Abstract
Background:
Currently, preoperative chemoradiotherapy, perioperative chemotherapy and preoperative chemotherapy are recommended by NCCN, ESMO and Japanese guidelines respectively for resectable esophageal and junctional cancer. However, these recommendations are mainly based on esophageal cancer research. Therefore, specific for esophagogastric junction cancer, we conducted the first systematic review and network meta-analysis to rank all potential treatments simultaneously and hierarchically.
Methods:
Record retrieval was conducted in PubMed, Web of Science, Cochrane Central Register of Controlled Trials, Embase, ASCO and ESMO Meeting Library from inception to September 2018. Regarding time-to-event survival data, randomized controlled trials featuring comparisons between different multimodal treatments against resectable esophagogastric junction cancer were eligible. Overall survival was the endpoint. Network calculation was based on a random-effects model and the relative ranking of each node was numerically indicated by P-score (CRD42018110369, registration identifier of the meta-analysis in PROSPERO.).
Results:
Eight studies were included in our systematic review, corresponding to 1218 patients. Regarding overall survival, ‘PreCRT’ (preoperative chemoradiotherapy) topped the hierarchy (HR 1.00, P-score = 0.823), better than ‘PeriCT’ (perioperative chemotherapy; HR 1.32, P-score = 0.591) and ‘PreCT’ (preoperative chemotherapy; HR 1.54, P-score = 0.428). In sensitivity analyses, irrespective of interchanging to fixed-effects model or removing potentially heterogeneous studies, relative rankings remained stable and ‘PreCRT’ was still the optimal node.
Conclusion:
Preoperative chemoradiotherapy could potentially be the optimal multimodal treatment, which displayed more overall survival benefits than perioperative chemotherapy and preoperative chemotherapy among resectable esophagogastric junction cancer patients. To further verify our pooled results, more randomized trials will be needed to compare preoperative chemoradiotherapy with perioperative chemotherapy (especially FLOT-based regimens).
Keywords: multimodal treatments, network meta-analysis, resectable esophagogastric junction cancer, preoperative chemoradiotherapy, systematic review
Introduction
According to the Siewert classification, esophagogastric junction (EGJ) cancer is a type of malignant tumor whose center is located at 5 cm proximal or distal to the junction, a histological transition area between esophageal and gastric epithelium.1 Unlike the decreasing trend of distal gastric cancer, the incidence of esophagogastric junction cancer has greatly increased among North American and European countries during the past decade, where the rise in male patients is the steepest among all solid malignancies.2–5
Like other solid tumors, surgery is the fundamental treatment against resectable esophagogastric junction cancer. However, despite curative operations, recurrence, especially systemic recurrence, is commonly observed, which greatly worsens the prognosis amid cancer sufferers.6,7 Therefore, multimodal treatments have been recommended against resectable esophagogastric junction cancer. Currently, Siewert type I/II and III EGJ cancer are treated as esophageal and gastric cancer, respectively.8 Nonetheless, this is only based on the location of the epicenter and pattern of lymph-node spread,9 which lacks support from direct and specific evidence. Moreover, even in terms of multimodal treatments against esophageal cancer, different organizations prefer diverse recommendations based on regional evidence, which further fuels the controversies over the therapeutic options for resectable EGJ cancer. In NCCN guidelines,8 preoperative chemoradiotherapy is the preferred strategy, while ESMO recommends both perioperative chemotherapy and preoperative chemoradiotherapy simultaneously.10 Similar uncertainties have also been found among Asian countries. In Japan,11 preoperative chemotherapy is the optimal choice while preoperative chemoradiotherapy is recommended according to Chinese guidelines.12 Inconsistent therapeutic strategies are a key problem for global gastric cancer treatments,4,13–15 without even accounting for the fact that recommendations are only based on general gastroesophageal analysis, not specifically referring to EGJ cancer.
Accordingly, we conducted the first systematic review and network meta-analysis of multimodal treatments against resectable esophagogastric junction cancer based on specific junctional data, aiming to hierarchically rank their therapeutic competencies and therefore offering valuable information for clinical decision-making and future design of randomized controlled trials (RCTs).
Methods
Registration and guidelines
The protocol of this systematic review and network meta-analysis had been published in PROSPERO (CRD42018110369). The design, conduct and writing of this systematic review and network meta-analysis was strictly in accordance with the requirements from the PRISMA Checklist for Network Meta-analysis and Cochrane Handbook 5.1. Each step was conducted by two investigators of our research group. Any discrepancy was judged and solved by the third investigator.
Search strategy
Electronic databases including PubMed, Web of Science, Cochrane Central Register of Controlled Trials and Embase were comprehensively examined. Additionally, we also thoroughly searched major databases for meeting abstracts, including the ASCO and ESMO Meeting Libraries. The search process started on 15 July 2018 and continued until 26 September 2018, covering the possible trials published from inception to September 2018. Both the abstracts and main texts of the retrieved entries were rigorously assessed in order to guarantee the accuracy of selection. The full search strategies are presented in Supplementary Materials.
