and whether they experience an aura or not. Exclusion criteria were overuse of acute medication (MOH) and accompanying headaches other than migraine. Healthy controls had no symptoms of any primary or secondary headache disorder and were free of psychiatric or neurological disorders. The demographic and clinical data of both groups can be found in Table 1.
Table 1.
Demographic and clinical characteristics.
| Patients | Healthy controls | |
|---|---|---|
| N | 54 | 54 |
| Gender (m/f) | 9/45 | 9/45 |
| Age (mean ± SD [min, max]) | 34.3 ± 11.9 [16, 59] | 32.6 ± 11.5 [18, 59] |
| Without Aura/with Aura/sometimes Aura | 40/13/1 | |
| Days with headache per month (mean ± SD [min, max]) | 11.8 ± 5.4 [4, 31] | |
| Episodic/chronic | 8/46 | |
| Duration of disease in years (mean ± SD [min, max]) | 18.1 ± 12.3 [0, 46] |
Patients were defined as ictal when they had a migraine attack during the experimental procedure and as pre-ictal (or post-ictal, respectively) for attacks 48 h prior (or after) the actual scan. This definition resulted in groups of 20 inter-ictal, three pre-ictal, 17 ictal, and 14 post-ictal patients. None of the participants was scanned twice to avoid conflicts in statistical comparisons (i.e. within- versus between-subject comparisons).
All volunteers participated in an experiment on trigeminal nociception following a standardized protocol of Stankewitz et al.31 The experiment consists of four conditions, namely (1) the transmission of highly concentrated gaseous ammonia, which induces a painful, trigeminal sensation, (2) the transmission of rose odor, and (3) air puffs mixed in a constant air flow into the left nostril. The duration of the stimulations was set to 0.8 s. (4) A further repetitive visual stimulation using a rotating circle with checkerboard-like pattern at 8 Hz (duration of 4 s) served as a visual control condition. During the experiment the volunteers received 15 stimulations of each condition in a randomized order.
Following 8–10 s after each stimulus presentation, participants were asked to rate the intensity of stimuli on a visual analogue scale from 0 to 100, as well as the unpleasantness of each stimulus (−50: very pleasant, 0: neutral, +50: very unpleasant). The stimulus interval was around 40 s but depended on the time consumption of the individual ratings. Further details on the experiment have been previously published multiple times and can be looked up by the interested reader.6,9,29,31–35
MR data acquisition
MR scanning used a Siemens Trio 3T scanner (Siemens, Erlangen, Germany) with a 32-channel head coil. We acquired a high-resolution T1-weighted structural image (voxel size 1 mm3, repetition time 2.3 s, echo time 2.98 ms, flip angle 9°, field of view 256 mm2, 240 axial slices, slice thickness 1 mm, gap 50%) using a magnetization-prepared rapid gradient echo sequence. Functional MR data acquisition used an echo planar imaging sequence (repetition time 2.62 s, echo time 30 ms, flip angle 80°, field of view 220 mm2, acceleration mode GRAPPA, spatial resolution 2.0 × 2.0 × 2.0 mm, acquisition matrix 110 × 110 × 40), covering the sTN in the brainstem, the cerebellum, the midbrain and most of the cortex up to the face’s representation in the somato-motoric homunculi. Each volume consisted of 40 axial slices (slice thickness 2 mm, gap 1 mm).
Processing of structural MR data
Prior analyzing structural cerebellar alterations, we assessed differences between healthy controls and migraineurs in gray matter volume (GMV) of cortical areas for comparison with and as reproduction of previous findings.11,12 Therefore, we used the CAT12 toolbox for SPM (C. Gaser, Structural Brain Mapping group, Jena University Hospital, http://www.neuro.uni-jena.de/cat/).36 T1-weighted images were corrected for field inhomogeneities, spatially normalized with the DARTEL algorithm,37 and segmented into GM, white matter as well as cerebrospinal fluid.38 This segmentation additionally accounted for partial volume effects.39–41 Afterwards all scans passed an automated quality check promoted in the manual of CAT12. After segmentation and normalization, modulated GM images were spatially smoothed by an 8 mm3 Gaussian kernel and tested by an unpaired t test together with covariates for total intracranial volume, age, and gender. Results for increased and decreased GMV are reported for a voxel-wise threshold of p < 0.001 and a minimum cluster extent of 20 voxel.
