Abstract
Background:
Mother-to-child transmission of HIV-1 occurs in a minority of HIV-infected mother-infant pairs, even without any interventions. The mechanisms that protect the majority of HIV-exposed infants from infection are unclear. T regulatory cells (Treg) have important immunomodulato-ry functions, but their role in the fetus as well as in mother-to-child transmission of HIV is under-studied.
Methods:
We studied available cryopreserved peripheral blood mononuclear cells from HIV-exposed infants from the Breastfeeding, Antiretrovirals and Nutrition (BAN) Study cohort in Malawi: 64 in-fants were HIV-uninfected and 28 infants were HIV-infected at birth. We quantified the frequency of Treg cells (CD4+CD25+FoxP3+), and activated CD4+ and CD8+ T cells (CD38+ HLADR+) by flow cytometry at birth, 6 weeks and 6, 9 and 12 months of age. Descriptive statistics were performed to describe the distributions of these lymphocyte markers according to the HIV infection status; and Student’s t tests and Wilcoxon-Rank Sum tests were performed to compare HIV- infected and unin-fected infants.
Results:
T cell activation increased rapidly in the first 6 weeks of life, more pronounced on CD8+ T cells; a further increase in activation was observed at the time of weaning from breastfeeding at 6 months of age. In contrast, the frequency of Treg was stable over the first 6 weeks of life (median, 0.5%), slightly decreased between 6 weeks and 6 months (median at 6 months, 0.3%) and then slight-ly increased between 6 months (time of weaning) and 12 months of age (median, 0.45%). HIV-infected infants had significantly higher frequencies of activated T cells than uninfected infants (P < 0.01). At the time of birth, HIV-exposed uninfected infants had higher levels of Treg, compared to in-fants infected in utero, even though this did not reach statistical significance in this small sample size (P = 0.08).
Conclusion:
This study provides initial evidence that Treg may play a role in preventing mother-to-child transmission of HIV, likely by suppressing immune activation in the fetus and infant, and needs to be substantiated in a larger study. Better characterization of the role of Treg in fetal and neonatal immunity may provide a valuable complementary approach to achieve eradication of mother-to-child transmission of HIV.
Keywords: HIV, mother-to-child transmission, Treg, regulatory T lymphocytes, neonate, infant
1. INTRODUCTION
Regulatory T cells (Treg) suppress the activation and proliferation of other T lymphocytes and are important in regulating the avoidance of autoimmune diseases, but also in modulating the course of infectious diseases [1-3]. There are conflicting data in the literature regarding the role of Treg in HIV infection [4]. In a study of adult patients with acute primary HIV infection, the frequency of Treg was lower than that in patients with chronic infection which decreased over time in untreated patients [5]. On the other hand, in chronically HIV-exposed individuals who remain uninfected, the proportion of Treg was found to be elevated, concurrent with low levels of immune activation [6]. Very few studies have investigated the role of Treg in pediatric HIV infection [7], despite the fact that Treg levels are highest in neonates and decrease with age [8]. In a study of Treg in cord blood, high levels of Treg and low levels of CD4+ and CD8+ T cell activation were found in the exposed uninfected neonates [9]. Treg may thus play a role in preventing, in utero, vertical transmission of HIV. We set out to examine this question, as well as to study Treg dynamics during the infants’ first year of life, among a cohort of infants born to HIV-infected mothers in the context of a randomized clinical trial in Lilongwe, Malawi.
2. Methods
2.1. Study Design
All infants were enrolled in the BAN (Breastfeeding, Antiretrovirals, and Nutrition) randomized, controlled clinical trial, which has been described in other studies as well [10, 11]. Briefly, pregnant women living with HIV infection who had CD4+ T cell counts > 250 cells/µL (> 200 cells/µL before 24 July 2006; the change was made to concur with Malawi Ministry of Health guidelines for treatment under these CD4+ count thresholds) were enrolled and followed in Lilongwe, Malawi, from April 2004 to January 2010.
