Watch a video presentation of this article
Watch the interview with the author
Answer questions and earn CME
Abbreviations
- ASA
American Society of Anesthesiologists
- ERCP
endoscopic retrograde cholangiopancreatography
- GETA
general (endotracheal) anesthesia
- MAC
monitored anesthesia care
Choosing an appropriate sedative in the setting of endoscopy in patients with cirrhosis is challenging for many reasons. Providers must take into account the severity of liver disease, underlying causative factor, medication metabolism and interactions, baseline hemodynamics, and history of opioid, benzodiazepine, or alcohol dependence. A reduction in hepatic blood flow, increased portosystemic shunting, and decreased function and number of hepatocytes have all been implicated in impaired drug metabolism.1 Evidence of decompensated liver disease, such as the presence of edema, ascites, hepatic encephalopathy, and hepatorenal syndrome, further influence pharmacokinetics of most medications used for sedation.2 Endoscopy in patients with cirrhosis is usually well tolerated, and several prospective controlled trials have confirmed acceptable safety profiles in upper and lower endoscopy as well as endoscopic retrograde cholangiopancreatography (ERCP).3, 4, 5, 6, 7, 8 However, controversy exists regarding which is safer—MAC or moderate sedation—and recent data from a retrospective study at our institution suggest that moderate sedation may be safer than MAC.9
Medications Used in Sedation for Endoscopy
A multitude of medications and approaches can be used in the context of sedation for endoscopy (Table 1). For example, some patients can be effectively sedated with only minimal sedation (also known as anxiolysis). This degree of sedation is highly desirable in patients who may have severe underlying medical disease and in whom other forms of sedation may be dangerous. Moderate sedation (also conscious sedation) for endoscopy typically uses a combination of an opioid and a benzodiazepine to achieve appropriate sedation. It is commonly used across a variety of different procedures and is generally considered to be highly safe and effective. It is often used in practice where rapid onset and clearing of sedative effects is desired. Deep sedation typically involves use of propofol as well as similar medications used in moderate sedation (i.e., a combination of opioid and benzodiazepine). However, because of the potential for loss of airway and ventilatory function, special training and credentialing is typically required for physicians using this level of sedation. General anesthesia requires endotracheal intubation and may be desirable in situations where it is vital to protect the patient’s airway, a prolonged procedure time is anticipated, or increased patient stillness or cooperation is required for a procedure.
Table 1.
Levels of Sedation
| Responsiveness | Airway | Spontaneous Ventilation | Cardiovascular Function | |
|---|---|---|---|---|
| Minimal sedation (anxiolysis) | Normal response to verbal stimulation | Unaffected | Unaffected | Unaffected |
| Moderate sedation | Purposeful response to verbal or tactile stimulation | No intervention required | Adequate | Usually maintained |
| Deep sedation | Purposeful response after repeated or painful stimulation | Intervention may be required | May be inadequate | Usually maintained |
| General anesthesia | Unarousable even with painful stimulus | Intervention often required | Frequently inadequate | May be impaired |
Adapted with permission from Annals of Emergency Medicine. 28 Copyright 2011, Elsevier.
Sedation in endoscopy uses a variety of different types of drugs with different characteristics (Table 2). Opiates are primarily metabolized via CYP450 (CYP2D6 and CYP3A4) and glucuronidation in hepatocytes and are therefore affected by both liver injury and disease.10, 11 Fentanyl is most commonly used because it has a faster onset of action, is rapidly cleared, and causes nausea less often than meperidine.12 The starting dose of fentanyl is 50 to 100 μg, and doses are repeated every 3 to 5 minutes until adequate sedation is achieved. Meperidine is commonly used for moderate sedation, although its use has been more limited because of the superior characteristics of fentanyl. The active metabolite of meperidine, normeperidine, is toxic and can result in tremors, delirium, and seizures; thus, we recommend that meperidine be avoided in patients with cirrhosis.13 Benzodiazepines such as midazolam have been demonstrated to have a prolonged half‐life in patients with cirrhosis,14 and thus the respiratory depressive and hemodynamic effects of benzodiazepines may be prolonged.10 It is also possible that benzodiazepines may trigger hepatic encephalopathy.7 It is therefore recommended that they be used with caution in patients with cirrhosis (Table 2). The starting dose of midazolam should be 1 to 2 mg, and adequate time between repeat dosing is recommended (3‐5 minutes) until adequate sedation is achieved. It is important to note that, although there are no dosage adjustments in the package labels for midazolam and fentanyl, clinicians should exercise caution because the dose needed for sedation may be significantly lower and the dosing interval needed to maintain sedation may be significantly longer than that required for patients without cirrhosis.
