Abstract
Background:
TRIP (Transmission Reduction Intervention Project) was a network-based, contact tracing approach to locate and link to care, mostly people who inject drugs (PWID) with recent HIV infection.
Objective:
We investigated whether sequences from HIV-infected participants with high viral load cluster together more frequently than what is expected by chance.
Methods:
Paired end reads were generated for 104 samples using Illumina MiSeq next-generation se-quencing.
Results:
63 sequences belonged to previously identified local transmission networks of PWID (LTNs) of an HIV outbreak in Athens, Greece. For two HIV-RNA cut-offs (105 and 106 IU/mL), HIV transmissions were more likely between PWID with similar levels of HIV-RNA (p<0.001). 10 of the 14 sequences (71.4%) from PWID with HIV-RNA >106 IU/mL were clustered in 5 pairs. For 4 of these clusters (80%), there was in each one of them at least one sequence from a recently HIV-infected PWID.
Conclusion:
We showed that transmissions are more likely among PWID with high viremia.
Keywords: HIV, recent infection, HIV transmission, PWID, HIV-RNA, TRIP
1. INTRODUCTION
Approximately 37 million people were living with HIV (PLHIV) by the end of 2017 (http://www.unaids.org/). A lot of different approaches, both behavioral and biomedical, have been used to reduce HIV transmission [1] but still around 2 million people are infected every year. The global community is now committed to end the HIV epidemic as a public health threat by 2030. UNAIDS has set the 90-90-90 target: 90% of all PLHIV to be aware of their HIV status (first 90); 90% of the HIV-diagnosed to receive antiretroviral treatment (ART) (second 90); and 90% of those on ART to achieve virological suppression (third 90).
Social network-based interventions to prevent HIV transmission have shown promising results [2-9]. The Transmission Reduction Intervention Project (TRIP) was a recently implemented network-based intervention to detect people who had acquired HIV in the past 6 months [10]. Identifying people with recent HIV infection is very
important. Increased HIV-RNA levels have been associated with heightened risk of HIV transmission [11-17]. Substantial reductions in HIV transmission or incidence have been observed or expected in settings with declines in individual or community viral load, respectively [12, 18-27]. Given that infectivity has been positively correlated with viral load, HIV transmission during the period of early infection (when viral load becomes very high) is extremely likely [28-41]. Phylogenetic studies estimate that almost half of the transmissions attributable to an HIV-infected person occur during the recent phase of his/her HIV infection [42-54].
Given the epidemiological associations described above, we aimed to investigate phylogenetically, using near full-length HIV sequences, whether HIV transmissions occur more frequently among persons with high viral load, which is a salient characteristic of recent HIV infection.
2. MATERIALS AND METHOD
2.1. Project Description
TRIP was a multi-site (Athens, Greece; Chicago, United States; and Odessa, Ukraine), network-based contact-tracing intervention to detect individuals with recent and/or undiagnosed HIV infection and link them to care. Details regarding the design and implementation of TRIP in Athens have been described elsewhere [10]. TRIP, Athens recruited 356 individuals (90.2% people who inject drugs - PWID). Of these, 149 participants (46.4%) tested HIV positive [10]. Demographics, epidemiological and clinical characteristics of the HIV positive participants in TRIP are presented in Table 1.
Table 1.
Demographics, epidemiological and clinical characteristics of: i) HIV-infected participants (N=149) of the Transmission Reduction Intervention Project (TRIP) and ii) a subset of the HIV positives (N=63 of 149) who were in local transmission networks of PWID in Athens, Greece.
| Characteristics | HIV Positives in TRIP | HIV Positives in PWID-LTNs | |
|---|---|---|---|
| Total [Ν (%)] | 149 (100) | 63 (100) | |
| Gender [Ν (%)] | Males | 113 (75.8) | 49 (77.8) |
| Females | 36 (24.2) | 14 (22.2) | |
| Median age [years (IQR)] | 34 (30-40) | 34 (30-39) | |
| Education level [Ν (%)] | Up to high school | 131 (87.9) | 59 (93.7) |
| Above high school | 18 (12.1) | 4 (6.3) | |
| Accommodation status in the past 6 months [Ν (%)] | Non-homeless | 97 (65.1) | 42 (66.7) |
| Homeless | 52 (34.9) | 21 (33.3) | |
| Drug injection in last 6 months [Ν (%)]a, b | Non- PWID | 5 (3.4) | 2 (3.2) |
| PWID | 141 (96.6) | 60 (96.8) | |
| Duration of injection [years (IQR)]c, d | 13 (7-17) | 12.5 (7-17) | |
| HIV-RNA [log IU/ml (IQR)]e | 5.4 (4.5-6.1) | 5.5 (5.0-6.1) |
IQR, interquartile range; PWID, people who inject drugs; aΝ=146 (of 149 HIV positives); bΝ=62 (of 63 HIV positives); cN= 140 (of 149 HIV positives); dΝ=60 (of 63 HIV positives); eN= 126 (of 149 HIV positives).