Mother-infant pairs were randomized within one week of birth to a maternal nutritional intervention and to an antiretroviral intervention that consisted of a triple-drug antiretroviral regimen for mothers (n = 849), daily nevirapine for infants (n = 852), or neither (control arm, n = 668). Irrespective of the study arm, all mothers when in labor and their newborns received a single dose of nevirapine and a 7-day course of zidovudine and lamivudine. The interventions began after delivery and were continued until breastfeeding cessation or for 28 weeks postnatal, whichever was first. Infants who were diagnosed with HIV-infection in the first 2 weeks of life were disenrolled from the study and referred for medical care. All mothers were counseled to breastfeed exclusively for the first 24 weeks of life, followed by a 4-week weaning period. Infants were tested for HIV infection at birth and at 2, 12, 28, and 48 weeks of age; if infected, they were referred for care.
All women provided written informed consent to participate in the study. The study protocol was approved by the Malawi National Health Science Research Committee and by Institutional Review Boards at the University of North Carolina and the Centers for Disease Control and Prevention.
2.2. Patient Samples
Infants were selected for the current analysis on the basis of the availability of serial samples of stored Peripheral Blood Mononuclear Cells (PBMC), and followed the same sampling strategy as that of a previous BAN substudy [5]. Serial samples from infants at birth, 12, 24, 36 and 48 weeks of age were desired for this analysis. The analysis included the 39 infants (19 males and 20 females) from the control arm who remained HIV uninfected till the end of the study and had available samples at 4 (n = 18) or 5 (n = 21) of the pre-specified timepoints as mentioned. In addition, 25 (17 males and 8 females) of the 93 BAN study infants who became HIV infected after 2 weeks of age (defined in this study as infected during breastfeeding) and had PBMC specimens from at least 2 of the pre-specified timepoints before infection and 1-time point after infection were included; these infants were from all BAN study arms. For time points before HIV infection was detected, these infants were considered HIV uninfected for the analyses. The study also included a random sample of 30 infants (14 males and 16 females) who were HIV-infected perinatally (positive HIV DNA PCR test result within the first 2 weeks of life) and had available PBMC at delivery. Twenty-six of the 30 perinatally-infected infants were diagnosed with HIV infection in the first 2 days of life and were thus considered to have acquired HIV in utero. As infants who were HIV-infected within the first two weeks of life were not followed in the BAN study [10], these infants had samples only at the time of birth.
2.3. Flow Cytometry
Lymphocyte immunophenotyping was performed by quickly thawing cryopreserved peripheral blood mononuclear cells (PBMCs), washing them twice with complete medium (RPMI 1640 containing 10% fetal bovine serum, penicillin/streptomycin, and L-glutamine), and resuspending them at 5 x 106/mL in FACS buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0.1% NaN3). An amount of 100 µL was stained with live/dead marker (Invitrogen, Carlsbad, CA) followed by surface markers at room temperature for 20 min and washed with FACS buffer. The Foxp3 tubes were fixed with 1X Fix/perm buffer (Biolegend, San Diego, CA) for 30 min at room temperature and then were washed with 1X perm buffer before staining for 30min with mAb to Foxp3. Cells were then washed with FACS buffer and finally re-suspended in 1% paraformaldehyde in PBS. Samples were acquired on LSR-II (BD Biosciences) and analyzed using FlowJo software (Tree Star, San Carlos, CA.) The panel of markers included T lymphocyte markers (CD3, CD4, and CD8), activation markers (CD38 and HLA-DR, CD25) and regulatory T cell markers Foxp3 (Forkhead box protein 3). Lymphocytes were gated on live cells. The complete staining panel of fluorochrome combinations used in the lymphocyte gate was: CD3/ CD4/CD8, CD4/HLA-DR/CD3/CD38, CD3/CD8/HLA-DR/ CD38, CD3/CD4/CD25/Foxp3. The monoclonal antibodies are commercially available and directly conjugated with flu- orescein isothiocyanate (FITC), PerCP (peridinin-chloro- phyll-protein), phycoerythrin (PE), or allophycocyanin (APC) (BD Biosciences). Appropriate isotypic controls (Mouse IgG1-PE and mouse IgG2b-APC) were used to evaluate non-specific staining. All monoclonal antibodies were obtained from BD Biosciences (San Jose, CA), except Foxp3 (Biolegend, San Diego, CA).
2.4. Statistical Analysis
Results are reported as median values and distributions (interquartile ranges, IQR). Statistical comparisons between HIV-infected and uninfected infants were performed with student’s t-test and with Wilcoxon Rank-Sum tests or exact Wilcoxon test. Spearman correlations (r and P values) were calculated to determine the relationships between the proportion of Treg cells with that of activated CD4+ and CD8+ T cells.
