Abstract
Background:
Cigarette smoking increases systemic oxidative stress, inflammation, and viral replication in individuals with HIV. Macrophages are infected during HIV infection and serve as an important reservoir throughout the process. Macrophages exist in two phenotypes, the classically acti-vated M1 macrophage and alternatively activated M2 macrophage. The expression of drug efflux transporters and metabolic enzymes, which have direct effects on intracellular drug concentrations, differ between the pro-inflammatory M1 macrophage and the anti-inflammatory M2 macrophage.
Objective:
To further explain the role of tobacco use in worsened outcomes in the HIV + population receiving antiretroviral therapy.
Methods:
Western blotting was used to examine macrophage polarization and expression of drug efflux transporters, CYP enzymes, and antioxidant enzymes. The arginase assay was used to measure arginase activity. Cytokine production was measured using the human multiplex inflammatory cytokine assay kit. The 8-OHdG DNA Damage Quantification Direct Kit was used to quantify DNA damage. Viral replication under the influence of tobacco and antiretroviral drug use was measured by p24 Elisa.
Results:
We observed phenotypic shifts from M1 to M2 with both individual and combination treatments with cigarette smoke condensate and the protease inhibitor antiretroviral drug lopinavir. These shifts lead to changes in cytokine production, the expression of CYP enzymes, anti-oxidant enzymes, and drug efflux transporters, as well as changes in viral replication.
Conclusion:
This data suggest a mechanism by which tobacco use impairs HIV antiretroviral therapy to increase intracellular drug concentrations in this important cellular reservoir.
Keywords: Macrophage phenotype, drug efflux transporter, CYP, Tobacco, HIV, oxidative stress
1. Introduction
Globally, over 35 million individuals have died of HIV since the beginning of the epidemic [1]. According to the CDC, approximately 1.2 million people are living with HIV in the United States, with 39,513 people diagnosed in 2015 alone [2]. Tobacco abuse is prevalent in this population [3]. It has been shown that tobacco use leads to a higher HIV viral load in mild-to-moderate smokers compared to HIV+ nonsmokers, and has negative effects on the time to progression to AIDS, as well as other key markers of disease progression. [4-7]. CYP pathways and cellular oxidative stress are implicated in the mediation of this outcome [4, 8, 9]. Cigarette components are metabolized through CYP enzyme pathways and produce reactive oxygen species (ROS) and reactive metabolites [10, 11]. ROS and reactive metabolites are capable of causing oxidative stress, DNA damage, and cell toxicity [9, 12, 13]. Antioxidant enzymes (AOE), on the other hand, play a role in mediating oxidative stress by quenching ROS [14]. In addition, smokers with HIV have been found to have poorer viral and immunological responses caused by the decreasing efficacy of antiretroviral therapy (ART) [6, 15]. Although ART enhances immune reconstitution, cigarette smoke deteriorates immunity [16, 17]. T-cells from tobacco smokers not only differ in proliferation response to T-cell mitogens, but also in numbers [16, 18, 19]. Both CD4+ and CD8+ T-cell functions have been shown to be impaired by smoking [19]. Macrophages, in addition to CD4+ and CD8 + cells, are essential players in immunity [20-22]. Macrophages are also affected by tobacco use [13, 23, 24]. These findings underscore the active role tobacco plays in exacerbating HIV pathogenesis in macrophages. The mechanisms, however, is not fully understood.
Macrophages are capable of being polarized to the M1 or M2 phenotype when activated by different stimuli [25-27]. Classically activated macrophages (M1) are polarized in response to interferon-gamma (IFNϒ) combined with bacterial lipopolysaccharide (LPS). M1 macrophages are involved in the activity of killing by producing a high amount of interleukin-12(IL-12), interleukin-23(IL-23), nitric oxide (NO), and reactive oxygen intermediates (ROI) [28]. M2 macrophages mediate immunosuppression and tissue repair by producing ornithine and polyamines [29-32]. M1 and M2 macrophages play an important role in a variety of disease states and inflammation. Polarized M1 macrophages stimulate a T helper cell type I-like response, produce pro-inflammatory cytokines, and maintain strong microbicidal activities. M2 macrophages, on the other hand, are implicated as having a central role in facilitating lung repair and remodeling in RSV-induced damages [33]. However, the role of macrophage polarization in HIV remains unclear [34-37]. Some researchers suggest that macrophages are polarized toward M1 phenotypes in the early stage of infection and serve as a major viral reservoir [38]. The cytokines interleukin (IL)-4/IL-13 then activate M2 macrophage polarization, which stops the expansion of viral reservoirs in the later stages of infection [39]. As an early target for infection, infected macrophages can persist for extended periods of time [21, 22, 40]. It has been shown that macrophages act as HIV reservoirs and permit viral replication, even after the initiation of antiretroviral therapy [21, 40-43]. Furthermore, polarized macrophages are more responsive to Toll-like receptor (TLR) stimuli caused by HIV infection [37].
Macrophages are major targets of drugs including antibiotics and antiretrovirals [44-47]. Drug efflux transporters, which have historically been investigated as a cause for decreased absorption and low plasma concentrations of drugs, may also have effects on drug concentrations inside macrophages [48, 49]. Drug efflux transporters, including P-glycoprotein (PGP) and multidrug resistance protein 1(MRP1) are able to transport a diverse collection of drugs and toxins for the purpose of maintaining barrier function of sanctuary site tissues [50]. Although ART is capable of suppressing detectable viral replication in the plasma, it cannot eradicate the virus in reservoirs [51-54]. It has been shown that protein expression levels of the drug efflux transporters MRP2 and BCRP were increased with ART-treated HIV+ patients. PGP expression was also significantly higher in HIV+ patients who received ART, showing that antiretrovirals can also influence transporter expression [55]. Intracellular antiretroviral levels might be changed due to the different drug efflux transporter expression levels.
In our previous research, we observed that drug efflux transporter levels varied between M1 and M2 phenotypes with a higher expression level of MRP1 but lower expression of PGP in M1 cells compared to M2 [48, 49]. Here, we aimed to clarify how a commonly utilized antiretroviral and cigarette smoke condensate interact in polarized macrophages, and how these influences change in the immunology of the M1 and M2 macrophages in terms of cytokine production and CYP enzyme dependent oxidative stress. We also examined alternations in drug efflux transporter expression and examined a drug efflux transporter based strategy to reduce viral replication.