Table 2.
Comparison of Medications
| Drug | Onset of Action (minutes) | Peak Effect (minutes) | Duration of Effect (minutes) | Dosing/Initial Dose | Maximum Dose | Significant Adverse Effects |
|---|---|---|---|---|---|---|
| Fentanyl | 1‐2 | 3‐5 | 30‐60 | 25‐100 μg | 400 μg | Respiratory depression, vomiting, transient hypotension |
| Midazolam | 1‐2 | 3‐4 | 15‐80 | 1‐2 mg | 10 mg | Respiratory depression, disinhibition |
| Propofol | <1 | 1‐2 | 4‐8 | 10‐40 μg | 400 μg | Respiratory depression, cardiovascular instability |
| Ketamine | <1 | 1 | 10‐15 | 0.5 mg/kg | Titrate to effect | Emergence reaction, laryngospasm |
Adapted with permission from Gastroenterology.28 Copyright 2007, Elsevier.
Propofol is used with monitored anesthesia care (MAC) sedation; this medication is a hypnotic agent that can be used to provide moderate or deep sedation during endoscopic procedures.4 It does not need dose adjustment in cirrhosis, and when compared with midazolam, has a faster onset of action, shorter effect, and faster recovery of motor and cognitive functions (Table 2). Pharmacokinetics and protein binding of propofol do not appear to be markedly affected by cirrhosis.15, 16, 17, 18 Recovery times are significantly longer, and patients with cirrhosis have a larger volume of distribution than patients without cirrhosis, but no differences in protein binding, clearance, or terminal elimination half‐life have been reported.15, 19, 20
General (endotracheal) anesthesia (GETA) has been used in some patients with cirrhosis, often in those in whom airway protection is important. The decision to use GETA most often depends on local practice patterns and the resources available at the time of the procedure. In our practice, GETA may be used to protect the airway in patients with gastrointestinal hemorrhage or other diseases that place the patient at high risk for aspiration. Again, this varies greatly depending on local practice patterns. GETA often uses combinations of many different agents, a discussion of which is beyond the scope of this review. Notably, the inhalation anesthetics (sevoflurane, desflurane, and isoflurane) are infrequently used in endoscopy. These agents undergo minimal hepatic metabolism and are considered safe for use in GETA.21 Desflurane is thought to be the ideal volatile agent because it is the least metabolized and provides the quickest emergence while also relatively preserving hepatic blood flow and cardiac output.22 The flurane family of drugs reduces mean arterial pressure and cardiac output and, consequentially, reduces hepatic blood flow. Halothane, no longer available in the United States, has been associated with hepatotoxicity and should not be used in patients with cirrhosis.23
Ketamine has also been used for endoscopic sedation. It produces dissociative anesthesia and has a combination of sedative, amnestic, and analgesic effects without suppressing the reticular activating system. Laryngeal reflexes remain intact, and cardiorespiratory function is not depressed.24, 25 It has the benefit of increasing blood pressure and cardiac output, the exact mechanism of which is currently not entirely understood. Furthermore, cardiac function, breathing, and airway reflexes typically remain functionally intact in contrast with many of the side effects of benzodiazepines and opioids. These properties, coupled with the rapid onset and short duration of action, make ketamine an ideal sedative agent for short procedures, especially in hemodynamically unstable patients such as those with cirrhosis, who typically have a lower baseline blood pressure, and in those suffering from acute gastrointestinal hemorrhage. Ketamine is metabolized and excreted in the liver, where it undergoes N‐demethylation by the P450 system to norketamine, which retains a fraction of the potency of the parent compound, followed by hydroxylation, glucuronidation, and renal elimination. No hepatic dosing is required.26 (Table 2) Finally, ketamine has been demonstrated to have little influence on arterial or portal blood flow in experimental models.26, 27 Ketamine may be a beneficial agent in patients with hypotension, such as patients with cirrhosis in general, as well as those who are actively bleeding, because it has the benefit of increasing blood pressure. It may also benefit patients who are opioid or benzodiazepine tolerant and have a history of requiring high doses of standard moderate sedation medications.