3. Results
3.1. Lymphocyte Activation Levels in HIV-infected and HIV-exposed but Uninfected Infants
Not all infant samples yielded enough viable cells for immunophenotyping after thawing; this was particularly a
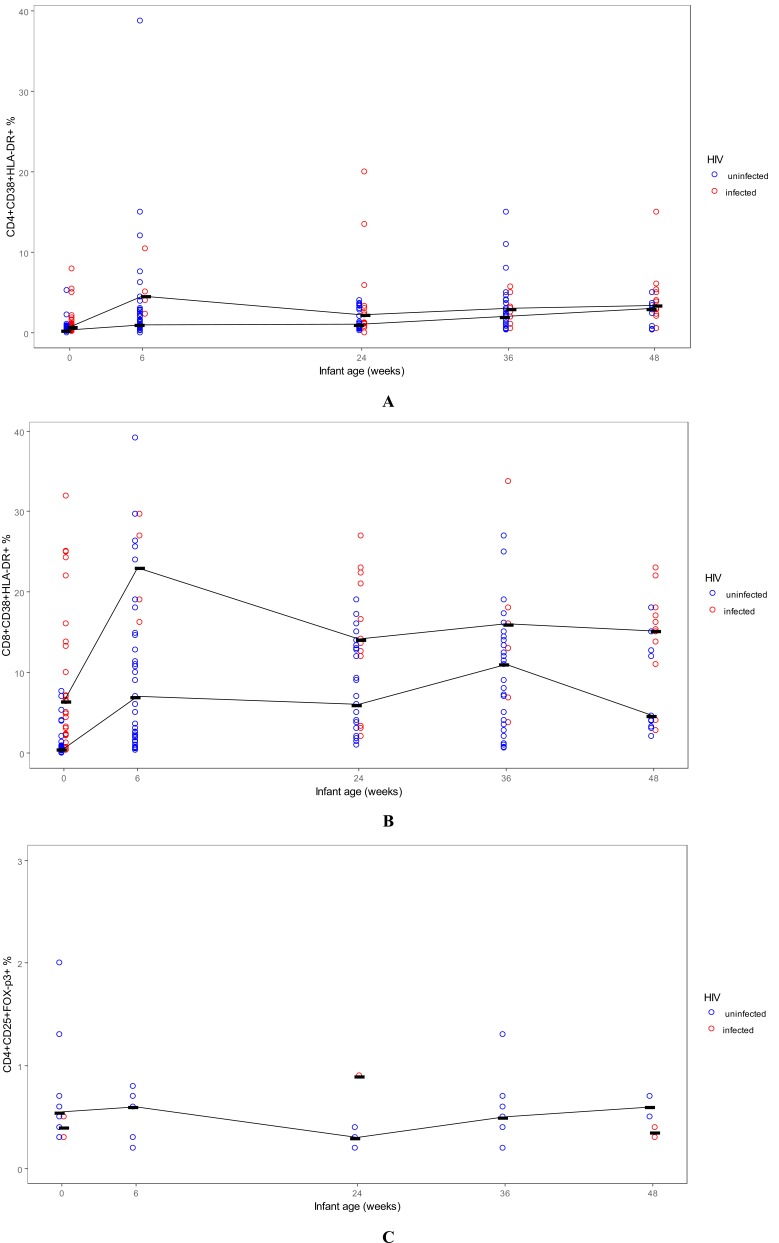
limitation for analysis of the Foxp3 marker. Among CD4+ and CD8+ T cells, the expression of activation markers increased rapidly after birth, peaking at week 6 (Table 1, Fig. 1A, 1B), more pronounced on CD8+ T cells; a further increase in activation was observed at the time of weaning from breastfeeding at 6 months of age. Infants who were HIV-infected at birth had significantly higher levels of activated CD4+ T lymphocytes (HLADR+ CD38+), compared with HIV-exposed uninfected infants at all time points studied; however, this difference reached statistical significance in our small sample at birth and 24 weeks of age (Table 1). Infants HIV-infected at birth had also significantly higher levels of activated CD8+ T lymphocytes (HLADR+ CD38+), compared to HIV-exposed but uninfected infants, at all time points followed, and this difference reached statistical significance at birth, 6, 12, 24 and 48 weeks of age (Table 1).
Table 1.