2.2. HIV-1 Testing
Blood samples were tested for HIV antibodies by AxSYM HIV-1/2 gO (Abbott) and confirmed by Western Blot (MP Diagnostics). Recent HIV-1 infection was determined using the Limiting Antigen Avidity assay [55]. HIV-RNA in plasma was quantified using Artus HI Virus-1 RT-PCR (Qiagen), according to the manufacturer’s recommendations.
2.3. HIV-1 Sequencing and Subtyping
104 near full-length genomic sequences were generated using methods developed under the Infection Response through Virus Genomics (ICONIC) project [56, 57]. Nucleotide sequences were aligned using the HIVAlign tool available on the Los Alamos HIV sequence database (http://hiv.lanl.gov/).
HIV-1 subtypes were identified for 104 sequences using the online automated HIV-1 subtyping tool COMET v.0.2 (COntext-based Modeling for Expeditious Typing) (http://comet.retrovirology.lu/) and the REGA HIV-1 subtyping tool. Subtyping results were further confirmed by using references to perform phylogenetic analysis.
These references were representative of all known HIV-1 subtypes and of most of the Circulating Recombinant Forms (CRFs), and were available on the Los Alamos HIV-1 sequence database. The presence of recombination was tested by bootscanning analysis as implemented in Simplot v3.5.1 [58] using pure subtypes and CRFs as references. Bootscanning analysis was run for a sliding window of 400 bps moving in steps of 50 bps. Putative recombinants were confirmed by phylogenetic analysis in separate genomic fragments with discordant phylogenetic clustering. Tree visualization and annotation were done using the FigTree v1.4 program (http://tree.bio.ed.ac.uk/software/figtree/).
2.4. Hypothesis Testing
We performed phylogenetic analyses to examine whether transmissions occur more often among persons with high plasma HIV-RNA levels (106 or 105 IU/mL) [59], a marker of recent HIV infection. Phylogenetic analysis was performed using the maximum likelihood method (ML) under the Generalized Time Reversible (GTR) model of nucleotide substitution including a Gamma-distributed rate of heterogeneity among sites as implemented in RAxMLv8.0 [60] and FastTree v2.1 [61]. HIV-1 sequences that were found outside the local transmission networks (LTNs) of PWID [62] or the Unique Recombinant Forms (URFs) were excluded from the analysis. Short length sequences were also excluded (< 4,000 bps). Phylogenetic trees were derived from non-recombinant near full-length genome alignments that belonged to the 4 PWID LTNs (CRF14_BG/B/CRF14_BG, CRF35_AD/A/ CRF35_AD, subtypes A and B).
We performed additional analysis to test whether transmissions among PWID with high HIV-RNA are frequent. The hypothesis that transmissions occur frequently among persons with high plasma HIV-RNA values (106 or 105 IU/mL) was investigated by reconstructing ancestral states assigned at the tips (high or low HIV-RNA values), and using the criterion of parsimony to estimate the total number of character changes across the phylogeny. In this case, character changes correspond to transmissions between persons with different levels of HIV-RNA (“low” and “high”). The analysis was performed on 300 bootstrap-reconstructed trees as estimated in RAxML version 8.0, using Mesquite program version 3.5 [63]. The estimated number of events corresponds to the “observed number of transmissions among persons with different HIV-RNA values”. The null hypothesis that corresponds to a random distribution of the characters states at the tips was simulated after a random reshuffling of the characters on the full set of bootstrap trees.
We also tested if HIV sequences from persons with high viral loads (>106 IU/mL) formed significant phylogenetic clusters receiving Shimodaira-Hasegawa (SH) value > 0.9 or bootstrap support > 75%.
2.5. Statistical Analysis
The non-parametric, one-sided Mann-Whitney test was used to compare the distribution of the total number of character changes (transmissions between different groups of HIV-RNA) between the original bootstrap trees and the trees that were randomly reshuffled at the tips (STATA 14 - StataCorp LP).
3. RESULTS
HIV-1 subtyping and recombination analysis showed that sequences from 63 individuals (60.6%) (Table 1) fell within the LTNs of PWID: CRF14_BG/B/CRF14_BG (n=35, 33.7%); CRF35_AD/A/CRF35_AD (n=17, 16.3%); subtype B (n=8, 7.7%); subtype A (n=3, 2.9%). The rest of the sequences were classified as subtypes A (n=4, 3.8%) and B (n=1, 1%) that did not belong to PWID-LTN, and as URFs (n=36, 34.6%). HIV-1 sequences available in protease (PR) and partial reverse transcriptase (RT) that were classified initially as CRF35_AD and CRF14_BG [62,64], were later identified as unique recombinants with identical patterns (CRF35_AD/A/CRF35_AD and CRF14_BG/B/CRF14_BG, respectively, in their complete genome).
