Abstract
The peculiar ability of skeletal muscle tissue to operate adaptive changes during post-natal de-velopment and adulthood has been associated with the existence of adult somatic stem cells. Satellite cells, occupying an exclusive niche within the adult muscle tissue, are considered bona fide stem cells with both stem-like properties and myogenic activities. Indeed, satellite cells retain the capability to both maintain the quiescence in uninjured muscles and to be promptly activated in response to growth or re-generative signals, re-engaging the cell cycle. Activated cells can undergo myogenic differentiation or self-renewal moving back to the quiescent state. Satellite cells behavior and their fate decision are finely controlled by mechanisms involving both cell-autonomous and external stimuli. Alterations in these regu-latory networks profoundly affect muscle homeostasis and the dynamic response to tissue damage, con-tributing to the decline of skeletal muscle that occurs under physio-pathologic conditions. Although the clear myogenic activity of satellite cells has been described and their pivotal role in muscle growth and regeneration has been reported, a comprehensive picture of inter-related mechanisms guiding muscle stem cell activity has still to be defined. Here, we reviewed the main regulatory networks determining satellite cell behavior. In particular, we focused on genetic and epigenetic mechanisms underlining satel-lite cell maintenance and commitment. Besides intrinsic regulations, we reported current evidences about the influence of environmental stimuli, derived from other cell populations within muscle tissue, on satel-lite cell biology.
Keywords: Skeletal muscle, Satellite cells, Regeneration, Muscle growth, Quiescence, Activation, Myogenic differentiation, Tissue niche
1. INTRODUCTION
Muscle development is a well-coordinated process in which the segmental derivatives of the paraxial mesoderm, namely the somites, give rise to the dermomyotome from which progenitor cells undergo differentiation to form multinucleated myofibers [1-3].
The existence of multipotent cells was thought to be restricted to tissue development; however, a suite of somatic stem cells has been recognized in early post-natal and mature skeletal muscle, significantly contributing to muscle growth, homeostatic maintenance, and regeneration [4].
Indeed, although myofibers are terminally differentiated, adult muscles retain the capability to increase in size upon hypertrophic stimuli and to efficiently regenerate after injury. This exceptional plasticity has been generally associated with the presence of adult stem cells, namely Satellite Cells (SCs), within mature cell populations. Satellite cells, surrounding myofibers, are considered the primary stem cells in adult skeletal muscle playing a central role in tissue growth and regeneration [5-7]. These undifferentiated cells are maintained under quiescent conditions in healthy muscles, being able to repristinate their proliferative activity upon proper stimulation. Satellite cells that are induced to leave the quiescence re-enter the cell cycle, undergoing multiple divisions. Retaining stem cell properties, these cells can engage both symmetric and asymmetric divisions, guaranteeing the maintenance of the stem cell pool and the production of committed cells, which contribute to muscle plasticity [5, 7]. Starting from their identification, satellite cell functions have been extensively studied; however, the contribution of these muscle stem cells to muscle growth and the exact mechanism regulating their behavior during regeneration still lacks a comprehensive picture.
Here, we reported an integrated overview of genetic and epigenetic mechanisms underlining satellite cell biology, reporting current advances in understanding regulatory networks characterizing every single stage of SCs activity during muscle regeneration. Furthermore, we described the interplay between myogenic and non-myogenic cells in regenerating muscle environment, providing evidences about the impact of the different cell components on the efficacy of tissue healing. In particular, we discuss the impact of both intrinsic and non-cell autonomous mechanisms regulating muscle regeneration with a focus on the alteration of this homeostatic mechanism in aging and disease.
2. SATELLITE CELLS IN MUSCLE PLASTICITY: FROM GROWTH TO REGENERATION
Skeletal muscle stem cell population was firstly identified by Mauro A. [8] and Katz B. [9] in 1961 by electron microscopy approach on frog and rat muscle samples, respectively. The denomination of Satellite Cells derived from the peculiar position within muscle tissue, in close association with single myofibers. As emerged from the first observation by Mauro and confirmed over time by more detailed studies, SCs occupied a protected location between the basal lamina and the sarcolemma, an exclusive niche preserving and regulating stem cell survival and behavior.
Besides the physical definition of the anatomical location, satellite cells have been also characterized by the expression of specific markers such as: paired box transcription factors (Pax3 and Pax7) [10, 11]; Neural cell adhesion molecule (NCAM) [12]; M-cadherin [13]; Forkhead box protein K (FoxK) [14]; tyrosine-protein kinase Met (c-Met) [15]; VCAM-1 [16]; CD34 [17]; Syndecan 3 and 4 [18]; Sox 8 [19]; Sox 15 [20]; Integrin α7 and β1 [21]; caveolin-1 [22]; Calcitonin receptor (CTR) [23]; Lamin A/C, Emerin [24]; Hairy/enhancer-of-split related with YRPW motif proteins Hey1 and Heyl [25]; Dystrophin [26].
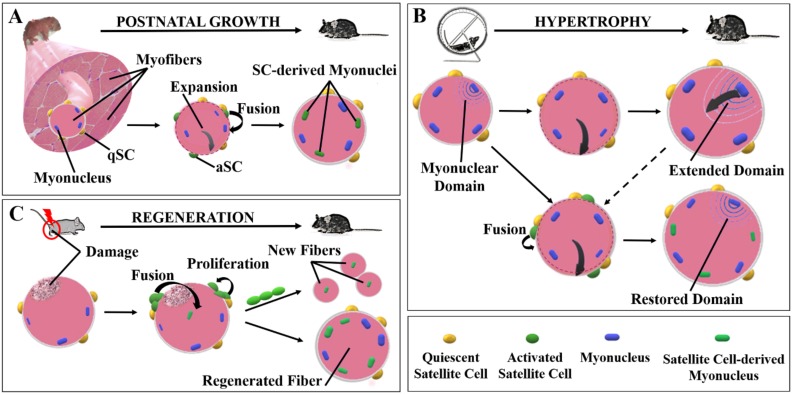
Starting by the observation that cells surrounding single myofibers can proliferate and differentiate in vitro, and taking the advantage of specific markers as Pax7, mounting information about the role of SCs in skeletal muscle have been provided. Satellite cells are thought to be responsible for muscle growth during post-natal life and of tissue regeneration after proper stimuli (Fig. 1). Transgenic mice depleted of SCs displayed a significant impairment in skeletal muscle mass, myofiber size and a general decrease of total body weight, confirming the role of satellite cells in muscle growth during post-natal development [10]. Accordingly, the number of cells, constituting the myogenic stem cell pool, showed a significant decrease during the first months of age in mice, from 30% observed at birth to 5% recognized at two months of age, then remaining constant through adulthood [27]. This is consistent with the implementation of skeletal muscle mass occurring early in post-natal life. Indeed, satellite cells can actively proliferate, probably in response to mechanical stimuli dictated by muscle enlargement and can fuse to existing myofibers, contributing to muscle fibers growth and maturation (Fig. 1A).
Muscle growth is not restricted to the first stages of life; myofibers can also enhance their diameter and length during the adult period. Although the increased myofiber size could derive from enhanced protein synthesis and sarcomeric adjunction, several evidences support the involvement of satellite cells in muscle hypertrophy [28]. In fact, the expansion of the cytosolic portion of muscle fibers in response to sport training has been related to the enhanced number of nuclei through the “myonuclear domain hypothesis” [29]. Each nucleus in a muscle fiber seems to retain the genomic control of a certain area of cytoplasm. During muscle hypertrophy, when the activation of anabolic pathways leads to the enhanced production of structural proteins, myofibers undergo physical expansion and the addition of new nuclei are thought to be necessary to govern an improved cytosolic domain, allowing fiber growth (Fig. 1B). Discording with the theory of myonuclear domain, a recent study using a Cre-lox approach to conditionally ablate satellite cells in mice, revealed that Pax7 positive cells are not necessary, at least at short term, to induce a significative increase of muscle mass during overload-related hypertrophy (Fig. 1B) [30]. However, long term depletion of SCs have been related to a reduced muscle hypertrophy, suggesting that myofiber plasticity can support the growing process by extending the cytosolic domain of each myonucleus in overloaded fibers, whereas this compensatory mechanism seems to be not able to efficiently support muscle growth under the conditions of long-term SC absence [30, 31]. Moreover, it has been reported, in murine models, that the requirement of satellite cell-derived myonuclei for a substantial fiber growth during muscle overload depends on the age of animals. Satellite cells are necessary to guarantee the gain of muscle mass in young-growing mice, whereas adult-mature SCnull mice, depleted of SCs, underwent muscle hypertrophy as well as SCpos. mice [32]. Of note, aged mice failed to activate a hypertrophic response to mechanical overload independently on satellite cells [33]. The impaired growth of myofibers observed in aged mice could be related to a general reduction of Pax7 positive cells in muscles of old mice compared to young, and/or to altered biochemical and mechanical signals in aged tissue microenvironment, inducing satellite cell dysfunction and impairing their ability to respond to physiologic stimuli [34–36].
If the involvement of satellite cells in muscle growth during adulthood is still debated, their pivotal role in muscle regeneration has been extensively described. Satellite cells are maintained in their quiescence state thanks to the exclusive niche in which they reside. Nevertheless, SCs are able to rapidly respond to external stimuli, such as mechanical signals, injury, or homeostatic factors, leaving the quiescence and re-entering the cell cycle [37]. Then SCs can either fuse with pre-existing fibers, or alternatively fuse each other to regenerate the injured muscle or to contribute to muscle growth (Fig. 1C) [38]. Of note, using a genetic approach to label and ablate Pax7 gene, it has been demonstrated that lack of SCs totally abolishes muscle regeneration [39].