GM alterations within the cerebellum and brainstem were identified using the SUIT toolbox10,42 for normalization. Modulated GM images (using the ‘preserve’ option of SUIT) are here smoothed with a 4 mm3 Gaussian kernel and again results are presented for a voxel-wise threshold of p < 0.001 and a minimum cluster extent of 20 voxel, also taking age and gender as covariates of no interest into account, and using a small volume correction within the regions defined by the SUIT atlas.
Furthermore, for the patient group we assessed cerebellar and brainstem GMV changes comodulating with the duration of the disease, i.e. years since the onset of the migraine, and the severity, i.e. the attack frequency measured as days with headache per month, in two separate analyses. Result had to pass the same thresholds stated in the previous paragraph.
Processing of functional MR data
To investigate neuronal activity in response to the painful trigeminal stimulation, individual T1-images were coregistered with the functional images. Thereafter, brainstem and cerebellum were isolated and normalized to SUIT space using the SUIT toolbox for SPM12.10,42 The functional images were realigned and slice time corrected to the middle slice of each volume. Following the protocol of the SUIT toolbox the first level analyses were performed in the individual subjects’ space. Only beta- and contrast images were normalized and afterwards smoothed by a 4 mm3 Gaussian kernel. For the first level analysis all five conditions (Pain, Air, Rose-odor, repetitive visual stimulation and all button presses) were modeled by convolving a stick-function at stimulus onset with a canonical hemodynamic response function. The general linear model (GLM) included further six regressors, computed in the previous alignment step, to attenuate movements. The highpass filters’ cutoff frequency was set to 128 s (∼0.008 Hz).
Nociception in the cerebellum
For the analysis of the main effect of trigeminal nociception we conducted an unpaired t test and used a brainstem and cerebellum specific mask provided by the SUIT toolbox. The threshold was set to p < 0.01 for a voxel-level family-wise error (FWE)-correction and a minimum cluster extent of 20 voxel. This conservative threshold was chosen, though we expected high effect sizes as shown in our previous work.29
Differences in cerebellar and brainstem activity between healthy controls and migraineurs were calculated by a two-sample, unpaired t test with an uncorrected threshold of p < 0.001. Small volume correction in cerebellar activation used regions defined by the SUIT atlas while for brainstem regions we corrected with coordinates originating from previous publications33 used as centers of a sphere with 8 mm radius.
Functional connectivity analysis
Cerebellar areas showing different activity between migraineurs and healthy controls entered a functional connectivity analysis, namely a physio-psychological interaction (PPI) analysis,43 providing insights into the alterations of connectivity during nociceptive processing.29,32,33,35 This PPI was calculated separately for the brainstem only—though intra-cerebellar connectivity is anatomically impossible—as well as for a whole-brain analysis, where preprocessed data was normalized to Montreal Neurological Institute (MNI) space using standard routines of SPM12 and smoothed with a 8 mm3 Gaussian kernel. Connectivity alterations are presented here for voxel-wise thresholds of p < 0.001 and minimum cluster extents of 10 voxel for both the brainstem specific and whole-brain analyses.
Modulation by subjects’ rating
An additional analysis was performed to gain insights into the cerebellars’ and brainstems’ parametric modulation of the painful stimulation of the trigeminal nerve. Therefore, the subject’s intensity and unpleasantness ratings for each of the 15 painful trials were centered and then included into separate first level analyses as additional regressors. Results of the t statistics comparing the healthy controls and migraineurs are presented for voxel passing a threshold of p < 0.001 uncorrected) and being part of clusters with a minimum extent of 10 voxel.
