Abstract
Background:
Primary and recurrent giant cell tumor of bone is typically benign; however, rarely giant cell tumor of bone can undergo malignant transformation. Malignancy in giant cell tumor of bone may be primary (adjacent to benign giant cell tumor of bone at first diagnosis) or secondary (at the site of previously treated giant cell tumor of bone). Malignant giant cell tumor of bone has a poor prognosis; it is important to distinguish malignant from benign lesions to facilitate appropriate management. The true incidence of malignant giant cell tumor of bone is not known, probably owing to inaccurate diagnosis and inconsistent nomenclature. We have analyzed current data to provide a robust estimate of the incidence of malignancy in giant cell tumor of bone.
Methods:
A literature search was performed to source published reports of primary and secondary cases of malignant giant cell tumor of bone. Studies that reported a denominator were used to estimate the incidence of malignancy.
Results:
We identified 4 large series of patients with malignant giant cell tumor of bone that provided data on 2315 patients with giant cell tumor of bone. Across these studies, the cumulative incidence of malignancy was 4.0%; the cumulative incidence of primary malignancy was 1.6% compared with 2.4% for secondary malignancy. Our analyses confirmed that most malignant giant cell tumor of bone is secondary and occurs following radiation. In addition, data from 8 small series showed that 4.8% of patients with giant cell tumor of bone who received radiation therapy developed secondary malignancy.
Conclusions:
Malignant giant cell tumor of bone is rare, and its identification is hindered by a lack of clear diagnostic criteria. For optimal care of patients with giant cell tumor of bone, we recommend: comprehensive histologic sampling to ensure accurate diagnoses; watchful follow-up, particularly for patients treated with radiation; and timely treatment of local recurrence.
Keywords: bone tumors, clinical features, diagnosis, incidence, prognosis, rare disease
Introduction
Giant cell tumor of bone (GCTB) accounts for approximately 5% to 6% of primary bone tumors.1,2 It usually occurs in the meta-epiphyseal region of long bones but may also occur in the axial skeleton or small bones of the hands and feet.3 Giant cell tumor of bone is typically benign but can be locally aggressive; bone disruption can be particularly problematic around joints, compromising joint function and mobility.2 Local benign GCTB recurrence occurs in approximately 25% of patients,4,5 with the highest rates reported (up to 50%) following curettage.5 Synchronous occurrence of benign GCTB at multiple sites is rare.6 Approximately 1% to 6% of benign tumors will metastasize, most commonly to lung.1 Although normally indolent, benign metastases can compromise pulmonary function and occasionally be lethal.1,3,7
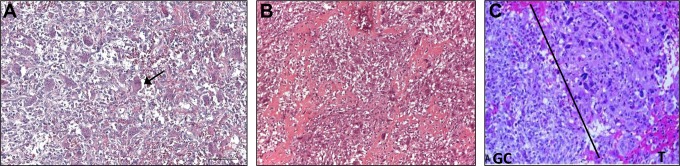
Rarely, GCTB undergoes sarcomatous transformation into a malignant tumor, which may be an osteosarcoma, fibrosarcoma, or undifferentiated pleomorphic sarcoma (historically known as malignant fibrous histiocytoma).8 Malignant tissue can be considered as either primary or secondary malignant GCTB. Primary malignant GCTBs are evident at first diagnosis of GCTB and contain an area or a nodule of highly pleomorphic mononuclear cells present within an otherwise conventional GCTB. Secondary malignancy GCTBs occur at the site of previously treated GCTB, and the preexisting GCBT may or may not be evident.9-12 Most malignant GCTB is secondary and typically follows radiotherapy,13 but it can follow surgery without adjuvant radiotherapy.7 Primary malignant GCTB is considered to be very rare;14 differential diagnoses include giant cell-rich sarcomas (eg, osteosarcoma, undifferentiated pleomorphic sarcoma, and leiomyosarcoma) and benign GCTB. A detailed histologic examination is required to confirm malignant tissue and initiate appropriate treatment (Figure 1).
Figure 1.
Histologic differences between benign giant cell tumor of bone (GCTB), giant cell-rich osteosarcoma, and malignant GCTB. (A) Benign GCTB consists of two cell types: stromal mononuclear spindle cells and multinucleated giant cells (GCs). Arrow shows a GC. (B) Giant cell-rich osteosarcoma consists of GCs, oval or spindle mononuclear cells with atypical nuclei, fibrovascular stroma, and new woven bone. (C) Malignant GCTB consists of a GC tumor component with mononuclear cells with typical nuclei, side by side with a pleomorphic malignant spindle cell component (T). Panels A and B are reproduced with permission from Dr Palmerini. Panel C has been adapted with permission from Bertoni et al.14
Malignant GCTB was first described nearly 80 years ago;15 however, estimates of malignant GCTB frequency have been confounded by lack of consensus on definition and diagnosis such that terminology has been inconsistently used in the literature.16 Furthermore, owing to the juxtaposition of benign and malignant tissue in primary malignant GCTB, failure to comprehensively sample tumors may lead to misdiagnoses of benign GCTB. If malignant tissue is detected during later sampling, primary malignant GCTB could be misdiagnosed as secondary malignancy.17
To evaluate the impact of treatments on the risk of GCTB malignant transformation, accurate data on its incidence are needed. This review summarizes published clinical experience of primary and secondary malignant GCTB, with consideration of the challenges associated with its diagnosis.