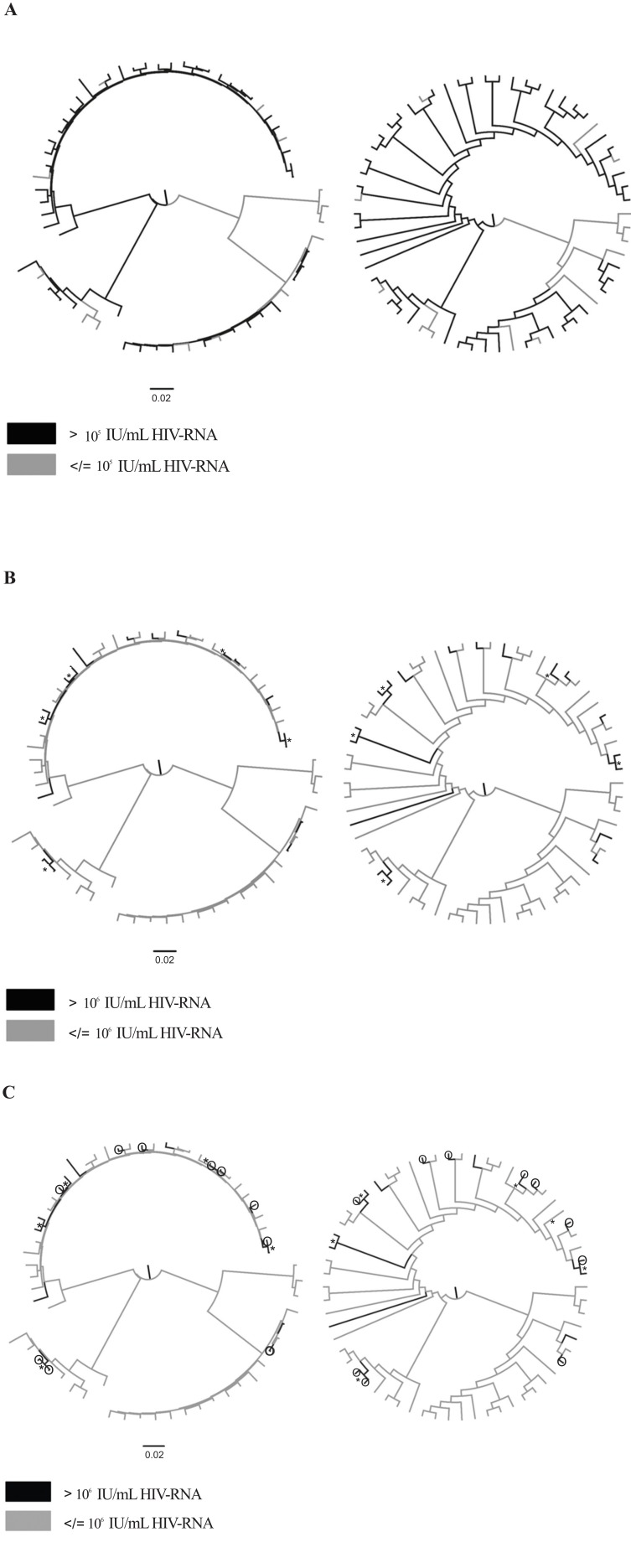
Phylogenetic analysis using the 62 near-complete HIV-1 sequences that belonged to the 4 PWID-LTNs (CRF14_ BG/B/CRF14_BG, CRF35_AD/A/CRF35_AD, subtypes A and B) was conducted to investigate whether transmissions occur more often among individuals with high HIV-RNA levels. One sequence was excluded due to its short length (< 4,000 bps). Phylogenetic trees that were inferred using the 62 sequences are shown in Fig. (1). The sequences from persons with high HIV-RNA (> 105 IU/mL or > 106 IU/mL) are highlighted in different colors (Figs. 1A and 1B).
Fig. (1).
Maximum likelihood phylogenetic trees of near full-length genomic sequencing found in local transmission networks (LTNs) of people who inject drugs (PWID) shown with branch lengths and without branch lengths. (A) PWID with HIV-RNA > 105 IU/mL are highlighted in black. (B) PWID with HIV-RNA > 106 IU/mL are highlighted in black. (C) PWID with HIV-RNA > 106 IU/mL are highlighted in black and PWID with HIV-RNA > 106 IU/mL and documented recent infection (< 6 months) are indicated by white circles with black outlines. Highly supported nodes (SH-support > 0.9 or bootstrap support > 75%) including viral sequences from PWID with HIV-RNA > 106 IU/mL are indicated by an asterisk.