2. Material and Methods
2.1. Cell Culture
The human monocytic cell lines U937 and U1 were cultured in RPMI 1640 medium supplemented with 10% FBS. U937 was purchased from American Type Culture Collection (ATCC Manassas, VA) and U1 cell lines were obtained from NIH AIDS Reagent Program (Germantown, MD). U937 and U1 cells were treated with cytokines to effect polarization for 48 hours. LPS (100 ng/ml, E. Coli origin, Sigma-Aldrich, L2630, St. Louis, Mo, USA) combined with IFN-γ (20ng/ml, Life Technologies, PHC4031, Carlsbad, CA, USA) were used for M1 polarization. LPS (100 ng/mL) with IL-4 (10 ng/mL, CST, 8919SF, Danvers, MA, USA) and IL-13 (10 ng/mL, CST, 8905SF, Danvers, MA, USA) were used to stimulate M2 polarization. No cytokines were added to unstimulated control cells. Cells were treated with cigarette smoke condensate (CSC) (40 μg/ml, Murty Pharmaceuticals, KY, USA), a concentration which approximates concentrations observed in smokers, [13, 56] with or without the antiretroviral protease inhibitor lopinavir (Sigma Aldrich, 1370101, St. Louis, MO, USA) (LPV) for 24 h before harvesting.
2.2. Western Blotting
Cellular protein expression levels were analyzed by western blot. Cells were harvested and lysed in cold RIPA buffer supplemented with a protease inhibitor cocktail (Roche, 11697498001, Indianapolis, IN, USA) after 24 h treatment for whole cell lysates. Cell membrane protein was isolated using a cell fraction kit (CST, 9038S, Massachusetts, USA); this protein was harvested for MRP1 detection. BCA assay (Pierce, 23225, Rockford, IL, USA) was used to estimate protein concentration. 5 to 35 μg of protein sample was loaded on PAGE-SDS mini-gel (8% separation gel and 4% stacking gel). After 1.5 h running at 120v, gels were transferred to nitrocellulose membranes (Bio-Rad, Hercules, 1620112, CA, USA). The membranes were blocked in 5% non-fat dry milk at room temperature for 1 h, followed by incubation at 4°C with the following respective primary antibodies overnight: anti-ARG1 (Cell Signaling, Massachusetts, USA, 1:1,000), anti-BCRP primary antibody (Abcam, Cambridge, UK, 1:2,000), anti-catalase (Santa Cruz, California, USA, 1:1,000), anti-CYP1B1 (Santa Cruz, California, USA,1:400), anti-CYP2A6 (Santa Cruz, California, USA,1:200), anti-iNOS antibody (Cayman, Ann Arbor, MI, USA, 1:1,000), anti-MRP1 antibody (Abcam, Cambridge, UK, 1:25), anti-PGP primary antibodies (Abcam, Cambridge, UK, 1:1,000), anti-PRDX6 (LSBio, Seattle, USA,1:400), anti-SOD1 (Santa Cruz, California, USA,1:1,000), and anti-SOD2 (Santa Cruz, California, USA, 1:1,000). Whole cellular protein was normalized using GAPDH (Cell Signaling, Danvers, MA, USA, 1:1,000). Membrane protein was normalized with K-ATPase antibody (Abcam, Cambridge, UK, 1:2,000). The membrane was incubated with the secondary antibody (IRDye® 800CW goat anti-rabbit or IRDye® 680RD Goat anti-Mouse) [1:15,000] in the dark at room temperature for 45 minutes. Dual-channel infrared scan and quantification of immunoblots were performed using the Odyssey Sa infrared imaging system with Image Studio (Ver. 3.1.4) (LI-COR, Lincoln, NE, USA).
2.3. Arginase Activity
Arginase activity of each sample was measured using the arginase assay, which determines urea production as described [57, 58]. Urea is the byproduct of the reaction of arginine to ornithine. Cells were lysed in 0.1% Triton X-100. 50 μL of cell lysates were then mixed and heated with 25 μL of 10mM MnCl2 in 50 mM Tris HCl at 55°C. After 10 minute incubation, 50 μL of 0.5 M arginine solution was added as substrate. The mixture was incubated at 37°C overnight. An acid solution consisting of H2SO4, H3PO4 and water (1:3:7) was added to terminate the reaction on the second day. The mixture was heated with 25 μL of 9% isonitrosopropiophenone (ISPF) at 100°C for 45 min. The absorbance of each sample was measured using a μQuant spectrophotometer (Bio-Tek, Winooski, VT, USA) at 540 nm. Arginase activity was calculated by comparing to a standard curve of known urea concentrations followed by normalization to cell counts. Arginase activity was then normalized to the unstimulated control condition.
2.4. Quantification of 8-Hydroxy-2′-deoxyguanosine (8-OHdG) Content
The EpiQuik 8-OHdG DNA Damage Quantification Direct Kit (Fluorometric) (Epigentek, p-6003, Farmingdale, NY) was used to quantify the 8-OHdG content of DNA in U937. Isolated DNA from each sample was added into wells of a plate that had a high DNA binding affinity. Capture and detection antibodies were used to detect 8-OHdG. Signal was enhanced by enhancer solution and followed by quantification. Measurements were obtained using a fluorescence microplate reader, which was equipped with an excitation filter of 530 nm and an emission filter of 590 nm. The amounts of 8-OHdG were calculated by comparing measurements to a standard curve.
2.5. Multiplex Cytokine Assay
Cytokine production in U937 and U1 cells were measured using the human multiplex inflammatory cytokine assay kit (Bio-Rad, m50000007a, CA, USA). Cell culture supernatant was collected after 24 h of treatment. Three volumes of sample diluents were then added. Magnetic beads were added into 50 μL of samples or standards. The mixtures were incubated on a shaker at room temperature for 30 minutes. Streptavidin-PE conjugate (50 μL) was added after washes. After 10 minutes incubation, 125 μL of assay buffer was added. Samples were analyzed using Biorad Bioplex HTS (Bio-Rad, CA, USA). Bio-Plex manager 5 using 5-PL statistics was used to estimate cytokine concentrations.
2.6. HIV-1 p24 ELISA
p24 antibody-coated plates (Zeptometrix Corporation, 0801111, Buffalo, NY) were washed 5 times with 1X wash buffer before use. Samples were collected and lysed with lysis buffer. 200 μL of sample were added into each well followed by 37°C incubation overnight. p24 antibody was added after 5 washes the next day. The plate was incubated at 37°C for 1 hr. After washing, streptavidin-peroxide working solution was added into each well. Plates were washed again. 100 μL of substrate working solution was added immediately after the last wash. The color was developed at room temperature for 30 minutes. Stop solution was added and the sample was measured at 450nm immediately. The amount of HIV-1 p24 antigen was compared and determined against the standard curve and normalized to the amount of protein present in the cell lysates.