Classification of GCTB
Historically, GCTB was classified as grade I (benign; no appreciable stromal cell abnormality), grade II (intermediate; slight or more marked stromal cell abnormality), or grade III (malignant; obvious signs of malignancy).8 This classification system is of limited clinical value and is no longer used.8
Radiographically, benign GCTB comprises an eccentric lytic lesion with nonsclerotic and sharply defined margins.18 It can show aggressive features including a wide zone of transition, cortical thinning and remodeling, and cortical bone destruction with an associated soft tissue mass.18 Histologically, GCTB comprises neoplastic cells (osteoclast precursor and spindle-shaped stromal cells) and reactive cells, including large, multinucleated, osteoclast-like giant cells (Figure 1).3
Malignant GCTB is a high-grade sarcoma.8 Primary malignant GCTB comprises sarcomatous growth juxtaposed to benign GCTB, whereas secondary malignant GCTB comprises sarcomatous growth at the site of previously documented benign GCTB.9-11
Methodology Used to Identify Published Evidence on Malignant GCTB
A structured literature search was performed to identify studies of interest for the review that reported the numbers of nonmalignant and malignant GCTB cases. Reports were selected for inclusion if they (1) reported the total number of patients (denominator for incidence calculation), (2) categorized the malignancy as primary or secondary, and (3) provided the previous treatment for secondary malignancy cases. Case reports of malignant GCTB were also considered. Reports were included irrespective of the diagnostic criteria for GCTB used in each study.
The literature search identified 32 publications that met the predefined criteria. The results included 8 publications from 4 centers reporting data from large patient series; 2 of these centers (the IRCCS Istituto Ortopedico Rizzoli Italy14,19 and Mayo Clinic, Rochester, Minnesota, USA2,11,20,21) were responsible for multiple publications, and, to avoid duplication, only the most recent reports from each center, with supporting information gleaned from the older publications as appropriate, were included in the review (Table 1).2,11,14,19-23 There were 8 publications that reported data from smaller series of patients who had received radiotherapy (Table 2).24-31 Eleven publications were identified that reported data on patients who had undergone curettage and grafting (without radiotherapy; Table 3).32-42 Finally, there were 5 additional publications that reported cases of secondary malignancy that were unrelated to treatment (Table 4).8,16,43-45
Table 1.
Results of Literature Review: Large Series.a
| Center | Reference | Patients | Mean (Range) Follow-Up | Malignancies, n (%) | Primary | Secondary | Mean (Range) Time to Secondary Malignancy | Prior Radiotherapy in Secondary Malignant Casesb | Prior Surgery Alone in Secondary Malignant Casesc |
|---|---|---|---|---|---|---|---|---|---|
| IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy | Bertoni et al14 | 924 cases | NR | 17 (1.8%) | 5 (4 OS, 1 MFH) | 12 (9 OS, 2 FS, 1 MFH) | 9 (3-15) years (postradiation) | 6 (50.0%) | 6 (50.0%) |
| Campanacci et al19 | 293 patients | NR (<2 to 44 years) | 10 (3.4%) | – | 10 (5 MFH, 3 FS, 2 OS) | NR (2.5-15 years postradiotherapy, 1.5-2 years postsurgery) | 8 (80.0%) | 2 (20.0%) | |
| Cancer Hospital, Kolkata, India | Mondal et al22 | 445 cases (39 prior radiotherapy) | NR (≤21 years) | 5 (1.1%) | – | 5 (3 FS, 1 MFH, 1 OS) | 11 years | 5 (100.0%) | – |
| Mayo Clinic, USA | Unni and Inwards2 | 671 cases | NR | 39 (5.8%) | 5 | 34 | NR | 26 (76.5%) | 8 (23.5%) |
| McDonald et al20 | 221 patients | 7 years (2-21 years) | 5 (2.3%) | 2 | 3 | NR | 2 (66.7%) | – | |
| Rock et al11 | 407 patients | NR | 26 (6.4%) | 7 | 19 (14 FS, 5 OS) | 12.3 years | 18 (94.7%) | 1 (5.3%) | |
| Dahlin21 | 407 patients | NR | 24 (5.9%) | 5 | 23 | >13 years (postradiation) | 21 (91.3%) | 2 (8.7%) | |
| Memorial Sloan Kettering Cancer Center, New York, NY, USA | Domovitov and Healey23 | 275 patients | 147 months (9-377 months for 26 primary malignancy cases) | 31 (11.3%) | 26 | 5 (2 OS, 3 MFH) | 2 years (3 months-4 years) | 5 (19%) | – |
Abbreviations: FS, fibrosarcoma; MFH, malignant fibrous histiocytoma; NR, not reported; OS, osteosarcoma.
aBertoni et al14 and Campanacci et al19 reported overlapping series from the same institution. Unni and Inwards2, McDonald et al20, Rock et al11, and Dahlin21 reported overlapping series from the same institution.
bPercentage represents patients who received radiotherapy and developed a secondary malignancy as a proportion of all secondary malignancy cases.
cPercentage represents patients who received surgery alone and developed a secondary malignancy as a proportion of all secondary malignancy cases.
Table 2.