Selection criteria
Studies that simultaneously met the following criteria were eligible for inclusion (PICOS framework).
Participant: patients with previously untreated resectable esophagogastric junction cancer, not including specific pathological type, targeted positivity or resectable superficial lesions.
Intervention: different multimodal treatments against resectable esophagogastric junction cancer, including preoperative, postoperative and perioperative chemotherapy, radiotherapy or chemoradiotherapy. Targeted medications among unselected patients were also eligible. In terms of chemotherapeutic types, since intraperitoneal chemotherapy was a controversial technique among different countries, we only included oral and intravenous chemotherapeutic regimens. Additionally, the comparisons between different regimens of chemotherapy were qualified while the comparisons between different dosages or methods of administration by the same chemotherapeutic regimen were not eligible. Comparisons between surgical techniques (such as open versus minimally invasive), or auxiliary therapeutics (such as anti-inflammatory medications, nutritional supportive methods, unspecified herbal medicine and immunomodulators) were also not qualified.
Comparator: ‘PreCRT’ (preoperative chemoradiotherapy) was the common comparator node in the network meta-analysis.
Outcome: time-to-event overall survival data (hazard ratio or Kaplan–Meier curves) on junctional cases were mandatory; time-to-event recurrence-free survival data or safety analysis on junctional cases were dispensable.
Study design: phase II and phase III RCTs reported from inception to September 2018 without language limitations.
Studies were excluded from systematic review due to the following reasons:
Interim or repetitive reports from the same registered study (we only included the one with the longest follow-up period).
Risk of bias assessment
The quality of each eligible study was evaluated by The Cochrane Risk of Bias Tool. The entire scale was constituted by seven domains, namely random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias.16 According to the criteria of the Cochrane Handbook 5.1, each domain could be judged as any of the three levels: low risk, unclear risk or high risk of bias. If the majority of items were judged as low risk of bias, then the entire methodological design of network meta-analysis was regarded as low risk of bias, and vice versa. Here, studies were defined to be low-quality if four or more items were scored as high risk of bias.
Data extraction
Predesigned forms were utilized to collect and organize the original data. Baseline information and survival data were extracted from the main text, tables, panels of subgroup analysis or supplementary materials, which had been cross-checked by two different investigators in our team before quantitative incorporations.
Baseline parameters and endpoints
All possible baseline parameters that could influence the clinical characteristics of each study were included and analyzed in our systematic review, including multimodal treatment, systemic regimens, performance status and Siewert classification (Table 1).
Table 1.
Baseline features of eligible studies in systematic review.
| Study | Registration | Phase | Enrollment | Treatment | Systemic regimens | Network nodes | Sample size | PS (0/1) | Siewert classification (%) | OS-HR |
|---|---|---|---|---|---|---|---|---|---|---|
| Cats et al.17 | NCT00407186 | III | 2007–2015 | Surgery plus perioperative chemoradiotherapy | Capecitabine plus cisplatin/oxaliplatin plus epirubicin | PeriCRT | 67 | 0–1 | II versus III (NA) | 1.12 (95% CI 0.61–2.06) |
| Surgery plus perioperative chemotherapy | Capecitabine plus cisplatin/oxaliplatin plus epirubicin | PeriCT | 68 | |||||||
| Fuchs et al.18 | NCT00052910 | III | 2002–2009 | Surgery plus adjuvant chemoradiotherapy | 5-FU plus cisplatin plus epirubicin | Not included | 55 | 0–2 | NA (NA) | 1.02 (95% CI 0.62–1.68) |
| Surgery plus adjuvant chemoradiotherapy | 5-FU plus leucovorin | 65 | ||||||||
| Stahl et al.2 | NA | III | 2000–2005 | Surgery plus preoperative chemoradiotherapy | 5-FU plus cisplatin | PreCRT | 60 | 33/24 | I versus II (55% versus 45%) | 0.65 (95% CI 0.42–1.01) |
| Surgery plus preoperative chemotherapy | 5-FU plus cisplatin | PreCT | 59 | 38/17 | I versus II (54% versus 46%) | |||||
| Cunningham et al.19 | NCT00450203 | III | 2007–2014 | Surgery plus perioperative chemotherapy | Capecitabine plus cisplatin plus epirubicin plus bevacizumab | PeriCTT | 271 | 0–1 | I versus II versus III (24% versus 35% versus 41%) | 0.98 (95% CI 0.77–1.25) |
| Surgery plus perioperative chemotherapy | Capecitabine plus cisplatin plus epirubicin | PeriCT | 265 | I versus II versus III (23% versus 39% versus 38%) | ||||||
| Noh et al.20 | NCT00411229 | III | 2006–2009 | Surgery plus adjuvant chemotherapy | Capecitabine plus oxaliplatin | PostCT | 24 | 0–2 | NA (NA) | 0.63 (95% CI 0.09–4.45) |
| Surgery | None | S | ||||||||
| Ychou et al.3 | NCT00002883 | III | 1995–2003 | Surgery plus perioperative chemotherapy | 5-FU plus cisplatin | PeriCT | 70 | 0–1 | NA (NA) | 0.57 (95% CI 0.39–0.83) |
| Surgery | None | S | 74 | |||||||
| Cunningham et al.21 | ISRCTN93793971 | III | 1994–2002 | Surgery plus perioperative chemotherapy | 5-FU plus cisplatin plus epirubicin | PeriCT | 28 | 0–1 | NA (NA) | 0.48 (95% CI 0.27–0.86) |
| Surgery | None | S | 30 | |||||||
| MRCOCWG22 | NA | III | 1992–1998 | Surgery plus preoperative chemotherapy | 5-FU plus cisplatin | PreCT | 40 | 0–1 | NA (NA) | 0.63 (95% CI 0.38–1.06) |
| Surgery | None | S | 42 |
MRCOCWG: Medical Research Council Oesophageal Cancer Working Group; NA: not available; OS-HR: overall survival (hazard ratio).