3. THE BEHAVIOR OF SATELLITE CELLS IS REGULATED BY GENETIC AND EPIGENETIC MECHANISMS
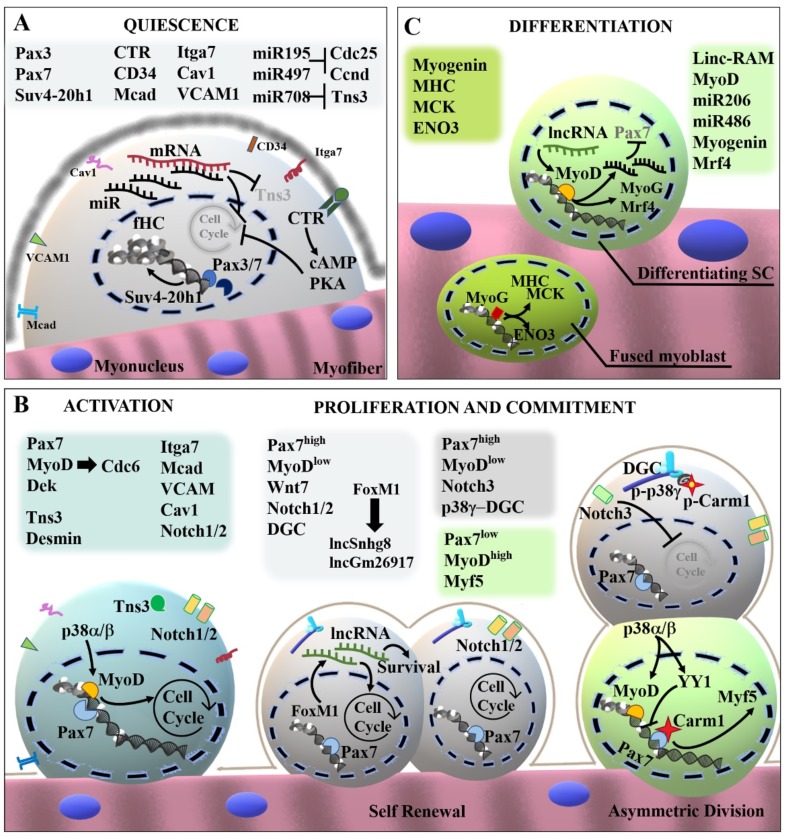
Satellite cells, in intimate association with intact and mature myofibers, are known to persist in a quiescent state with a low transcriptional activity evidenced by the presence of more heterochromatin compared to post-mitotic myonuclei. It has been shown that oval-shaped nuclei of muscle stem cells presented facultative heterochromatin (fHC) [40]. Unlike constitutive heterochromatin, fHC can be dynamically converted in euchromatin, reactivating the expression of silenced genes and this mechanism seems to be important for the regulation of satellite cell functions. It has been demonstrated that the activity of the epigenetic enzyme Suv4-20h1, an H4K20 dimethyltransferase able to induce the condensation of chromatin, was related to the maintenance of SCs quiescence (Fig. 2A) [40]. Interestingly, the absence of Suv4-20h1 results in loss of fHC by muscle stem cells, which undergo massive activation and proliferation, leading to the exhaustion of the myogenic stem cell pool and altered regeneration. This represents a pathologic feature of chronic and degenerative diseases such as muscular dystrophies. Thus, understanding the molecular mechanisms regulating stem cell quiescence might represent a critical issue to design therapeutic approaches. A genome-wide analysis, comparing the gene expression profile of quiescent and proliferating satellite cells, identified a suite of genes up-regulated in quiescent satellite cells (qSCs) which included negative regulators of proliferation and myogenesis and genes involved in cell adhesion, extracellular matrix formation, copper and iron homeostasis, and lipid transportation [23]. Moreover, it has been reported that only quiescent satellite cells expressed the calcitonin receptor (CTR) which is thought to be involved in the preservation of the muscle stem cell pool. In fact, the stimulation with calcitonin induced a significant impairment of satellite cell activation in vitro, by inhibiting the ability of qSCs to re-enter the cell cycle (Fig. 2A) [23]. Accordingly, studies performed using a CTR conditional knock-out mouse model confirmed that the calcitonin signaling, via cAMP/PKA axis, plays a determinant role in preserving the adult stem cell pool in skeletal muscle tissue [41]. The molecular profile associated with quiescent SCs has been defined in a recent elegant work [42]. This profile was used to select factors as Elcatonin, Forskolin and Somatostatin, able to maintain the dormant potent state of SCs in vitro, mimicking signals from their native environment [42].
Satellite cell behavior is also known to be under active epigenetic control exerted by non-coding RNAs-dependent regulatory networks. The genetic ablation of Dicer in Pax3pos./ Pax7pos. cells resulted in the activation of adult SCs [43]. Since Dicer is the endonuclease principally involved in the biogenesis of small non-coding RNAs, microRNAs (miRNAs) have been suggested as possible regulatory elements in SCs activity. Among miRNAs enriched in adult SCs, Sato and colleagues identified miR-195 and miR-497 as important mediators regulating SCs quiescence [43]. The regulatory action of these miRNAs on the maintenance of the undifferentiated state of adult SCs was associated with a direct impact on the cell cycle. Indeed, miR-195/497 are able to target the transcript of important cell cycle activators as Cdc25 and Ccnd, thus inhibiting SCs proliferation (Fig. 2A). Moreover, the inhibition of miR-195/497 in adult SCs resulted in the progression of cell cycle and in the reduced expression of Pax7, whereas the overexpression of these post-transcriptional regulators induced a quiescent stem cell profile characterized by withdrawal from cell cycle, expression of Pax7 and the down-modulation of MyoD [43]. In addition, miR489 has been identified as a positive modulator of adult SCs quiescence through the post-transcriptional suppression of the oncogene Dek, which is involved in the proliferative expansion of myogenic progenitors [44]. Another microRNA highly expressed in qSCs is miR-708, a short hairpin intron miRNA (mirtron), recently described as a positive modulator of SC quiescence [45, 46]. miR-708 inhibits cell migration by targeting the transcript of Tensin3, a focal-adhesion associated protein. Through this mechanism, miR-708 supports the persistence of SCs in the dormant state and its down-modulation has been associated with SC activation [46].
3.1. Satellite Cell Activation and Self-renewal
A traumatic event in skeletal muscle tissue induces the activation of a multi-step coordinated program, leading to a complete tissue repair under physiologic conditions.
The essential prerequisite for proper muscle regeneration is the activation of the resident SCs pool, which expresses relevant markers of proliferating satellite cells such as desmin, Myogenic factor 5 (Myf-5), Myoblast determination protein (MyoD), and Proliferating cell nuclear antigen (PCNA) [47–49]. Although the mRNAs of myogenic factors as MyoD and Myf5 have been already detected in quiescent SCs, the transcriptional activity of these and other mediators is required for the progression from the dormant to the activated state [50, 51]. It has been reported that MyoD is able to regulate the expression of Cdc6 early after activation, driving SCs to re-engage the cell cycle (Fig. 2B) [52]. Indeed, upon proper stimulation satellite cells undergo active proliferation through asymmetric divisions, giving rise to both committed and stem cell populations. Depending on MyoD activity, activated satellite cells (aSCs) can follow one of two fates: they may down-regulate MyoD and self-renew, guaranteeing the maintenance of a pool of quiescent Pax7pos. satellite cells; alternatively, satellite cells maintain MyoD expression but down-regulate Pax7 and activate myogenin expression, thus committing to differentiation (Fig. 2B) [53]. Recent findings highlighted a role for the dystrophin-associated glycoprotein complex (DGC) as a structural regulator of the stem cell fate [54]. The expression of DGC is required for the establishment of a correct SCs polarity during divisions, guaranteeing an efficient generation of committed myoblasts and daughter cells exerting stem cell properties [26]. This macromolecular complex and in particular β1-syntrophin is involved in the recruitment of p38γ mitogen-activated protein kinase (MAPK) which is, in turn, able to phosphorylate the Histone-arginine methyltransferase Carm1 and prevent its nuclear translocation in the stem cell daughter during asymmetric division. Indeed, nuclear Carm1 induced the transcription of Myf5, promoting the myogenic commitment [54]. Other members of the p38 MAP kinases family, which are considered precocious markers of activated satellite cells, play an opposite role in the asymmetric division. p38α is responsible for the progressive repression of Pax7 expression during SCs proliferation, by interacting with important transcriptional repressors as YY1 and Polycomb Repressive Complex 2 (PRC2) [55–57]. In addition, the activation of p38 α/β MAPK signaling plays an important role in the promotion of the early differentiative program, since it has been associated to the induction of the transcriptional activity of MyoD (Fig. 2B). Since the regulatory actions of MyoD are known to be context-dependent, it promotes, in proliferating SCs, the expression of muscle-specific miRNAs as miR206 and miR133, inducing the withdrawal from the cell cycle and thus driving myogenic differentiation (Fig. 2C) [58, 59].
The maintenance of the muscle stem cell pool during adulthood is guaranteed by the homeostatic mechanism of self-renewal. Indeed, aSCs are able to generate not only differentiating myoblasts but also a progeny of cells able to repristinate the quiescent state. During asymmetric divisions, daughter cells in which the p38 pathway is not active are not subject to the regulation of MyoD, evading the differentiative fate. A crucial regulator of SCs self-renewal and quiescence is the Notch signaling since the interference with this pathway led to a significant loss of the quiescent cell pool due to a massive differentiation [60]. It has been recently reported that Notch1 and Notch2 expression in activated satellite cells is essential for the maintenance of the stem cell pool, being required in self-renewal [61]. Notch1/2 inactivation in transgenic mice led to an imbalance between proliferating SCs and committed myoblasts, impairing muscle regeneration after injury [61]. On the contrary, Notch3 has been reported as a negative regulator of proliferation since its genetic ablation significantly increased SCs proliferation in Notch3null mice [61]. Another molecular regulator of SCs survival and proliferation is the Forkhead box protein M1 (FoxM1), a transcription factor involved in the regulation of the expression of long noncoding RNAs (lncRNAs) and factors associated with cell cycle progression and apoptosis. In particular, the FoxM1-dependent induction of lncRNA Snhg8 has been reported to mediate the proliferative action of FoxM1 in satellite cells, whereas the FoxM1/lncGm26917 axis is thought to promote SCs survival (Fig. 2B) [62]. Although mounting studies highlighted even more mechanisms regulating the balance between the progression of the myogenic program and the re-establishment of quiescence, the exact mechanisms underlining stem cell self-renewal have to be fully elucidated.
