Abstract
Background
Rheumatoid Arthritis (RA) is a devastating disease characterized by continual addition of leukocytes and T cells within the articular cavity causing inflammation and cartilage destruction. Withania somnifera is one of the most precious medicinal herbs, reported to have antioxidant, anti‐inflammatory, and immunomodulatory properties.
Objective
The purpose of this study was to evaluate anti-inflammatory activity of aqueous extract of Withania somnifera roots (WSAq) in Collagen Induced Arthritic (CIA) rats.
Methods
To achieve this, we assessed the level of inflammatory cytokines such as Tumor Necrosis Factor (TNF)-α, IL-1β, IL-6 and IL-10 in CIA rats. Further, transcription factor, oxidative stress parameters and CD+8 expressions were also analyzed in CIA rats.
Results
Arthritic rats showed a greater increase in the levels of pro inflammatory cytokines such as TNF-α, IL-1β, IL-6, transcription factor NF-κB and a decrease in IL-10 concentration than controls rats. Oral administration of WSAq at a dose of 300mg/kg.wt. (WSAq300) appreciably attenuated the production of these pro inflammatory cytokines. This anti-inflammatory activity of WSAq300 might be partly mediated through an increase in the secretion of IL-10 and inhibition of NF-κB activity. Further, arthritic rats also show increased oxidative stress as compared to control rats. This increased oxidative stress in the arthritic rats appears to be the outcome of both an activated pro-oxidant and a poor antioxidant defense system. Treatment with WSAq300 strongly ameliorates all these ROS parameters significantly to near normal. Additional, metalloproteinase MMP-8 levels were also measured and found to be increased in CIA rats, which after treatment with WSAq300 came down to near normal.
Conclusion
From the above results, it can be concluded that the use of WSAq300 may be a valuable supplement which can improve human arthritis.
Keywords: Collagen induced arthritis, inflammation, joint destruction, oxidative stress, rheumatoid arthritis, Withania somnifera
1. Introduction
Rheumatoid Arthritis (RA) is a chronic autoimmune inflammatory disease that primarily affects the synovium of diarthrodial joints [1]. Synovium is the protective covering made of synoviocytes that line the synovial cavity and are responsible for the smooth movement of the joints. Continuous infiltration of inflammatory cells, CD4+/CD8+ T cells and B cells in synovium of affected joints leads to synovial hyperplasia (pannus formation) that invades neighboring cartilage and subchondral bone and promotes articular destruction through secretion of various inflammatory cytokines such as Tumor necrosis factor alpha (TNF-α) and Interleukin-1 beta (IL-1β). These inflammatory cytokines in combination with Receptor Activator Nuclear Kappa Ligand (RANKL), may directly induce the synthesis of proteolytic enzyme, matrix metalloproteinase (MMPs), that contributes to cartilage and bone destruction [2-5]. In addition, these inflammatory cells also secrete ROS/RNS, which can compromise the cellular structure and function of a variety of significant biomolecules, such as proteins and membrane lipids in the joints of RA patients causing oxidative stress [6-8].
The uncontrolled expression of these inflammatory mediators can be governed by a set of pro-inflammatory signaling pathways in rheumatoid synovitis [9]. Among them, Nuclear Factor kappa B (NF-κB) has been acknowledged as a prime transcription factor that controls the expression of diverse proinflammatory cytokines and mediators like TNF-α IL-1β and iNOS [10]. Hence, the restraint of inflammation is not only a fundamental target for curing RA, but also crucial objectives for the development of new anti-inflammatory drugs. Hence, therapeutics that hold back the production of proinflammatory mediators, offer a new hope for treatment of RA.
Since, the last two decades or so, treatment strategy of RA has changed comprehensively, with considerable improvements in the quality and availability of accessible therapeutics. Currently available treatment modalities include Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), Disease-Modifying Antirheumatic Drugs (DMARDs) and biologics directed against immune abnormalities that allegedly drive synovial cell proliferation and cartilage erosion in RA. These drugs although efficient are still unable to induce remission or even cure in most patients and may cause some serious side effects on long term use [11-13]. These adverse reactions have propelled the search for new therapeutic options for RA. The present study was an attempt in such direction.
Methotrexate (MTX) is the most commonly used DMARDs and considered as a first-line disease-modifying drug in the treatment of RA. It inhibits the propagation of cells accountable for synovial inflammation by inhibiting the synthesis of purines and pyrimidines [14]. Although inhibition of the immune system may be beneficial in controlling autoimmune-mediated inflammatory responses, this potentially boosts the menace of infection in these patients.
Withania somnifera Dunal (Solanaceae) (WS) commonly known as Ashwagandha, is an important medicinal plant that has been used in Ayurvedic and indigenous medicine since long. Phytosteroids like Withaferin A and Withanloids are the main biologically active compound and most of the therapeutic properties of WS are attributed to them [15]. These compounds are reported to have anti-inflammatory, immunomodulatory, and anti-tumor properties. Recently various studies have shown that these compounds also inhibit the expression of MMPs and transcription factor NF-κB [16, 17]. Hence, the present investigation is aimed to assess the therapeutic efficacy of aqueous extract of Withania somnifera roots (WSAq) in Collagen Induced Arthritic (CIA) rats.