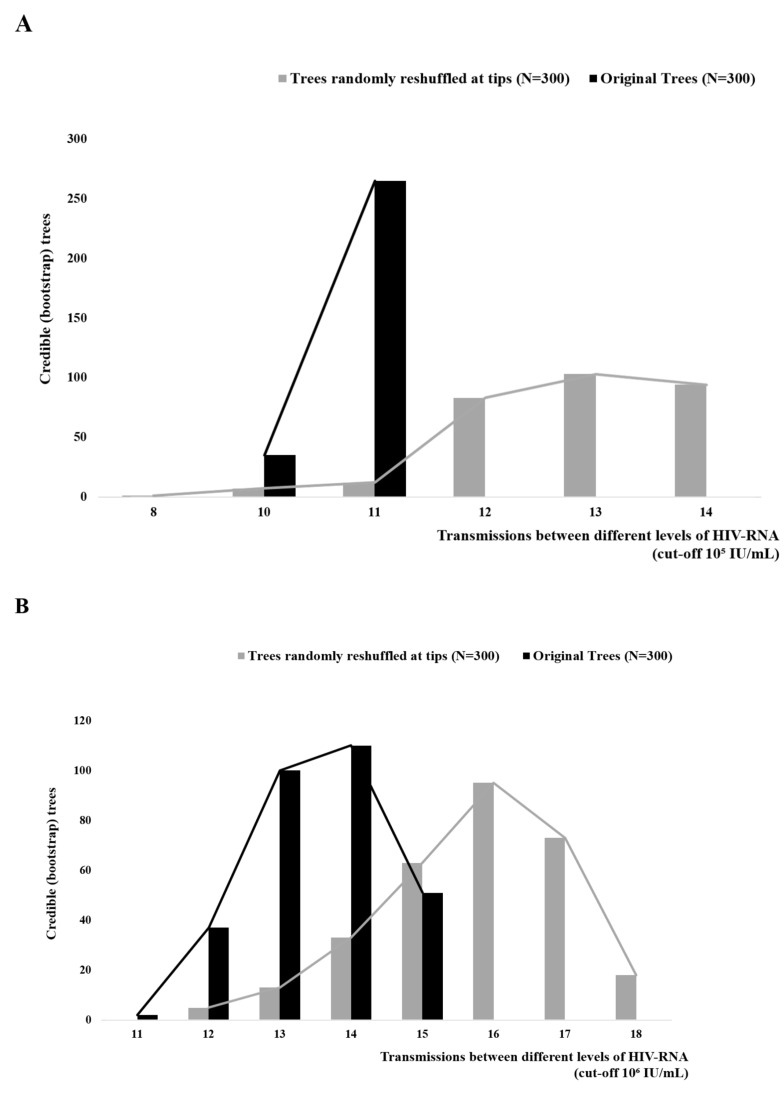
Additional analyses included the estimation of the total number of character changes between PWID with high and low HIV-RNA, which are indicative of transmissions between these two groups, and the examination of whether the number of transmissions is significantly lower than the number of changes expected by chance. For both HIV-RNA cut-offs (105 and 106 IU/mL), the number of transmissions between the two groups was significantly lower (p<0.001), suggesting that HIV transmissions occur more frequently between PWID with similar levels of HIV-RNA (Figs. 2A and 2B). Notably, most sequences from PWID with HIV-RNA > 106 IU/mL formed highly supported clusters (i.e., they received SH-support > 0.9 or bootstrap support > 75%) (Fig. 1B). For 14 of 18 PWID with HIV-RNA > 106 IU/mL, phylogenetic clustering with at least one sequence was highly supported. 10 of the 14 sequences (71.4%) from PWID with HIV-RNA > 106 IU/mL were clustered in 5 pairs (Fig. 1B).
Fig. (2).
Total number of character changes (transmissions between different levels of HIV-RNA) estimated between original bootstrap trees (black) and after a reshuffling at the tips (grey) (A) using a cut-off of 105 IU/mL and (B) using a cut-off of 106 IU/mL.
We also examined whether there were any PWID with documented recent infection (< 6 months) among the PWID with HIV-RNA > 106 IU/mL. For 4 of the 5 clusters (80%) of sequences (n=10) from PWID with high HIV- RNA, there was in each cluster at least one sequence from a recently HIV-infected PWID (Fig. 1C). Specifically, 6 of 10 transmissions (60%) among PWID with HIV-RNA > 106 IU/mL in transmission pairs originated from people with recent HIV infection. For one cluster, both sequences were from recently HIV-infected individuals (Fig. 1C).
4. DISCUSSION
This study examined, using molecular epidemiology methods, whether HIV transmissions occur more often among PWID with high HIV-RNA levels. Our analysis was based on near full-length genomic sequencing from HIV-infected PWID who participated in a network-based intervention (TRIP) in Athens, Greece. All analyses supported that transmissions among PWID with high HIV-RNA levels (> 105 IU/mL or > 106 IU/mL) occur at high rates. Given that very high HIV-RNA levels (> 106 IU/mL) are a marker for recent HIV infection, our results suggest that transmissions among people who acquired HIV recently are more frequent. The important role of recently HIV-infected individuals was further supported by our findings that in 80% of the clusters of PWID with HIV-RNA > 106 IU/mL, there was at least one PWID with documented recent infection. This suggests that those individuals who have recently been infected with HIV may be the source of HIV transmission within transmission pairs.
These findings are in accordance with those from other analyses in TRIP and also with evidence from previous studies. It has been shown in TRIP that the proportion of recently HIV-infected persons among the HIV positives in the networks of recently HIV-infected seeds was approximately 3 times the proportion of recently HIV-infected persons in the networks of seeds with long-term HIV infection [10]. Seeds refer to primary participants recruited by TRIP to start the network-based intervention. Brenner et al. have reported that almost 50% of viral sequences from primary infections in Quebec, Canada were clustered into groups of 2-17 sequences/cluster [43]. Pinkerton used mathematical modeling to show that the probability of HIV transmission during acute infection was approximately 42 times higher than that during chronic HIV infection [65]. The same analysis revealed that the acute phase was responsible for 89% of all transmission events in the first 20 months of follow-up [65]. Miller et al. also highlighted the important role of acute and early HIV infection (due to high levels of HIV-RNA during these stages), as opposed to chronic infection, in sexual transmission [66]. Similarly, several studies reported that during HIV outbreaks among PWID, a high number of individuals have been infected with genetically similar viruses [62, 64, 67-80] - a finding that supports the hypothesis of the involvement of recently HIV-infected people in HIV transmissions. The crucial role of the natural history of HIV-1 (high HIV-RNA) has also been investigated by simulation studies and was found to play a significant role (along with injection risk networks) during periods of high HIV prevalence among PWID [5].