3.2. Myogenic Differentiation of Activated Satellite Cells
The transition from satellite cells proliferation to differentiation involves the down-regulation of proliferative-associated genes and the activation of specific differentiating markers, such as myogenin, neonatal isoform of myosin heavy chain (MHC), slow-twitch skeletal muscle troponin T (Tnnt1), cardiac and slow-twitch skeletal muscle Ca2+-ATPase (Atp2a2), Insulin-like growth factor-2 (Igf-2), fibroblast growth factor receptor 4 (Fgfr4), nicotinic cholinergic receptor alpha polypeptide 1 (Chrna1), and cardiac/slow-twitch skeletal muscle troponin C (Tncc) [63, 64]. The permanent exit from the cell cycle is then ratified by the expression of p21 protein [65]. The expression of MyoD is not restricted to activated/proliferating SCs since it has been recognized as a master regulator of the myogenic program, modulating a suite of genetic and epigenetic mediators involved in myoblast differentiation (Fig. 2B-C). For instance, it has been described that MyoD induced the expression of miRNAs promoting myoblast differentiation, as miR 206 and miR486 (Fig. 2C). When proliferating myoblasts engage the differentiative program, miR 206/486 are involved in the downmodulation of Pax7 by directly targeting the 3' untranslated region (UTR) of its gene sequence [66]. Although MyoD exerts the genetic control of myogenic factors, even its function is tightly regulated. The transcriptional activity of MyoD is known to be dependent on the association with chromatin modifiers as Baf60c, connoting the epigenetic control of its function. In this context the importance of the muscle-specific long non-coding RNA Linc-RAM it has been recently highlighted. Yu and colleagues reported that Linc-RAM can function as a transcriptional enhancer of
MyoD, by promoting the formation of the MyoD-Baf60c-Brg1 complex, responsible for the expression of genes strictly required for myogenic differentiation [33]. Supporting this mechanistic model, Linc-RAM knock-out mice showed important defects in muscle regeneration due to an impaired muscle differentiation. Moreover, ChIP analysis in C2C12 myogenic cell line revealed that MyoD was significantly enriched in the promoter region of myogenin gene when Linc-RAM was over-expressed, indicating that this lncRNA can promote muscle differentiation [67]. In fact, the terminal differentiation of committed myoblasts requires the expression of both MyoD and myogenin, acting in a tightly regulated mechanism determining the expression of the myogenic regulatory factor 4 (Mrf 4), along with other markers of the terminally differentiated state [68].
Terminally differentiated myoblasts can fuse each other or to damaged myofibers to became part of a multinucleated muscle fiber. The differentiation program is then completed by the expression of protein associated with the mature muscle phenotype such as myosin heavy chain (MHC), enolase 3 (ENO3) and the muscle creatine kinase (MCK) (Fig. 2C).
Pax3/7: Paired box transcription factor 3/7; Suv4-20h1: Histone-lysine N-methyltransferase; Tns3: Tensin3; CTR: Calcitonin receptor; Mcad: M-cadherin; Itga7: Integrin α7; Cav1: Caveolin 1; VCAM: Vascular cell adhesion molecule; miR: microRNA; Cdc25/6: Cell Division Cycle 25/6; Ccnd: G1/S-specific cyclin-D1; cAMP: Cyclic adenosine monophosphate ; PKA: cAMP-dependent protein kinase catalytic subunit alpha; MyoD: Myoblast determination protein; DGC: dystrophin-associated glycoprotein complex; FoxM1: Forkhead box protein M1; lnc: long non-coding RNA; Myf5: Myogenic factor 5; YY1: Transcriptional repressor protein YY1; Mrf4: Myogenic regulatory factor 4; MHC: Myosin heavy chain; MCK: muscle creatine kinase; ENO3: Beta-enaolase.
4. CELLULAR AND MOLECULAR INTERACTORS IN MUSCLE STEM CELL NICHE
Although satellite cells represent the main actors in muscle plasticity, the complex mechanism of muscle regeneration cannot be considered a “one cell show”. Efficient healing of skeletal muscle occurs in five interrelated phases: i) muscle degeneration; ii) activation of the inflammatory response; iii) regeneration; iv) tissue remodeling and angiogenesis; v) muscle maturation and recovery of function. Each stage involves the active participation of different cell populations that play a critical role in regulating and supporting SCs activity, contributing to the establishment of a qualitative environment permissive for proper regeneration. Among cellular interactors in muscle regeneration immune cells, myogenic and non-myogenic progenitors, endothelial cells and fibroblasts create an intricated and tightly regulated network of mechanical and molecular signals within the stem cell niche. The concept of stem cell niche has been extended over time, by the definition of the anatomical compartment to the cohesive influence of a wealth of stimuli deriving from the environment surrounding SCs. Indeed, although residing in a privileged location, satellite cells convey the influence of other cell types within the muscle tissue which are responsible for the production of physical and molecular signals regulating their behavior. Supporting the concept of muscle regeneration as an intricate network of cellular and molecular mediators, a series of studies reported that the depletion of specific cell populations in skeletal muscle environment can profoundly affect the ability of SCs to repair damaged tissue. In fact, during muscle regeneration, the activation and differentiation of SCs are strictly accompanied by related homeostatic responses favoring and supporting the myogenic program.
The activation of an acute inflammation represents the first physiologic response to tissue damage, which is the trigger stimulus for muscle regeneration. Resident immune cells, as mast cells and neutrophils, can function as sentries at the site of the lesion being rapidly activated by necrotic cell-derived molecules. This first response is then reinforced by a subsequent wave of pro-inflammatory mediators released by activated neutrophils and degranulating mast cells. Among secreted factors, histamine is responsible for the enhanced vascular permeability facilitating immune cell infiltration, whereas Tumor necrosis factor α (TNFα), Interferon γ (IFNγ) and Interleukin-1β (IL-1β) contribute to the recruitment of peripheral myeloid cells. This pro-inflammatory milieu will summon a great number of neutrophils early after damage (2-24h), which are, in turn, responsible for the first phase of cell debris removal. The early action of neutrophils in triggering inflammation is required for proper muscle regeneration since neutropenia, experimentally induced in mice, impaired tissue regeneration after injury [69].
In addition, neutrophil-derived mediators as the soluble receptor of Interleukin-6 (sIL6R), are involved in the modulation of inflammation. Indeed, a different subtype of macrophages can exert various functions during tissue healing. M1 polarized cells present phagocytic activity and produce several Matrix Metalloproteinases (MMPs), contributing to the clearance of the necrotic area, thus promoting the establishment of a pro-regenerative environment [70]. Macrophages contribute to muscle regeneration also through the secretion of soluble mediators as TNFα and IL-6, which are necessary to promote myoblast proliferation and differentiation [71–73]. Besides the classical M1 activation, recruited monocytes can be alternatively activated acquiring the M2 anti-inflammatory phenotype. M2 cells can be divided into different subtypes which are induced by varied stimuli and exert immunomodulatory effects. M2a and M2b phenotype are known to be induced by IL-4/-13 and IL-1R agonists respectively, whereas M2c cells are induced by IL-10, a critical anti-inflammatory cytokine promoting the transition from classically to alternatively activated macrophages [74]. M2 macrophages, lacking the phagocytic activity, are involved in the resolution of the inflammatory response, the promotion of myoblast proliferation and in the differentiation of myotubes, contributing to the progression of the myogenic program in regenerating muscles [75, 76]. To support the pivotal role of macrophages in muscle regeneration, Liu and colleagues studied the impact of macrophagic depletion in a contusion model of muscle injury [77]. Mice treated with clodronate-containing liposomes, inducing apoptosis specifically in macrophages, showed a marked impairment in muscle regeneration with an accumulation of necrotic fibers and inflammatory cells at the site of the lesion 7 days after injury, when muscles from control animals mostly presented centronucleated fibers [77]. In addition, the damaged muscles depleted of macrophages presented a reduced expression of factors positively regulating tissue regeneration as a hepatocyte growth factor (HGF) and Insulin-like growth factor-1 (IGF-1). Satellite cells are responsive to IGF-1, a trophic factor implicated in many anabolic pathways in skeletal muscle [35]. IGF-1 is able to stimulate satellite cell proliferation by promoting the activation of the Akt/FoxO1 pathway, whilst in post-mitotic myoblasts can induce myogenic differentiation by modulating regulatory networks [78, 79]. Furthermore, IGF-1 has been reported as a modulator of the inflammatory response in injured muscles, playing a central role during regeneration [80]. Interfering with the macrophagic production of IGF-1, Tonkin and colleagues reported an imbalance between M1 and M2 populations in regenerating muscles, leading to the impaired tissue repair. Another soluble mediator released by activated macrophages and influencing satellite cell behavior is IL-6 [81, 82]. Transiently increased levels of IL-6 are physiologically required to induce satellite cell proliferation under regenerative conditions, and this homeostatic mechanism was found deregulated in transgenic mice lacking the expression of this specific myokine [83].
Both IGF-1 and IL-6, along with Follistatin and Wnt factors, are also produced and released by another peculiar cell population in skeletal muscle environment, namely fibro-adipogenic progenitors (FAPs) [84, 85], which are lineageneg./Sca1pos./CD34pos./α7integrinneg. cells. Satellite cells and Fibro-adipogenic Progenitors (FAPs) present a reciprocal regulation in muscle tissue, and their interactions are critical for an efficient regenerative process. Indeed, FAPs represent a multipotent cell population of non-myogenic progenitors with a pivotal role in supporting myogenic cells during muscle regeneration. In particular, FAPs are involved in the paracrine regulation of the initial stages of muscle healing through the secretion of trophic and pro-myogenic mediators, being rapidly mobilized when muscle damage occurs. In vitro studies reported that FAPs not only promoted SCs proliferation but also influenced the commitment of myoblasts to terminal differentiation [84, 86]. Culturing myogenic progenitors in the presence of FAPs resulted in the downmodulation of early markers of quiescent and activated SCs and in the enhanced expression of myogenic markers as MyoD and myogenin [84]. The influence of FAPs in modulating muscle environment and promoting reparative myogenesis is known to be transient and finely regulated. In fact, the excess of FAPs resulted from a physiologic regeneration is subject to clearance mechanisms mainly mediated by apoptotic stimuli derived from immune cells and satellite cells, whereas proliferating progenitors repristinated the quiescent state [86, 87]. Furthermore, FAPs can differentiate in myofibroblasts which are designated to the production of extracellular matrix components, constituting a proper scaffold for newly generated myofibers further allowing their alignment.
The production and remodeling of Extracellular Matrix (ECM) represent another critical step in muscle regeneration since the ECM components not only serve as physical support for regenerating fibers but also provide mechanical and biochemical signals for SCs, by influencing the retention and activity of secreted mediators in muscle milieu [88–90]. In this context the temporary action of FAPs in inducing regenerative fibrogenesis has been reported to be necessary for muscle regeneration [39, 91]. Accordingly, a negative impact of the genetic or pharmacologic ablation of FAPs on regenerative myogenesis has been described. Nilotinib, a tyrosine kinase inhibitor, has been used as a pharmacologic approach to inhibit FAPs proliferation during muscle regeneration. This treatment induced a significant reduction of the transient ECM deposition after acute damage and marked impairment of SCs proliferation, thus leading to a defective regeneration [91].
A large amount of ECM components, including collagen VI, derived from muscle connective tissue (MCT) fibroblasts which can be recognized by the expression of Tcf4. In particular, collagen VI has been involved in the prevention of myogenic commitment and in the promotion of self-renewal in activated satellite cells [92]. MCT fibroblasts have been detected in close association with satellite cells in vivo and are thought to influence their activity. In fact, SCs in murine muscles depleted of Tcf4pos. fibroblasts showed an impaired ability to proliferate upon traumatic stimuli, undergoing a premature differentiation [39].