Nodes: PeriCRT: perioperative chemoradiotherapy; PeriCT: perioperative chemotherapy; PreCRT: preoperative chemoradiotherapy; PreCT: preoperative chemotherapy; PeriCTT: perioperative chemotherapy plus targeted medication; PostCT: postoperative chemotherapy; S: surgery alone.
Since Fuchs and colleagues 2017 could not form a network with other studies, it was eliminated from the network calculation and was only narratively discussed.
Owing to limitations on the amount of original time-to-event survival data (most studies did not offer subgroup analysis on recurrence-free survival and safety profiles), overall survival was the only quantitative endpoint in our network meta-analysis. Consistent among all included trials, overall survival was defined as the time from randomization to death from any cause.
Statistical analysis
Our systematic review contained both narrative and quantitative synthesis. Those trials with high homogeneity as well as adequate original data were incorporated into network meta-analysis. Hazard ratio (HR) and its 95% confidential interval (95% CI) were used as the effect size for overall survival.
Transitivity was the key hypothesis for network meta-analysis. When the head-to-head results of A versus C and B versus C were respectively gained, then the hypothesis of transitivity also permitted a statistical comparison between A and B. However, it required comparable general features within each node as the prerequisite condition to eliminate selection bias and justify statistical connections among indirect arms.23 Both methodological designs (such as RCTs) and clinical features (such as region and systemic regimens) were crucial for assessment of transitivity. Statistical heterogeneity of the network meta-analysis was the overall degree of disparity within the same pairwise comparison.24 The I2 statistic was the chief indicator of statistical heterogeneity, with its value <25%, 25–50% and >50% indicating low, moderate and high heterogeneity respectively. The Q statistic of heterogeneity and its p value also facilitated the assessment of statistical heterogeneity. If the p value of the Q statistic was less than 0.05, it suggested that there was a significant heterogeneity within.
The consistency, another crucial assumption for network meta-analysis, referred to the statistically consistent results between direct and indirect effect sizes regarding the same comparison. Significant differences between direct and network calculations might indicate inconsistency within the network meta-analysis, while also suggesting the unsuitability for transitivity.25 Among closed loops of each network, we utilized a loop-specific method that assessed the mutual variance between direct and indirect results. The inconsistency factor (IF) was applied as the quantitative indicator that suggested the existence of inconsistency once its 95% CI excluded zero.26 Meanwhile, the Q statistic of inconsistency was another statistical indicator to numerically estimate the consistency within the comparisons, whose p value (<0.05) could suggest a significant inconsistency of network meta-analysis. Both consistency and homogeneity were crucial bases to offer reliable outcomes by network meta-analysis. If inconsistency or significant heterogeneity occurred, we deleted the original data from the most inconsistent or heterogeneous pairwise comparisons to examine whether the results remained unchanged; otherwise, it was not appropriate for pooled analysis.24,27
A network plot and comparison-adjusted funnel plot were applied to display the network structure and examine the publication bias across the included trials respectively, where the more symmetrical it was, the less probability of publication bias the merged results would have. We conducted the random-effects network meta-analysis based on a frequentist model. A network forest plot or league table was used for demonstrating the entire regimens with their relative confidential intervals. In addition, we also utilized P-score to rank all regimens based on their network estimates. The closer P-score approached to 1, the better the regimen could be. Sensitivity analysis was performed to detect the stability of pooled outcomes by deleting potentially heterogeneous studies. Quantitative network meta-analysis was conducted using R software 3.4.3, assisted by STATA 14.0 in terms of graphical functions. For some studies that offered forest plots of subgroup overall survival data, we use Engauge Digitizer 10.9 to obtain and estimate the HR before quantitative incorporation.