When to Engage Anesthesiology Consultation
The engagement of anesthesia professionals in endoscopy has now become common in practice. In general, anesthesia professionals are considered to be important for sedation of patients with severe comorbid disease (especially those with cardiopulmonary disease, morbid obesity, or neurological or neuromuscular disorders), usually with American Society of Anesthesiologists (ASA) classification 4 or 5 (Table 3). There are also several other situations in which involvement of an anesthesiologist would be considered appropriate, including patients with a history of alcohol or substance abuse who may be difficult to sedate with standard moderate sedation or patients who are uncooperative or delirious. In addition, anesthesiology consultation is typically required when propofol or other general anesthetic agents are used. Also, certain procedures that are likely better performed with the assistance of an anesthesiologist include ERCP, endoscopic ultrasound (EUS), and complex therapeutic procedures.12, 28 (Fig. 1)
Table 3.
ASA Classification
| Class | Description |
|---|---|
| I | Patient is normal and healthy. |
| II | Patient has mild systemic disease that does not limit activities (e.g., controlled hypertension or controlled diabetes without systemic sequelae). |
| III | Patient has moderate or severe systemic disease that does not limit the activities (e.g., stable angina or diabetes with systemic sequelae). |
| IV | Patient has severe systemic disease that is a constant threat to life (e.g., severe congestive heart failure, end‐stage renal failure). |
| V | Patient is morbid and at a substantial risk for death within 24 hours (with or without a procedure). |
| E | Emergency status: In addition to indicating the underlying ASA status (1‐5), any patient undergoing an emergency procedure is indicated by suffix “E.” |
Adapted with permission from Gastrointestinal Endoscopy.12 Copyright 2018, Elsevier.
Figure 1.
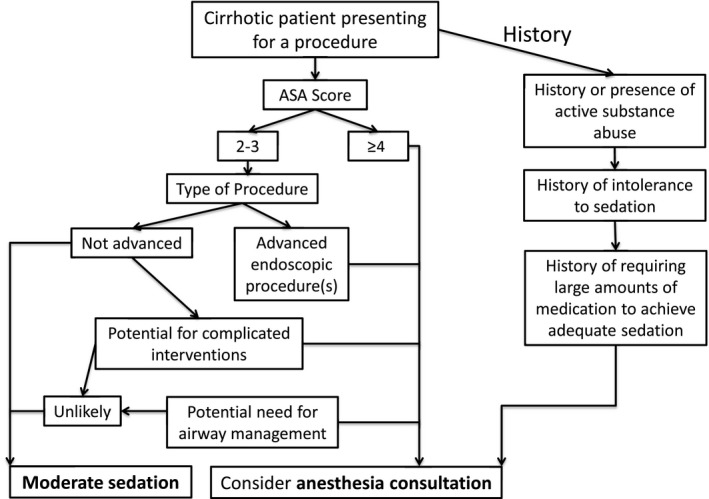
When to consider engaging anesthesiology for assistance in sedation.
Intraprocedural Monitoring
For moderate sedation, physiological parameters, such as blood pressure, heart rate, respiratory rate, and oxygen saturation, should be frequently assessed.29 Currently, the American Gastroenterological Association recommends continuous ECG monitoring for patients with a history of significant cardiovascular disease or arrhythmia.30 Hypoxemia from depressed respiratory function is the main risk factor for adverse respiratory events during sedation, and some experts advocate the use of capnography monitoring. However, there is insufficient evidence to support the possibility that its routine use improves patient safety.31 Current ASA guidelines recommend standard monitoring consisting of pulse oximetry, arterial blood pressure, and heart rate every 5 minutes and do not differentiate the monitoring of patients with cirrhosis when compared with those without cirrhosis, although the decision to increase the frequency or intensity of monitoring should be tailored to the specific clinical setting.32
Conclusions
Endoscopy in patients with cirrhosis is safe and should not be withheld. Recent data from a retrospective cohort study at our institution indicate that moderate sedation may be safer than MAC sedation and that the complication rate for patients with cirrhosis is similar to that of the general population.9 As such, the majority of endoscopic procedures can be completed safely with midazolam‐ and fentanyl‐based moderate sedation, and use of anesthesiology should be reserved for those patients with ASA classification 4 to 5 or those undergoing advanced endoscopic procedures such as ERCP or EUS.