Results of Literature Review: Small Series, Following Radiation Therapy.
| Reference | Patients | Mean (Range) Follow-Up | Malignancies, n (%) | Primary | Secondary | Mean (Range) Time to Malignancy | Prior Radiotherapy, n (%) |
Prior Surgery, n (%) |
|---|---|---|---|---|---|---|---|---|
| Boriani et al24 | 327 | NR (7 months- 10 years) | 10 (3%) | 10 (5 MFH, 3 FS, OS) | NR (2.5-15 years postradiation; 1-2 years postsurgery) | 8 (80%; 29% for dose >40 Gy) | 8 (80%) | |
| Ruka et al25 | 77 | NR | 2 (2.6%) | – | 2 | NR | 77 (100%) | NR |
| Feigenberg et al26 | 24 | 12 years (2.5-26 years) | 1 (4.2%) | – | 1 (UPS) | 22 years | 1 (4%) | NR |
| Shi et al27 | 34 | 16.8 years (1.4-33.7 years) | 1 (3%) | – | 1 (OS) | 52 months | 34 (100%) | 13 (38%) |
| Chakravarti et al28 | 20 | 9.3 years (3- 19 years) | (0%) | – | – | NA | 20 (100%) | NR |
| Nair and Jyothirmayi 199929 | 20 | 48 months | 0 (0%) | – | – | NA | 20 (100%) | NR |
| Caudell et al30 | 25 | 8.8 years (0.67-34 years) | 0 (0%) | – | – | NA | 25 (100%) | NR |
| Malone et al31 | 21 | 15.4 years (2-35 years) | 0 (0%) | – | – | NA | 21 (100%) | NR |
Abbreviations: FS, fibrosarcoma; MFH, malignant fibrous histiocytoma; NA, not applicable; NR, not reported; OS, osteosarcoma; UPS, undifferentiated pleomorphic sarcoma.
Table 3.
Results of Literature Review: Small Series, Following Curettage and Grafting.
| Reference | Patients | Mean (Range) Follow-Up | Malignancies, n | Primary | Secondary | Mean (Range) Time to Malignancy | Prior Surgery, n (%) |
|---|---|---|---|---|---|---|---|
| Picci et al32 | 12 | 19 years (6.5-28 years) | 12 | – | 12 (8 OS, 3 MFH, 1 FS) | NR | 12 (100%) |
| Takesako et al33 | 1 | 40 years | 1 | – | 1 (OS) | 40 years | 1 (100%) |
| Kadowaki et al34 | 1 | 41 years | 1 | – | 1 (OS) | 41 years | 1 (100%) |
| Li et al35 | 1 | 15 years | 1 | – | 1 (FS) | 15 years | 1 (100%) |
| Marui et al36 | 2 | 16.5 years | 2 | – | 2 (1 MFH, 1 OS) | 14 years | 2 (100%) |
| Saito et al37 | 1 | 6 months | 1 | – | 1 | 6 months | 1 (100%) |
| Mori et al38 | 1 | 29 years | 1 | – | 1 (MFH) | 25 years | 1 (100%) |
| Muramatsu et al39 | 1 | 38 years | 1 | – | 1 (MFH) | 38 years | 1 (100%) |
| Miller et al40 | 1 | 4 years | 1 | – | 1 (OS) | 4 years | 1 (100%) |
| Hashimoto et al41 | 1 | 10 years | 1 | – | 1 (OS) | 10 years | 1 (100%) |
| Machinami et al42 | 1 | 25 years | 1 | – | 1 (OS, SCC) | 25 years | 1 (100%) |
Abbreviations: FS, fibrosarcoma; MFH, malignant fibrous histiocytoma; NR, not reported; OS, osteosarcoma; SCC, squamous cell carcinoma; UPS, undifferentiated pleomorphic sarcoma.
Table 4.
Results of Literature Review: Case Studies of Secondary Malignancy in GCTB.
| Reference | Cases of Secondary Malignancy in GCTB | GCTB History |
|---|---|---|
| Brien et al43 | 1 | Lung metastases after 6 years |
| Wojcik et al44 | 6 | All after local recurrence, 2 with radiotherapy |
| Gong et al8 | 12 | All after local recurrence, none with radiotherapy |
| Grote et al16 | 1 | 10 years with no treatment |
| Oda et al45 | 2 | All after local recurrence, none with radiotherapy |
Abbreviation: GCTB, giant cell tumor of bone.
Frequency of Primary and Secondary Malignant GCTB: Pooled Analysis
The most recent data from the 4 large GCTB patient series showed that the frequency of malignancy was 1.1% to 11.3% (Table 1).2,14,22,23 The 4 series comprised 2315 patients, 92 diagnosed with primary or secondary malignant GCTB giving a cumulative incidence of 4.0%. Primary malignant GCTB was rare, with 36 cases reported among the 2315 patients (incidence of 1.6%). At the Rizzoli Institute, Bertoni and colleagues reported 5 cases of primary malignancy among 924 patients with GCTB (0.5%);14 four had osteosarcomas, and 1 had malignant fibrous histiocytoma.14 At the Mayo Clinic, of 671 patients with GCTB, only 5 (0.7%) had primary malignancies.2 The Cancer Hospital, Kolkata, reported the lowest primary malignancy incidence with no cases among the 445 patients with GCTB, despite a follow-up of up to 21 years. The highest rate of primary malignancy was reported at the Memorial Sloan Kettering Cancer Center (MSKCC) with 26 (9.5%) cases among 275 patients after a follow-up of up to 31 years.22,23 The design of the latter study differed from the others in that it retrospectively applied well-defined diagnostic criteria.23,46 This may, in part, explain the higher primary malignancy incidence reported from the MSKCC.