Co-modulation of nociception and biomarkers
Of special interest from a clinical point of view is whether the severity of the migraine and/or the phase within the migraine cycle has an impact on the cerebellars’ processing of trigeminal pain. Therefore we calculated a one-way ANOVA with the aforementioned grouping levels inter-ictal, ictal, pre-ictal, and post-ictal in the patients. We included migraine frequency, here measured as days with headache per month, as a covariate of interest into the statistical model. Age, gender, and whether an acute medication was taken at the day of the experiment were included into the model as further covariates of no interest. All covariates were centered. We then analyzed the migraine frequency as an F-contrast with a threshold of p < 0.05, voxel-wise FWE-corrected, and further post-hoc t statistics for the contrasts interictal versus ictal as well as ictal versus post-ictal. As the group of pre-ictal only consisted of three members, post-hoc tests for this group were neglected. Furthermore, we calculated if the attack frequency had an increasing or decreasing impact on the activation in brainstem and cerebellum by means of t tests. All results for the t tests were threshold at p < 0.01, uncorrected.
Results
Behavior
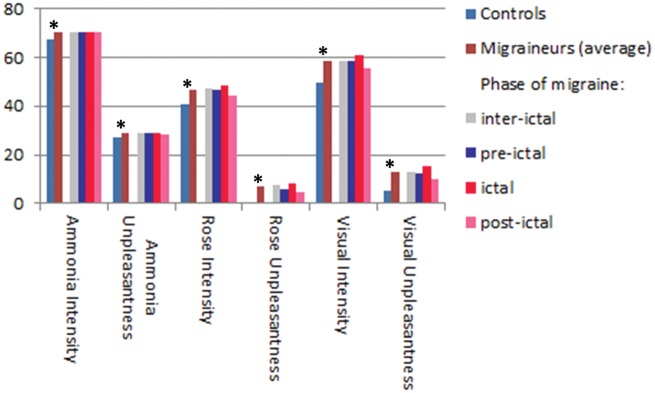
All ratings on intensity and unpleasantness for all experimental conditions were significantly different between migraineurs and controls (p < 0.001 in a two-tailed, unpaired t test) with higher values in migraine patients (see Figure 1). No significant difference with regard to the migraine phase (inter-ictal, pre-ictal, ictal, post-ictal) was found (one-way ANOVA, F(3,50), p > 0.1).
Figure 1.
Differences in intensity and unpleasantness ratings between healthy controls and migraineurs (and their subgroups separated by the phase of the migraine during the experiment). Asterisks mark significant (p < 0.01, unpaired, two-tailed t test) differences.
GMV alterations
Using the CAT12 toolbox for the whole brain, we found decreased GMV for the migraine group in bilateral precentral gyrus, right postcentral gyrus, left supramarginal, and a part of the left angular gyrus. A significant increase involved temporal occipital areas and angular gyrus on the right hand side as well as the pallidum on the left hand side.
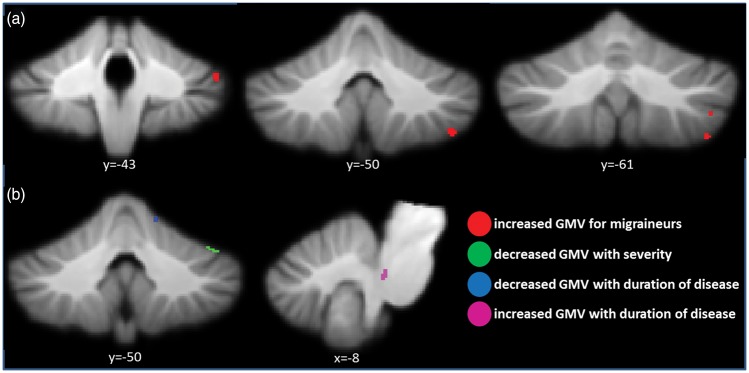
We interestingly neither observed significant decreases of GMV in the brainstem nor in the cerebellum of the migraineurs when restricting our analysis to cerebellum and brainstem with the SUIT toolbox. Instead there were four clusters on the right hand side of the cerebellum where migraineurs showed significant increases of GMV, including the cerebellar areas VI, VIIb, VIIIa, crus I, and crus II. Furthermore, analyzing correlations of severity and duration of the migraine in the patient group revealed a decrease of GMV in right VI with higher attack frequency and a decrease with right V with duration of disease. An increase of GMV correlating with duration of the migraine showed up in the left side of the rostral part of the pons in the brainstem. The structural changes of the cerebellum are sketched in Figure 2 and while the full picture including the cortical areas can be found in Supplementary Table 1.