Activated CD4+ and CD8+ T lymphocytes and Regulatory T cells in Infants, by Age and HIV Status at Study Visit.
| Marker, Infant Age | HIV-Infected | HIV-uninfected | P value | ||||
|---|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | ||||
| %CD4+CD38+HLADR+ | |||||||
| Delivery | 28 | 0.70 | (0.35-1.30) | 64 | 0.30 | (0.20-0.50) | <0.001 |
| 6 wk | 4 | 4.55 | (3.15-7.75) | 44 | 0.95 | (0.55-2.65) | 0.23 |
| 24 wk | 13 | 2.20 | (1.00-3.30) | 37 | 1.00 | (0.60-2.00) | 0.065 |
| 36 wk | 9 | 3.00 | (1.90-3.20) | 36 | 2.00 | (1.0-3.30) | .326 |
| 48 wk | 12 | 3.40 | (2.15-5.15) | 11 | 3.00 | (0.80-3.40) | .241 |
| %CD8+CD38+HLADR+ | |||||||
| Delivery | 27 | 6.40 | (2.20-16.0) | 55 | 0.50 | (0.20-0.80) | <0.001 |
| 6 wk | 4 | 23.0 | (17.6-28.4) | 37 | 7.00 | (2.00-12.8) | .012 |
| 24 wk | 13 | 14.1 | (12.0-21.0) | 29 | 6.00 | (2.00-13.0) | .018 |
| 36 wk | 7 | 16.0 | (6.80-18.0) | 31 | 11.0 | (4.00-15.0) | .187 |
| 48 wk | 12 | 15.2 | (12.4-17.5) | 11 | 4.60 | (3.20-12.7) | .029 |
| CD4+CD25+Foxp3+ | |||||||
| Delivery | 4 | 0.40 | (0.30-0.50) | 12 | 0.55 | (0.40-1.65) | 0.03 |
| 6 wk | 0 | - | - | 8 | 0.60 | (0.30-0.75) | - |
| 24 wk | 1 | 0.90 | (0.90-0.90) | 7 | 0.30 | (0.20-0.30) | 0.125 |
| 36 wk | 0 | - | - | 7 | 0.50 | (0.20-0.70) | - |
| 48 wk | 2 | 0.35 | (0.30-0.40) | 2 | 0.60 | (0.50-0.70) | 0.333 |