The data reported from these large patient series are consistent with most malignant GCTB being secondary; among the 92 malignant cases reported, 56 were secondary. Therefore, among the population of 2315 patients with GCTB, the cumulative incidence of secondary malignancy was 2.4%. In the Mayo Clinic series of 671 patients, 34 of the 39 malignant cases were secondary (5.1%), approximately three-quarters (26 cases) of which were treated with radiation and a quarter (8 cases) with surgery.2 In the series from the Rizzoli Institute, 12 (71%) of the 17 malignancies among the 924 patients were secondary; equal numbers were treated with radiation and surgery. There were 5 cases each of secondary malignancy at the Cancer Hospital, Kolkata (445 patients with GCTB) and the MSKCC (275 patients with GCTB); all cases were treated with radiation.22,23
These data also support that most secondary malignant GCTBs occur several years after radiotherapy and are considered to be radiation induced.16 Across the large patient series, only a quarter (14/56) of secondary malignancies followed bone graft/surgery without radiotherapy (cumulative incidence 0.6%; from 2315 GCTB cases), but it is unclear whether the use of surgical adjuvants (such as phenol or cement) in these cases may have influenced the course of the disease. Furthermore, the first of the 2 reports from the Rizzoli Institute showed a clear association between secondary malignancy and prior radiation dose: over a quarter of patients who received 4000 to 6000 rads adjuvant to surgery developed sarcoma, compared with none who received 3000 rads or less.19 The authors proposed that secondary malignancy cases with short latent periods may be a result of radiation-induced enhancement of the propensity for GCTB to malignant transformation. In secondary malignancy occurring after curettage and bone grafting, it has been postulated that reparative proliferation occurring at the border of dead bone could serve as the source of malignant transformation.17,32
When considering data from the pooled analysis of the large patient series, it should be noted that 1 population-based study from the Swedish Cancer Registry, which reported primary malignant GCTB incidences of 56.5% between 1958 and 1982 and 8% between 1983 and 2011, was excluded from our analyses.47 These high estimates likely arise because the Swedish Cancer Registry receives data from all Swedish clinics; thus, the diagnosis of primary GCTB may not have been verified by an expert. It is probable that many of these cases were giant cell-rich osteosarcomas.47
Although data from individual studies suggest that the mean time from GCTB diagnosis to malignant transformation is shorter in patients who receive radiotherapy than in those who receive surgical treatment alone, data from the 4 large patient series do not support a clear relationship. At the Rizzoli Institute, the mean latency following radiotherapy was 9 years compared with 19 years following bone grafts.14 Early analyses of data from the Mayo Clinic showed latencies of 4 to 38 years following radiotherapy, compared to 1.5 to 22 years following bone grafts.9,11 At the Cancer Hospital, Kolkata, all cases of secondary malignancy followed radiotherapy, and the average latency was 11 years.22
Finally, pooled data were derived from the small patient series comprising 206 patients with GCTB who had received radiotherapy, 12 (4.8%) of who developed secondary malignancy (Table 2).24-31 In total, 23 cases of secondary malignancy were identified in patients who underwent curettage and grafting without radiotherapy (Table 3).32-42 Malignant transformation without previous radiotherapy or surgery is considered to be rare: We identified 22 cases of secondary malignancy with no relation to treatment (combined with 14 cases identified in the large series, there were 36 in total; Table 4).8,16,43-45
A summary of the different incidences and causes, and other characteristics, of primary versus secondary malignant GCTB are summarized in Table 5.
Table 5.
Summary of the Characteristics of Primary and Secondary Malignant GCTB.
| Incidence | Histological Presentation | Differential Diagnosis | Causes | Prognosis | |
|---|---|---|---|---|---|
| Primary malignant GCTB | 1.6% (range 0%-9.5%)a | Sarcomatous growth next to benign GCTB | Giant cell-rich sarcomas (osteosarcoma, undifferentiated pleomorphic sarcoma, leiomyosarcoma) and benign GCTB | Sarcomatous/malignant transformation | Better than for secondary malignant GCTB, eg, 5-year survival 87%23 |
| Secondary malignant GCTB | 2.4% (range 1.1%-5.1%)a | Sarcomatous growth at the site of previously documented GCTB | Giant cell-rich sarcomas (osteosarcoma, undifferentiated pleomorphic sarcoma, leiomyosarcoma) | Typically follows radiotherapy (75%); effect is related to dose of radiation
used Follows bone graft/surgery without radiotherapy (∼25%) |
Worse than for primary malignant GCTB, eg, 5-year disease-free survival 32%11 |
Comparing Clinical Features of Primary Versus Secondary Malignant GCTB
The retrospective long-term study reported by Domovitov and Healey compared benign and primary malignant GCTB.23 Of the 31 malignant cases identified from 275 patients with GCTB, 5 cases of postradiation malignancy were excluded from the subsequent analysis. The resulting 26 primary malignant cases were compared to 244 benign cases. The data suggested that lesions described as Campanacci stage 1 (associated with a well-defined margin and a thin rim of mature bone48) may predict a low hazard of malignancy. The skeletal distribution and epidemiological characteristics of malignant and benign tumors were similar. The recurrence rate was higher for malignant than for benign GCTB (20% vs 9%) although this was not statistically significant. The 5-year survival rate for malignant GCTB was 87%, with a total mortality of 16%; this was interpreted to reflect the low grade of the malignancies that grow and metastasize at a slow rate. Only 1 death was recorded in the benign group (undetermined cause).23