Other important players in muscle regeneration are endothelial cells (ECs) of blood vessels since angiogenesis contributes to muscle plasticity and changes in vascular system are observed during regenerative processes [93, 94]. In vivo and in vitro studies revealed that ECs exert a pro-myogenic activity on muscle progenitor cells (MPCs) by stimulating their migration, proliferation and terminal differentiation [94]. The reciprocal stimulation between ECs and MPCs has been associated to the secretion of molecular mediators as Apelin (APLN) that promotes myogenesis/angiogenesis, Oncostatin M (OSM) which exerts both stimulatory and inhibitory actions on angiogenesis and myogenesis, and Periostin (POSTN) stimulating angiogenesis and later stages of myogenic differentiation. Indeed, inhibitors of APLN, OSM and POSTN impaired both angiogenesis and myogenesis in vitro and muscle regeneration in vivo [94]. The effective restoration of functional muscle tissue after the injury occurs when regenerated myofibers are terminally differentiated and when the vascular bed and functional connections with nerves have been restored. The restoration of neuro-muscular junctions occurs within few weeks after injury and represents the final stage of muscle regeneration/maturation. This process must be also finely regulated and occurs only when the regenerating myofibers completed the differentiative program. Satellite cells also play a critical role in this process since they have been identified as a source of semaphorin 3A (Sema3A). This neural chemorepellent factor avoids a premature motor neuron reattachment inhibiting the establishment of a synaptic contact on damaged or not completely mature myofibers [95]. On the other hand, Sema3A promotes the myogenic differentiation of satellite cells [95–97], suggesting an SC-mediated control of tissue repair exerted by preventing an improper innervation and thus regulating the spatiotemporal progression of muscle regeneration.
4.1. Other Myogenic Populations Contributing to Muscle Regeneration
Satellite cells are considered the primary players of regenerative myogenesis. However, it has been proposed that other non-muscle stem cells and progenitors, including endothelial-associated cells [98], interstitial cells [99-101], vessel-associated stem cells [102, 103], and bone marrow-derived stem cells [104, 105] can contribute to muscle regeneration, supporting the activity of satellite cells.
In particular, a specific subpopulation of circulating hematopoietic/endothelial progenitors has been identified in human blood. These stem cells, expressing AC133 antigen, have been shown the capability to differentiate into myoblasts in vitro and to occupy the satellite cell niche in vivo [106]. In another work, a skeletal muscle side population (SP), has been recognized in muscle tissue and identified as CD45neg./c-Kit neg./Sca-1 pos./Abcg2 pos. cells [104, 107-110]. Skeletal muscle SP cells, exerting myogenic functions in muscle tissue, mostly originate from somites and can be divided into interstitial SP cells and satellite-SP cells, according to their specific anatomic location [110]. It has been observed that SP cells residing in muscular interstitium can contribute to muscle repair after injury, although they lack spontaneous myogenic activity in vitro [107]. In contrast, satellite-SP cells have been indicated as possible progenitors of satellite cells residing in close association with mature myofibers and maintaining their location even after single fiber isolation [110]. Other multipotent progenitors involved in adult myogenesis are vessel-associated stem cells with the mesodermal origin and elevated myogenic activity. In particular mesoangioblasts (MABs), originally isolated from the embryonic dorsal aorta, have been recognized in postnatal skeletal muscle and heart as multipotent progenitors associated with small vessels [102, 103]. These progenitor/stem cells are known to express endothelial-vascular and pericytes markers and have been shown to share a common signature with FAPs [103, 111, 112]. However, MABs exhibited a clear myogenic activity both in vitro and in vivo, differentiating into skeletal and smooth muscle [102, 113, 114]. In addition, aorta-derived stem cells have shown to exert a therapeutic potential in preclinical and clinical studies of muscle pathologies [113-115]. A class of progenitors correlated with MABs are pericytes, which are thought to derive from prenatal mesoangioblasts. It has been suggested that pericyte cells represent the interstitial progeny of prenatal MABs which can leave their endothelial location during development, acquiring a pericyte-like phenotype [111]. Pericytes exert myogenic activity in vitro [116, 117] and are known to contribute to postnatal muscle growth and regenerative myogenesis, being also able to join the satellite cell compartment [116-119]. However, pericytes might also represent a heterogeneous population. Birbrair and colleagues identified two subtypes of pericytes in the skeletal muscle interstitium: type-1 pericytes, expressing NG2 marker and negative for Nestin; type-2 cells Nestinpos./NG2pos. [119]. In particular, while type-1 subtype is involved in fatty tissue accumulation and lacks myogenic activity in vitro and in vivo, type-2 cells have been identified as the myogenic subpopulation of pericytes in skeletal muscle [119].
5. INTRINSIC AND EXTRINSIC ALTERATIONS OF STEM CELL BIOLOGY IN AGING AND DISEASES
Skeletal muscle tissue is known to retain elevated adaptive capabilities, being able to dynamically respond to metabolic changes and physical insults. However, it has been extensively described that the ability of muscle tissue to support homeostatic changes and regenerative processes is impaired during aging and diseases [120, 121]. Aged muscles present a decline in the ability to respond to hypertrophic stimuli and to recovery after traumatic events. Since the SCs reservoir is mainly involved in muscle growth and repair, the intrinsic alteration of satellite cells accumulated throughout lifespan has been proposed as a key contributor to muscle weakening during aging. Genome-wide analysis on SCs derived from a range of healthy individuals, from 21 to 78 years of age, revealed that SCs accumulated a number of mutations every year [122]. These mutations occurring during aging seem to affect genes involved in SCs activity. In particular, it has been reported that SCs from human biopsies showed an impaired myogenic differentiation in vitro, which was directly correlated with the average number of carrying mutations, suggesting a novel mechanism contributing to the loss of muscle mass maintenance in elderly [122].
Another proposed factor involved in the intrinsic alteration of SCs behaviour is telomere shortening that occurs at every cell division. Reduced levels in adult cells of telomerase, the enzyme responsible for telomeric DNA adjunction, implicated that subsequent SCs division throughout life could progressively reduce telomeres, until reaching the Hayflik minimum and thus proliferative senescence [123-126]. In spite of what reported in healthy individuals, a significant reduction of satellite cell telomeres has been described in patients affected by Duchenne muscular dystrophy (DMD) [126, 127]. DMD is a genetic disease due to the absence of a functional dystrophin protein and is characterized by extensive muscle degeneration, inflammation and impaired regeneration. The extent of necrosis in dystrophic muscle continuously stimulates regenerative processes which resulted in an inefficient tissue repair and led to the progressive exhaustion of the SCs pool [82, 128]. In this context, several studies ascribed, at least to some extent, the impaired regenerative potential of SCs to premature senescence induced by their excessive proliferation. Indeed, SCs deriving from DMD patients showed a marked reduction of telomeres fragment length compared to healthy subjects. In addition, the progressive shortening of telomeres during the disease course was correlated with the delay of regenerative foci in older DMD patients, suggesting that satellite cell senescence could be involved in the abortive regeneration observed in dystrophic muscles at later stages of the pathology [126, 127].
As previously mentioned, dystrophin protein is involved in the regulation of the stem cell fate during asymmetric division, determining the localization of p38γ/Carm1 complex. Thus, the absence of this structural protein in dystrophic SCs resulted in the alteration of cell polarity reducing the generation of committed myoblasts [54]. On the contrary, in aged muscles where the stem cell pool is known to be progressively reduced, SCs dysfunction has been associated to the impairment of the symmetric divisions generating self-renewal stem cells [129, 130]. Aged SCs lost the tuned balance between commitment and self-renewal, preferentially undergoing asymmetric division [130]. This defect has been described as heightened in geriatric SCs that showed replicative senescence, caused by activation of p16 (INK4a) [131].
While intrinsic alterations in satellite cell biology have been observed in aging and diseases, several studies indicated that impaired muscle regeneration can be related to the deteriorating tissue environment induced by local and systemic mediators. Supporting this hypothesis, aged progenitors repristinate their myogenic activity when exposed to a young systemic environment, during heterochronic experiments performed by muscle transplantation or parabiosis [36, 132–134]. Otherwise, muscles from young donors showed an impaired ability to regenerate when transplanted in old hosts, further evidencing the key role of a permissive environment to support the physiological activity of muscle stem cells. A critical contributor to the altered regenerative capability of aged muscles is the persistent inflammatory status established during aging. The so-called inflamm-aging represents a chronic low-grade inflammation due to the deregulated production of pro-inflammatory mediators in elderly. Among factors increased in serum during aging, IL-6, TNFα and osteopontin (OPN) can play a detrimental role in skeletal muscle homeostasis contributing to SCs dysfunction and impairing muscle regeneration [135-137]. Elevated levels of IL-6 and TNFα negatively affect muscle mass in old individuals by inducing catabolic pathways and inhibiting the production of critical anabolic mediators as IGF-1 [138]. OPN, as well as IL-6, has been described to play positive roles in muscle regeneration when transiently expressed; however, its persistent production can undermine muscle differentiation and regeneration in vitro and in vivo [139-141]. The age-related decline of trophic mediators as IGF-1 in skeletal muscle could be also ascribed to a reduced number of FAPs, which are a source of this anabolic factor. Formicola and colleagues [85] reported that in young limb muscles FAPs and SCs were represented in equal percentages, while in elderly the number of FAPs was markedly reduced probably leading to the loss of pro-myogenic stimuli for SCs. Accordingly, young extraocular muscles, that are spared by aging- and disease-related alterations, presented an elevated percentage of FAPs which are maintained in a 2:1 ratio with SCs also in aged mice [85]. Since FAPs are a source of trophic and pro-myogenic mediators as IGF-1, Follistatin and IL-33, their progressive decline during aging can contribute to the alteration of SC function [85, 142]. Nevertheless, the activity of FAPs must be finely regulated. In an efficient muscle regenerative process, satellite cells produce factors that inhibit FAPs differentiation and activate apoptosis, avoiding FAPs accumulation [143]. In contrast, in several pathologic conditions, such as muscular dystrophy or aging sarcopenia, satellite cells are defective in the production of factors that control FAPs activity, leading to FAPs persistence within muscle tissue, where they can readily differentiate into adipocytes or fibrocytes [144].
Indeed, FAPs are highly responsive to stimuli derived from the niche as highlighted by transplantation experiments evidencing that a healthy muscle environment inhibits FAP adipogenic differentiation whereas a deregulated milieu can induce their conversion into adipocytes [84].