Figure 2.
Cerebellar gray matter volume (GMV) alterations of migraineurs. (a) Comparison of migraineurs and healthy controls. (b) Patients’ GMV co-modulations with severity, i.e. days of headaches per month, and with duration of their disease.
Nociception in the cerebellum
At the conservative threshold of p < 0.01 (voxel-wise, FWE-corrected) the resulting activated voxel formed one dominant cluster including numerous cerebellar and brainstem regions listed in Supplementary Table 2, which are in accordance with previously published results.29 As expected29,33 one further cluster was found in the left sTN ipsilateral to the stimulated. Results are visualized in Figure 3.
Figure 3.
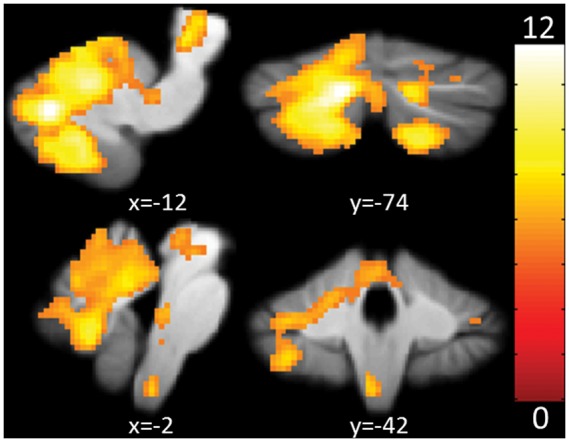
Main effect of trigeminal nociception. Shown are activations in cerebellum and brainstem at a voxel-wise, FWE-corrected threshold of p < 0.01. The color bar signifies T values. FWE: family-wise error.
Compared to controls, migraineurs showed increased activity in the periaqueductal gray (PAG) and left crus I (ipsilateral to the stimulation) at an uncorrected threshold of 0.001 (see Supplementary Table 3 and Figure 4). Small volume correction became significant for both locations. Using covariates to control for age and gender did change these results only slightly and both clusters still yielded significant activation.
Figure 4.
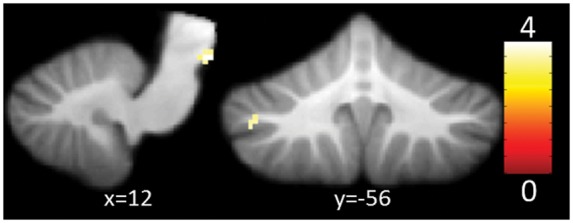
Increased activity of cerebellum and brainstem for migraineurs when compared to pairwise age and gender matched healthy controls in response to trigeminal nociception at a voxel-wise, uncorrected threshold of p < 0.001. The color bar signifies T values.
Estimating the difference in functional connectivity of the left crus I showed decreases with left thalamus, bilateral occipital areas, and the right fusiformis gyrus for migraineurs. Brainstem areas showed neither increased nor decreased differences in functional connectivity (see Supplementary Table 4 for further details).
Modulation by subjects’ rating
We found one cluster in the right PAG (coordinates 8,-14,-11 xyz/mm; cluster extent: 28 voxel, T value: 4.31) where healthy subjects showed less modulation by intensity ratings than migraineurs on a single trial level. We could not find significant differences in the parametric modulation for the unpleasantness ratings (voxel-wise threshold of p < 0.01, uncorrected, with a minimum cluster extent of 10 voxel).
