Version Changes
Revised. Amendments from Version 1
We added how the secondary structure was calculated and the citation to the RNAfold tool
We added the precursor size to the locus panel A-right
We added help explaining how to modify the abundance profile in panel B-left
We changed the sequences abundances to show normalized values in panel C
We updated figure 1 to show the previous changes and increased the resolution
Abstract
The study of small RNAs provides us with a deeper understanding of the complexity of gene regulation within cells. Of the different types of small RNAs, the most important in mammals are miRNA, tRNA fragments and piRNAs. Using small RNA-seq analysis, we can study all small RNA types simultaneously, with the potential to detect novel small RNA types. We describe SeqclusterViz, an interactive HTML-javascript webpage for visualizing small noncoding RNAs (small RNAs) detected by Seqcluster. The SeqclusterViz tool allows users to visualize known and novel small RNA types in model or non-model organisms, and to select small RNA candidates for further validation. SeqclusterViz is divided into three panels: i) query-ready tables showing detected small RNA clusters and their genomic locations, ii) the expression profile over the precursor for all the samples together with RNA secondary structures, and iii) the mostly highly expressed sequences. Here, we show the capabilities of the visualization tool and its validation using human brain samples from patients with Parkinson’s disease.
Keywords: small RNA, miRNA, tRNA, snoRNA, sequencing, visualization, report
Introduction
Small RNAs are 18-36-nt-long RNA molecules that are involved in gene regulation, chromatin structure, and transposon element repression. The most well known small RNAs are miRNAs, endo-siRNAs and piRNAs 1. They are typically processed from double-stranded RNA molecules or single-stranded RNA molecules with a hairpin structure 2. They bind to members of the Argonaute (AGO) protein family to form the RNA-induced silencing complex that regulates other RNA molecules and plays a key role in gene silencing 3, 4. Small RNAs can also regulate chromatin states through histone modification and methylation 5, 6. Next generation sequencing technologies have enabled a deeper understanding of miRNAs, and other small RNA types have been detected. For instance, it is now known that miRNA genes generate several mature variants called isomiRs that have been detected in multiple conditions, tissues and species 7. Other small RNAs can arise from mature tRNAs (tRNA fragments) or small nucleolar RNAs 8, 9. While the biogenesis of these molecules is not well understood, studies suggest that they bind to AGO proteins and perform similar functions 10, 11.
High-throughput sequencing is a powerful technique for detecting and quantifying small RNAs. The analysis of small RNA data involves multiple steps for detection, annotation, quantification, and de novo discovery of putative small RNA molecules. In general, tools focus on the annotation of known miRNAs 12, but new methods to detect other functional types of small RNAs are becoming increasingly important to understand the complex roles of small RNAs. Some tools have been developed to address this challenge 13– 15 but few of them produce a visual and interactive report 16, 17, and many depend on the use of a remote web server 18– 21.
We previously developed seqcluster, a genome-wide small RNA characterization tool that detects units of transcripts (clusters) using a heuristic iterative algorithm to deal with multi-mapped events 22. It quantifies all types of small RNAs in non-redundant manner, and extracts patterns of expression in biologically defined groups. This allows us to study any small RNA cluster detected in the samples, including novel regions not previously discovered or small RNAs in species with poorly curated annotations. Here we describe seqclusterViz 23, an interactive web-app that reports the output of seqcluster, visualizing small RNA biological features to better understand their putative functions. It allows the user to browse lists of detected small RNAs, shows the precursor secondary structures and the small RNA expression on the precursor, allowing for more in-depth characterization of isomiRs, tRNA fragments, and any other small RNAs detected.
seqcluster and seqclusterViz are integrated into bcbio-nextgen, a community-based Python framework for fully automated high throughput sequencing analysis.
Methods
Implementation
seqclusterViz 23 is developed in HTML, CSS and JavaScript programming languages. It is a stand-alone tool without external dependencies. It runs locally on one’s computer making it portable and independent. It uses an SQLite JavaScript library to load all the information from a file created by the seqcluster tool 22.