Role of the funding source
The sponsors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
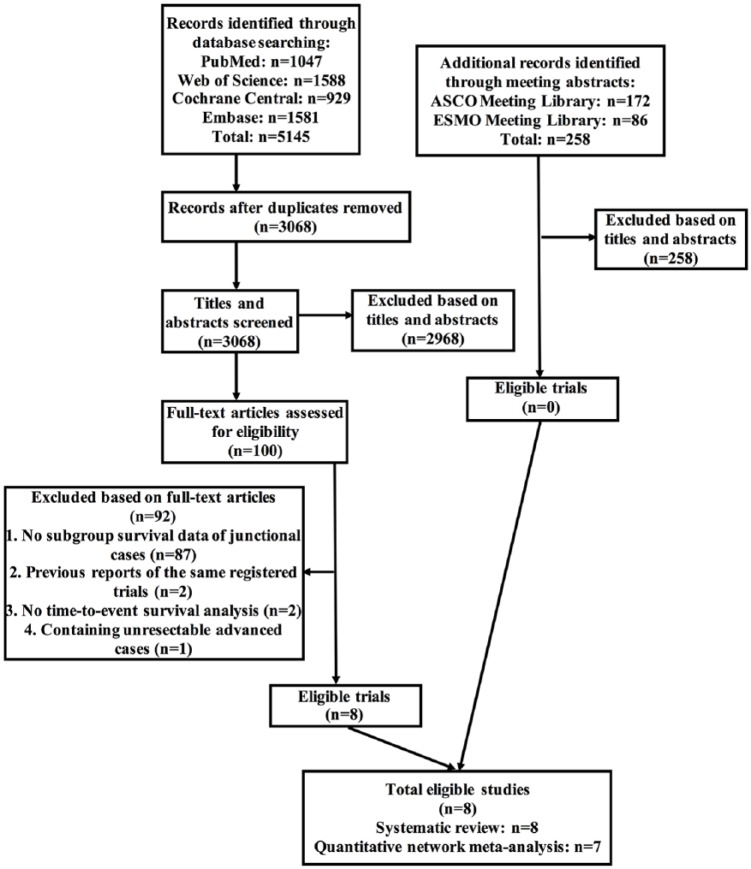
After screening through 5403 preliminary records (5145 from databases and 258 from meeting libraries), a total of eight RCTs were eligible for inclusion into our systematic review, corresponding to 1218 participants (Figure 1 and Table 1). Reasons of ineligibility by full-text assessment were described in Supplementary Table 1.
Figure 1.
Selection flow chart for the systematic review.
Overall, seven studies were based on western populations while only one eligible trial originated from eastern countries. All studies were phase III RCTs and six of them reported registration protocols ahead of trial enrollment. Four trials featured comparisons between multimodal strategies against surgery alone, while the remaining investigations focused on comparisons between different multimodal treatments. Since only one trial specifically reported junctional cases, the other seven studies contained both junctional and gastric or esophageal cancer patients; therefore the median age and gender ratio of junctional cases across different studies could not be precisely compared. However, based on the results of their general analysis, we still believed that their ages and gender ratios were comparable. Predominantly, studies only recruited patients with a performance status of either 0 or 1. All studies made general enrollment of junctional cases without indication of certain Siewert types. Therefore, the demographic characteristics of included trials were generally comparable (Table 1).
Risk of bias
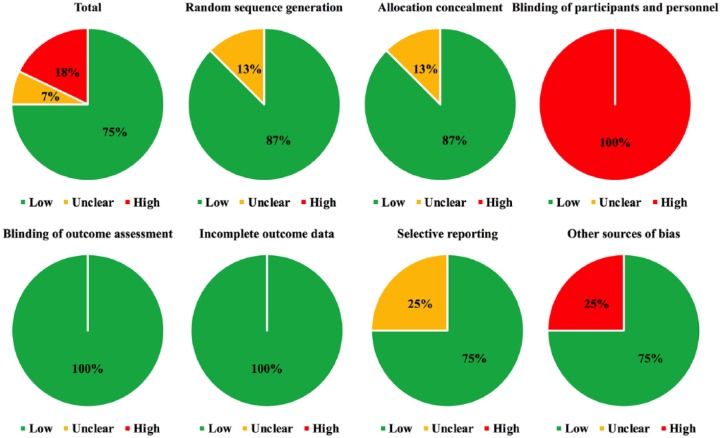
Overall, the included studies had low risk of bias since more than half of the assessment parameters were scored as low risk of bias (75%), while unclear risk (7%) or high risk of bias (18%) took up relatively small proportions (Figure 2). Individually, none of the eligible studies had a high risk of bias concerning methodological design (Supplementary Table 2).
Figure 2.
Risk of bias assessment.
Specifically, since the majority of trials were centrally allocated and adequately randomized, 87% and 87% of the studies were evaluated as low risk of bias concerning random sequence generation and allocation concealment respectively, while no high risk of bias was reported in these two key domains. Due to the open-label design and impossibility for treatment masking with greatly differently administered arms, all of the included trials were scored as high risk of bias in terms of blinding or participants and personnel. Since overall survival was the only endpoint in our network meta-analysis that was relatively objective and unlikely to be affected by artificial bias, all studies were scored as low risk of bias in terms of blinding of outcome assessment. In addition, since overall survival analyses of all studies were based on intent-to-treat population while the majority of them had reported enough endpoints, 100% and 75% of the eligible trials had low risk of bias regarding incomplete outcome data and selective reporting, respectively. Moreover, since most of studies were completed, without early termination, and also described adequate baseline details, more than half of the studies were appraised as low risk of bias with respect to other sources of bias (75%) (Figure 2).