IQR: Interquartile Range.
Fig. (1).
Lymphocyte subsets in infants over the first year of life, BAN study, Lilongwe, Malawi. A: Activated CD4+ T lymphocytes; B: Activated CD8+ T lymphocytes; C: Treg lymphocytes. Lines connect the median values for each group at each time point.
3.2. Regulatory T Lymphocyte Levels in HIV-infected and HIV-exposed but Uninfected Infants
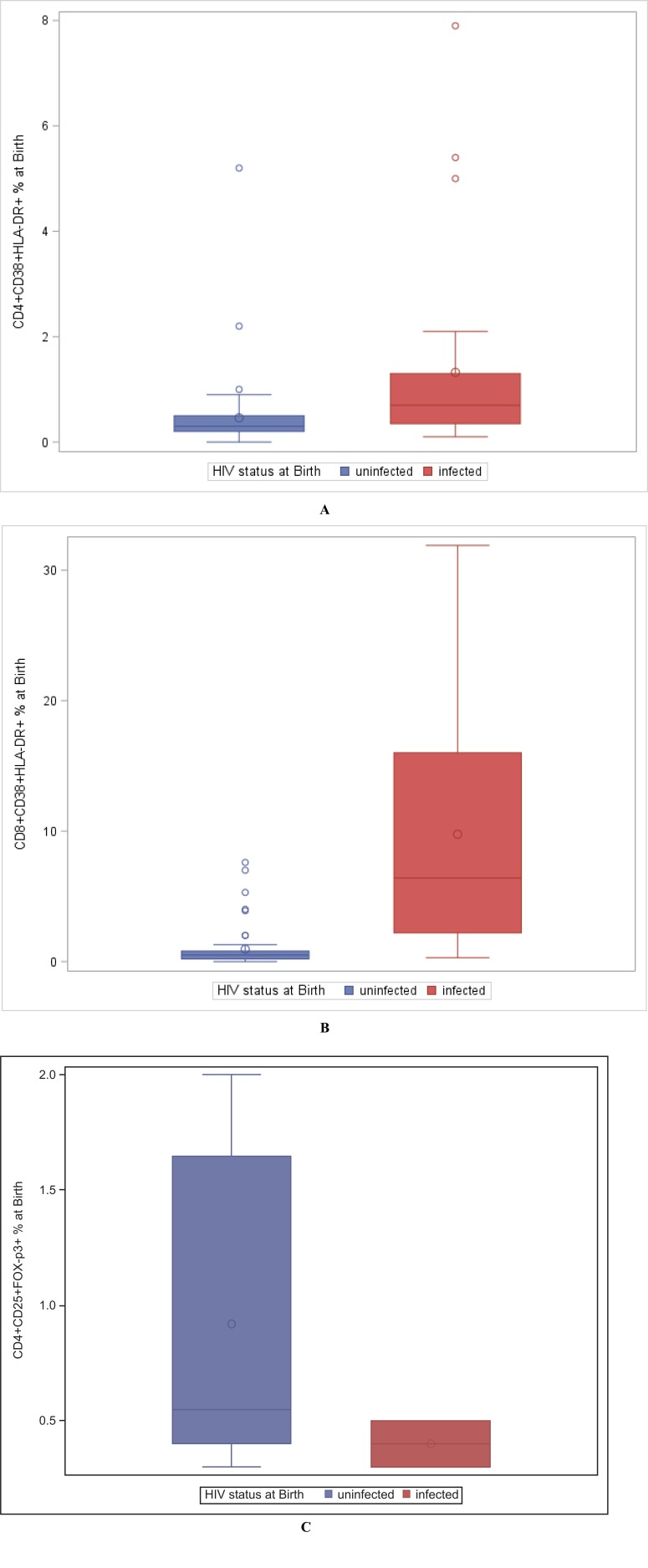
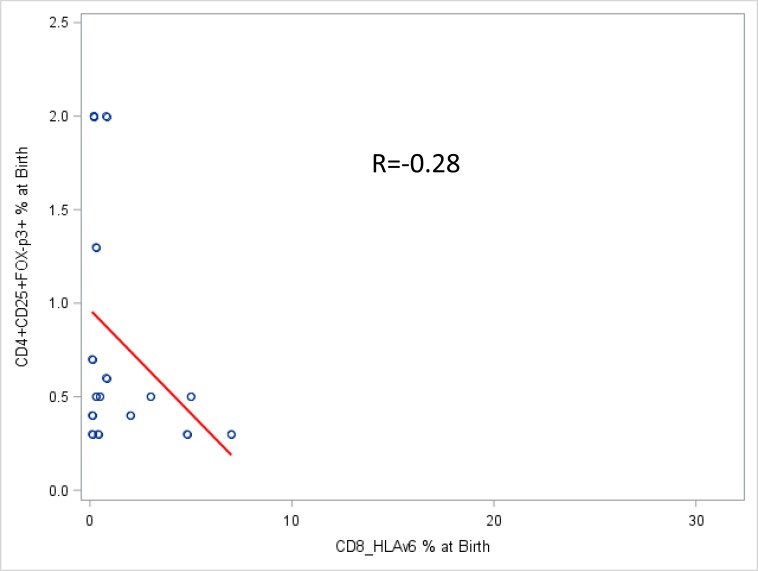
At the time of birth, HIV-exposed uninfected infants had higher levels of Treg, compared to infants infected in utero, even though this did not reach statistical significance (Fig. 2C, P = 0.08). The frequency of Treg was stable over the first 6 weeks of life (median, 0.5% of CD4+ T cells), slightly decreased between 6 weeks and 6 months (median at 6 months, 0.3%) and then slightly increased between 6 months (time of weaning) and 12 months of age (median, 0.45%) (Table 1, Fig. 1C). There was a modest negative correlation of Treg with CD8 T cell activation (Spearman R=-0.28) (Fig. 3).
Fig. (2).
Comparison of activated CD4+ (A), CD8+ T lymphocytes (B) and Treg (C) in HIV-exposed uninfected and HIV-infected neonates at the time of birth.
Fig. (3).
Correlation of activated CD8+ T lymphocytes with Treg in the blood of infants at birth.