Operation
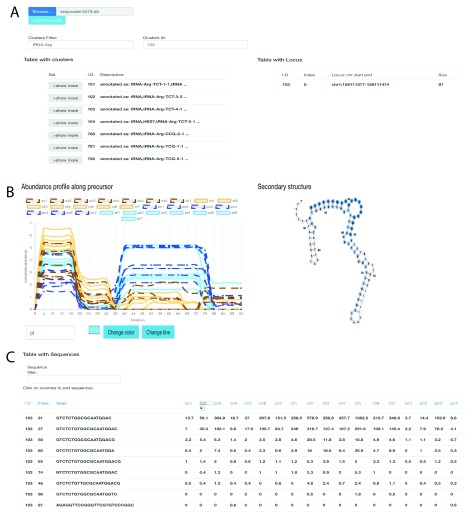
seqclusterViz 23 works on Opera >44.0, Firefox >52.0 and Chrome >57.0. It requires a seqcluster report as input. An Internet connection is not required. The tool can be downloaded from its home page ( https://github.com/lpantano/seqclusterViz/archive/master.zip). After extracting the ZIP file content, the user can open the index.html file with the desired web browser. The user first clicks the ’UPLOAD’ button and then selects the seqcluster.db file. Once the data has been uploaded, the top-left panel displays all of the small RNA transcripts detected. Each small RNA transcript is clickable to obtain more information ( 1A). After selecting a small RNA transcript, the top-right panel shows the genomic locations for that transcript. The middle-left panel displays the abundance profile along the precursor ( 1B); the middle-right displays the RNA secondary structure ( 1B); as calculated by seqcluster with RNAfold and default parameters 24; and the bottom table shows the top 50 most abundant sequences. This table can be sorted and searched using text queries ( 1C).
Figure 1. seqclusterViz features.
( A) Top panel with table showing the list of small RNAs detected (left) and genomic location (right). ( B) Middle panel shows abundance profile over the precursor (left), and secondary structure (right). This is an example of batch effect at the 3’ end (blue higher than brown) and disease effect at the 5’ end (solid lines higher than dashed lines). ( C) Bottom panel shows a table with the top most expressed sequences on the selected small RNA transcript. The index column is the sequence identifier that links the results to the original seqcluster output files.
The tool provides a number of formatting options to emphasize differences between groups and/or samples and to customize figures. Figures can be exported by right-clicking on it. This provides an easy and quick option to generate publication-ready material.
Use cases
We used public data from 14 human brain samples at pre-motor (PT) and motor (CT) stages of Parkinson’s disease (GEO accession number GSE97285) and 14 healthy human brain samples (pre-motor controls - PC and motor stages control - CC) 22. Data was analyzed with bcbio-nextgen using piDNA to detect the adapter 25, cutadapt to remove it 26, STAR to align against the hg19 genome assembly 27, and seqcluster to detect small RNA transcripts 22. We used the output seqcluster.db from seqcluster report command to test seqclusterViz 23. It took four seconds to upload this 28 MB file to the web page. This dataset is affected by a batch effect for the two Parkinson’s groups due to the groups being sequenced at different read lengths. PC and CC samples were derived from the same RNA extraction, and were expected to show similar expression profiles. However, there is a clear difference by batch (brown versus blue) that is visually apparent in the abundance profile of the tRNA-Arg-TCT RNA across the length of the transcript in ( 1B). Longer reads allow for detection of longer small RNAs since the 3’ adapter can be recognized during the analysis (there is a requirement to include adapter sequences in the seqcluster tool). The longer reads from the PC/PT samples (blue) permitted detection of longer small RNAs at the end of the precursor, generating the batch difference in the abundance profile. Moreover, there is a difference in expression at the 5’ end of the precursor, where Parkinson’s samples (solid lines) are higher than their respective controls (dashed lines). The secondary structure of this small RNA shows a pre-miRNA-like hairpin structure (with a stem-bulge-stem and a terminal-loop) that is normally required to be processed into 18-33-nt mature molecules, where the stem-bulge-stem section encodes the mature sequence 28, 29. Although the structure is larger than typical pre-miRNAs, it is still possible to process with the miRNA machinery. Thus the secondary structure of the molecule can serve as an additional feature to evaluate when seeking candidates for further experimental validation. Quantitative polymerase chain reaction (qPCR) or small RNA transfection technologies are often used to validate small RNA stability and function. To do so, a single small RNA needs to be used as the target sequence for these assays. The table at the bottom of the page ( 1C) allows users to select the most abundant sequence in the current small RNA that can be used for such experiments.