Potential conflict of interest
Nothing to report.
Disclaimer: The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of the Air Force, and Department of Defense or the US government.
References
- 1. Verbeeck R. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 2008;64:1147‐1161. [DOI] [PubMed] [Google Scholar]
- 2. Le Couteur DG, Fraser R, Hilmer S, Rivory LP, McLean AJ. The hepatic sinusoid in aging and cirrhosis: effects on hepatic substrate disposition and drug clearance. Clin Pharmacokinet 2005;44:187‐200. [DOI] [PubMed] [Google Scholar]
- 3. Agrawal A, Sharma BC, Sharma P, Uppal R, Sarin SK. Randomized controlled trial for endoscopy with propofol versus midazolam on psychometric tests and critical flicker frequency in people with cirrhosis. J Gastroenterol Hepatol 2012;27:1726‐1732. [DOI] [PubMed] [Google Scholar]
- 4. Correia LM, Bonilha DQ, Gomes GF, et al. Sedation during upper GI endoscopy in cirrhotic outpatients: a randomized, controlled trial comparing propofol and fentanyl with midazolam and fentanyl. Gastrointest Endosc 2011;73:45‐51, 51.e1. [DOI] [PubMed] [Google Scholar]
- 5. Faga E, De Cento M, Giordanino C, et al. Safety of propofol in cirrhotic patients undergoing colonoscopy and endoscopic retrograde cholangiography: results of a prospective controlled study. Eur J Gastroenterol Hepatol 2012;24:70‐76. [DOI] [PubMed] [Google Scholar]
- 6. Khamaysi I, William N, Olga A, et al. Sub‐clinical hepatic encephalopathy in cirrhotic patients is not aggravated by sedation with propofol compared to midazolam: a randomized controlled study. J Hepatol 2011;54:72‐77. [DOI] [PubMed] [Google Scholar]
- 7. Riphaus A, Lechowicz I, Frenz MB, Wehrmann T. Propofol sedation for upper gastrointestinal endoscopy in patients with liver cirrhosis as an alternative to midazolam to avoid acute deterioration of minimal encephalopathy: a randomized, controlled study. Scand J Gastroenterol 2009;44:1244‐1251. [DOI] [PubMed] [Google Scholar]
- 8. Weston BR, Chadalawada V, Chalasani N, et al. Nurse‐administered propofol versus midazolam and meperidine for upper endoscopy in cirrhotic patients. Am J Gastroenterol 2003;98:2440‐2447. [DOI] [PubMed] [Google Scholar]
- 9. Suarez AL, Edelson JC, Zhang J, Rockey DC. Sedation during endoscopy in hospitalized patients with cirrhosis: safety profile and predictors of adverse events. Gastroenterology 2017;152:S1142. [DOI] [PubMed] [Google Scholar]
- 10. Dwyer JP, Jayasekera C, Nicoll A. Analgesia for the cirrhotic patient: a literature review and recommendations. J Gastroenterol Hepatol 2014;29:1356‐1360. [DOI] [PubMed] [Google Scholar]
- 11. Hoyumpa AM, Schenker S. Is glucuronidation truly preserved in patients with liver disease? Hepatology 1991;13:786‐795. [PubMed] [Google Scholar]
- 12. Early DS, Lightdale JR, Vargo JJ 2nd, et al. Guidelines for sedation and anesthesia in GI endoscopy: ASGE Standards of Practice Committee. Gastrointest Endosc 2018;87:327‐337. [DOI] [PubMed] [Google Scholar]
- 13. Soleimanpour H, Safari S, Shahsavari Nia K, Sanaie S, Alavian SM. Opioid drugs in patients with liver disease: a systematic review. Hepat Mon 2016;16:e32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacGilchrist A, Birnie G, Cook A, et al. Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut 1986;27:190‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tsai HC, Lin YC, Ko CL, et al. Propofol versus midazolam for upper gastrointestinal endoscopy in cirrhotic patients: a meta‐analysis of randomized controlled trials. PLoS One 2015;10:e0117585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Servin F, Cockshott ID, Farinotti R, Haberer JP, Winckler C, Desmonts JM. Pharmacokinetics of propofol infusions in patients with cirrhosis. Br J Anaesth 1990;65:177‐183. [DOI] [PubMed] [Google Scholar]