4. Discussion
Among our population of HIV-exposed infants in Malawi, the expression of lymphocyte activation markers increased rapidly after birth, peaking at week 6 of life, and was more pronounced on CD8+ T cells; a further increase in activation was observed at the time of weaning from breastfeeding at 6 months of age. As a reminder, infants in our study weaned from breastfeeding at around 6 months of age and were introduced to other foods, which, along with increasing mobility and exposure of the infant to the environment, may induce immune activation at that age through increased exposure to many new environmental stimuli [11]. As expected, HIV-infected infants had higher levels of immune activation, compared with those uninfected, and this was more noted among CD8+ T lymphocytes. Other studies have also demonstrated that infants with HIV infection have higher levels of T lymphocyte activation compared to their uninfected counterparts [12-14]. However, HIV-exposed
uninfected infants also have high levels of immune activation [12-15] and this was evidenced in our study as well. The expression of activation markers on CD4+ and CD8+ T cells increases rapidly from birth in the first few weeks of life in the HIV-exposed infants [16, 17]. Such high immune activation has been hypothesized to contribute to ongoing inflammation and poor health outcomes of such infants [18], possibly mediated through intestinal mucosal damage [11, 17]
Our finding of higher Treg levels in HIV-exposed uninfected neonates is in agreement with that by Legrand et al. [9], who hypothesized that in utero Treg cells may be contributing to lack of HIV transmission by decreasing T cell activation. Treg cells are highest early in gestation and decrease with gestational age in the fetus [8]. Indeed, regulatory T cells (Tregs) suppress T cell activation and HIV-1 replication [19, 20]. In early infection, Treg help may help the establishment of viral infection by suppressing the anti-HIV response, whereas in chronic infection, Treg suppress hyperimmune activation, and the anti-HIV response, which may favor HIV reservoir persistence [19, 21]. However, before the active establishment of infection, Treg may play a role in the prevention of the infection by the same mechanism, particularly in the fetus/infant when their number is highest [9]. This is a critical question, as deciphering very early events that can determine whether an infection is established or not is important in devising appropriate therapies that can prevent a chronic infection from taking hold.
We observed a negative correlation of Treg with lymphocyte activation in infants in our study. This is consistent with what is known about the functional role of Treg and has been demonstrated also in another study [20]. Even though our study is limited by its small sample size, which limits its statistical power, serial blood samples from neonates exposed in utero to HIV are difficult to obtain, particularly in the setting of lack of exposure to antiretroviral therapy antenatally. One other study that was able to obtain serial samples from the infants has shown relative preservation of Treg among HIV-infected infants, compared to conventional CD4+ T cells [7].
conclusion
In conclusion, our findings concur with those of one previous study [9] and indicate that Treg cell activity might partially determine the vulnerability of infants to HIV or other chronic viral infections in utero. These results may have important implications for understanding maternal and fetal immunity against HIV during pregnancy, as well as for the development of neonatal vaccines and other immune-based approaches to prevent mother to child transmission of HIV or other chronic viral infections, and establishment of infection at critical known points of introduction more generally.
Acknowledgements
I am particularly thankful to Ms. Surinder Kaur for expert laboratory assistance and Dr Chris Ibegbu at Emory University School of Medicine who provided me with the immunophenotypic data and assisted me with the analysis/and interpretation. We are grateful to the BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project in Lilongwe: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Nicole Davis, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, Chifundo Zimba. Finally and most especially, all the women and infants that have agreed to participate in the study.
The Breastfeeding, Antiretrovirals, and Nutrition Study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01); the National Institute of Allergy and Infectious Diseases, the University of North Carolina Center for AIDS Research (P30-AI50410); the Carolina Population Center (R24 HD050924); the National Institutes of Health Fogarty International Programs [AIDS International Training and Research Program (D43 TW001039) and Scholars and Fellows Program (R24 TW007988); the American Recovery and Reinvestment Act]; and the Bill and Melinda Gates Foundation (Grant # OPP53107).
The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development.
Ethics Approval and Consent to Participate
The study protocol was approved by the Malawi National Health Science Research Committee, and by Institutional Review Boards at the University of North Carolina, and the Centers for Disease Control and Prevention, USA.
Human and Animal Rights
No animals were used in the study. All humans research procedures followed were in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
Consent for Publication
All women provided written informed consent to participate in the study.
Conflict of Interest
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Vignali D., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez A.M., Yang Y. The role of natural regulatory T cells in infection. Immunol. Res. 2011;49:124–134. doi: 10.1007/s12026-010-8176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veiga-Parga T., Sehrawat S., Rouse B.T. Role of regulatory T cells during virus infection. Immunol. Rev. 2013;255:182–196. doi: 10.1111/imr.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonetta F, Bourgeois C.