2.7. Statistical Analysis
Western blot densitometry data was obtained from one of three representative blots. Other data were collected from three independent experiments and are shown as mean ±SD. GraphPad Prism (GraphPad Software, La Jolla, CA, USA) was used to perform the analysis. ANOVA with Tukey’s multiple comparison tests was used to determine statistical significance.
3. Results
3.1. CSC and LPV Treatments Shift Macrophage Polarization
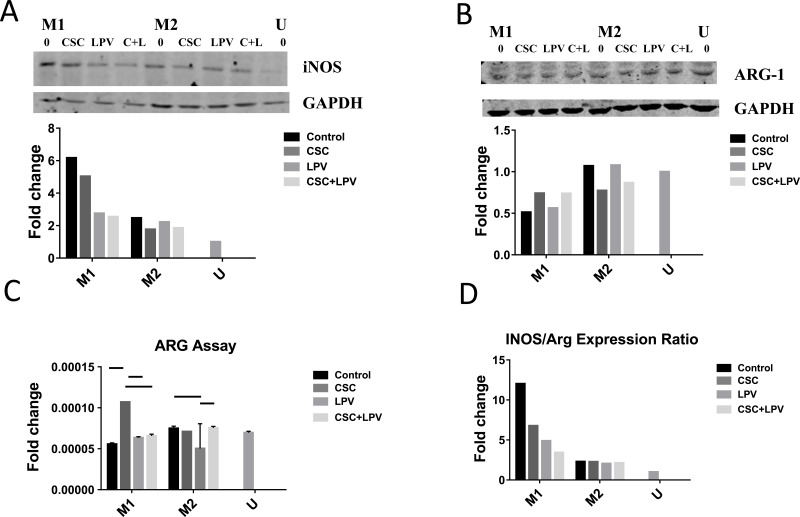
We first assessed how macrophage polarization was affected by treatment with CSC and LPV. Western blots with the U937 cell lysates were performed. The expression levels of the M1 marker inducible nitric oxide synthase (iNOS) and the M2 marker arginase 1 (Arg1) were analyzed. As expected, the M1 phenotype expressed threefold higher levels of iNOS, but only had 50% of the expression levels of Arg1 than the M2 phenotype (Fig. 1). Cells treated with CSC, LPV, and both CSC+LPV showed decreased iNOS protein expression in the M1-treated cells, by approximately 20% with ethanol, and by half in the LPV and CSC+LPV conditions, while having very little effect on iNOS expression in the M2-treated cells (Fig. 1A). Higher Arg1 expression was observed in the M2 cells than the M1 cells. (Fig. 1B). Similarly, a significant increase and a non-significant trend for arginase activity in the CSC and CSC+LPV treatment groups in M1 were observed, respectively (Fig. 1C). Finally, we assessed the ratio of iNOS to Arg1 expression, as a general marker of macrophage polarization. A halving in this ratio is consistent with a shift away from the M1 phenotype towards the M2 phenotype. CSC and CSC+LPV exposure greatly decreased the iNOS/Arg1 ratio in the M1 cells, while having no effect on cells already polarized to the M2 phenotype (Fig. 1D).
Fig. (1).
Treatment with CSC and LPV shifts polarization of M1 cells while having no effect on M2 polarized cells. U937cells were treated with either IFNγ + LPS or IL4+IL13+LPS to polarize to M1 or M2 respectively for 48 h. Cells were then treated with CSC, LPV or CSC+LPV for 24 h. Each western blot data set (A-B) shows a representative blot from three replicates. Blot density was normalized to the housekeeping protein and to the unstimulated control for (A) and (B) data. (A) Western blot and densitometry for iNOS expression. (B) Western blot and densitometry for Arg1 expression. (C) Arginase Assay. Arginase activity was quantified as the L-arginine to urea conversion after cell collection. Samples were normalized to cell counts followed by normalization to the unstimulated control. Data represent an average of three replicates from a representative arginase assay. (D) iNOS/Arg1 ratio. The ratio of iNOS/Arg1 expression in a representative blot was assessed for all conditions. A higher ratio is consistent with higher M1 polarization. Arginase samples were analyzed using the ANOVA with Tukey’s multiple comparison test. Bars represent p <0.05.
3.2. Cytokine Production
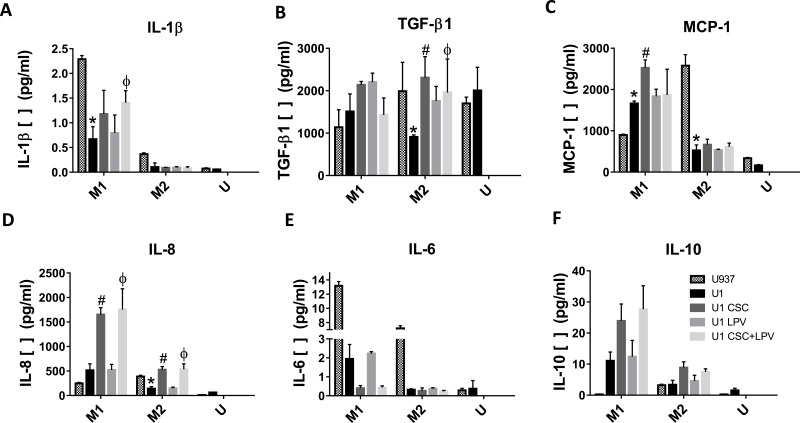
Next, we utilized U937 and U1 cells to examine the effects of polarization shift on the cytokines production. IL-6, a key cytokine of pro-inflammatory was significantly decreased while IL-10, an essential anti-inflammatory cytokine was significantly increased in M1 with CSC and CSC+LPV treatments (Fig. 2D and 2F). The changes in cytokine production with the CSC and LPV treatments in the M2 macrophages were minor (Fig. 2D and 2F). This is consistent with the polarization data that CSC and CSC+LPV treatments shift the M1 macrophage towards M2 while having minimal effects on the M2 macrophages. Other cytokines produced by M1 and M2 cells were also affected by CSC treatment with more pronounced significant changes in the M1 than the M2 macrophages, such as IL-8, IL-1β and MCP-1(Fig. 2A, 2B, 2C). In M2 cells, Only CSC and CSC+LPV treatments increased IL-8 and TGF-β1 expression significantly (Fig. 2A and 2E). Interestingly, CSC-treated M1 macrophages were shown to have significantly higher production of pro-inflammatory cytokines IL-8, IL-1β, and MCP-1. (Fig. 2A, 2B, and 2C).
Fig. (2).