These data indicated that the local tissue environment, which is a source of signals guiding SC activity, can be modulated by non-myogenic progenitors that are itself influenced by environmental stimuli. Thus, FAPs might promote efficient muscle regeneration in a permissive milieu whilst can contribute to fibro-adipogenic events under deregulated conditions, strengthening the concept of tissue niche as a critical determinant of SC behavior and muscle regeneration [35].
The pivotal influence of muscle milieu on regenerative processes has been also elucidated in pathologic contexts as DMD. Our recent works demonstrated that the modulation of specific niche factors in dystrophic mice (mdx) is sufficient to induce an amelioration or exacerbation of the muscle phenotype, markedly influencing regenerative events. In particular, the over-expression of the local isoform of IGF-1 in mdx muscles (mdx/mIGF-1), not only induced significant muscle hypertrophy but also stimulated the maturation of the myogenic program, further supporting the maintenance of the differentiated phenotype [145, 146]. On the other side, inducing the expression of elevated circulating levels of IL-6 in mdx mice (mdx/IL-6) we obtained a significant worsening of dystrophic muscles, generating a more severe mouse model of DMD pathology [128, 147-149]. Although IL-6 is a positive regulator of SCs under physiologic conditions, its over-production dramatically impaired muscle differentiation in vitro and induced a progressive depletion of the SC pool in mdx mice at later stages of the pathology. In mdx/IL-6 muscles we also observed an increased percentage of FAPs which are known to undergo extensive proliferation under pathologic conditions prevailing on satellite cells, losing their pro-myogenic activity thereby promoting fibrotic and fatty tissue deposition [128]. The detrimental impact on muscle regeneration of a sustained pro-inflammatory milieu promoted by IL-6 in DMD has been further demonstrated through the interference with IL-6 signalling. The inhibition of the IL-6 receptor alpha, mainly mediating the pro-inflammatory actions of IL-6, in mdx mice, promoted the homeostatic maintenance of dystrophic muscles, favouring the maturation of the myogenic program [150]. In particular, the modulation of the inflammatory status in mdx muscles was accompanied by the upregulation of myogenic factors involved in myoblast differentiation/fusion as MyoD, myogenin and IL-4, along with molecular markers of terminally differentiated myofibers, including miR24 [150].
CONCLUSION
A proper activity of the muscle stem cell pool is critical for the maintenance of a healthy functional skeletal muscle tissue during adulthood, participating in the growth and regenerative processes. The homeostatic balance between quiescent and activated satellite cells is a tightly regulated mechanism involving the action of genetic and epigenetic controllers, as transcription factors and non-coding RNAs. Intrinsic signals are required to establish the persistence of SCs in a quiescent state or to determine their activation and the subsequent commitment to the myogenic program. However, satellite cell behavior is known to be also influenced by signals deriving from the tissue environment. While satellite cells are central players in skeletal muscle homeostasis and regeneration, the functional interaction with non-myogenic cell populations in muscle niche guarantees the establishment of a permissive environment necessary to support satellite cell activity in achieving functional results. In fact, the deregulation of skeletal muscle environment that occurs under physio-pathologic condition, as aging and muscular dystrophies, significantly affects satellite cell functions contributing to the loss of a functional muscle tissue. Thus, a comprehensive understanding of mechanisms regulating satellite cell behavior can allow important advances in the field of muscle diseases. However, although satellite cells were identified more than 50 years ago and a wealth of knowledges has been accumulated during these years, a clear picture of markers and molecular pathways underlining every single stage of satellite cell activity has still to be provided. Further advances will be necessary to bring into focus the intricate network of signals and fate determinants influencing the dynamics of satellite cell behavior.
Fig. (1).
Satellite cells are involved in muscle growth and regeneration. The adjunction of satellite cell-derived myonuclei occurs during different homeostatic responses underlining muscle plasticity: (A) During post-natal growth early after birth, the physiologic expansion of myofibers results in the activation of quiescent satellite cells, which are able to fuse into growing myofibers contributing to muscle enlargement. (B) In mature myofibers, each myonucleus can govern a specific cytosolic domain. Under hypertrophic conditions, when myofibers undergo physical expansion, the myonuclear domain can be extended allowing myofiber growth without the involvement of myonuclear accretion. However, the enlargement of myofibers can also induce the activation and fusion of satellite cells, leading to the restoration of the original domain. (C) Satellite cells are absolutely necessary to repair injured muscle. During muscle regeneration, activated satellite cells can fuse either to damaged fibers or each other to generate new myofibers driving skeletal muscle healing. qSC: quiescent satellite cell; aSC: activated satellite cell.
Fig. (2).
Molecular markers and mechanisms underlining satellite cell behavior. (A) Quiescent satellite cells are characterized by the presence of facultative heterochromatin and thus by a minimal transcriptional activity, being in a dormant state in steady-state muscles. (B) Upon proper stimulation, satellite cells become activated, express MyoD and re-engage the cell cycle. Activated cells can undergo both symmetric and asymmetric division replenishing the stem cell pool and generating committed myoblasts. Daughter cells expressing elevated levels of Pax7 and low levels of MyoD (Pax7high/MyoDlow) maintain the stem-like phenotype, whilst cells highly inducing MyoD and down-modulating Pax7 (Pax7low/MyoDhigh) enter the differentiative program. (C) Differentiating cells express later molecular mediators of the myogenic program as myogenin and Mrf4. Myoblasts can fuse to pre-existing myofibers and express markers of the terminally differentiated phenotype as Myosin heavy chain, muscle creatine kinase and beta enolase.
Acknowledgements
We apologize to colleagues whose studies were not cited. The work in the authors' laboratory has supported by ASI and Progetto Finalizzata.
LIST OF ABBREVIATIONS
- Abcg2
ATP binding cassette subfamily G member 2
- AC133
CD133 Antigen, also known as prominin-1
- Akt
RAC-Alpha serine/threonine-protein Kinase
- aSC
Activated Satellite Cell
- Baf60c
SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily D member 3
- Brg1
Transcription activator BRG1
- Ccnd
G1/S-specific cyclin-D1
- CD34
Hematopoietic progenitor cell antigen CD34
- CD45
leukocyte common antigen
- Cdc25
Cell Division Cycle 25
- c-Kit
KIT proto-oncogene receptor tyrosine kinase
- Foxo1
Forkhead box protein O1
- NG2
Neural/glial antigen 2
- qSC
Quiescent satellite cell
- Sca1
Stem cell antigen 1
- SCs
Satellite Cells
CONTRIBUTIONS
Antonio Musarò conceptualized the study; Antonio Musarò and Laura Forcina wrote the original draft; Laura Pelosi and Carmen Miano wrote/reviewed and edited the text and figures.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Tajbakhsh S., Cossu G. Establishing myogenic identity during somitogenesis. Curr. Opin. Genet. Dev. 1997;7(5):634–641. doi: 10.1016/s0959-437x(97)80011-1. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D., Relaix F. The formation of skeletal muscle: from somite to limb. J. Anat. 2003;202(1):59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musumeci G., Castrogiovanni P., Coleman R., Szychlinska M.A., Salvatorelli L., Parenti R., Magro G., Imbesi R. Somitogenesis: From somite to skeletal muscle. Acta Histochem. 2015;117(4-5):313–328. doi: 10.1016/j.acthis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Chargè S.B.P., Rudnicki M.A. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84(1):209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 5.Kuang S., Kuroda K., Le Grand F., Rudnicki M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129(5):999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dumont N.A., Bentzinger C.F., Sincennes M-C., Rudnicki M.A. Satellite cells and skeletal muscle regeneration. Compr. Physiol. 2015;5(3):1027–1059. doi: 10.1002/cphy.c140068. [DOI] [PubMed] [Google Scholar]
- 7.Feige P., Brun C.E., Ritso M., Rudnicki M.A. Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell. 2018;23(5):653–664. doi: 10.1016/j.stem.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mauro A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961;9(2):493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz B. The terminations of the afferent nerve fibre in the muscle spindle of the frog. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1961;243(703):221–240. [Google Scholar]
- 10.Seale P., Sabourin L.A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M.A. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102(6):777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 11.Relaix F., Montarras D., Zaffran S., Gayraud-Morel B., Rocancourt D., Tajbakhsh S., Mansouri A., Cumano A., Buckingham M. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 2006;172(1):91–102. doi: 10.1083/jcb.200508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mechtersheimer G., Staudter M., Möller P. Expression of the natural killer cell-associated antigens CD56 and CD57 in human neural and striated muscle cells and in their tumors. Cancer Res. 1991;51(4):1300–1307. [PubMed] [Google Scholar]
- 13.Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev. Dyn. 1994;199(4):326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- 14.Garry D.J., Yang Q., Bassel-Duby R., Williams R.S. Persistent expression of MNF identifies myogenic stem cells in postnatal muscles. Dev. Biol. 1997;188(2):280–294. doi: 10.1006/dbio.1997.8657. [DOI] [PubMed] [Google Scholar]
- 15.Tatsumi R., Anderson J.E., Nevoret C.J., Halevy O., Allen R.E. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev. Biol. 1998;194(1):114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- 16.Jesse T.L., LaChance R., Iademarco M.F., Dean D.C. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J. Cell Biol. 1998;140(5):1265–1276. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauchamp J.R., Heslop L., Yu D.S., Tajbakhsh S., Kelly R.G., Wernig A., Buckingham M.E., Partridge T.A., Zammit P.S. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J. Cell Biol. 2000;151(6):1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelison D.D.W., Filla M.S., Stanley H.M., Rapraeger A.C., Olwin B.B. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev. Biol. 2001;239(1):79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt K., Glaser G., Wernig A., Wegner M., Rosorius O. Sox8 is a specific marker for muscle satellite cells and inhibits myogenesis. J. Biol. Chem. 2003;278(32):29769–29775. doi: 10.1074/jbc.M301539200. [DOI] [PubMed] [Google Scholar]