- 5.Kared H., Lelievre J.D., Donkova-Petrini V., et al. Regulatory T cells are associated with higher CD4 counts in primary infection. AIDS. 2008;22:2451–2460. doi: 10.1097/QAD.0b013e328319edc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card C.M., McLaren P.J., Wachihi C., et al. Decreased immune activation in resistance to HiV-1 infection is associated with an elevated frequency of Cd4+CD25+FOXP3+ regulatory T cells. J. Infect. Dis. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 7.Degaffe G., Zakhour R., Zhang W., et al. Forkhead box protein 3+ regulatory T cells and Helios+ subset in perinatally acquired HIV. Clin. Exp. Immunol. 2014;180:108–117. doi: 10.1111/cei.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsaknaridis L., Spencer L., Culbertson N., et al. Functional assay for human CD4+CD25+ T reg cells reveals an age-dependent loss of suppressive activity. J. Neurosci. Res. 2003;74:296–308. doi: 10.1002/jnr.10766. [DOI] [PubMed] [Google Scholar]
- 9.Legrand F.A., Nixon D.F., Loo C.P., et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One. 2006;1:e102. doi: 10.1371/journal.pone.0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamieson D.J., Chasela C.S., Hudgens M.G., et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48 week follow-up of the BAN randomized controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kourtis A.P., Ibegbu C.C., Wiener J., et al. Role of intestinal mucosal integrity in HIV transmission to infants through breast-feeding: The BAN study. J. Infect. Dis. 2013;208:653–661. doi: 10.1093/infdis/jit221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plaeger-Marshall S., Hultin P., Bertolli J., et al. Activation and differentiation antigens on T cells of healthy, at-risk, and HIV-infected children. J. Acquir. Immune Defic. Syndr. 1993;6:984–993. [PubMed] [Google Scholar]
- 13.Ono E., Nune dos Santos A.M., Menezes Succi R.C., et al. Imbalance of naïve and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz. J. Med. Biol. Res. 2008;41(8):700–708. doi: 10.1590/s0100-879x2008000800011. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto M., Govea A., Ono E., Succi R., Pahwa S., Moraes-Pinto M. Immune development in HIV-exposed uninfected children born to HIV-infected women. Rev. Inst. Med. Trop. São Paulo. 2017;59:e30. doi: 10.1590/S1678-9946201759030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerici M., Saresella M., Colombo F., et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–3871. [PubMed] [Google Scholar]
- 16.McFarland E.J., Powell T.M., Onyango-Makumbi C., et al. Ontogeny of CD4+ T lymphocytes with phenotypic susceptibility to HIV-1 during exclusive and nonexclusive breastfeeding in HIV-1-exposed Ugandan infants. J. Infect. Dis. 2017;215:368–377. doi: 10.1093/infdis/jiw553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prendergast A.J., Chasekwa B., Rokobo S., et al. Intestinal damage and inflammatory biomarkers in HIV-exposed and HIV-infected Zimbabwean infants. J. Infect. Dis. 2017;216:651–661. doi: 10.1093/infdis/jix367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans C., Jones C.E., Prendergast A.J. HIV-exposed, uninfected infants: new global challenges in the era of pediatric HIV elimination. Lancet Infect. Dis. 2016;16:e92–e107. doi: 10.1016/S1473-3099(16)00055-4. [DOI] [PubMed] [Google Scholar]
- 19.Li G., Nunoya J., Cheng L., et al. Regulatory T cells contribute to HIV-1 reservoir in CD4+ T cells through cyclic adenosine monophosphate-dependent mechanisms in humanized mice in vivo. J. Infect. Dis. 2017;216(5):1579–1591. doi: 10.1093/infdis/jix547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freguja R., Gianesin K., Mosconi I., et al. Regulatory T cells and chronic immune activation in human immunodeficiency virus 1 (HIV-1)-infected children. Clin. Exp. Immunol. 2011;164:373–380. doi: 10.1111/j.1365-2249.2011.04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes D., Jiang Q., Zhang L., Su L. Foxp3 and Treg cells in HIV-1 infection and immune-pathogenesis. Immunol. Res. 2008;41:248–266. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]