Cytokine production of U1 and U937 macrophages with CSC and LPV treatment. U1 and U937 cells were polarized to M1 or M2 with either IFNγ + LPS or IL4+IL13+LPS treatment for 48 h. Cells were then treated with CSC, LPV or CSC+LPV for the next 24 hrs. Cell culture supernatants were collected and cytokine levels were measured for the following cytokines: (A) IL-8, (B) IL-1β, (C) MCP-1, (D) IL-6, (E) TGF-β1, (F) IL-10. Data represent an average of three replicates. The p values (* represents U1 vs U937, # represents U1 CSC vs U1, and Φ represents U1 CSC+LPV vs U1) were calculated using one-way-ANOVA with Tukey’s post-test, with significance determined with a p < 0.05.
3.3. CYP enzymes, Metabolic Stress and DNA Damage
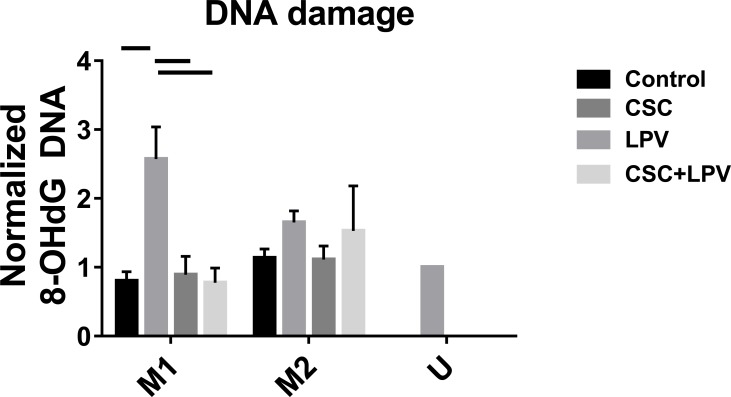
We next assessed DNA damage, DNA damage relevant CYP enzyme expression, and metabolic stress in the cells. The amount of 8-OHdG, a marker for DNA damage, was assessed in U937 cells. In M1 cells, DNA damages were similar among all treatment conditions except for CSC treatment group in which DNA damage was significantly increased (Fig. 3). In M2 cells, DNA damage was non-significantly increased with CSC and CSC+LPV treatments (Fig. 3). As expected, this increase was more pronounced in M1 compared to M2 cells, consistent with the cytokine production data (Fig. 3).
Fig. (3).
Oxidative stress measurement in M1, M2 and unstimulated U937 cells. U937 cells were treated with either IFNγ + LPS or IL4+IL13+LPS to polarize to M1 or M2, respectively, for 48 h. Cells were then treated with CSC, LPV or CSC+LPV for 24 h. The amount of 8-OHdG from each sample was captured and detected by detection antibody, and measured by fluorescence microplate reader. Data represent an average of three replicates. Samples were analyzed using the ANOVA with Tukey’s multiple comparison test. Bars represent p <0.05.
Since DNA damage is CYP enzyme pathway dependent, we then examined the expression of two therapeutically relevant CYP enzymes, CYP1B1 and CYP2A6, as expressed in U937 cells, via Western blotting. CSC, LPV and CSC+LPV induced CYP1B1 in M1 cells at least two-fold while in M2 cells, the increase was most pronounced with CSC treatment, with an almost 3 fold increase (Fig. 4A). Inductions were observed in CYP2A6 expression with CSC, LPV and CSC+LPV in M1 cells, with the largest increase observed in the CSC+LPV condition (Fig. 4B). However, in M2 cells, all treatment conditions had minimal effects on CYP2A6 (Fig. 4B). In M1 cells, more pronounced increases were observed for CYP1B1 compared to CYP2A6 (Fig. 4A and Fig. 4B).
Fig. (4).
CYP and antioxidant enzyme expression level with CSC and LPV treatments. U937 cells were treated with either IFNγ + LPS or IL4+IL13+LPS to polarize to M1 or M2, respectively, for 48 h. Cells were then treated with CSC, LPV or CSC+LPV for 24 h. Each western blot data set shows a representative blot from three replicates. Blot density was normalized to the housekeeping protein and to the unstimulated control. (A) Western blot and densitometry for CYP 1B1expression. (B) Western blot and densitometry for CYP 2A6 expression. Blot density was normalized to the housekeeping protein and to the unstimulated control. (C) Western blot and densitometry for catalase expression. Blot density was normalized to the housekeeping protein and to the unstimulated control. (D) Western blot and densitometry for SOD2 expression. Blot density was normalized to the housekeeping protein and to the unstimulated control.
We then determined whether the AOEs catalase, and SOD2, which decrease oxidative stress and DNA damage, were changed by CSC and LPV treatments. When we looked at catalase expression levels in U937, the changes in the M1 cells were minimal (Fig. 4C). We then assessed SOD2, a specific AOE enzyme, levels in U937, CSC+LPV increased
SOD2 expression 50% in M1 macrophages (Fig. 4D). In M2 cells, SOD2 expression was upregulated at least 3-fold by all treatment conditions (Fig. 4D).
3.4. Drug Efflux Transporters
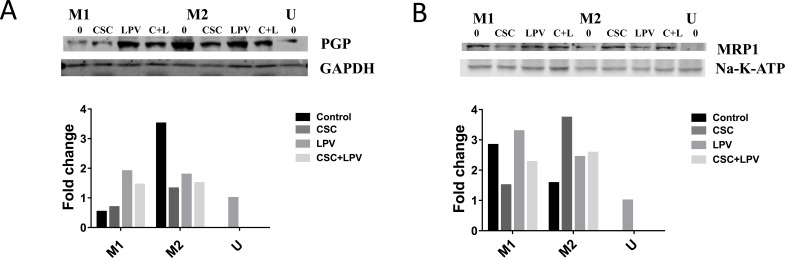
We also examined the effects of polarization shift caused by CSC, LPV and CSC+LPV on the drug efflux transporters PGP and MRP1 in U937 cells using Western blot. As expected, M1 untreated cells expressed lower levels of PGP, but higher levels of MRP1 than did M2-untreated cells (Fig. 5A and Fig. 5B). PGP expression was increased by 30% in CSC treated and 2.5-fold in by CSC+LPV in M1 cells (Fig. 5A). When we next examined MRP1 expression, we observed that CSC and CSC+LPV decreased MRP1 in M1 cells (Fig. 5B). In M2 cells, MRP1 expression levels were increased in all treatment conditions, with the most pronounced increase in the CSC treated cells (Fig. 5B). From our previous research that PGP has higher expression level in the M2 macrophages while MRP1 has higher expression level in the M1 macrophages, the changes of drug efflux transporter expression caused by CSC and CSC+LPV further support that CSC and CSC+LPV treatment shifts macrophage expression towards the M2 phenotype (Fig. 5A and Fig. 5B) [48, 49].