Challenges Associated With Diagnosis of Malignant GCTB
Estimates of malignant GCTB frequency have been confounded by a lack of consensus on definition and diagnosis such that terminology has been inconsistently used in the literature.16 The challenges in the accurate diagnosis of malignant GCTB have been highlighted here by virtue of the range of definitions applied in the reviewed studies (Table 6).2,11,14,19-23,46 The radiologic features of primary malignant GCTB are similar to those of the benign tumor, making it difficult to diagnose using conventional imaging (Table 5).49 Histologic analyses are complicated because the high-grade sarcoma of primary malignant GCTB often exists next to histologically benign GCTB.8 Errors in sampling larger tumors owing to biopsies missing coexistent sarcoma have been well documented.14,17,50 Primary malignancy may be detected only retrospectively when specimens are reevaluated.17,51 Therefore, multiple biopsy specimens should be analyzed before excluding primary malignancy17 to avoid misdiagnoses of secondary malignancy later on; cases of secondary malignancy with short latencies may indicate missed primary malignancy.14 There is also a need to standardize diagnostic criteria for malignant GCTB. The studies we examined often did not report details on the criteria used to diagnose GCTB, and the criteria that were reported were inconsistent across studies. Notably, no studies reviewed here reported misdiagnosis of primary malignant GTCB. Given that misdiagnosis is a recognized challenge in primary malignant GCTB,8,52 this may suggest that there are limitations to the relevance of these data to real-world practice. Alternatively, it could reflect another challenge known to be associated with primary malignant GCTB, in that many diagnoses are missed.8,52 Complementary to pathologic evaluation in GCTB diagnosis is radiology. Bertoni and colleagues reported that primary malignant cases (and certain secondary malignant cases) were impossible to distinguish from benign lesions on plain films, and computerized tomography (CT)/magnetic resonance imaging (MRI) data were often unavailable.14 Domovitov and Healey noted that radiologic diagnosis was complicated by the lack of specific malignant features; aggressive features could be seen in benign lesions. Furthermore, these authors reported that CT/MRI also failed to provide specific signs.23 Upon imaging, secondary malignant GCTB usually presents as an aggressive osteolytic tumor with cortical destruction and soft-tissue extension.16 Postsurgical and radiotherapy-induced secondary malignancy are believed to have different etiologies but cannot be distinguished by radiographic and histologic presentation.14
Table 6.
Definition and Diagnostic Criteria for GCTB and Malignant GCTB Used in 4 Large Case Series.
| Center and References | Diagnosis of Benign GCTB | Definition of Malignant GCTB |
|---|---|---|
| IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy Bertoni et al14 Campanacci et al19 |
Based on clinical data, radiographs, and histologic slides | GCTB was classified as primary or secondary according to Hutter et al10 and Dahlin et al9 Primary malignant GCTB is a high-grade sarcoma that arises side by side with giant cell tumors of bone. Secondary malignant GCTB is a high-grade sarcoma that occurs at the site where a giant cell tumor was previously treated either by radiotherapy (radiation-induced form) or one of various types of surgery (evolutionary form) |
| Cancer Hospital, Kolkata, India Mondal et al22 |
NAC followed by open biopsy was performed for all patients as part of the diagnostic process. Computerized tomography-guided NAC was used for deep-seated lesions such as those occurring in the vertebrae and pelvic bones. The histopathologic diagnosis of GCTB was verified in three separate orthopedic centers by three separate histopathologists after a clinical meeting with orthopedic surgeons and radiologists | A primary malignant GCTB is composed of sarcomatous growth juxtaposed to zones of typical benign GCTB. A secondary malignant GCTB is a sarcomatous growth that occurs at the site of previously documented benign giant cell tumor of bone. Histopathologic examination, verified by three separate histopathologists, was performed for all suspected cases of malignant GCTB |
| Mayo Clinic, Rochester, Minnesota, USA Unni and Inwards2 McDonald et al20 Rock et al11 Dahlin21 |
The diagnosis of benign GCTB was confirmed by a review of histologic sections | GCTB was classified as primary or secondary according to Hutter et al10 and Dahlin et al.9 Primary malignant GCTB is a malignant tumor of bone that is composed of sarcomatous growth juxtaposed to zones of typical benign GCTB tumor, and a secondary malignant GCTB is a sarcomatous growth that occurs at the site of a previously documented benign GCTB. For cases designated as malignant GCTB, the pathologic, radiographic, and clinical aspects were reviewed |
| Memorial Sloan Kettering Cancer Center, New York, NY, USA Domovitov23 |
GCTB was confirmed histologically | Malignancy in GCTB was defined according to Huvos:46 “primary malignant GCTB shows a characteristic pattern of giant cells and
stromal cells in which the appearance of the stromal cells in many microscopic
fields gives the tissue a clearly sarcomatous picture.” Large areas of collagenous
deposits within the lesion and mitotic figures alone were insufficient to make the
diagnosis of malignant GCTB Diagnosis of malignancy of GCTBs was established by means of an open biopsy for most tumors; a needle biopsy was used for spinal tumors. Some cases of malignancy were identified from curettage specimens after treatment of what was suspected to be benign GCTB. All cases were reviewed and confirmed in a weekly tumor board meeting by clinical, radiologic, and histologic correlation |
Abbreviations: GCTB, giant cell tumor of bone, NAC, needle aspiration cytology.