Summary
seqclusterViz 23 helps users to explore the expression profiles of detected small RNAs across the length of the precursor, the secondary structure of the small RNA, and the annotation. We show the importance of visualizing small RNAseq data to prioritize candidate small RNAs for further experimental validation or functional analysis. The user can modify the figure format and export it for publication or presentation purposes. It is also possible to select the most highly expressed sequence of a transcript cluster that can be used for qPCR or for cell transfection assays.
Data availability
Data to reproduce this analysis is available from the Parkinson project page.
Data from 14 healthy human brain samples were originally reported by Pantano et al. 22. Data from 14 human brain samples at pre-motor (PT) and motor (CT) stages of Parkinson’s disease are available at GEO, accession number GSE97285.
The web-tool can be tested at GitHub pages. Click on Load Example to start using the tool with the example data set.
Software availability
seqclusterViz is downloaded from: https://github.com/lpantano/seqclusterViz/archive/v0.1.2.zip.
Source code available from: https://github.com/lpantano/seqclusterViz.
Link to source code as at time of publication: url https://doi.org/10.5281/zenodo.3250205 23.
License: MIT License.
Acknowledgments
The authors would like to thank researchers who helped to improve this tool: Aron Gyuris, Mira Pavkovic, Maria Mavrikaki. Thank you also to Amanda King for edits.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- 1. Martens-Uzunova ES, Olvedy M, Jenster G: Beyond microRNA--novel RNAs derived from small non-coding RNA and their implication in cancer. Cancer Lett. 2013;340(2):201–211. 10.1016/j.canlet.2012.11.058 [DOI] [PubMed] [Google Scholar]
- 2. Kim VN, Han J, Siomi MC: Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10(2):126–139. 10.1038/nrm2632 [DOI] [PubMed] [Google Scholar]
- 3. Kim DH, Saetrom P, Snøve O, Jr, et al. : MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc Natl Acad Sci U S A. 2008;105(42):16230–16235. 10.1073/pnas.0808830105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamura K, Lai EC: Endogenous small interfering RNAs in animals. Nat Rev Mol Cell Biol. 2008;9(9):673–678. 10.1038/nrm2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moazed D: Small RNAs in transcriptional gene silencing and genome defence. Nature. 2009;457(7228):413–420. 10.1038/nature07756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gonzalez S, Pisano DG, Serrano M: Mechanistic principles of chromatin remodeling guided by siRNAs and miRNAs. Cell Cycle. 2008;7(16):2601–2608. 10.4161/cc.7.16.6541 [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y, Zang Q, Xu B, et al. : IsomiR Bank: a research resource for tracking IsomiRs. Bioinformatics. 2016;32(13):2069–2071. 10.1093/bioinformatics/btw070 [DOI] [PubMed] [Google Scholar]
- 8. Kawaji H, Nakamura M, Takahashi Y, et al. : Hidden layers of human small RNAs. BMC Genomics. 2008;9(1):157. 10.1186/1471-2164-9-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Telonis AG, Loher P, Honda S, et al. : Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget. 2015;6(28):24797–822. 10.18632/oncotarget.4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole C, Sobala A, Lu C, et al. : Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15(12):2147–2160. 10.1261/rna.1738409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brameier M, Herwig A, Reinhardt R, et al. : Human box C/D snoRNAs with miRNA like functions: expanding the range of regulatory RNAs. Nucleic Acids Res. 2011;39(2):675–686. 