Co-modulation of nociception and biomarkers
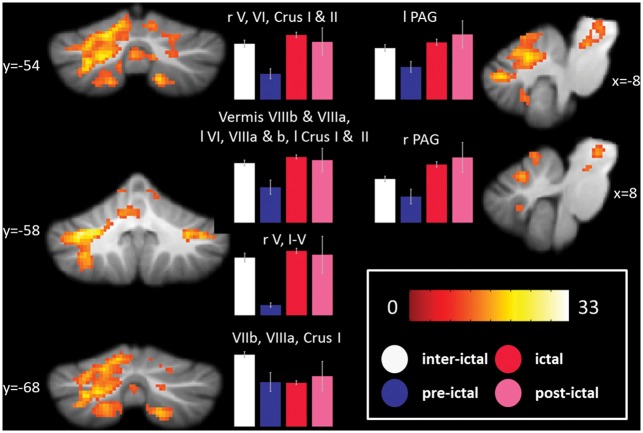
The migraine phase modulated the cerebellar activation to nociceptive trigeminal input in six clusters (one-way ANOVA, F test at a threshold of p < 0.05, voxel-wise FWE corrected, and a minimum cluster extent of 10 voxel). The most dominant cluster comprised of vermis VIIIb, vermis VIIIa, VI, VIIIa, VIIb, crus I, crus II, V, vermis VIIb, VIIIb, I–IV, vermis IX, and vermis VI on the left hemisphere. Three smaller clusters on the right hand side of the cerebellum included cerebellar areas V, VI, and VIIb. Furthermore the brainstem was modulated by the migraine phase in the left as well as in the right hand side of the PAG. Post-hoc tests revealed that ictal patients have lower (i.e. inter-ictal patients have a higher) activity in two clusters on the right hand side of the cerebellum, namely VIIIa and VIIb. A summary of the described results is given in Figure 5 and details in Supplementary Table 5.
Figure 5.
Results of the ANOVA testing the co-modulation of current migraine phase and BOLD response to trigeminal nociceptive input. The color bar reflects F values at a threshold of p < 0.05, voxel-wise FWE-corrected. The bar plots show average signal changes of the indicated clusters separate for the phases of the migraine with error bars signifying the standard error of the mean. ANOVA: analysis of variance; BOLD: blood oxygenation level dependent; FWE: family-wise error.
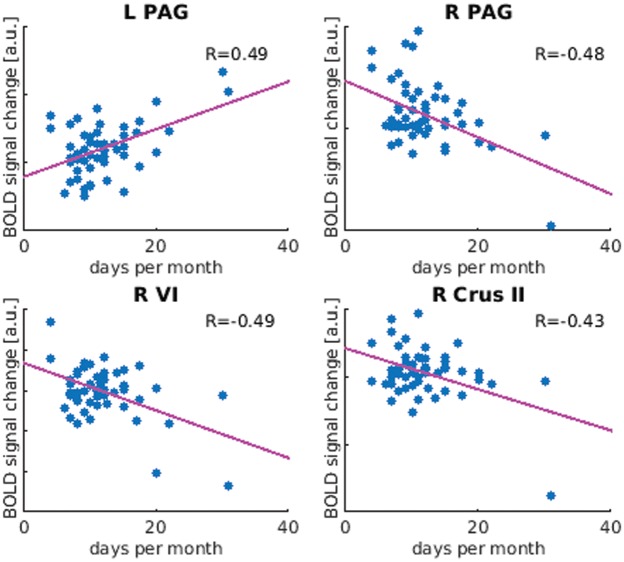
Severity of migraine by means of headache days per month correlated positively to neuronal activity in the left PAG and negatively in the right PAG as well as in right lateralized cerebellar regions VI and crus II. These correlations are sketched in Figure 6 and in detail reported in Supplementary Table 5.
Figure 6.
Significant (p < 0.05) correlation (Pearson) of BOLD response to trigeminal pain with headache frequency (days per month). R: correlation coefficient; BOLD: blood oxygenation level dependent.
Discussion
The main result of this study is a straight-forward replication of our previous findings29 with higher statistical precision due to the high number of 108 participants, emphasizing the important role of the cerebellum in nociception. Given this robust result it is highly interesting that we found a functional but also structural difference between migraineurs and controls. Functionally, migraineurs showed increased activity in the PAG and left crus I (ipsilateral to the stimulation), compared to controls. Structurally, migraineurs showed significant increases of GMV in four clusters, namely the cerebellar areas VI, VIIb, VIIIa, crus I, and crus II. We note that these findings were coinciding in the crus I.