- 20.Lee H-J., Göring W., Ochs M., Mühlfeld C., Steding G., Paprotta I., Engel W., Adham I.M. Sox15 is required for skeletal muscle regeneration. Mol. Cell. Biol. 2004;24(19):8428–8436. doi: 10.1128/MCB.24.19.8428-8436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherwood R.I., Christensen J.L., Conboy I.M., Conboy M.J., Rando T.A., Weissman I.L., Wagers A.J. Isolation of adult mouse myogenic progenitors. Cell. 2004;119(4):543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Volonte D., Liu Y., Galbiati F. The modulation of caveolin-1 expression controls satellite cell activation during muscle repair. FASEB J. 2005;19(2):237–239. doi: 10.1096/fj.04-2215fje. [DOI] [PubMed] [Google Scholar]
- 23.Fukada S., Uezumi A., Ikemoto M., Masuda S., Segawa M., Tanimura N., Yamamoto H., Miyagoe-Suzuki Y., Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25(10):2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 24.Gnocchi V.F., White R.B., Ono Y., Ellis J.A., Zammit P.S. Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One. 2009;4(4):e5205. doi: 10.1371/journal.pone.0005205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukada S-i., Yamaguchi M., Kokubo H., Ogawa R., Uezumi A., Yoneda T., Matev M.M., Motohashi N., Ito T., Zolkiewska A., Johnson R.L., Saga Y., Miyagoe-Suzuki Y., Tsujikawa K., Takeda S., Yamamoto H. Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development. 2011;138(21):4609–4619. doi: 10.1242/dev.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumont N.A., Wang Y.X., von Maltzahn J., Pasut A., Bentzinger C.F., Brun C.E., Rudnicki M.A. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med. 2015;21(12):1455–1463. doi: 10.1038/nm.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bischoff R., Heintz C. Enhancement of skeletal muscle regeneration. Dev. Dyn. 1994;201(1):41–54. doi: 10.1002/aja.1002010105. [DOI] [PubMed] [Google Scholar]
- 28.Blaauw B., Reggiani C. The role of satellite cells in muscle hypertrophy. J. Muscle Res. Cell Motil. 2014;35(1):3–10. doi: 10.1007/s10974-014-9376-y. [DOI] [PubMed] [Google Scholar]
- 29.Murach K.A., Englund D.A., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Myonuclear domain flexibility challenges rigid assumptions on satellite cell contribution to skeletal muscle fiber hypertrophy. Front. Physiol. 2018;9:635. doi: 10.3389/fphys.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry C.S., Lee J.D., Jackson J.R., Kirby T.J., Stasko S.A., Liu H., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014;28(4):1654–1665. doi: 10.1096/fj.13-239426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCarthy J.J., Mula J., Miyazaki M., Erfani R., Garrison K., Farooqui A.B., Srikuea R., Lawson B.A., Grimes B., Keller C., Van Zant G., Campbell K.S., Esser K.A., Dupont-Versteegden E.E., Peterson C.A. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138(17):3657–3666. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murach K.A., White S.H., Wen Y., Ho A., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Differential requirement for satellite cells during overload-induced muscle hypertrophy in growing versus mature mice. Skelet. Muscle. 2017;7(1):14. doi: 10.1186/s13395-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J.D., Fry C.S., Mula J., Kirby T.J., Jackson J.R., Liu F., Yang L., Dupont-Versteegden E.E., McCarthy J.J., Peterson C.A. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71(4):461–467. doi: 10.1093/gerona/glv033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shefer G., Van de Mark D.P., Richardson J.B., Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev. Biol. 2006;294(1):50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scicchitano B.M., Sica G., Musarò A. Stem Cells and Tissue Niche: Two Faces of the Same Coin of Muscle Regeneration. Eur. J. Transl. Myol. 2016;26(4):6125. doi: 10.4081/ejtm.2016.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barberi L., Scicchitano B.M., De Rossi M., Bigot A., Duguez S., Wielgosik A., Stewart C., McPhee J., Conte M., Narici M., Franceschi C., Mouly V., Butler-Browne G., Musarò A. Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology. 2013;14(3):273–292. doi: 10.1007/s10522-013-9429-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bischoff R. Interaction between satellite cells and skeletal muscle fibers. Development. 1990;109(4):943–952. doi: 10.1242/dev.109.4.943. [DOI] [PubMed] [Google Scholar]
- 38.Xu X., Wilschut K.J., Kouklis G., Tian H., Hesse R., Garland C., Sbitany H., Hansen S., Seth R., Knott P.D., Hoffman W.Y., Pomerantz J.H. Human satellite cell transplantation and regeneration from diverse skeletal muscles. Stem Cell Reports. 2015;5(3):419–434. doi: 10.1016/j.stemcr.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy M.M., Lawson J.A., Mathew S.J., Hutcheson D.A., Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138(17):3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boonsanay V., Zhang T., Georgieva A., Kostin S., Qi H., Yuan X., Zhou Y., Braun T. Regulation of skeletal muscle stem cell quiescence by Suv4-20h1-dependent facultative heterochromatin formation. Cell Stem Cell. 2016;18(2):229–242. doi: 10.1016/j.stem.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi M., Watanabe Y., Ohtani T., Uezumi A., Mikami N., Nakamura M., Sato T., Ikawa M., Hoshino M., Tsuchida K., Miyagoe-Suzuki Y., Tsujikawa K., Takeda S., Yamamoto H., Fukada S. Calcitonin receptor signaling inhibits muscle stem cells from escaping the quiescent state and the niche. Cell Reports. 2015;13(2):302–314. doi: 10.1016/j.celrep.2015.08.083. [DOI] [PubMed] [Google Scholar]
- 42.Quarta M., Brett J.O., DiMarco R., De Morree A., Boutet S.C., Chacon R., Gibbons M.C., Garcia V.A., Su J., Shrager J.B., Heilshorn S., Rando T.A. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol. 2016;34(7):752–759. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato T., Yamamoto T., Sehara-Fujisawa A. miR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat. Commun. 2014;5(1):4597. doi: 10.1038/ncomms5597. [DOI] [PubMed] [Google Scholar]
- 44.Cheung T.H., Quach N.L., Charville G.W., Liu L., Park L., Edalati A., Yoo B., Hoang P., Rando T.A. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature. 2012;482(7386):524–528. doi: 10.1038/nature10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westholm J.O., Lai E.C. Mirtrons: microRNA biogenesis via splicing. Biochimie. 2011;93(11):1897–1904. doi: 10.1016/j.biochi.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baghdadi M.B., Firmino J., Soni K., Evano B., Di Girolamo D., Mourikis P., Castel D., Tajbakhsh S. Notch-Induced miR-708 Antagonizes satellite cell migration and maintains quiescence. Cell Stem Cell. 2018;23:1–10. doi: 10.1016/j.stem.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Scharner J., Zammit P.S. The muscle satellite cell at 50: the formative years. Skelet. Muscle. 2011;1(1):28. doi: 10.1186/2044-5040-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Creuzet S., Lescaudron L., Li Z., Fontaine-Pérus J., Myo D. Myogenin, and Desmin-nls-lacZ Transgene emphasize the distinct patterns of satellite cell activation in growth and regeneration. Exp. Cell Res. 1998;243(2):241–253. doi: 10.1006/excr.1998.4100. [DOI] [PubMed] [Google Scholar]
- 49.Yablonka-Reuveni Z., Rivera A.J. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev. Biol. 1994;164(2):588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Velthoven C.T.J., de Morree A., Egner I.M., Brett J.O., Rando T.A. Transcriptional profiling of quiescent muscle stem cells in vivo. Cell Reports. 2017;21(7):1994–2004. doi: 10.1016/j.celrep.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu X., Wang H., Hu P. Stem cell activation in skeletal muscle regeneration. Cell. Mol. Life Sci. 2015;72(9):1663–1677. doi: 10.1007/s00018-014-1819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang K., Sha J., Harter M.L. Activation of Cdc6 by MyoD is associated with the expansion of quiescent myogenic satellite cells. J. Cell Biol. 2010;188(1):39–48. doi: 10.1083/jcb.200904144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Relaix F., Zammit P.S. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139(16):2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- 54.Chang N.C., Sincennes M-C., Chevalier F.P., Brun C.E., Lacaria M., Segalés J., Muñoz-Cánoves P., Ming H., Rudnicki M.A. The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Stem Cell. 2018;22(5):755–768. doi: 10.1016/j.stem.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palacios D., Mozzetta C., Consalvi S., Caretti G., Saccone V., Proserpio V., Marquez V.E., Valente S., Mai A., Forcales S.V., Sartorelli V., Puri P.L. TNF/p38α/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7(4):455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mozzetta C., Consalvi S., Saccone V., Forcales S.V., Puri P.L., Palacios D. Selective control of Pax7 expression by TNF-activated p38α/polycomb repressive complex 2 (PRC2) signaling during muscle satellite cell differentiation. Cell Cycle. 2011;10(2):191–198. doi: 10.4161/cc.10.2.14441. [DOI] [PubMed] [Google Scholar]
- 57.Ding S., Swennen G.N.M., Messmer T., Gagliardi M., Molin D.G.M., Li C., Zhou G., Post M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018;8(1):10808. doi: 10.1038/s41598-018-28746-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J-F., Mandel E.M., Thomson J.M., Wu Q., Callis T.E., Hammond S.M., Conlon F.L., Wang D-Z. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat. Genet. 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H.K., Lee Y.S., Sivaprasad U., Malhotra A., Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J. Cell Biol. 2006;174(5):677–687. doi: 10.1083/jcb.200603008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baghdadi M.B., Castel D., Machado L., Fukada S., Birk D.E., Relaix F., Tajbakhsh S., Mourikis P. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature. 2018;557(7707):714–718. doi: 10.1038/s41586-018-0144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujimaki S., Seko D., Kitajima Y., Yoshioka K., Tsuchiya Y., Masuda S., Ono Y. Notch1 and Notch2 coordinately regulate stem cell function in the quiescent and activated states of muscle satellite cells. Stem Cells. 2018;36(2):278–285. doi: 10.1002/stem.2743. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z., Bu N., Qiao X., Zuo Z., Shu Y., Liu Z., Qian Z., Chen J., Hou Y. Forkhead box M1 transcriptionally regulates the expression of long noncoding RNAs Snhg8 and Gm26917 to promote proliferation and survival of muscle satellite cells. Stem Cells. 2018;36(7):1097–1108. doi: 10.1002/stem.2824. [DOI] [PubMed] [Google Scholar]