10.1093/nar/gkq776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lukasik A, Wójcikowski M, Zielenkiewicz P: Tools4miRs - one place to gather all the tools for miRNA analysis. Bioinformatics. 2016;32(17):2722–4. 10.1093/bioinformatics/btw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baras AS, Mitchell CJ, Myers JR, et al. : miRge - A Multiplexed Method of Processing Small RNA-Seq Data to Determine MicroRNA Entropy. PLoS One. 2015;10(11):e0143066. 10.1371/journal.pone.0143066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beckers M, Mohorianu I, Stocks M, et al. : Comprehensive processing of high-throughput small RNA sequencing data including quality checking, normalization, and differential expression analysis using the UEA sRNA Workbench. RNA. 2017;23(6):823–835. 10.1261/rna.059360.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giurato G, De Filippo MR, Rinaldi A, et al. : iMir: an integrated pipeline for high-throughput analysis of small non-coding RNA data obtained by smallRNA-Seq. BMC Bioinformatics. 2013;14:362. 10.1186/1471-2105-14-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stocks MB, Moxon S, Mapleson D, et al. : The UEA sRNA workbench: a suite of tools for analysing and visualizing next generation sequencing microRNA and small RNA datasets. Bioinformatics. 2012;28(15):2059–2061. 10.1093/bioinformatics/bts311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Quek C, Jung CH, Bellingham SA, et al. : iSRAP - a one-touch research tool for rapid profiling of small RNA-seq data. J Extracell Vesicles. 2015;4:29454. 10.3402/jev.v4.29454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rueda A, Barturen G, Lebrón R, et al. : sRNAtoolbox: an integrated collection of small RNA research tools. Nucleic Acids Res. 2015;43(W1):W467–73. 10.1093/nar/gkv555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Xu B, Yang Y, et al. : CPSS: a computational platform for the analysis of small RNA deep sequencing data. Bioinformatics. 2012;28(14):1925–1927. 10.1093/bioinformatics/bts282 [DOI] [PubMed] [Google Scholar]
- 20. Yang JH, Qu LH: DeepBase: annotation and discovery of microRNAs and other noncoding RNAs from deep-sequencing data. Methods Mol Biol. 2012;822:233–248. 10.1007/978-1-61779-427-8_16 [DOI] [PubMed] [Google Scholar]
- 21. Huang PJ, Liu YC, Lee CC, et al. : DSAP: deep-sequencing small RNA analysis pipeline. Nucleic Acids Res. 2010;38(Web Server issue):W385–91. 10.1093/nar/gkq392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pantano L, Friedländer MR, Escaramís G, et al. : Specific small-RNA signatures in the amygdala at premotor and motor stages of Parkinson's disease revealed by deep sequencing analysis. Bioinformatics. 2016;32(5):673–681. 10.1093/bioinformatics/btv632 [DOI] [PubMed] [Google Scholar]
- 23. Pantano L, franpantano: lpantano/seqclusterviz: v0.1.2.2019. 10.5281/zenodo.3250205 [DOI] [Google Scholar]
- 24. Lorenz R, Bernhart SH, Höner Zu Siederdissen C, et al. : Viennarna Package 2.0. Algorithms Mol Biol. 2011;6(1):26. 10.1186/1748-7188-6-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuji J, Weng Z: DNApi: A De Novo Adapter Prediction Algorithm for Small RNA Sequencing Data. PLoS One. 2016;11(10):e0164228. 10.1371/journal.pone.0164228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin M: Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 27. Dobin A, Davis CA, Schlesinger F, et al. : STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartel DP: MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 29. Feng Y, Zhang X, Graves P, et al. : A comprehensive analysis of precursor microRNA cleavage by human Dicer. RNA. 2012;18(11):2083–92. 10.1261/rna.033688.112 [DOI] [PMC free article] [PubMed] [Google Scholar]