CONCLUSION
In the current study, using molecular epidemiology methods, we generated additional evidence that transmissions were more likely to have occurred among PWID who had high HIV-RNA at the time the study was conducted. We also found that in 80% of the transmission pairs of PWID with very high viral load, there was at least one recent infection.
Given these findings, early diagnosis soon after infection and immediate treatment initiation to reduce viral load should receive attention as a major HIV prevention strategy.
Acknowledgements
The study was supported by the United States (US) National Institute on Drug Abuse [DP1 DA034989], the Hellenic Scientific Society for the Study of AIDS and STDs, and the WT/DoH ICONIC grant. The funding agencies had no role in study design, collection, analysis or interpretation of data or in the decision to submit the article for publication.
LIST OF ABBREVIATIONS
- ART
Antiretroviral treatment
- CRFs
Circulating Recombinant Forms
- GTR
Generalized Time Reversible
- HIV
Human immunodeficiency virus
- ICONIC
Infection Response through Virus Genomics
- LTNs
Local transmission networks
- PLHIV
People living with HIV
- PR
Protease
- PWID
People who inject drugs
- RT
Reverse transcriptase
- SH
Shimodaira-Hasegawa
- TRIP
Transmission Reduction Intervention Project
- URFs
Unique Recombinant Forms.
Ethics Approval and Consent to Participate
The study was approved by the Institutional Review Boards of the Hellenic Scientific Society for the study of AIDS and Sexually Transmitted Diseases (Athens, Greece), and of the National Development and Research Institutes (New York City, US).
Human and Animal Rights
No animals were used in the study. All humans research procedures followed were in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2008 (http://www.wma.net/en/20activities/10ethics/10helsinki/).
Consent for Publication
Each participant of TRIP gave informed consent.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Author Contributions
Conceptualization, D.P., E.N., A.Hat., S.R.F. and G.K.N.
Methodology, D.P. and G.K.N. designed the study
D.F., B.F., J.R., P.G. and Z.K. generated the full-length sequencing data
E.P. conducted the interviews with TRIP participants and facilitated blood collection
Formal analysis, E.-G.K., K.P., A.Had. and L.D.W.
Writing-original draft preparation, E.-G.K., D.F. and D.P.
Writing-review and editing, G.K.N., A.Hat., A.Had., S.R.F., L.D.W., K.P., B.F., J.R., P.G., Z.K., E.N. and E.P.
Supervision, D.P., E.N. and G.K.N.
REFERENCES
- 1.Bekker L-G., Beyrer C., Quinn T.C. Behavioral and biomedical combination strategies for HIV prevention. Cold Spring Harb. Perspect. Med. 2012;2:a007435. doi: 10.1101/cshperspect.a007435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latkin C.A., Davey-Rothwell M.A., Knowlton A.R., et al. Social network approaches to recruitment, HIV prevention, medical care, and medication adherence. J. Acquir. Immune Defic. Syndr. 2013;63(Suppl. 1):S54–S58. doi: 10.1097/QAI.0b013e3182928e2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh D., Krishnan A., Gibson B., et al. Social network strategies to address HIV prevention and treatment continuum of care among at-risk and HIV-infected substance users: A systematic scoping review. AIDS Behav. 2017;21:1183–1207. doi: 10.1007/s10461-016-1413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman S.R., Kottiri B.J., Neaigus A., et al. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am. J. Epidemiol. 2000;152:913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 5.Dombrowski K., Khan B., Habecker P., et al. The interaction of risk network structures and virus natural history in the non-spreading of HIV among people who inject drugs in the early stages of the epidemic. AIDS Behav. 2017;21:1004–1015. doi: 10.1007/s10461-016-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman S.R., Neaigus A., Jose B., et al. Sociometric risk networks and risk for HIV infection. Am. J. Public Health. 1997;87:1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson B.T., Redding C.A., DiClemente R.J., et al. A Network-individual-resource model for HIV prevention. AIDS Behav. 2010;14:204–221. doi: 10.1007/s10461-010-9803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amirkhanian Y.A., Kelly J.A., Kabakchieva E., et al. A randomized social network HIV prevention trial with young men who have sex with men in Russia and Bulgaria. AIDS. 2005;19:1897–1905. doi: 10.1097/01.aids.0000189867.74806.fb. [DOI] [PubMed] [Google Scholar]
- 9.Latkin C.A., Knowlton A.R. Micro-social structural approaches to HIV prevention: a social ecological perspective. AIDS Care. 2005;17:102–113. doi: 10.1080/09540120500121185. [DOI] [PubMed] [Google Scholar]
- 10.Nikolopoulos G., Pavlitina E., Muth S., et al. A network intervention that locates and intervenes with recently HIV-infected persons: The Transmission Reduction Intervention Project (TRIP). Sci. Rep. 2016;6:38100. doi: 10.1038/srep38100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hull M.W., Montaner J. Antiretroviral therapy: A key component of a comprehensive HIV prevention strategy. Curr. HIV/AIDS Rep. 2011;8:85–93. doi: 10.1007/s11904-011-0076-6. [DOI] [PubMed] [Google Scholar]