Overall survival
Network geometry
There were totally seven RCTs merged into the quantitative analysis, corresponding to seven network nodes (Supplementary Figure 1 and Table 1). Due to failure to form a network with other studies, the 2017 study by Fuchs and colleagues18 was removed from the quantitative analysis. The results are described in the Discussion section.
Transitivity
We had reorganized and categorized studies with the same nodes into comparisons (Supplementary Table 3). Since all included trials were RCTs with relatively low risk of bias concerning study design, the overall methodological heterogeneity was considered to be of a low level. Generally, all included trials were comparable concerning clinical features. Moreover, all surgical operations were radically conducted with enough resection margin, lymph-node dissection and standardized administration of systemic regimens (see Supplementary Materials). However, among the ‘PeriCT’ node, only Ychou and colleagues3 reported fluoropyrimidine plus platinum doublet regimen, while the other three studies featured fluoropyrimidine plus platinum plus epirubicin triplet regimen. Meanwhile, Noh and colleagues20 was the only trial based on an eastern population. Therefore, we decided to delete these two studies in the sensitivity analysis to enhance the homogeneity as well as to detect the outcome stability.
Consistency and heterogeneity
Since no closed loop had been found within the network, consistency of the network could not be analyzed. In terms of statistical heterogeneity, both the I2 (I2 = 0%) and Q statistic (Q-heterogeneity: p = 0.632) implied that there was no significant heterogeneity across the network.
Publication bias
There was no publication bias among the included studies due to symmetrical distribution of effect sizes inside the funnel plot (Supplementary Figure 2).
Network calculation
Since preoperative chemoradiotherapy has recently been recommended as the preferred regimen by the NCCN guidelines, ‘PreCRT’ was selected as the common comparator. Based on P-score ranking of the network meta-analysis, ‘PreCRT’ (network HR 1.00, P-score = 0.823) was the best ranking node, followed by ‘PeriCTT’ (network HR 95% CI: 1.30 (0.60–2.682), P-score = 0.622) and ‘PeriCT’ (network HR 95% CI: 1.32 (0.63–2.77), P-score = 0.591). The network forest plot and league table are demonstrated in Figures 3 and 4 respectively.
Figure 3.
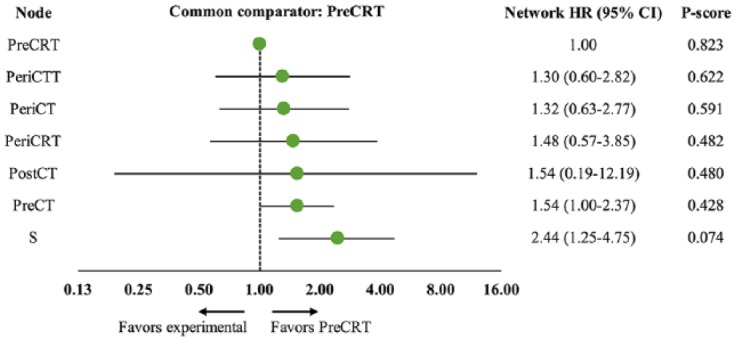
Network forest plot of overall survival.
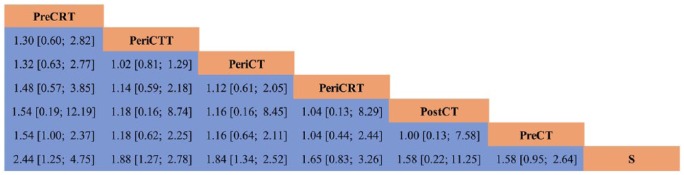
Figure 4.
Network league table of overall survival.
Treatments are hierarchically ranked according to their P-score. The higher position in the table a regimen locates at, the better survival benefits it could offer. Values situated at the intersection of a specific column and row are the network effect sizes (HR and 95% CI) of lower-situated regimen versus higher-situated regimen.
Sensitivity analysis
First, we used a fixed-effects model instead of a random-effects model to detect outcome stability, which demonstrated similar ranking and network results of each node and ‘PreCRT’ still ruled the entire hierarchy (Supplementary Figure 3). Second, as previously mentioned, the studies by Ychou and colleagues and Noh and colleagues were removed from the network due to possibly heterogeneous clinical features, which did not alter the ultimate ranking among the involved nodes where ‘PreCRT’ was the optimal option (Supplementary Figure 4). Therefore, the results of the network meta-analysis were stable.
Discussion
EGJ cancer is a type of malignancy located in the transition part of the esophagus and stomach, for which smoking, obesity and gastroesophageal reflux disease appear to be significant risk factors.28 Due to its rising incidence during recent years, especially in western countries, there is an urgent need to figure out the best therapeutic strategy. Unfortunately, there is a lack of a specific systematic summary of potential multimodal treatments for EGJ cancer, let alone a hierarchical comparison of efficacy that could benefit clinical decision-making and future design of RCTs.