- 17. Servin F, Desmonts JM, Haberer JP, Cockshott ID, Plummer GF, Farinotti R. Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology 1988;69:887‐891. [DOI] [PubMed] [Google Scholar]
- 18. Servin F, Desmonts J, Farinotti R, Haberer J, Winckler C. Pharmacokinetics of propofol administered by continuous infusion in patients with cirrhosis, preliminary results. Anesthesia 1988;43(Suppl.):23‐24. [DOI] [PubMed] [Google Scholar]
- 19. Wu J, Huang SQ, Chen QL, Zheng SS. The influence of the severity of chronic virus‐related liver disease on propofol requirements during propofol‐remifentanil anesthesia. Yonsei Med J 2013;54:231‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Costela JL, Jimenez R, Calvo R, Suarez E, Carlos R. Serum protein binding of propofol in patients with renal failure or hepatic cirrhosis. Acta Anaesthesiol Scand 1996;40:741‐745. [DOI] [PubMed] [Google Scholar]
- 21. Zaleski L, Abello D, Gold M. Desflurane versus isoflurane in patients with chronic hepatic and renal disease. Anesthes Analg 1993;76:353‐356. [PubMed] [Google Scholar]
- 22. Vaja R, McNicol L, Sisley I. Anaesthesia for patients with liver disease. Contin Educ Anaesth Crit Care Pain 2010;10:15‐19. [Google Scholar]
- 23. Soleimanpour H, Safari S, Rahmani F, Ameli H, Alavian SM. The role of inhalational anesthetic drugs in patients with hepatic dysfunction: a review article. Anesth Pain Med 2015;5:e23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kotwal R, Butler F, Edgar E, Shackelford S, Bennett D, Bailey J. Saving lives on the battlefield: a joint trauma system review of pre‐hospital trauma care in combined joint operating area? Afghanistan (CJOA‐A) Executive Summary. J Spec Oper Med 2013;13:77‐85. [PubMed] [Google Scholar]
- 25. Sener S, Eken C, Schultz CH, Serinken M, Ozsarac M. Ketamine with and without midazolam for emergency department sedation in adults: a randomized controlled trial. Ann Emerg Med 2011;57:109‐114.e102. [DOI] [PubMed] [Google Scholar]
- 26. Mion G, Villevieille T. Ketamine pharmacology: an update (pharmacodynamics and molecular aspects, recent findings). CNS Neurosci Ther 2013;19:370‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomson I, Fitch W, Campbell D, Watson R. Effects of ketamine on liver blood flow and hepatic oxygen consumption. Studies in the anaesthetised greyhound. Acta Anaesthesiol Scand 1988;32:10‐14. [DOI] [PubMed] [Google Scholar]
- 28. Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology 2007;133:675‐701. [DOI] [PubMed] [Google Scholar]
- 29. Calderwood AH, Chapman FJ, Cohen J, et al. Guidelines for safety in the gastrointestinal endoscopy unit: ASGE Ensuring Safety in the Gastrointestinal Endoscopy Unit Task Force. Gastrointest Endosc 2014;79:363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waring J, Baron T, Hirota W, et al. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc 2003;58:317‐322. [DOI] [PubMed] [Google Scholar]
- 31. Friedrich‐Rust M, Welte M, Welte C, et al. Capnographic monitoring of propofol‐based sedation during colonoscopy. Endoscopy 2014;46:236‐244. [DOI] [PubMed] [Google Scholar]
- 32. Practice Guidelines for Moderate Procedural Sedation and Analgesia 2018: A Report by the American Society of Anesthesiologists Task Force on Moderate Procedural Sedation and Analgesia, the American Association of Oral and Maxillofacial Surgeons, American College of Radiology, American Dental Association, American Society of Dentist Anesthesiologists, and Society of Interventional Radiology. Anesthesiology 2018;128:437‐479. [DOI] [PubMed] [Google Scholar]