Fig. (5).
Drug efflux transporter expression with CSC and LPV treatments. U937 cells were treated with either IFNγ + LPS or IL4+IL13+LPS to polarize to M1 or M2 respectively for 48 h. Cells were then treated with CSC, LPV or CSC+LPV for 24 h. Each western blot data set shows a representative blot from three replicates. Blot density was normalized to the housekeeping protein and to the unstimulated control for all data. (A) Western blot and densitometry for PGP expression. (B) Western blot and densitometry for MRP1expression. Each western blot data set shows a representative blot from three replicates.
3.5. Viral Replication
Drug efflux transporters play an important role in influencing drug concentrations, both systemically and intracellularly. Decreased efficacy of HIV protease inhibitors, such as indinavir, saquinavir, and ritonavir, has been reported in CD4 lymphocytes due to high expression of PGP [59]. Finally, we assessed viral replication in CSC exposed polarized U1 treated with an antiretroviral and a small molecule inhibitor of PGP cells via the production of p24. LPV reduces viral replication by preventing viral proteins cleavage into mature functional proteins. The majority of HIV protease inhibitors including LPV are PGP substrates [60]. We also examined the strategy of reducing viral replication under the treatment of CSC by inhibiting PGP transporter considering the polarization shift from M1 to M2 and a higher expression of PGP in the M2 compared to M1. Cells were treated with the PGP inhibitor elacridar (El) [61]. LPV decreased viral replication significantly in M1 cells with or without elacridar (Fig. 6A). In the M2 cells, CSC significantly increased while LPV significantly reduced CSC dependent p24 viral replication (Fig. 6B). In the M1 cells, the addition of elacridar to the LPV and CSC+LPV treatment groups had no effect on viral replication compared with LPV and CSC+LPV groups (Fig. 6A). While in the M2 cells, elacridar reduced p24 production significantly in the LPV+El and CSC+LPV+El treatment groups compared with LPV and CSC+LPV groups, respectively (Fig. 6B). This indicates the increased efficacy of LPV in reducing p24 viral replication with the addition of PGP inhibitor elacridar in conditions where PGP expression is high. These results suggest that inhibiting the function or expression of relevant drug efflux transporter might be a valuable way to reduce viral replication in cellular reservoir, and supports a need to develop strategies to inhibit viral replication in cellular reservoirs of macrophages across their spectrum of activation.
Fig. (6).
p24 replication of U1 with CSC, LPV and elacridar treatment. U1 cells were treated either with IFNγ + LPS or IL4+IL13+LPS to polarize to M1 or M2, respectively, for 48 h. Cells were next treated with CSC, LPV or CSC+LPV for 24 h, as well as the PGP inhibitor elacridar. HIV-1 p24 antigen levels of (A) M1 and (B) M2. Data represent an average from three replicates. Bars represent p <0.05 between groups and were calculated via one-way ANOVA with Tukey’s post-test.
4. Discussion
In this paper, we have shown the effects of CSC on shifting macrophage polarization. As shown by a decrease in the iNOS/Arg1 ratio, M1 macrophages were shifted towards M2 with CSC and CSC+LPV treatments. This shift induced the expression of the drug efflux transporter PGP, and the CYP enzymes CYP1B1 and CYP2A6. The M1 to M2 shift caused by CSC and CSC+LPV also led to changes in the immunoregulatory response of macrophages: the expression of the pro-inflammatory cytokine IL-6 was decreased while the anti-inflammatory cytokine IL-10 was increased. It has been reported that HIV protease inhibitors increase IL-6 and TNF- α expression through increasing mRNA stabilities, accomplished by translocating and enhancing RNA-binding protein HuR to 3’UTR of IL-6 and TNF- α [62]. Thus, changes caused by a shift in polarization might be counteracted by the effects of LPV itself on the cells.
Our results are consistent with others’ observation that CSC shifts macrophage polarization from M1 to M2. The IL-4 receptor α mediates recruitment of STAT6, activating arginase 1 transcription in M2 cells [63]. It has been shown that prenatal exposure to smoking activates STAT6 in mice [64]. In addition, secondhand tobacco smoke induces airway hyperactivity (AHR) and Th2 lung inflammation in a murine allergic asthma model. Furthermore, prenatal mice exposed to cigarette smoke showed increased GATA3, which was associated with Th2 polarization and suppression of T-bet, which promotes Th1 polarization [64]. When activated, macrophages are able to polarize to a spectrum of states between M1 and M2 phenotypes [27, 29, 36, 65-68]. The M1 phenotype expresses high levels of pro-inflammatory cytokines, while M2 is more involved in tissue repair. The phenotypes of macrophages are reversible and plasticity is a crucial feature of macrophages [67, 69]. The decreased iNOS/ arginase ratio by treatments in M1 cells suggests a shift towards M2 polarization. M2 polarization occurs by IL-4 and IL-13, which are produced under a Th2 cytokine polarization response [58, 70]. CSC, LPV and CSC+LPV shifted M1 cells towards the M2 phenotype independent of M2-polarizing cytokines, as observed through the decreased iNOS/Arg1 ratio. Though no effects were observed on arginase expression and activity in M1 cells with LPV treatment, the iNOS/Arg1 ratio was decreased due to the reduction of iNOS expression. Macrophages retain their plasticity and respond to a combination of stimuli. When polarized to a specific phenotype, macrophages still maintain some characteristics of the other phenotype [25, 71, 72]. This may explain the unchanged level of arginase expression and activity, but decreased iNOS, in M1 cells treated with LPV compared to the M1 control. This also might be the reason for the increased pro-inflammatory cytokines, such as IL-1β, MCP-1 and IL-8 with CSC treatment.
Both IL-6 and IL-10 are important effector cytokines produced by polarized macrophages. IL-6, which plays a pro-inflammatory role, was decreased by CSC and CSC+LPV treatments, while the anti-inflammatory cytokine IL-10 was increased with the same treatments in M1 cells. This indicates a shift in M1 cells towards M2 polarization due to CSC and LPV treatments. Changes in cytokine production, in turn, further the shift of polarization. By activating the transcription factor STAT3, IL-10 promotes the M2 phenotype [23, 73, 74]. Both IL-6 and IL-10 are capable of activating STAT3; however, some research suggests a higher sensitivity of IL-10 toward activation of STAT3. In this paper, decreased IL-6 and increased IL-10 in U1 cells compared with U937 supports that HIV infection shifted cells toward an M2-like phenotype. Unlike the phenotypic shift observed in M1, CSC, LPV and CSC+LPV drove the unstimulated U937 more towards M1 with increased iNOS/Arg1 ratio.