Giant cell-rich osteosarcoma is characterized by an abundance of osteoclast-like giant cells and lack of tumor osteoid, which can be misdiagnosed as GCTB;52 similar to GCTB, it also occurs at epiphyses.52 Bertoni and colleagues suggested that the clinical importance of differentiating primary malignant GCTB from giant cell-rich osteosarcoma may be limited, but the need to differentiate both diseases from benign GCTB is complicated by the subtlety of pathologic evidence of malignancy (Table 5).14 Malignant GCTB is also confused with other giant cell-containing tumors, such as angiosarcoma, fibrosarcoma, and chondrosarcoma.14
A problem with the definition of malignant GCTB is the occurrence of benign metastasizing GCTB; pulmonary metastases are the most common.3 Of the 671 patients with GCTB at the Mayo Clinic, 20 developed benign pulmonary metastases and 2 had tumor-related deaths (4 died from other causes).2 Of the 293 patients at the Rizzoli Institute, only 3 developed confirmed benign pulmonary metastases, and none died from their disease.19 While GCTB pulmonary metastases tend to be indolent and can be treated with surgery,3,7 sarcomatous pulmonary metastases are often fatal.7
Mutational testing is under evaluation as an approach to assist in the diagnosis of GCTB, with recent research suggesting a potential role for histone 3.3 gene mutations.53 In particular, H3F3A mutations may help to differentiate GCTB from giant cell-rich sarcomas. In a recent study, 24 of the 25 GCTBs evaluated had an H3F3A mutation compared with 5 of 35 giant cell-rich sarcomas; all of the sarcomas with the mutation were secondary malignant GCTB.53 Research is ongoing to further evaluate the potential use of mutational analysis in GCTB diagnosis and to understand whether specific mutations are retained or lost when GCTBs undergo malignant transformation.
Prognosis
The prognosis of malignant GCTB is not fully understood owing to the lack of long-term follow-up data.8 In the large case series, the prognosis was better among patients with primary than secondary malignancy. At the Rizzoli Institute, 1 patient with primary malignancy died at 8 months, another was alive at 40 months, and the remaining 3 had no sign of disease at 2, 15, and 161 months.14 At the MSKCC, 5-year survival with primary malignancy was 87%.23 At the Rizzoli Institute, however, all patients with secondary malignancy following radiotherapy died (after 5-148 months), and half of those with secondary malignancy following bone graft died.14 At the Mayo Clinic, 5-year disease-free survival in patients with secondary malignancy was 32%.11 At the Cancer Hospital, Kolkata, all patients who developed postradiation sarcoma died within a few months, owing to sarcomatous lung metastasis.22
It is generally accepted that malignant GCTB is a high-grade sarcoma;1,14 however, data from the MSKCC show that malignant GCTB behaves like a low- or intermediate-grade sarcoma. The difference in GCTB grades reported in this study may reflect that most malignant cases at this center were primary rather than secondary. As discussed, primary malignancies appear to be associated with better prognoses than secondary malignancies (Table 5).
Conclusions
Malignant GCTB is rare; among 2315 patients with GCTB, our analysis identified an incidence of 1.6% for primary malignancy and 2.4% for secondary malignancy. Although current data appear to confirm that most secondary malignancies follow radiation, some large series do not provide complete treatment data, making it difficult to determine secondary malignancy risk associated with specific previous GCTB treatment. Additional studies on treatment patterns and malignancy incidence would help to clarify malignancy risk associated with each treatment. To ensure optimal care of patients with GCTB, we recommend comprehensive histologic sampling to establish an accurate diagnosis; watchful follow-up, particularly for patients treated with radiation; and timely treatment of local recurrence.
Acknowledgments
The authors would like to thank Daniela Niepel for her useful insights and critical review of the manuscript. Medical writing support, funded by Amgen (Europe) GmbH, was provided by Kelly Soady, PhD (Oxford PharmaGenesis, Oxford, UK). Editorial support was provided by Stéphane Gamboni of Amgen (Europe) GmbH.
Abbreviations
- CT
computerized tomography
- MRI
magnetic resonance imaging
- MSKCC
Memorial Sloan Kettering Cancer Center.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: E. Palmerini has received consulting fees from Amgen, Daiichi Sankyo, and Lilly, and research support from Bristol-Myers Squibb, Pfizer, and PharmaMar. P. Picci has received travel support or consulting fees from Amgen, Lilly, Pharmamar, and Takeda. P. Reichardt has received grants from Novartis, and honoraria from Amgen, AstraZeneca, Bayer, Clinigen, Deciphera, Lilly, Pfizer, and PharmaMar. G. Downey was an employee of and owned stock in Amgen, at the time the research was conducted.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for medical writing support was provided by Amgen (Europe) GmbH (Rotkreuz, Switzerland).