This is the first systematic review and network meta-analysis specific for survival comparisons among different multimodal treatments for resectable EGJ cancer. In terms of overall survival, ‘PreCRT’ was the top node in the hierarchy, displaying insignificant superiority against other counterparts such as ‘PreCT’ and ‘PeriCT’, which were recommended by Japanese11 and ESMO10 guidelines, respectively. This result was partially contributed by, and consistent with, the original data of Stahl and colleagues,2 which reported that preoperative chemoradiotherapy was comparable with preoperative chemotherapy in terms of overall survival among resectable EGJ cancer patients, but demonstrating a statistical boundary advantage (HR = 0.65, p = 0.055). Meanwhile, our pooled result was also in accord with the recommendation by NCCN guidelines,8 despite the fact that their decision was mainly based on esophageal cancer and was not specific to EGJ cancer. Since most of the original data were extracted from subgroup analyses in which overall survival instead of recurrence and safety profiles was the most likely endpoint to be reported, we failed to obtain enough data for recurrence and safety comparisons. Only Stahl and colleagues2 reported that local progression-free survival by preoperative chemoradiotherapy was significantly better than that of preoperative chemotherapy only (HR 0.37, p = 0.01), as well as histological tumor response rate (p = 0.03). As for safety profiles, although there was inadequate specific data, based on general gastroesophageal rather than specific EGJ cases, adding radiation treatment to chemotherapy seemed to result in comparable tolerability compared to chemotherapy alone.17,29 All these results hinted that preoperative chemoradiotherapy might be the most survival-beneficial treatment against resectable EGJ cancer.
Regarding the work by Fuchs and colleagues,18 although it could not be incorporated into the network calculation, we believe that the removal of it may not have substantial impact on our final results since it featured a comparison between two different systemic regimens of postoperative chemoradiotherapy without showing statistically positive outcome. Also, postoperative chemoradiotherapy is not believed to be the mainstream treatment against resectable EGJ cancer currently. Recently, perioperative FLOT-based (5-FU plus leucovorin plus oxaliplatin plus docetaxel) chemotherapy displayed significantly better survival benefits than perioperative EPF-based (fluoropyrimidine plus platinum plus epirubicin) chemotherapy among resectable esophagogastric cancer patients (FLOT4-AIO trial).30,31 However, since it did not report subgroup survival data specific on EGJ cases, we could not include the trial in our systematic review. However, based on their subgroup analysis of complete tumor response among EGJ cases, the FLOT regimen was also significantly superior to the EPF regimen (10/61 versus 3/78, p = 0.01),30 which hinted at a potential survival superiority of FLOT regimen among resectable EGJ cases. Since all studies included into our network meta-analysis with perioperative chemotherapy were based on EPF regimens, the survival comparison between preoperative chemoradiotherapy and perioperative FLOT-based chemotherapy could be an interesting topic in future updates of our systematic review or design of pairwise RCTs.
Although our systematic review and network meta-analysis were rigorously designed and conducted, there were still some limitations. First, there were only seven studies qualified for the quantitative analysis, which limited the statistical power and clinical significance. The restricted number of studies resulted in inadequate pairwise comparisons between any two of the seven nodes, which meant we were unable to evaluate network consistency of our pooled results. Besides the pairwise comparison with ‘PreCT’, there was no RCT featuring the comparison between ‘PreCRT’ and any other regimen, such as surgery alone or perioperative chemotherapies. Among the seven studies, six offered only subgroup data of the EGJ cases and failed to further provide survival information based on different Siewert types that could allow us to make more specific subgroup comparisons. Besides, the majority of the studies were based on western populations, which diminished the value of our conclusion for utilization in eastern countries. Meanwhile, inadequate data also limited our calculation to the endpoint of overall survival, while results for recurrence and safety profile could not be established. Second, despite that all included trials were proven to be clinically comparable, without significant heterogeneity, and sensitivity analysis had also been conducted, underlying heterogeneity could not be fully eliminated. Apart from clinical and methodological heterogeneity, since the HR was a relative endpoint, the baseline survival rate of studies among the same node is a critical indicator for determination of homogeneity as well as the rationality of quantitative incorporation. However, since the original data of most studies derived from subgroup analysis, we could not further obtain the baseline survival rates of EGJ cases. Therefore, future updates, especially individual patient data (IPD) meta-analysis, are to be welcomed.
Taken together, our systematic review and network meta-analysis provided the first and most specific evidence regarding therapeutic treatments against resectable EGJ cancer. Our findings suggest that preoperative chemoradiotherapy could potentially be the optimal multimodal treatment as it showed more overall survival benefits than perioperative chemotherapy and preoperative chemotherapy among resectable esophagogastric junction cancer patients. Therefore, a global (both eastern and western populations) RCT is needed to confirm our results, which should compare preoperative chemoradiotherapy with perioperative chemotherapy (especially FLOT-based regimens), in terms of overall survival, recurrence and safety profile, as well as different subgroup data on Siewert types (Table 2).
Table 2.