- 63.Yan Z., Choi S., Liu X., Zhang M., Schageman J.J., Lee S.Y., Hart R., Lin L., Thurmond F.A., Williams R.S. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J. Biol. Chem. 2003;278(10):8826–8836. doi: 10.1074/jbc.M209879200. [DOI] [PubMed] [Google Scholar]
- 64.Musarò A. The basis of muscle regeneration. Adv. Biol. 2014:1–16. [Google Scholar]
- 65.Halevy O., Novitch B.G., Spicer D.B., Skapek S.X., Rhee J., Hannon G.J., Beach D., Lassar A.B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 66.Dey B.K., Gagan J., Dutta A. miR-206 and -486 induce myoblast differentiation by downregulating Pax7. Mol. Cell. Biol. 2011;31(1):203–214. doi: 10.1128/MCB.01009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu X., Zhang Y., Li T., Ma Z., Jia H., Chen Q., Zhao Y., Zhai L., Zhong R., Li C., Zou X., Meng J., Chen A.K., Puri P.L., Chen M., Zhu D. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat. Commun. 2017;8:14016. doi: 10.1038/ncomms14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernández-Hernández J.M., García-González E.G., Brun C.E., Rudnicki M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Teixeira C.F.P., Chaves F., Zamunér S.R., Fernandes C.M., Zuliani J.P., Cruz-Hofling M.A., Fernandes I., Gutiérrez J.M. Effects of neutrophil depletion in the local pathological alterations and muscle regeneration in mice injected with Bothrops jararaca snake venom. Int. J. Exp. Pathol. 2005;86(2):107–115. doi: 10.1111/j.0959-9673.2005.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W-C., Sala-Newby G.B., Susana A., Johnson J.L., Newby A.C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS One. 2012;7(8):e42507. doi: 10.1371/journal.pone.0042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen S-E., Jin B., Li Y-P. TNF-alpha regulates myogenesis and muscle regeneration by activating p38 MAPK. Am. J. Physiol. Cell Physiol. 2007;292(5):C1660–C1671. doi: 10.1152/ajpcell.00486.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peterson J.M., Bakkar N., Guttridge D.C. NF-κB signaling in skeletal muscle health and disease. Curr. Top. Dev. Biol. 2011;96:85–119. doi: 10.1016/B978-0-12-385940-2.00004-8. [DOI] [PubMed] [Google Scholar]
- 73.Muñoz-Cánoves P., Scheele C., Pedersen B.K., Serrano A.L. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280(17):4131–4148. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng B., Wehling-Henricks M., Villalta S.A., Wang Y., Tidball J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012;189(7):3669–3680. doi: 10.4049/jimmunol.1103180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arnold L., Henry A., Poron F., Baba-Amer Y., van Rooijen N., Plonquet A., Gherardi R.K., Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ruffell D., Mourkioti F., Gambardella A., Kirstetter P., Lopez R.G., Rosenthal N., Nerlov C.A. CREB-C/EBPbeta cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA. 2009;106(41):17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X., Liu Y., Zhao L., Zeng Z., Xiao W., Chen P. Macrophage depletion impairs skeletal muscle regeneration: The roles of regulatory factors for muscle regeneration. Cell Biol. Int. 2017;41(3):228–238. doi: 10.1002/cbin.10705. [DOI] [PubMed] [Google Scholar]
- 78.Engert J.C., Berglund E.B., Rosenthal N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 1996;135(2):431–440. doi: 10.1083/jcb.135.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Musarò A., Rosenthal N. Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol. Cell. Biol. 1999;19(4):3115–3124. doi: 10.1128/mcb.19.4.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tonkin J., Temmerman L., Sampson R.D., Gallego-Colon E., Barberi L., Bilbao D., Schneider M.D., Musarò A., Rosenthal N. Monocyte/Macrophage-derived IGF-1 Orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol. Ther. 2015;23(7):1189–1200. doi: 10.1038/mt.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang C., Li Y., Wu Y., Wang L., Wang X., Du J. Interleukin-6/STAT3 pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J. Biol. Chem. 2013;288(3):1489–1499. doi: 10.1074/jbc.M112.419788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Forcina L., Miano C., Musarò A. The physiopathologic interplay between stem cells and tissue niche in muscle regeneration and the role of IL-6 on muscle homeostasis and diseases. Cytokine Growth Factor Rev. 2018;41:1–9. doi: 10.1016/j.cytogfr.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 83.Serrano A.L., Baeza-Raja B., Perdiguero E., Jardí M., Muñoz-Cánoves P. Interleukin-6 Is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 84.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., Rossi F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Formicola L., Marazzi G., Sassoon D.A. The extraocular muscle stem cell niche is resistant to ageing and disease. Front. Aging Neurosci. 2014;6:328. doi: 10.3389/fnagi.2014.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 87.Lemos D.R., Babaeijandaghi F., Low M., Chang C-K., Lee S.T., Fiore D., Zhang R-H., Natarajan A., Nedospasov S.A., Rossi F.M.V. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat. Med. 2015;21(7):786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 88.Shin J., McFarland D.C., Velleman S.G. Heparan sulfate proteoglycans, syndecan-4 and glypican-1, differentially regulate myogenic regulatory transcription factors and paired box 7 expression during turkey satellite cell myogenesis: Implications for muscle growth. Poult. Sci. 2012;91(1):201–207. doi: 10.3382/ps.2011-01695. [DOI] [PubMed] [Google Scholar]
- 89.Harthan L.B., McFarland D.C., Velleman S.G. The effect of syndecan-4 and glypican-1 expression on age-related changes in myogenic satellite cell proliferation, differentiation, and fibroblast growth factor 2 responsiveness. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013;166(4):590–602. doi: 10.1016/j.cbpa.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 90.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93(1):23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fiore D., Judson R.N., Low M., Lee S., Zhang E., Hopkins C., Xu P., Lenzi A., Rossi F.M.V., Lemos D.R. Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration. Stem Cell Res. (Amst.) 2016;17(1):161–169. doi: 10.1016/j.scr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 92.Urciuolo A., Quarta M., Morbidoni V., Gattazzo F., Molon S., Grumati P., Montemurro F., Tedesco F.S., Blaauw B., Cossu G., Vozzi G., Rando T.A., Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013;4(1):1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thépenier C., Pascal Q., Guguin A., Gayraud-Morel B., Cavaillon J-M., Tajbakhsh S., Rocheteau P., Chrétien F. Comparative study of injury models for studying muscle regeneration in mice. PLoS One. 2016;11(1):e0147198. doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Latroche C., Weiss-Gayet M., Muller L., Gitiaux C., Leblanc P., Liot S., Ben-Larbi S., Abou-Khalil R., Verger N., Bardot P., Magnan M., Chrétien F., Mounier R., Germain S., Chazaud B. Coupling between myogenesis and angiogenesis during skeletal muscle regeneration is stimulated by restorative macrophages. Stem Cell Reports. 2017;9(6):2018–2033. doi: 10.1016/j.stemcr.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qahar M., Takuma Y., Mizunoya W., Tatsumi R., Ikeuchi Y., Nakamura M. Semaphorin 3A promotes activation of Pax7, Myf5, and MyoD through inhibition of emerin expression in activated satellite cells. FEBS Open Bio. 2016;6(6):529–539. doi: 10.1002/2211-5463.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tatsumi R., Sankoda Y., Anderson J.E., Sato Y., Mizunoya W., Shimizu N., Suzuki T., Yamada M., Rhoads R.P., Ikeuchi Y., Allen R.E., Furuse M., Ikcuchi Y., Nishimura T., Yagi T. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am. J. Physiol. 2009;297(2):C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- 97.Tatsumi R., Suzuki T., Do M.Q., Ohya Y., Anderson J.E., Shibata A., Kawaguchi M., Ohya S., Ohtsubo H., Mizunoya W., Sawano S., Komiya Y., Ichitsubo R., Ojima K., Nishimatsu S.I., Nohno T., Ohsawa Y., Sunada Y., Nakamura M., Furuse M., Ikeuchi Y., Nishimura T., Yagi T., Allen R.E. Slow-myofiber commitment by semaphorin 3A secreted from myogenic stem cells. Stem Cells. 2017;35(7):1815–1834. doi: 10.1002/stem.2639. [DOI] [PubMed] [Google Scholar]
- 98.De Angelis L., Berghella L., Coletta M., Lattanzi L., Zanchi M., Cusella-De Angelis M.G., Ponzetto C., Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J. Cell Biol. 1999;147(4):869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tamaki T., Akatsuka A., Ando K., Nakamura Y., Matsuzawa H., Hotta T., Roy R.R., Edgerton V.R. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J. Cell Biol. 2002;157(4):571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Polesskaya A., Seale P., Rudnicki M.A. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113(7):841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 101.Kuang S., Chargé S.B., Seale P., Huh M., Rudnicki M.A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 2006;172(1):103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minasi M.G., Riminucci M., De Angelis L., Borello U., Berarducci B., Innocenzi A., Caprioli A., Sirabella D., Baiocchi M., De Maria R., Boratto R., Jaffredo T., Broccoli V., Bianco P., Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129(11):2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 103.Tonlorenzi R., Dellavalle A., Schnapp E., Cossu G., Sampaolesi M. Isolation and characterization of mesoangioblasts from mouse, dog, and human tissues. 2007. [DOI] [PubMed]
- 104.Gussoni E., Soneoka Y., Strickland C.D., Buzney E.A., Khan M.K., Flint A.F., Kunkel L.M., Mulligan R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401(6751):390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 105.Asakura A., Rudnicki M.A. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp. Hematol. 2002;30(11):1339–1345. doi: 10.1016/s0301-472x(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 106.Torrente Y., Belicchi M., Sampaolesi M., Pisati F., Meregalli M., D’Antona G., Tonlorenzi R., Porretti L., Gavina M., Mamchaoui K., Pellegrino M.A., Furling D., Mouly V., Butler-Browne G.S., Bottinelli R., Cossu G., Bresolin N. Human circulating AC133+ stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 2004;114(2):182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meeson A.P., Hawke T.J., Graham S., Jiang N., Elterman J., Hutcheson K., DiMaio J.M., Gallardo T.D., Garry D.J. Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells. 2004;22(7):1305–1320. doi: 10.1634/stemcells.2004-0077. [DOI] [PubMed] [Google Scholar]
- 108.Montanaro F., Liadaki K., Schienda J., Flint A., Gussoni E., Kunkel L.M. Demystifying SP cell purification: viability, yield, and phenotype are defined by isolation parameters. Exp. Cell Res. 2004;298(1):144–154. doi: 10.1016/j.yexcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 109.Rivier F., Alkan O., Flint A.F., Muskiewicz K., Allen P.D., Leboulch P., Gussoni E. Role of bone marrow cell trafficking in replenishing skeletal muscle SP and MP cell populations. J. Cell Sci. 2004;117(10):1979–1988. doi: 10.1242/jcs.01051. [DOI] [PubMed] [Google Scholar]