ORCID iD: Emanuela Palmerini  https://orcid.org/0000-0003-3406-6705
https://orcid.org/0000-0003-3406-6705
References
- 1. López-Pousa A, Martín Broto J, Garrido T, Vázquez J. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015;17(6):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Unni KK, Inwards C. Dahlin’s Bone Tumours: General Aspects and Data on 10165 Cases, Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2010. [Google Scholar]
- 3. van der Heijden L, Dijkstra PD, van de Sande MA, et al. The clinical approach toward giant cell tumor of bone. Oncologist. 2014;19(5):550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanerkin NG. Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer. 1980;46(7):1641–1649. [DOI] [PubMed] [Google Scholar]
- 5. Hu P, Zhao L, Zhang H, et al. Recurrence rates and risk factors for primary giant cell tumors around the knee: a multicentre retrospective study in China. Sci Rep. 2016;6:36332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sobti A, Agrawal P, Agarwala S, Agarwal M. Giant cell tumor of bone - An overview. Arch Bone Jt Surg. 2016;4(1):2–9. [PMC free article] [PubMed] [Google Scholar]
- 7. Unni KK. How to diagnose malignant giant cell tumor. Pathol Case Rev. 2001;6(1):33–37. [Google Scholar]
- 8. Gong L, Liu W, Sun X, et al. Histological and clinical characteristics of malignant giant cell tumor of bone. Virchows Arch. 2012;460(3):327–334. [DOI] [PubMed] [Google Scholar]
- 9. Dahlin DC, Cupps RE, Johnson EW., Jr Giant-cell tumor: a study of 195 cases. Cancer. 1970;25(5):1061–1070. [DOI] [PubMed] [Google Scholar]
- 10. Hutter RV, Worcester JN, Jr, Francis KC, Foote FW, Jr, Stewart FW. Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer. 1962;15:653–690. [DOI] [PubMed] [Google Scholar]
- 11. Rock MG, Sim FH, Unni KK, et al. Secondary malignant giant-cell tumor of bone. Clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68(7):1073–1079. [PubMed] [Google Scholar]
- 12. Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone, 4 ed Vol. 5 2013, Lyon, France: IARC; http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4005. Accessed March 19, 2019. [Google Scholar]
- 13. Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014;15(3):507–518. [DOI] [PubMed] [Google Scholar]
- 14. Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor of bone. Cancer. 2003;97(10):2520–2529. [DOI] [PubMed] [Google Scholar]
- 15. Stewart FW, Coley BL, Farrow JH. Malignant giant cell tumor of bone. Am J Pathol. 1938;14(5):515–536. [PMC free article] [PubMed] [Google Scholar]
- 16. Grote HJ, Braun M, Kalinski T, et al. Spontaneous malignant transformation of conventional giant cell tumor. Skeletal Radiol. 2004;33(3):169–175. [DOI] [PubMed] [Google Scholar]
- 17. Sakkers RJ, van der Heul RO, Kroon HM, Taminiau AH, Hogendoorn PC. Late malignant transformation of a benign giant-cell tumor of bone. A case report. J Bone Joint Surg Am. 1997;79(2):259–262. [DOI] [PubMed] [Google Scholar]
- 18. Chakarun CJ, Forrester DM, Gottsegen CJ, Patel DB, White EA, Matcuk GR., Jr Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. 2013;33(1):197–211. [DOI] [PubMed] [Google Scholar]
- 19. Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69(1):106–114. [PubMed] [Google Scholar]
- 20. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235–242. [PubMed] [Google Scholar]
- 21. Dahlin DC. Caldwell Lecture. Giant cell tumor of bone: highlights of 407 cases. AJR Am J Roentgenol. 1985;144(5):955–960. [DOI] [PubMed] [Google Scholar]
- 22. Mondal A, Kundu B, Gupta S, Biswas J. Secondary malignant giant cell tumour of bone—a study of five cases with short review of literature. Indian J Pathol Microbiol. 2002;45(3):273–275. [PubMed] [Google Scholar]
- 23. Domovitov SV, Healey JH. Primary malignant giant-cell tumor of bone has high survival rate. Ann Surg Oncol. 2010;17(3):694–701. [DOI] [PubMed] [Google Scholar]
- 24. Boriani S, Sudanese A, Baldini N, Picci P. Sarcomatous degeneration of giant cell tumours. Ital J Orthop Traumatol. 1986;12(2):191–199. [PubMed] [Google Scholar]
- 25. Ruka W, Rutkowski P, Morysinski T, et al. The megavoltage radiation therapy in treatment of patients with advanced or difficult giant cell tumors of bone. Int J Radiat Oncol Biol Phys. 2010;78(2):494–498. [DOI] [PubMed] [Google Scholar]
- 26. Feigenberg SJ, Marcus RB, Jr, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Radiation therapy for giant cell tumors of bone. Clin Orthop Relat Res. 2003;411:207–216. [DOI] [PubMed] [Google Scholar]
- 27. Shi W, Indelicato DJ, Reith J, et al. Radiotherapy in the management of giant cell tumor of bone. Am J Clin Oncol. 2013;36(5):505–508. [DOI] [PubMed] [Google Scholar]
- 28. Chakravarti A, Spiro IJ, Hug EB, Mankin HJ, Efird JT, Suit HD. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J Bone Joint Surg Am. 1999;81(11):1566–1573. [DOI] [PubMed] [Google Scholar]
- 29. Nair MK, Jyothirmayi R. Radiation therapy in the treatment of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 1999;43(5):1065–1069. [DOI] [PubMed] [Google Scholar]