- 12.Hull M.W., Montaner J.S.G. HIV treatment as prevention: The key to an AIDS-free generation. Yao Wu Shi Pin Fen Xi. 2013;21:S95–S101. doi: 10.1016/j.jfda.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tymejczyk O., Jamison K., Pathela P., et al. HIV Care and Viral Load Suppression After Sexual Health Clinic Visits by Out-of-Care HIV-Positive Persons. AIDS Patient Care STDS. 2018;32:390–398. doi: 10.1089/apc.2018.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn T.C., Wawer M.J., Sewankambo N., et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 15.Tovanabutra S., Robison V., Wongtrakul J., et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J. Acquir. Immune Defic. Syndr. 2002;29:275–283. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hughes J.P., Baeten J.M., Lingappa J.R., et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J. Infect. Dis. 2012;205:358–365. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Z., Li Y., Jiang Y., et al. Population HIV transmission risk for serodiscordant couples in Guangxi, Southern China: A cohort study. Medicine (Baltimore) 2018;97(36):e12077. doi: 10.1097/MD.0000000000012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingappa J.R., Hughes J.P., Wang R.S., et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5:e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montain J., Ti L., Hayashi K., et al. Impact of length of injecting career on HIV incidence among people who inject drugs. Addict. Behav. 2016;58:90–94. doi: 10.1016/j.addbeh.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood E., Kerr T., Marshall B.D.L., et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Romero J., Río I., Castilla J., et al. Absence of transmission from HIV-infected individuals with HAART to their heterosexual serodiscordant partners. Enferm. Infecc. Microbiol. Clin. 2015;33:666–672. doi: 10.1016/j.eimc.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Pedraza M.A., del Romero J., Roldán F., et al. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J. Acquir. Immune Defic. Syndr. 1999;21:120–125. [PubMed] [Google Scholar]
- 23.Donnell D., Baeten J.M., Kiarie J., et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murnane P.M., Hughes J.P., Celum C., et al. Using plasma viral load to guide antiretroviral therapy initiation to prevent HIV-1 transmission. PLoS One. 2012;7:e51192. doi: 10.1371/journal.pone.0051192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loutfy M.R., Wu W., Letchumanan M., et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS One. 2013;8:e55747. doi: 10.1371/journal.pone.0055747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spittal P.M., Craib K.J.P., Wood E., et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ. 2002;166:894–899. [PMC free article] [PubMed] [Google Scholar]
- 27.Kerr T., Marshall B.D.L., Milloy M-J., et al. Patterns of heroin and cocaine injection and plasma HIV-1 RNA suppression among a long-term cohort of injection drug users. Drug Alcohol Depend. 2012;124:108–112. doi: 10.1016/j.drugalcdep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wawer M.J., Gray R.H., Sewankambo N.K., et al. Rates of HIV‐1 transmission per coital act, by stage of HIV‐1 infection, in Rakai, Uganda. J. Infect. Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 29.Cohen M.S., Smith M.K., Muessig K.E., et al. Antiretroviral treatment of HIV-1 prevents transmission of HIV-1: where do we go from here? Lancet. 2013;382:1515–1524. doi: 10.1016/S0140-6736(13)61998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown A., Gill O., Delpech V. HIV treatment as prevention among men who have sex with men in the UK: is transmission controlled by universal access to HIV treatment and care? HIV Med. 2013;14:563–570. doi: 10.1111/hiv.12066. [DOI] [PubMed] [Google Scholar]
- 31.Anglemyer A., Horvath T., Rutherford G. Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA. 2013;310:1619. doi: 10.1001/jama.2013.278328. [DOI] [PubMed] [Google Scholar]
- 32.Solomon S.S., Mehta S.H., McFall A.M., et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. Lancet HIV. 2016;3:e183–e190. doi: 10.1016/S2352-3018(16)00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen M.S., Gay C.L. Treatment to prevent transmission of HIV‐1. Clin. Infect. Dis. 2010;50:S85–S95. doi: 10.1086/651478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiebig E., Wright D., Rawal B., et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2013;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 35.Hollingsworth T.D., Anderson R.M., Fraser C. HIV-1 transmission, by stage of infection. J. Infect. Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 36.Leynaert B n d., Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: Variability of infectivity throughout the course of infection. Am. J. Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 37.Bellan S.E., Dushoff J., Galvani A.P., et al. Reassessment of HIV-1 acute phase infectivity: Accounting for heterogeneity and study design with simulated cohorts. PLoS Med. 2015;12:e1001801. doi: 10.1371/journal.pmed.1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray R.H., Wawer M.J., Brookmeyer R., et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 39.Serwadda D., Gray R.H., Wawer M.J., et al. The social dynamics of HIV transmission as reflected through discordant couples in rural Uganda. AIDS. 1995;9:745–750. doi: 10.1097/00002030-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 40.Kiwanuka N., Laeyendecker O., Quinn T.C., et al. HIV-1 subtypes and differences in heterosexual HIV transmission among HIV-discordant couples in Rakai, Uganda. AIDS. 2009;23:2479–2484. doi: 10.1097/QAD.0b013e328330cc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safren S.A., Mayer K.H., Ou S-S., et al. Adherence to early antiretroviral therapy: Results from HPTN 052, a phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. J. Acquir. Immune Defic. Syndr. 2015;69:234–240. doi: 10.1097/QAI.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrosioni J., Junier T., Delhumeau C., et al. Impact of highly active antiretroviral therapy on the molecular epidemiology of newly diagnosed HIV infections. AIDS. 2012;26:2079–2086. doi: 10.1097/QAD.0b013e32835805b6. [DOI] [PubMed] [Google Scholar]