CYP enzymes are the phase I enzymes which detoxify the parent compound [75]. Reactive oxygen species (ROS) are produced through CYP enzyme-mediated tobacco constituent metabolism [4, 76, 77]. It has recently been shown significant expression of CYP enzymes in monocytic cells, and their potential role in smoking-mediated cytotoxicity and HIV pathogenesis [13]. CSC treatment increased CYP enzyme expression while it maintained general antioxidant enzyme level in M1 cells. This leads to higher oxidative stress, which subsequently increases damage in M1 cells. However, in the CSC+LPV treatment group, both CYP enzyme and the anti-oxidative enzyme expression were increased in M1 cells. This might explain the lack of difference in DNA damage between CSC+LPV and control in M1 cells.
There are, however, limitations associated with our findings. In our viral replication studies, viral replication was reduced by adding elacridar to both LPV+elacridar and CSC+LPV+Elacridar groups. However, some small molecule inhibitors are substrates of multiple transporters. Elacridar here is an inhibitor for both PGP and BCRP with a 40-fold higher IC50 for BCRp [78]. Although LPV is a substrate for PGP but not BCRP, off-target effects cannot be entirely eliminated due to increased BCRP and decreased PGP with LPV treatment [79].
In addition to the major drug efflux transporters PGP and MRP1, other drug efflux and influx transporters also contribute to drug concentrations inside of cells [80]. In this paper, we examined the expressions of two efflux transporters, and in future experiments, it will be of interest to assess other important transporters such as solute carrier (SLC) transporters and organic anion transporters. A better understanding of factors that have effects on drug concentrations will provide valuable knowledge in the mechanisms of increasing drug concentrations, thereby controlling virus replication.
Here, we have utilized LPV primarily as a probe, due to its relatively clean status as a substrate of PGP. However, effective antiretroviral therapy requires at least three agents. How multiple antiretroviral agents interact in influencing transporter expression, and how this alters the concentrations of antiretrovirals and resultant changes in viral replication, is a continuing interest for our laboratory and for other research groups worldwide.
Conclusion
Our study suggests the contribution of a shift in macrophage phenotype on regulating drug efflux transporters, metabolic enzyme expressions, oxidative stress, and DNA damage with tobacco use and ART treatment. Regulating drug efflux transporters may directly or indirectly influence drug concentrations inside cells, which effects therapeutic outcomes. By increasing antioxidant enzyme expression, LPV decreased DNA damage in the CSC+LPV group compared with the CSC treatment group. This suggests another method to increase therapeutic efficacy. These findings may also provide a valuable method and information for assessing other drug transporters, including the influx and efflux varieties, and for understanding the effects and mechanisms of other drugs that have been widely abused by HIV+ smokers.
Acknowledgements
This study was supported by the UTHSC College of Pharmacy and by the National Institutes of Health (R01 AA022063)
Ethics Approval and Consent to Participate
Not applicable.
Human and Animal Rights
No Animals/Humans were used for studies that are the basis of this research.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.WHO 2015 http://www.who.int/gho/hiv/en/
- 2.CDC HIV in the United States: At A Glance. 2016 https://www.cdc.gov/hiv/statistics/overview/ ataglance.html
- 3.Reynolds N.R. Cigarette smoking and HIV: more evidence for action. AIDS Educ. Prev. 2009;21(3) Suppl.:106–121. doi: 10.1521/aeap.2009.21.3_supp.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ande A., McArthur C., Ayuk L., et al. Effect of mild-to-moderate smoking on viral load, cytokines, oxidative stress, and cytochrome P450 enzymes in HIV-infected individuals. PLoS One. 2015;10(4):e0122402. doi: 10.1371/journal.pone.0122402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crothers K., Griffith T.A., McGinnis K.A., et al. The impact of cigarette smoking on mortality, quality of life, and comorbid illness among HIV-positive veterans. J. Gen. Intern. Med. 2005;20(12):1142–1145. doi: 10.1111/j.1525-1497.2005.0255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman J.G., Minkoff H., Schneider M.F., et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the women’s interagency HIV study. Am. J. Public Health. 2006;96(6):1060–1065. doi: 10.2105/AJPH.2005.062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nieman R.B., Fleming J., Coker R.J., Harris J.R., Mitchell D.M. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. AIDS. 1993;7(5):705–710. doi: 10.1097/00002030-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Czekaj P., Wiaderkiewicz A., Florek E., Wiaderkiewicz R. Tobacco smoke-dependent changes in cytochrome P450 1A1, 1A2, and 2E1 protein expressions in fetuses, newborns, pregnant rats, and human placenta. Arch. Toxicol. 2005;79(1):13–24. doi: 10.1007/s00204-004-0607-7. [DOI] [PubMed] [Google Scholar]
- 9.Ande A., McArthur C., Kumar A., Kumar S. Tobacco smoking effect on HIV-1 pathogenesis: role of cytochrome P450 isozymes. Expert Opin. Drug Metab. Toxicol. 2013;9(11):1453–1464. doi: 10.1517/17425255.2013.816285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi Y., Nasu F., Harada A., Kunitomo M. Oxidants in the gas phase of cigarette smoke pass through the lung alveolar wall and raise systemic oxidative stress. J. Pharmacol. Sci. 2007;103(3):275–282. doi: 10.1254/jphs.fp0061055. [DOI] [PubMed] [Google Scholar]
- 11.Hodge-Bell K.C., Lee K.M., Renne R.A., Gideon K.M., Harbo S.J., McKinney W.J. Pulmonary inflammation in mice exposed to mainstream cigarette smoke. Inhal. Toxicol. 2007;19(4):361–376. doi: 10.1080/08958370601144076. [DOI] [PubMed] [Google Scholar]
- 12.Jin M., Earla R., Shah A., et al. A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV + smokers. J. Neuroimmune Pharmacol. 2012;7(1):289–299. doi: 10.1007/s11481-011-9283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao P., Ande A., Sinha N., Kumar A., Kumar S., et al. Effects of cigarette smoke condensate on oxidative stress, apoptotic cell death, and hiv replication in human monocytic cells. PLoS One. 2016;11(5):e0155791. doi: 10.1371/journal.pone.0155791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollace V., Salvemini D., Riley D.P., et al. The contribution of oxidative stress in apoptosis of human-cultured astroglial cells induced by supernatants of HIV-1-infected macrophages. J. Leukoc. Biol. 2002;71(1):65–72. [PubMed] [Google Scholar]