Ongoing randomized controlled trials on multimodal treatments for resectable esophagogastric junction cancer.
| ClinicalTrials.gov identifier | Phase | Disease | Siewert type | Location | Status | Estimated completion date | Experimental arm | Control arm |
|---|---|---|---|---|---|---|---|---|
| NCT01962246 | II/III | Resectable gastroesophageal junction cancer | II–III | China | Active | December 2018 | Surgery plus perioperative chemoradiotherapy (capecitabine plus oxaliplatin) | Surgery plus postoperative chemotherapy (capecitabine plus oxaliplatin) |
| NCT01523015 | II/III | Resectable gastroesophageal junction cancer | I–III | Poland | Recruiting | December 2020 | Surgery plus preoperative chemoradiotherapy (5-FU plus oxaliplatin plus docetaxel) for Siewert I and II; surgery plus preoperative chemotherapy (5-FU plus oxaliplatin plus docetaxel) for Siewert III | Surgery alone |
| NCT02193594 | II/III | Resectable upper gastric or gastroesophageal junction cancer | NA | China | Recruiting | May 2020 | Surgery plus perioperative chemoradiotherapy (S-1 plus oxaliplatin) | Surgery plus postoperative chemotherapy (S-1 plus oxaliplatin) |
| NCT03349866 | II | HER2-negative resectable gastroesophageal junction cancer | II–III | China | Recruiting | June 2020 | Surgery plus preoperative chemoradiotherapy (capecitabine plus oxaliplatin) plus apatinib | Surgery plus preoperative chemoradiotherapy (capecitabine plus oxaliplatin) |
| NCT03421288 | II | Resectable gastric or gastroesophageal junction cancer | I–III | Germany | Recruiting | February 2025 | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel) plus atezolizumab | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel) |
| NCT02509286 | III | Resectable esophageal or gastroesophageal junction cancer | NA | Germany | Recruiting | June 2024 | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel) | Surgery plus preoperative chemoradiotherapy (carboplatin plus paclitaxel) |
| NCT02205047 | II | HER2-positive resectable gastric or gastroesophageal junction cancer | I–III | Multiple European countries | Recruiting | September 2024 | Surgery plus perioperative chemotherapy (5-FU/capecitabine plus cisplatin) plus trastuzumab plus pertuzumab; surgery plus perioperative chemotherapy (5-FU/capecitabine plus cisplatin) plus trastuzumab | Surgery plus perioperative chemotherapy (5-FU/capecitabine plus cisplatin) |
| NCT01196390 | III | HER2-positive resectable esophageal or gastroesophageal junction cancer | I–III | USA | Active | April 2019 | Surgery plus preoperative chemoradiotherapy (carboplatin plus paclitaxel) plus perioperative trastuzumab | Surgery plus preoperative chemoradiotherapy (carboplatin plus paclitaxel) |
| NCT01726452 | III | Resectable esophageal or gastroesophageal junction cancer | NA | Denmark, France, Ireland, UK | Recruiting | January 2024 | Surgery plus perioperative chemotherapy (5-FU/capecitabine plus cisplatin plus epirubicin) | Surgery plus preoperative chemoradiotherapy (carboplatin plus paclitaxel) |
| NCT01404156 | II/III | Resectable esophageal or gastroesophageal junction cancer | I–II | Canada | Recruiting | April 2021 | Surgery plus preoperative chemoradiotherapy (carboplatin plus paclitaxel) | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel OR 5-FU/capecitabine plus cisplatin plus epirubicin) |
| NCT03013010 | III | Resectable gastric or gastroesophageal junction cancer | II–III | China | Recruiting | December 2023 | Surgery plus perioperative chemoradiotherapy (S-1 plus oxaliplatin) | Surgery plus perioperative chemotherapy (S-1 plus oxaliplatin) |
| NCT02512380 | III | Resectable gastric or gastroesophageal junction cancer | NA | China | Recruiting | July 2021 | Surgery plus perioperative chemotherapy (S-1 plus oxaliplatin plus docetaxel) | Surgery plus perioperative chemotherapy (S-1 plus oxaliplatin) |
| NCT02581462 | II/III | HER2-positive resectable gastric or gastroesophageal junction cancer | I–III | Germany | Active | March 2021 | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel) plus trastuzumab/pertuzumab | Surgery plus perioperative chemotherapy (5-FU plus leucovorin plus oxaliplatin plus docetaxel) |
The search of ongoing trials was conducted on ClinicalTrials.gov. Only phase II and phase III randomized controlled trials with a status of ‘recruiting’ or ‘active’ were included.
Supplemental Material
Supplemental material, Revised_supplementary_Materials for Multimodal treatments for resectable esophagogastric junction cancer: a systematic review and network meta-analysis by Ji Cheng, Ming Cai, Xiaoming Shuai, Jinbo Gao, Guobin Wang and Kaixiong Tao in Therapeutic Advances in Medical Oncology
Acknowledgments
We thank all staff in our department for providing clinical and methodological advice during the performance of our meta-analysis.