- 43.Brenner B.G., Roger M., Routy J., et al. High rates of forward transmission events after acute/early HIV‐1 infection. J. Infect. Dis. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 44.Escudero D.J., Lurie M.N., Mayer K.H., et al. The risk of HIV transmission at each step of the HIV care continuum among people who inject drugs: a modeling study. BMC Public Health. 2017;17:614. doi: 10.1186/s12889-017-4528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paraskevis D., Kostaki E., Nikolopoulos G.K., et al. Molecular tracing of the geographical origin of human immunodeficiency virus type 1 infection and patterns of epidemic spread among migrants who inject drugs in athens. Clin. Infect. Dis. 2017;65:2078–2084. doi: 10.1093/cid/cix717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paraskevis D., Kostaki E., Magiorkinis G., et al. Prevalence of drug resistance among HIV-1 treatment-naive patients in Greece during 2003-2015: Transmitted drug resistance is due to onward transmissions. Infect. Genet. Evol. 2017;54:183–191. doi: 10.1016/j.meegid.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Vasylyeva T.I., Friedman S.R., Lourenco J., et al. Reducing HIV infection in people who inject drugs is impossible without targeting recently-infected subjects. AIDS. 2016;30:2885–2890. doi: 10.1097/QAD.0000000000001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drescher S.M., von Wyl V., Yang W-L., et al. Treatment-naive individuals are the major source of transmitted HIV-1 drug resistance in men who have sex with men in the swiss HIV cohort study. Clin. Infect. Dis. 2014;58:285–294. doi: 10.1093/cid/cit694. [DOI] [PubMed] [Google Scholar]
- 49.Yerly S., Junier T., Gayet-Ageron A., et al. The impact of transmission clusters on primary drug resistance in newly diagnosed HIV-1 infection. AIDS. 2009;23:1415–1423. doi: 10.1097/QAD.0b013e32832d40ad. [DOI] [PubMed] [Google Scholar]
- 50.Paraskevis D., Nikolopoulos G.K., Magiorkinis G., et al. The application of HIV molecular epidemiology to public health. Infect. Genet. Evol. 2016;46:159–168. doi: 10.1016/j.meegid.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Audelin A.M., Cowan S.A., Obel N., et al. Phylogenetics of the Danish HIV epidemic. J. Acquir. Immune Defic. Syndr. 2013;62:102–108. doi: 10.1097/QAI.0b013e318276becc. [DOI] [PubMed] [Google Scholar]
- 52.Kouyos R.D., von Wyl V., Yerly S., et al. Molecular epidemiology reveals long‐term changes in HIV type 1 subtype B transmission in Switzerland. J. Infect. Dis. 2010;201:1488–1497. doi: 10.1086/651951. [DOI] [PubMed] [Google Scholar]
- 53.Lourenço L., Colley G., Nosyk B., et al. High levels of heterogeneity in the HIV cascade of care across different population subgroups in British Columbia, Canada. PLoS One. 2014;9:e115277. doi: 10.1371/journal.pone.0115277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasylyeva T.I., Liulchuk M., Friedman S.R., et al. Molecular epidemiology reveals the role of war in the spread of HIV in Ukraine. Proc. Natl. Acad. Sci. USA. 2018;115:1051–1056. doi: 10.1073/pnas.1701447115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikolopoulos G.K., Katsoulidou A., Kantzanou M., et al. Evaluation of the limiting antigen avidity EIA (LAg) in people who inject drugs in Greece. Epidemiol. Infect. 2017;145:401–412. doi: 10.1017/S0950268816002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yebra G., Frampton D., Gallo Cassarino T., et al. A high HIV-1 strain variability in London, UK, revealed by full-genome analysis: Results from the ICONIC project. PLoS One. 2018;13:e0192081. doi: 10.1371/journal.pone.0192081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yebra G., Hodcroft E.B., Ragonnet-Cronin M.L., et al. Using nearly full-genome HIV sequence data improves phylogeny reconstruction in a simulated epidemic. Sci. Rep. 2016;6:39489. doi: 10.1038/srep39489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lole K.S., Bollinger R.C., Paranjape R.S., et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chu C., Selwyn P. Diagnosis and initial management of acute HIV infection. Am. Fam. Physician. 2010;81:1239–1244. [PubMed] [Google Scholar]
- 60.Stamatakis A. Raxml version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Price M.N., Dehal P.S., Arkin A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kostaki E., Magiorkinis G., Psichogiou M., et al. Detailed molecular surveillance of the HIV-1 outbreak among people who inject drugs (PWID) in Athens during a period of four years. Curr. HIV Res. 2017;15(6):396–404. doi: 10.2174/1570162X15666171120104048. [DOI] [PubMed] [Google Scholar]
- 63.Maddison W. Mesquite: A modular system for evolutionary analysis. 2018.