Crus I and crus II are areas closely linked to the association cortices,44 especially prefrontal and posterior parietal cortical areas.45 The crus II is a major recipient area for frontal lobe afferents45 and also activates during cognitive tasks like mental rotation and remembering.46 Crus I and crus II are thought to hold cognitive and emotional representations1,2,47 and show overlapping activity between aversive and heat pain.2 This emotional and cognitive hub of the cerebellum also relates to the acuteness of the migraine attack, i.e. the migraine phase: The crus shows highest activity in nociception in the ictal, and lowest in the pre-ictal phase while inter- and post-ictal phase show moderate activity. We note that the picture of neuronal response (or lack of it) to nociception between ictal and interictal migraine phase is incomplete, given the functional importance of the pre-ictal phase raised by concurrent publications.35 Unfortunately, the current group of patients in the pre-ictal phase was too small to be statistically analyzed in the current study.
Only migraineurs exhibit a decreased functional connectivity between the crus I and parts of the fusiformis gyrus and the thalamus. The fusiform gyrus is known to be involved in cognitive pain processing48–50 and has been reported to be hyperactive in episodic migraineurs.8 Given the mainly inhibitory role of the cerebellum also in nociception,4 one could speculate, that the crus in migraineurs is more activated than in controls because its signal to the thalamus is reduced due to a diminished functional connectivity. The crus thus increases activity to maintain its inhibitory function but this may be ineffective. Because the cerebellar inhibitory signal does not reach the thalamus, the gating51 of external signals becomes disturbed and a cascade of events, involving cortical areas such as the fusiform gyrus, may trigger susceptibility for migraine attacks.
We note, that our morphometric analysis provides also very robust results and shows comparable results to recently published meta-analyses on cortical alterations in migraineurs.11,12 We can affirm that migraine patients have a decreased GMV in precentral gyri as well as in postcentral gyrus52 and temporal lobe.52–54 Nevertheless, the consistency of voxel-based morphometry (VBM) studies in migraineurs is still under debate.55 Especially, only few findings about GMV increases in migraineurs have been published, possibly because these changes are more subtle and need higher numbers of participants and/or more refined methods.
Using methods specifically developed for research on brainstem and cerebellum,10,42 we identified an increase in the posterior part of the cerebellum (see above) and the posterior part of the brainstem,30,35,56 while a decrease of GMV could neither be found in the brainstem nor in the cerebellum of the migraineurs.57 It is of interest that just like the previously reported cortical studies52 all of these cerebellar and brainstem GMV changes correlated positively to severity and disease duration.
Taken together, the cerebellum of migraineurs and controls differs functionally but also structurally. GMV as well as neuronal activity in response to trigeminal pain was increased in the posterior part of the cerebellum, namely the crus, and its activity modulated by migraine severity and the migraine phase. As migraine patients also exhibit a decreased connectivity to the thalamus and higher cortical areas, these data suggest an abnormally decreased inhibitory involvement of the migraine cerebellum on gating and evaluation of trigeminal nociception.
Supplementary Material
Acknowledgment
We would like to thank Julia Hebestreit and Inga Kröger for help acquiring the data and Dagmar Timmann-Braun for discussing the content.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the German Research Foundation, SFB936/A5 and DFG 1862/12-1 to AM.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author’s contributions
AM designed the study. JM analyzed the data. JM and AM prepared and revised the manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Moulton EA, Schmahmann JD, Becerra L, et al. The cerebellum and pain: passive integrator or active participator? Brain Res Rev 2010; 65: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moulton EA, Elman I, Pendse G, et al. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci 2011; 31: 3795–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borsook D, Moulton EA, Tully S, et al. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum 2008; 7: 252–272. [DOI] [PubMed] [Google Scholar]