Footnotes
Author contributions: Study design: Ji Cheng, Guobin Wang and Kaixiong Tao. Manuscript writing and revision: Ji Cheng and Kaixiong Tao. Literature retrieval: Ji Cheng and Ming Cai. Decision on eligibility: Ji Cheng and Ming Cai. Quality assessment: Ji Cheng and Jinbo Gao. Data extraction: Ji Cheng and Xiaoming Shuai. Statistical analysis: Ji Cheng and Kaixiong Tao.
Funding: The meta-analysis was funded by Scientific Research Training Program for Young Talents (Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) to Ji Cheng and National Natural Science Foundation of China (grant no. 81572413) to Kaixiong Tao.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
ORCID iD: Ji Cheng  https://orcid.org/0000-0002-7673-9157
https://orcid.org/0000-0002-7673-9157
Contributor Information
Ji Cheng, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No.1277 Jiefang Avenue, Wuhan 430022, China Department of Pathology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, USA.
Ming Cai, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Xiaoming Shuai, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Jinbo Gao, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Guobin Wang, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Kaixiong Tao, Department of Gastrointestinal Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No.1277 Jiefang Avenue, Wuhan 430022, China.
References
- 1. Rudiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2000; 232: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stahl M, Walz MK, Riera-Knorrenschild J, et al. Preoperative chemotherapy versus chemoradiotherapy in locally advanced adenocarcinomas of the oesophagogastric junction (POET): long-term results of a controlled randomised trial. Eur J Cancer 2017; 81: 183–190. [DOI] [PubMed] [Google Scholar]
- 3. Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 4. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 6. Peyre CG, Hagen JA, DeMeester SR, et al. Predicting systemic disease in patients with esophageal cancer after esophagectomy: a multinational study on the significance of the number of involved lymph nodes. Ann Surg 2008; 248: 979–985. [DOI] [PubMed] [Google Scholar]
- 7. Gee DW, Rattner DW. Management of gastroesophageal tumors. Oncologist 2007; 12: 175–185. [DOI] [PubMed] [Google Scholar]
- 8. National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Esophageal and esophagogastric junction cancer, Version 2. 2018, https://www.nccn.org (2018, accessed May 22, 2018).
- 9. Chevallay M, Bollschweiler E, Chandramohan SM, et al. Cancer of the gastroesophageal junction: a diagnosis, classification, and management review. Ann N Y Acad Sci 2018; 1434: 132–138. [DOI] [PubMed] [Google Scholar]
- 10. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v50–v57. [DOI] [PubMed] [Google Scholar]
- 11. Japan Esophageal Society. Japanese esophageal cancer treatment guidelines, Version 5, http://www.esophagus.jp. 2018. [Google Scholar]
- 12. Committee CGC. Chinese guidelines on the management of esophageal cancer (2018 edition), http://test.csco.org.cn. 2018. [Google Scholar]
- 13. Committee CGC. Chinese guidelines on the management of gastric cancer (2018 edition), http://test.csco.org.cn. 2018. [Google Scholar]
- 14. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines, Version 5. 2018, http://www.jgca.jp. [Google Scholar]
- 15. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v38–v49. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cats A, Jansen EPM, van Grieken NCT, et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19: 616–628. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant chemoradiotherapy with epirubicin, cisplatin, and fluorouracil compared with adjuvant chemoradiotherapy with fluorouracil and leucovorin after curative resection of gastric cancer: results from CALGB 80101 (Alliance). J Clin Oncol 2017; 35: 3671–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2–3 trial. Lancet Oncol 2017; 18: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 21. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 22. Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; 359: 1727–1733. [DOI] [PubMed] [Google Scholar]
- 23. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385: 2047–2056. [DOI] [PubMed] [Google Scholar]
- 24. Fujii T, Le Du F, Xiao L, et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer: a systematic review and network meta-analysis. JAMA Oncol 2015; 1: 1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010; 29: 932–944. [DOI] [PubMed] [Google Scholar]
- 26. Cheng J, Cai M, Shuai X, et al. Comparative efficacy and tolerability of antiemetic prophylaxis for adult highly emetogenic chemotherapy: a network meta-analysis of 143 randomized controlled trials. Int J Cancer 2018; 142: 1067–1076. [DOI] [PubMed] [Google Scholar]
- 27. Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol 2013; 42: 332–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol 2013; 23: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park SH, Sohn TS, Lee J, et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J Clin Oncol 2015; 33: 3130–3136. [DOI] [PubMed] [Google Scholar]
- 30. Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016; 17: 1697–1708. [DOI] [PubMed] [Google Scholar]
- 31. Al-Batran SE, Pauligk C, Homann N, et al. LBA-008Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) as perioperative treatment of resectable gastric or gastro-esophageal junction adenocarcinoma: the multicenter, randomized phase 3 FLOT4 trial (German Gastric Group at AIO). Ann Oncol 2017; 28 Epub ahead of print July 3, 2017. DOI: 10.1093/annonc/mdx302.007. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Revised_supplementary_Materials for Multimodal treatments for resectable esophagogastric junction cancer: a systematic review and network meta-analysis by Ji Cheng, Ming Cai, Xiaoming Shuai, Jinbo Gao, Guobin Wang and Kaixiong Tao in Therapeutic Advances in Medical Oncology