- 15.Valiathan R., Miguez M.J., Patel B., Arheart K.L., Asthana D., et al. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: a cross-sectional pilot study. PLoS One. 2014;9(5):e97698. doi: 10.1371/journal.pone.0097698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt P.G. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42(4):241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sopori M.L., Kozak W. Immunomodulatory effects of cigarette smoke. J. Neuroimmunol. 1998;83(1-2):148–156. doi: 10.1016/s0165-5728(97)00231-2. [DOI] [PubMed] [Google Scholar]
- 18.Tollerud D.J., Clark J.W., Brown L.M., et al. The effects of cigarette smoking on T cell subsets. A population-based survey of healthy caucasians. Am. Rev. Respir. Dis. 1989;139(6):1446–1451. doi: 10.1164/ajrccm/139.6.1446. [DOI] [PubMed] [Google Scholar]
- 19.Zeidel A., Beilin B., Yardeni I., Mayburd E., Smirnov G., Bessler H. Immune response in asymptomatic smokers. Acta Anaesthesiol. Scand. 2002;46(8):959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuroda M.J. Macrophages: do they impact AIDS progression more than CD4 T cells? J. Leukoc. Biol. 2010;87(4):569–573. doi: 10.1189/jlb.0909626. [DOI] [PubMed] [Google Scholar]
- 21.Sattentau Q.J., Stevenson M. Macrophages and HIV-1: An unhealthy constellation. Cell Host Microbe. 2016;19(3):304–310. doi: 10.1016/j.chom.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honeycutt J.B., Thayer W.O., Baker C.E., et al. HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat. Med. 2017;23(5):638–643. doi: 10.1038/nm.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan F. Fu X2, Shi H, Chen G, Dong P, Zhang W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS One. 2014;9(9):e107063. doi: 10.1371/journal.pone.0107063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbud R.A., Finegan C.K., Guay L.A., Rich E.A. Enhanced production of human immunodeficiency virus type 1 by in vitro-infected alveolar macrophages from otherwise healthy cigarette smokers. J. Infect. Dis. 1995;172(3):859–863. doi: 10.1093/infdis/172.3.859. [DOI] [PubMed] [Google Scholar]
- 25.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Hodge S., Matthews G., Mukaro V., et al. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am. J. Respir. Cell Mol. Biol. 2011;44(5):673–681. doi: 10.1165/rcmb.2009-0459OC. [DOI] [PubMed] [Google Scholar]
- 27.Mege J.L., Mehraj V., Capo C. Macrophage polarization and bacterial infections. Curr. Opin. Infect. Dis. 2011;24(3):230–234. doi: 10.1097/QCO.0b013e328344b73e. [DOI] [PubMed] [Google Scholar]
- 28.Verreck F.A., de Boer T., Langenberg D.M., et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA. 2004;101(13):4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mills C.D. M1 and M2 Macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 30.Mosser D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003;73(2):209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 31.Krysko O., Holtappels G., Zhang N., et al. Alternatively activated macrophages and impaired phagocytosis of S. aureus in chronic rhinosinusitis. Allergy. 2011;66(3):396–403. doi: 10.1111/j.1398-9995.2010.02498.x. [DOI] [PubMed] [Google Scholar]
- 32.Chihara T., Hashimoto M., Osman A., et al. HIV-1 proteins preferentially activate anti-inflammatory M2-type macrophages. J. Immunol. 2012;188(8):3620–3627. doi: 10.4049/jimmunol.1101593. [DOI] [PubMed] [Google Scholar]
- 33.Shirey K.A., Pletneva L.M., Puche A.C., et al. Control of RSV-induced lung injury by alternatively activated macrophages is IL-4R alpha-, TLR4-, and IFN-beta-dependent. Mucosal Immunol. 2010;3(3):291–300. doi: 10.1038/mi.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cobos-Jiménez V. HIV-1 infection in polarized primary macrophages. Retrovirology. 2011;8:1–1. [Google Scholar]
- 35.Cassol E., Cassetta L., Rizzi C., Gabuzda D., Alfano M., Poli G. Dendritic cell-specific ICAM-3 grabbing nonintegrin mediates HIV-1 infection of and transmission by M2a-polarized macrophages in vitro. AIDS. 2013;27(5):707–716. doi: 10.1097/QAD.0b013e32835cfc82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfano M., Graziano F., Genovese L., Poli G. Macrophage polarization at the crossroad between HIV-1 infection and cancer development. Arterioscler. Thromb. Vasc. Biol. 2013;33(6):1145–1152. doi: 10.1161/ATVBAHA.112.300171. [DOI] [PubMed] [Google Scholar]
- 37.Schlaepfer E. Mary-Aude Rochat, Li Duo, Roberto F. Speck. Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J. Virol. 2014;88(17):9769–9781. doi: 10.1128/JVI.01053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbein G., Gras G., Khan K.A., Abbas W. Macrophage signaling in HIV-1 infection. Retrovirology. 2010;7:34. doi: 10.1186/1742-4690-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbein G., Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues V., Ruffin N., San-Roman M., Benaroch P. Myeloid Cell Interaction with HIV: A Complex Relationship. Front. Immunol. 2017;8:1698. doi: 10.3389/fimmu.2017.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zalar A., Figueroa M.I., Ruibal-Ares B., et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antiviral Res. 2010;87(2):269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Kumar A., Abbas W., Herbein G. HIV-1 latency in monocytes/macrophages. Viruses. 2014;6(4):1837–1860. doi: 10.3390/v6041837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arainga M., Edagwa B., Mosley R.L., Poluektova L.Y., Gorantla S., Gendelman H.E. A mature macrophage is a principal HIV-1 cellular reservoir in humanized mice after treatment with long acting antiretroviral therapy. Retrovirology. 2017;14(1):17. doi: 10.1186/s12977-017-0344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tacke F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017;66(6):1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 45.van der Valk F.M., van Wijk D.F., Lobatto M.E., et al. Prednisolone-containing liposomes accumulate in human atherosclerotic macrophages upon intravenous administration. Nanomedicine (Lond.) 2015;11(5):1039–1046. doi: 10.1016/j.nano.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mu H., Tang J., Liu Q., Sun C., Wang T., Duan J. Potent antibacterial nanoparticles against biofilm and intracellular bacteria. Sci. Rep. 2016;6:18877. doi: 10.1038/srep18877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su F.Y., Srinivasan S., Lee B., et al. Macrophage-targeted drugamers with enzyme-cleavable linkers deliver high intracellular drug dosing and sustained drug pharmacokinetics against alveolar pulmonary infections. J. Control. Release. 2018;287:1–11. doi: 10.1016/j.jconrel.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He H., Buckley M., Britton B., et al. Polarized macrophage subsets differentially express the drug efflux transporters MRP1 and BCRP, resulting in altered HIV production. Antivir. Chem. Chemother. 