- 110.Tanaka K.K., Hall J.K., Troy A.A., Cornelison D.D.W., Majka S.M., Olwin B.B. Syndecan-4-expressing muscle progenitor cells in the SP engraft as satellite cells during muscle regeneration. Cell Stem Cell. 2009;4(3):217–225. doi: 10.1016/j.stem.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sancricca C., Mirabella M., Gliubizzi C., Broccolini A., Gidaro T., Morosetti R. Vessel-associated stem cells from skeletal muscle: From biology to future uses in cell therapy. World J. Stem Cells. 2010;2(3):39. doi: 10.4252/wjsc.v2.i3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quattrocelli M., Palazzolo G., Perini I., Crippa S., Cassano M., Sampaolesi M. Mouse and human mesoangioblasts: Isolation and characterization from adult skeletal muscles. Methods Mol. Biol. 2012;798:65–76. doi: 10.1007/978-1-61779-343-1_4. [DOI] [PubMed] [Google Scholar]
- 113.Sampaolesi M., Torrente Y., Innocenzi A., Tonlorenzi R., D’Antona G., Pellegrino M.A., Barresi R., Bresolin N., De Angelis M.G.C., Campbell K.P., Bottinelli R., Cossu G. Cell therapy of -sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301(5632):487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- 114.Sampaolesi M., Blot S., D’Antona G., Granger N., Tonlorenzi R., Innocenzi A., Mognol P., Thibaud J-L., Galvez B.G., Barthélémy I., Perani L., Mantero S., Guttinger M., Pansarasa O., Rinaldi C., Cusella De Angelis M.G., Torrente Y., Bordignon C., Bottinelli R., Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444(7119):574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- 115.Cossu G., Previtali S.C., Napolitano S., Cicalese M.P., Tedesco F.S., Nicastro F., Noviello M., Roostalu U., Natali Sora M.G., Scarlato M., De Pellegrin M., Godi C., Giuliani S., Ciotti F., Tonlorenzi R., Lorenzetti I., Rivellini C., Benedetti S., Gatti R., Marktel S., Mazzi B., Tettamanti A., Ragazzi M., Imro M.A., Marano G., Ambrosi A., Fiori R., Sormani M.P., Bonini C., Venturini M., Politi L.S., Torrente Y., Ciceri F. Intra-arterial transplantation of HLA-matched donor mesoangioblasts in Duchenne muscular dystrophy. EMBO Mol. Med. 2015;7(12):1513–1528. doi: 10.15252/emmm.201505636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B.G., Messina G., Morosetti R., Li S., Belicchi M., Peretti G., Chamberlain J.S., Wright W.E., Torrente Y., Ferrari S., Bianco P., Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 117.Dellavalle A., Maroli G., Covarello D., Azzoni E., Innocenzi A., Perani L., Antonini S., Sambasivan R., Brunelli S., Tajbakhsh S., Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat. Commun. 2011;2(1):499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- 118.Crisan M., Yap S., Casteilla L., Chen C-W., Corselli M., Park T.S., Andriolo G., Sun B., Zheng B., Zhang L., Norotte C., Teng P-N., Traas J., Schugar R., Deasy B.M., Badylak S., Bűhring H-J., Giacobino J-P., Lazzari L., Huard J., Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Birbrair A., Zhang T., Wang Z-M., Messi M.L., Enikolopov G.N., Mintz A., Delbono O. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 2013;22(16):2298–2314. doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Scicchitano B.M., Dobrowolny G., Sica G., Musaro A. Molecular insights into muscle homeostasis, atrophy and wasting. Curr. Genomics. 2018;19(5):356–369. doi: 10.2174/1389202919666180101153911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Scicchitano B.M., Pelosi L., Sica G., Musarò A. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev. 2018;170:37–44. doi: 10.1016/j.mad.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Franco I., Johansson A., Olsson K., Vrtačnik P., Lundin P., Helgadottir H.T., Larsson M., Revêchon G., Bosia C., Pagnani A., Provero P., Gustafsson T., Fischer H., Eriksson M. Somatic mutagenesis in satellite cells associates with human skeletal muscle aging. Nat. Commun. 2018;9(1):800. doi: 10.1038/s41467-018-03244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965;37(3):614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 124.George T., Velloso C.P., Alsharidah M., Lazarus N.R., Harridge S.D.R. Sera from young and older humans equally sustain proliferation and differentiation of human myoblasts. Exp. Gerontol. 2010;45(11):875–881. doi: 10.1016/j.exger.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 125.Hikida R.S. Aging changes in satellite cells and their functions. Curr. Aging Sci. 2011;4(3):279–297. doi: 10.2174/1874609811104030279. [DOI] [PubMed] [Google Scholar]
- 126.Tichy E.D., Sidibe D.K., Tierney M.T., Stec M.J., Sharifi-Sanjani M., Hosalkar H., Mubarak S., Johnson F.B., Sacco A., Mourkioti F. Single stem cell imaging and analysis reveals telomere length differences in diseased human and mouse skeletal muscles. Stem Cell Reports. 2017;9(4):1328–1341. doi: 10.1016/j.stemcr.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Decary S., Hamida C.B., Mouly V., Barbet J.P., Hentati F., Butler-Browne G.S. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul. Disord. 2000;10(2):113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 128.Pelosi L., Berardinelli M.G., Forcina L., Spelta E., Rizzuto E., Nicoletti C., Camilli C., Testa E., Catizone A., De Benedetti F., Musarò A. Increased levels of interleukin-6 exacerbate the dystrophic phenotype in mdx mice. Hum. Mol. Genet. 2015;24(21):6041–6053. doi: 10.1093/hmg/ddv323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bernet J.D., Doles J.D., Hall J.K., Kelly Tanaka K., Carter T.A., Olwin B.B. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 2014;20(3):265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Price F.D., von Maltzahn J., Bentzinger C.F., Dumont N.A., Yin H., Chang N.C., Wilson D.H., Frenette J., Rudnicki M.A. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat. Med. 2014;20(10):1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sousa-Victor P., Gutarra S., García-Prat L., Rodriguez-Ubreva J., Ortet L., Ruiz-Bonilla V., Jardí M., Ballestar E., González S., Serrano A.L., Perdiguero E., Muñoz-Cánoves P. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506(7488):316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 132.Carlson B.M., Faulkner J.A. Muscle transplantation between young and old rats: age of host determines recovery. Am. J. Physiol. 1989;256(6 Pt 1):C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 133.Carlson B.M., Dedkov E.I., Borisov A.B., Faulkner J.A. Skeletal muscle regeneration in very old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56(5):B224–B233. doi: 10.1093/gerona/56.5.b224. [DOI] [PubMed] [Google Scholar]
- 134.Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 135.Daynes R.A., Araneo B.A., Ershler W.B., Maloney C., Li G.Z., Ryu S.Y. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J. Immunol. 1993;150(12):5219–5230. [PubMed] [Google Scholar]
- 136.Paliwal P., Pishesha N., Wijaya D., Conboy I.M. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany N.Y.) 2012;4(8):553–566. doi: 10.18632/aging.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McKay B.R., Ogborn D.I., Baker J.M., Toth K.G., Tarnopolsky M.A., Parise G. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am. J. Physiol. Cell Physiol. 2013;304(8):C717–C728. doi: 10.1152/ajpcell.00305.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barbieri M., Ferrucci L., Ragno E., Corsi A., Bandinelli S., Bonafè M., Olivieri F., Giovagnetti S., Franceschi C., Guralnik J.M., Paolisso G. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am. J. Physiol. Endocrinol. Metab. 2003;284(3):E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 139.Hirata A., Masuda S., Tamura T., Kai K., Ojima K., Fukase A., Motoyoshi K., Kamakura K., Miyagoe-Suzuki Y., Takeda S. Expression profiling of cytokines and related genes in regenerating skeletal muscle after cardiotoxin injection. Am. J. Pathol. 2003;163(1):203–215. doi: 10.1016/S0002-9440(10)63644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Uaesoontrachoon K., Yoo H-J., Tudor E.M., Pike R.N., Mackie E.J., Pagel C.N. Osteopontin and skeletal muscle myoblasts: Association with muscle regeneration and regulation of myoblast function in vitro. Int. J. Biochem. Cell Biol. 2008;40(10):2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 141.Vetrone S.A., Montecino-Rodriguez E., Kudryashova E., Kramerova I., Hoffman E.P., Liu S.D., Miceli M.C., Spencer M.J. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J. Clin. Invest. 2009;119(6):1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kuswanto W., Burzyn D., Panduro M., Wang K.K., Jang Y.C., Wagers A.J., Benoist C., Mathis D. Poor repair of skeletal muscle in aging mice reflects a defect in local, interleukin-33-dependent accumulation of regulatory T cells. Immunity. 2016;44(2):355–367. doi: 10.1016/j.immuni.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uezumi A., Fukada S., Yamamoto N., Takeda S., Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 144.Uezumi A., Ito T., Morikawa D., Shimizu N., Yoneda T., Segawa M., Yamaguchi M., Ogawa R., Matev M.M., Miyagoe-Suzuki Y., Takeda S., Tsujikawa K., Tsuchida K., Yamamoto H., Fukada S-i. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011;124(21):3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 145.Pelosi L., Coggi A., Forcina L., Musarò A. MicroRNAs modulated by local mIGF-1 expression in mdx dystrophic mice. Front. Aging Neurosci. 2015;7:69. doi: 10.3389/fnagi.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Barton E.R., Morris L., Musaro A., Rosenthal N., Sweeney H.L. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J. Cell Biol. 2002;157(1):137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Petrillo S., Pelosi L., Piemonte F., Travaglini L., Forcina L., Catteruccia M., Petrini S., Verardo M., D’Amico A., Musarò A., Bertini E. Oxidative stress in Duchenne muscular dystrophy: focus on the NRF2 redox pathway. Hum. Mol. Genet. 2017;26(14):2781–2790. doi: 10.1093/hmg/ddx173. [DOI] [PubMed] [Google Scholar]
- 148.Pelosi L., Forcina L., Nicoletti C., Scicchitano B.M., Musarò A. Increased circulating levels of interleukin-6 induce perturbation in redox-regulated signaling cascades in muscle of dystrophic mice. Oxid. Med. Cell. Longev. 2017;2017:1–10. doi: 10.1155/2017/1987218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Forcina L., Pelosi L., Miano C., Musarò A., Forcina L., Pelosi L., Miano C., Musarò A. Insights into the pathogenic secondary symptoms caused by the primary loss of dystrophin. J. Funct. Morphol. Kinesiol. 2017;2(4):44. [Google Scholar]
- 150.Pelosi L., Berardinelli M.G., De Pasquale L., Nicoletti C., D’Amico A., Carvello F., Moneta G.M., Catizone A., Bertini E., De Benedetti F., Musarò A. Functional and morphological improvement of dystrophic muscle by interleukin 6 receptor blockade. EBioMed. 2015;2(4):285–293. doi: 10.1016/j.ebiom.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]