- 4.Ruscheweyh R, Kühnel M, Filippopulos F, et al. Altered experimental pain perception after cerebellar infarction. Pain 2014; 155: 1303–1312. [DOI] [PubMed] [Google Scholar]
- 5.Moulton EA, Becerra L, Maleki N, et al. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 2011; 21: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stankewitz A, Aderjan D, Eippert F, et al. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J Neurosci 2011; 31: 1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo A, Tessitore A, Esposito F, et al. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J Neurol 2012; 259: 1903–1912. [DOI] [PubMed] [Google Scholar]
- 8.Schwedt TJ, Chong CD, Chiang C-C, et al. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia 2014; 34: 947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stankewitz A, May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology 2011; 77: 476–482. [DOI] [PubMed] [Google Scholar]
- 10.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage 2006; 33: 127–138. [DOI] [PubMed] [Google Scholar]
- 11.Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. NeuroImage 2017; 14: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu W, Guo J, Chen N, et al. A meta-analysis of voxel-based morphometric studies on migraine. Int J Clin Exp Med 2015; 8: 4311–4319. [PMC free article] [PubMed] [Google Scholar]
- 13.Bilgiç B, Kocaman G, Arslan AB, et al. Volumetric differences suggest involvement of cerebellum and brainstem in chronic migraine. Cephalalgia 2016; 36: 301–308. [DOI] [PubMed] [Google Scholar]
- 14.Schmitz N, Admiraal-Behloul F, Arkink EB, et al. Attack frequency and disease duration as indicators for brain damage in migraine. Headache 2008; 48: 1044–1055. [DOI] [PubMed] [Google Scholar]
- 15.Grimaldi D, Tonon C, Cevoli S, et al. Clinical and neuroimaging evidence of interictal cerebellar dysfunction in FHM2. Cephalalgia 2010; 30: 552–559. [DOI] [PubMed] [Google Scholar]
- 16.Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum 2012; 11: 336–351. [DOI] [PubMed] [Google Scholar]
- 17.Gerwig M, Rauschen L, Gaul C, et al. Subclinical cerebellar dysfunction in patients with migraine: evidence from eyeblink conditioning. Cephalalgia 2014; 34: 904–913. [DOI] [PubMed] [Google Scholar]
- 18.Koppen H, Boele H-J, Palm-Meinders IH, et al. Cerebellar function and ischemic brain lesions in migraine patients from the general population. Cephalalgia 2017; 37: 177–190. [DOI] [PMC free article] [PubMed]
- 19.Edvinsson L, Eftekhari S, Salvatore CA, et al. Cerebellar distribution of calcitonin gene-related peptide (CGRP) and its receptor components calcitonin receptor-like receptor (CLR) and receptor activity modifying protein 1 (RAMP1) in rat. Mol Cell Neurosci 2011; 46: 333–339. [DOI] [PubMed] [Google Scholar]
- 20.Eftekhari S, Salvatore CA, Gaspar RC, et al. Localization of CGRP receptor components, CGRP, and receptor binding sites in human and rhesus cerebellar cortex. Cerebellum 2013; 12: 937–949. [DOI] [PubMed] [Google Scholar]
- 21.Buzzi MG, Bonamini M, Moskowitz MA. Neurogenic model of migraine. Cephalalgia 1995; 15: 277–280. [DOI] [PubMed] [Google Scholar]
- 22.Edvinsson L. CGRP receptor antagonists and antibodies against CGRP and its receptor in migraine treatment. Br J Clin Pharmacol 2015; 80: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edvinsson L. The journey to establish CGRP as a migraine target: a retrospective view. Headache 2015; 55: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 24.Edvinsson L. Blockade of CGRP receptors in the intracranial vasculature: a new target in the treatment of headache. Cephalalgia 2004; 24: 611–622. [DOI] [PubMed] [Google Scholar]
- 25.Edvinsson L, Ho TW. CGRP receptor antagonism and migraine. Neurotherapeutics 2010; 7: 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tepper SJ, Stillman MJ. Clinical and preclinical rationale for CGRP-receptor antagonists in the treatment of migraine. Headache 2008; 48: 1259–1268. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter MB, Hanna GR. Fiber projections from the spinal trigeminal nucleus in the cat. J Comp Neurol 1961; 117: 117–131. [DOI] [PubMed] [Google Scholar]
- 28.Huerta MF, Frankfurter A, Harting JK. Studies of the principal sensory and spinal trigeminal nuclei of the rat: projections to the superior colliculus, inferior olive, and cerebellum. J Comp Neurol 1983; 220: 147–167. [DOI] [PubMed] [Google Scholar]