- 30. Caudell JJ, Ballo MT, Zagars GK, et al. Radiotherapy in the management of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 2003;57(1):158–165. [DOI] [PubMed] [Google Scholar]
- 31. Malone S, O’Sullivan B, Catton C, Bell R, Fornasier V, Davis A. Long-term follow-up of efficacy and safety of megavoltage radiotherapy in high-risk giant cell tumors of bone. Int J Radiat Oncol Biol Phys. 1995;33(3):689–694. [DOI] [PubMed] [Google Scholar]
- 32. Picci P, Sieberova G, Alberghini M, et al. Late sarcoma development after curettage and bone grafting of benign bone tumors. Eur J Radiol. 2011;77(1):19–25. [DOI] [PubMed] [Google Scholar]
- 33. Takesako H, Osaka E, Yoshida Y, Sugitani M, Tokuhashi Y. Secondary malignant giant cell tumor of bone due to malignant transformation 40 years after surgery without radiation therapy, presenting as fever of unknown origin: a case report. J Med Case Rep. 2016;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadowaki M, Yamamoto S, Uchio Y. Late malignant transformation of giant cell tumor of bone 41 years after primary surgery. Orthopedics. 2012;35(10): e1566–1570. [DOI] [PubMed] [Google Scholar]
- 35. Li J, Zhu Y, Wei Y. Fibrosarcoma development 15 years after curettage and bone grafting of giant cell tumor of bone. Orthopedics. 2014;37(5): e512–516. [DOI] [PubMed] [Google Scholar]
- 36. Marui T, Yamamoto T, Yoshihara H, Kurosaka M, Mizuno K, Akamatsu T. De novo malignant transformation of giant cell tumor of bone. Skeletal Radiol. 2001;30(2):104–108. [DOI] [PubMed] [Google Scholar]
- 37. Saito T, Mitomi H, Suehara Y, et al. A case of de novo secondary malignant giant-cell tumor of bone with loss of heterozygosity of p53 gene that transformed within a short-term follow-up. Pathol Res Pract. 2011;207(10):664–669. [DOI] [PubMed] [Google Scholar]
- 38. Mori Y, Tsuchiya H, Karita M, Nonomura A, Nojima T, Tomita K. Malignant transformation of a giant cell tumor 25 years after initial treatment. Clin Orthop Relat Res. 2000;381:185–191. [DOI] [PubMed] [Google Scholar]
- 39. Muramatsu K, Ihara K, Miyoshi T, Kawakami Y, Nakashima D, Taguchi T. Late development of malignant fibrous histiocytoma at the site of a giant cell tumour 38 years after initial surgery. Acta Orthop Belg. 2012;78(2):279–284. [PubMed] [Google Scholar]
- 40. Miller IJ, Blank A, Yin SM, McNickle A, Gray R, Gitelis S. A case of recurrent giant cell tumor of bone with malignant transformation and benign pulmonary metastases. Diagn Pathol. 2010;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto K, Hatori M, Hosaka M, Watanabe M, Hasegawa T, Kokubun S. Osteosarcoma arising from giant cell tumor of bone ten years after primary surgery: a case report and review of the literature. Tohoku J Exp Med. 2006;208(2):157–162. [DOI] [PubMed] [Google Scholar]
- 42. Machinami R, Nishida K, Ishida T, et al. Carcinosarcomatous malignancy, osteosarcoma and squamous cell carcinoma, in giant cell tumor of the right distal femur. Pathol Res Pract. 2008;204(8):583–588. [DOI] [PubMed] [Google Scholar]
- 43. Brien EW, Mirra JM, Kessler S, Suen M, Ho JK, Yang WT. Benign giant cell tumor of bone with osteosarcomatous transformation (“dedifferentiated” primary malignant GCT): report of two cases. Skeletal Radiol. 1997;26(4):246–255. [DOI] [PubMed] [Google Scholar]
- 44. Wojcik J, Rosenberg AE, Bredella MA, et al. Denosumab-treated giant cell tumor of bone exhibits morphologic overlap with malignant giant cell tumor of bone. Am J Surg Pathol. 2016;40(1):72–80. [DOI] [PubMed] [Google Scholar]
- 45. Oda Y, Sakamoto A, Saito T, et al. Secondary malignant giant-cell tumour of bone: molecular abnormalities of p53 and H-ras gene correlated with malignant transformation. Histopathology. 2001;39(6):629–637. [DOI] [PubMed] [Google Scholar]
- 46. Huvos AG. Bone Tumors: Diagnosis, Treatment and Prognosis. New York, New York: W.B. Saunders CBS Educ. and Professional Publ; 1987. [Google Scholar]
- 47. Rockberg J, Bach BA, Amelio J, et al. Incidence trends in the diagnosis of giant cell tumor of bone in Sweden since 1958. J Bone Joint Surg Am. 2015;97(21):1756–1766. [DOI] [PubMed] [Google Scholar]
- 48. Campanacci M, Giunti A, Olmi R. Metaphyseal and diaphyseal localization of giant cell tumors. Chir Organi Mov. 1975;62(1):29–34. [PubMed] [Google Scholar]
- 49. Chen L, Ding XY, Wang CS, Si MJ, Du LJ, Lu Y. Triple-phase dynamic MRI: a new clue to predict malignant transformation of giant cell tumor of bone. Eur J Radiol. 2014;83(2):354–359. [DOI] [PubMed] [Google Scholar]
- 50. Heffernan EJ, O’Sullivan PJ, Adibeig M, et al. Primary malignant transformation of giant cell tumor of bone. Eur J Radiol. 2007;62(3):89–93. [Google Scholar]
- 51. Raymond AK, Jaffe N. Conditions that mimic osteosarcoma. Cancer Treat Res. 2009;152:85–121. [DOI] [PubMed] [Google Scholar]
- 52. Chow LT. Fibular giant cell-rich osteosarcoma virtually indistinguishable radiographically and histopathologically from giant cell tumor—analysis of subtle differentiating features. APMIS. 2015;123(6):530–539. [DOI] [PubMed] [Google Scholar]
- 53. Righi A, Mancini I, Gambarotti M, et al. Histone 3.3 mutations in giant cell tumor and giant cell-rich sarcomas of bone. Hum Pathol. 2017;68:128–135. [DOI] [PubMed] [Google Scholar]