2018;26:1–7. doi: 10.1177/2040206617745168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cory T.J., He H., Winchester L.C., Kumar S., Fletcher C.V. Alterations in P-Glycoprotein expression and function between macrophage subsets. Pharm. Res. 2016;33(11):2713–2721. doi: 10.1007/s11095-016-1998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leslie E.M., Deeley R.G., Cole S.P. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204(3):216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Rothenberger M.K., Keele B.F., Wietgrefe S.W., et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc. Natl. Acad. Sci. USA. 2015;112(10):E1126–E1134. doi: 10.1073/pnas.1414926112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kline C., Ndjomou J., Franks T., et al. Persistence of viral reservoirs in multiple tissues after antiretroviral therapy suppression in a macaque RT-SHIV model. PLoS One. 2013;8(12):e84275. doi: 10.1371/journal.pone.0084275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kauffman R.C., Villalobos A., Bowen J.H., Adamson L., Schinazi R.F. Residual viremia in an RT-SHIV rhesus macaque HAART model marked by the presence of a predominant plasma clone and a lack of viral evolution. PLoS One. 2014;9(2):e88258. doi: 10.1371/journal.pone.0088258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chun T.W., Moir S., Fauci A.S. HIV reservoirs as obstacles and opportunities for an HIV cure. Nat. Immunol. 2015;16(6):584–589. doi: 10.1038/ni.3152. [DOI] [PubMed] [Google Scholar]
- 55.Kis O., Sankaran-Walters S., Hoque M.T., Walmsley S.L., Dandekar S., Bendayan R. HIV-1 Alters intestinal expression of drug transporters and metabolic enzymes: implications for antiretroviral drug disposition. Antimicrob. Agents Chemother. 2016;60(5):2771–2781. doi: 10.1128/AAC.02278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kulkarni R., Rampersaud R., Aguilar J.L., Randis T.M., Kreindler J.L., Ratner A.J. Cigarette smoke inhibits airway epithelial cell innate immune responses to bacteria. Infect. Immun. 2010;78(5):2146–2152. doi: 10.1128/IAI.01410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cory T.J., Birket S.E., Murphy B.S., Mattingly C., Breslow-Deckman J.M., Feola D.J. Azithromycin increases in vitro fibronectin production through interactions between macrophages and fibroblasts stimulated with Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2013;68(4):840–851. doi: 10.1093/jac/dks476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murphy B.S., Sundareshan V., Cory T.J., Hayes D., Jr, Anstead M.I., Feola D.J. Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 2008;61(3):554–560. doi: 10.1093/jac/dkn007. [DOI] [PubMed] [Google Scholar]
- 59.Lee C.G., et al. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37(11):3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 60.Gantt S., Casper C., Ambinder R.F. Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention. Curr. Opin. Oncol. 2013;25(5):495–502. doi: 10.1097/CCO.0b013e328363dfee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward K.W., Azzarano L.M. Preclinical pharmacokinetic properties of the P-glycoprotein inhibitor GF120918A (HCl salt of GF120918, 9,10-dihydro-5-methoxy-9-oxo-N-[4-[2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquino linyl)ethyl]phenyl]-4-acridine-carboxamide) in the mouse, rat, dog, and monkey. J. Pharmacol. Exp. Ther. 2004;310(2):703–709. doi: 10.1124/jpet.104.068288. [DOI] [PubMed] [Google Scholar]
- 62.Chen L., Jarujaron S., Wu X., et al. HIV protease inhibitor lopinavir-induced TNF-alpha and IL-6 expression is coupled to the unfolded protein response and ERK signaling pathways in macrophages. Biochem. Pharmacol. 2009;78(1):70–77. doi: 10.1016/j.bcp.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 64.Singh S.P., Gundavarapu S., Peña-Philippides J.C., et al. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J. Immunol. 2011;187(9):4542–4552. doi: 10.4049/jimmunol.1101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benoit M., Desnues B., Mege J.L. Macrophage polarization in bacterial infections. J. Immunol. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 67.Cassol E., Cassetta L., Rizzi C., Alfano M., Poli G. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 2009;182(10):6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 68.Lugo-Villarino G., Vérollet C., Maridonneau-Parini I., Neyrolles O. Macrophage polarization: convergence point targeted by mycobacterium tuberculosis and HIV. Front. Immunol. 2011;2:43. doi: 10.3389/fimmu.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labonte A.C., Tosello-Trampont A.C., Hahn Y.S. The role of macrophage polarization in infectious and inflammatory diseases. Mol. Cells. 2014;37(4):275–285. doi: 10.14348/molcells.2014.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Muraille E., Leo O., Moser M. TH1/TH2 paradigm extended: macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 2014;5:603. doi: 10.3389/fimmu.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gordon S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 72.Mantovani A., Garlanda C., Locati M. Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler. Thromb. Vasc. Biol. 2009;29(10):1419–1423. doi: 10.1161/ATVBAHA.108.180497. [DOI] [PubMed] [Google Scholar]
- 73.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu X., Chen J., Wang L., Ivashkiv L.B. Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 2007;82(2):237–243. doi: 10.1189/jlb.1206763. [DOI] [PubMed] [Google Scholar]
- 75.Lakehal F., Wendum D., Barbu V., et al. Phase I and phase II drug-metabolizing enzymes are expressed and heterogeneously distributed in the biliary epithelium. Hepatology. 1999;30(6):1498–1506. doi: 10.1002/hep.510300619. [DOI] [PubMed] [Google Scholar]
- 76.Rahal A., Kumar A., Singh V., et al. Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Earla R., Ande A., McArthur C., Kumar A., Kumar S. Enhanced nicotine metabolism in HIV-1-positive smokers compared with HIV-negative smokers: simultaneous determination of nicotine and its four metabolites in their plasma using a simple and sensitive electrospray ionization liquid chromatography-tandem mass spectrometry technique. Drug Metab. Dispos. 2014;42(2):282–293. doi: 10.1124/dmd.113.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Bruin M., Miyake K., Litman T., Robey R., Bates S.E. Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett. 1999;146(2):117–126. doi: 10.1016/s0304-3835(99)00182-2. [DOI] [PubMed] [Google Scholar]
- 79.Janneh O., Jones E., Chandler B., Owen A., Khoo S.H. Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J. Antimicrob. Chemother. 2007;60(5):987–993. doi: 10.1093/jac/dkm353. [DOI] [PubMed] [Google Scholar]
- 80.Kis O., Robillard K., Chan G.N., Bendayan R. The complexities of antiretroviral drug-drug interactions: role of ABC and SLC transporters. Trends Pharmacol. Sci. 2010;31(1):22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]