- 64.Paraskevis D., Nikolopoulos G., Fotiou A., et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pinkerton S.D. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav. 2008;12:677–684. doi: 10.1007/s10461-007-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller W.C., Rosenberg N.E., Rutstein S.E., et al. Role of acute and early HIV infection in the sexual transmission of HIV. Curr. Opin. HIV AIDS. 2010;5:277–282. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell E.M., Jia H., Shankar A., et al. Detailed transmission network analysis of a large opiate-driven outbreak of HIV infection in the United States. J. Infect. Dis. 2017;216:1053–1062. doi: 10.1093/infdis/jix307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niculescu I., Paraschiv S., Paraskevis D., et al. Recent HIV-1 outbreak among intravenous drug users in romania: Evidence for cocirculation of CRF14_BG and subtype F1 strains. AIDS Res. Hum. Retroviruses. 2015;31:488–495. doi: 10.1089/aid.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ragonnet-Cronin M., Jackson C., Bradley-Stewart A., et al. Recent and rapid transmission of HIV among people who inject drugs in scotland revealed through phylogenetic analysis. J. Infect. Dis. 2018;217:1875–1882. doi: 10.1093/infdis/jiy130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paraskevis D, Nikolopoulos GK, Sypsa V, et al. Molecular investigation of HIV-1 cross-group transmissions during an outbreak among people who inject drugs (2011-2014) in Athens, Greece. . Infect Genet Evol . [DOI] [PMC free article] [PubMed]
- 71.Paraskevis D, Nikolopoulos G, Tsiara C, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. . EuroSurveill . 2011. [DOI] [PubMed]
- 72.Kostaki E-G., Nikolopoulos G.K., Pavlitina E., et al. Molecular analysis of human immunodeficiency virus type 1 (HIV-1)-infected individuals in a network-based intervention (Transmission Reduction Intervention Project): Phylogenetics identify HIV-1-infected individuals with social links. J. Infect. Dis. 2018;218:707–715. doi: 10.1093/infdis/jiy239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kostaki E-G., Karamitros T., Bobkova M., et al. Spatiotemporal characteristics of the HIV-1 CRF02_AG/CRF63_02A1 epidemic in Russia and central Asia. AIDS Res. Hum. Retroviruses. 2018;34:415–420. doi: 10.1089/AID.2017.0233. [DOI] [PubMed] [Google Scholar]
- 74.Nikolopoulos G.K., Kostaki E-G., Paraskevis D. Overview of HIV molecular epidemiology among people who inject drugs in Europe and Asia. Infect. Genet. Evol. 2016;46:256–268. doi: 10.1016/j.meegid.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sallam M., Esbjörnsson J., Baldvinsdóttir G., et al. Molecular epidemiology of HIV-1 in Iceland: Early introductions, transmission dynamics and recent outbreaks among injection drug users. Infect. Genet. Evol. 2017;49:157–163. doi: 10.1016/j.meegid.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 76.Paraschiv S., Banica L., Nicolae I., et al. Epidemic dispersion of HIV and HCV in a population of co-infected Romanian injecting drug users. PLoS One. 2017;12:e0185866. doi: 10.1371/journal.pone.0185866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mozhgani S-H., Ebrahimian S.A., Gudarzi H., et al. CRF35-AD as the main circulating genotype of human immunodeficiency virus type 1 infection in Iran: A phylogenetic and demographic-based study. Intervirology. 2017;60:144–148. doi: 10.1159/000484691. [DOI] [PubMed] [Google Scholar]
- 78.Alexiev I., Shankar A., Dimitrova R., et al. Origin and spread of HIV-1 in persons who inject drugs in Bulgaria. Infect. Genet. Evol. 2016;46:269–278. doi: 10.1016/j.meegid.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peters P.J., Pontones P., Hoover K.W., et al. HIV Infection linked to injection use of oxymorphone in Indiana, 2014-2015. N. Engl. J. Med. 2016;375:229–239. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 80.da Silva França DD, Del-Rios NHA. HIV-1 infection among crack cocaine users in a region far from the epicenter of the HIV epidemic in Brazil: Prevalence and molecular characteristics. PLoS One. 2018;13:e0199606. doi: 10.1371/journal.pone.0199606. [DOI] [PMC free article] [PubMed] [Google Scholar]