- 29.Mehnert J, Schulte L, Timmann D, et al. Activity and connectivity of the cerebellum in trigeminal nociception. Neuroimage 2017; 150: 112–118. [DOI] [PubMed] [Google Scholar]
- 30.Schulte LH and May A. Of generators, networks and migraine attacks. Curr Opin Neurol 2017; 30: 241–245. [DOI] [PubMed]
- 31.Stankewitz A, Voit HL, Bingel U, et al. A new trigemino-nociceptive stimulation model for event-related fMRI. Cephalalgia 2010; 30: 475–485. [DOI] [PubMed] [Google Scholar]
- 32.Kröger IL, May A. Triptan-induced disruption of trigemino-cortical connectivity. Neurology 2015; 84: 2124–2131. [DOI] [PubMed] [Google Scholar]
- 33.Schulte LH, Sprenger C, May A. Physiological brainstem mechanisms of trigeminal nociception: an fMRI study at 3T. NeuroImage 2016; 124: 518–525. [DOI] [PubMed] [Google Scholar]
- 34.Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia 2013; 33: 256–265. [DOI] [PubMed] [Google Scholar]
- 35.Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain 2016; 139: 1987–1993. [DOI] [PubMed] [Google Scholar]
- 36.Besteher B, Gaser C, Langbein K, et al. Effects of subclinical depression, anxiety and somatization on brain structure in healthy subjects. J Affect Disord 2017; 215: 111–117. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- 38.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005; 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 39.Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. NeuroImage 2004; 23: 84–97. [DOI] [PubMed] [Google Scholar]
- 40.Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE Trans Med Imaging 1997; 16: 176–186. [DOI] [PubMed] [Google Scholar]
- 41.Cuadra MB, Cammoun L, Butz T, et al. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE Trans Med Imaging 2005; 24: 1548–1565. [DOI] [PubMed] [Google Scholar]
- 42.Diedrichsen J, Balsters JH, Flavell J, et al. A probabilistic MR atlas of the human cerebellum. NeuroImage 2009; 46: 39–46. [DOI] [PubMed] [Google Scholar]
- 43.Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 1997; 6: 218–229. [DOI] [PubMed] [Google Scholar]
- 44.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 2011; 46: 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blatt DGJ, Oblak DAL, Schmahmann DJD. Cerebellar connections with limbic circuits: anatomy and functional implications. In: Manto M, Schmahmann JD, Rossi F, Gruol DL, et al. (eds). Handbook of the cerebellum and cerebellar disorders, Netherlands: Springer, 2013, pp. 479–496. [Google Scholar]
- 46.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. Neuroimage 2012; 59: 1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Timmann D, Drepper J, Frings M, et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010; 46: 845–857. [DOI] [PubMed] [Google Scholar]
- 48.Ter Minassian A, Ricalens E, Humbert S, et al. Dissociating anticipation from perception: acute pain activates default mode network. Hum Brain Mapp 2013; 34: 2228–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glass JM, Williams DA, Fernandez-Sanchez M-L, et al. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J Pain 2011; 12: 1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimo K, Ueno T, Younger J, et al. Visualization of painful experiences believed to trigger the activation of affective and emotional brain regions in subjects with low back pain. PLoS ONE 2011; 6: e26681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol 2013; 109: 5–12. [DOI] [PubMed] [Google Scholar]
- 52.Maleki N, Becerra L, Brawn J, et al. Concurrent functional and structural cortical alterations in migraine. Cephalalgia 2012; 32: 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rocca MA, Messina R, Colombo B, et al. Structural brain MRI abnormalities in pediatric patients with migraine. J Neurol 2014; 261: 350–357. [DOI] [PubMed] [Google Scholar]
- 54.Valfrè W, Rainero I, Bergui M, et al. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache 2008; 48: 109–117. [DOI] [PubMed] [Google Scholar]
- 55.Hougaard A, Amin FM, Ashina M. Migraine and structural abnormalities in the brain. Curr Opin Neurol 2014; 27: 309–314. [DOI] [PubMed] [Google Scholar]
- 56.Dahlem MA. Migraine generator network and spreading depression dynamics as neuromodulation targets in episodic migraine. Chaos 2013; 23: 046101. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt-Wilcke T, Gänßbauer S, Neuner T, et al. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 2008; 28: 1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.