2. Materials and Methods
2.1. Chemical and Reagents
Complete Freund’s Adjuvant (CFA) and bovine collagen type II (CII) were purchased from Sigma-Aldrich, St. Louis, MO, USA. Immunoassay kits for TNF-α, IL-1β, IL-6 and IL-10 were purchased from Pierce Biotechnology, Rockford, USA. Diaminobenzidine (DAB), HRP conjugated secondary antibody, was obtained from Thermo scientific, USA. NF-κB and MMP-8 assay kits were supplied by Cusa Biotech, Hubei Province, China and Ray biotech, Norcross, Georgia USA respectively. Super Oxide Dismutase (SOD), Catalse (CAT), and Glutathione Peroxidise (GPx) assay kits were purchased from Bioassay Systems, Hayward, CA, USA. Polyclonol Primary antibodies for CD8+ were purchased from Santa Cruz USA and those for IL-1β, TNF-α and MMP-8 were purchased from Novus biological South park Way, USA. Withaferin- A, Withanone and Withanolide A standards were obtained from Sigma–Aldrich, St. Louis, MO, USA. Trizol reagent was purchased from invitrogen, Carlsbad CA, USA. Maxima SYBR Green/ROX qPCR Master Mix (2X) and Verso cDNA Synthesis Kit were purchased from Thermo Scientific, Waltham, MA USA. All other chemicals used were of analytical grade and obtained from either Sisco research laboratories, Mumbai, India or Qualigens fine chemicals, Mumbai, India.
2.2. Plant Material
Fresh roots of WS, were obtained from the local market of Delhi in the month of March and authenticated by Dr. Sunita Garg [Chief Scientist & Head, Raw Materials and Herbarium, National Institute of Science Communication and Information Resources (NISCAIR), New Delhi]. The voucher specimen (NISCAIR/RHMD/2013/2198/204) was deposited in the raw material and herbarium museum, NISCAIR, Delhi.
2.3. Preparation of Extract and High Performance Thin Layer Chromatography (HPTLC) Analysis
Soxhlet extraction was performed using Soxhlet apparatus. The dried root power (50g) first refluxed with water (500 ml) in a Soxhlet Apparatus for about 14 hours at room temperature. After extraction, the extract was pooled, concentrated in a vacuum evaporator and stored in refrigerator at 4°C.
The HPTLC analysis was performed on Aluminum precoated silica gel aluminum plate G60F254 (E.MERCK, Germany, 0.20 mm thickness) to confirm the presence of active steroidal constituents of Withania somnifera. The stock solution of standards (Withaferin A, Withanone and Withanoloid A) and sample were prepared in the concentration of 10mg/ml in methanol and aliquot was further diluted into volume with methanol to obtain a working stock solution of 10μg/ml. Both sample and standard solutions (8 μl-10 μl each) were applied on 20cmx10cm plates as 6mm band width using CAMAG Linomet V semiautomatic sample applicator with a 100 μl HPTLC Hamilton syringe under constant flow of nitrogen with a steady application rate of 100nL/sec. Mobile phase consisted of chloroform and ethyl acetate in a ratio of 1:1 (v/v). The chromatogram was developed in a chromatographic twin-trough vertical glass chamber for 20 min at room temperature up to the distance of 80mm and air dried at room temperature. The presence or absence of the investigated compounds was determined according to their Rf values with the corresponding spot of Standards.
2.4. Test Animals
Male Wistar albino rats of body weight 140-150 g were obtained from the Central Animal House facility of the Institute. The animals were allowed to acclimatize for one week before the initiation of the experiment. All animals received humane care in compliance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC Approval No. – IAEC/2014-104 dated 6th March, 2014).
2.5. Induction of Arthritis
Arthritis in rats was induced by immunization with bovine type II collagen (CII) as described by Campo et al. [18]. Collagen was dissolved in 0.1 M acetic acid (2mg/ml) by gently stirring overnight at 4°C and emulsified with an equal amount of Complete Freund’s Adjuvant (CFA). Each rat was immunized with 0.2 ml (200 μg) of the emulsion by subcutaneous injection at the base of the tail. To ensure a high incidence and severity of arthritis, a booster injection with 0.2 ml (200 μg) emulsion was given 14 days after immunization. After the second immunization, the development of arthritic symptoms such as redness, swelling of each joint was monitored macroscopically.
2.6. Grouping and Treatment
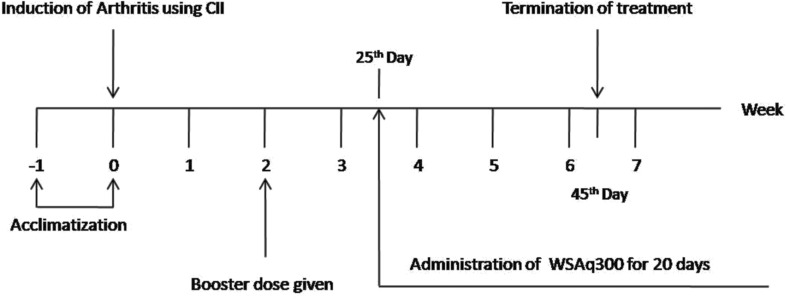
The induced animals were randomly divided into 4 groups (6 animals each). The dose of WSAq was selected after literature survey and preliminary studies. Various treatments were started on the 25th day and continued for subsequent 20 days (Fig. 1).
Fig. (1).
Treatment protocol.
Group I – Control (non-induced + Normal saline).
Group II – CIA (treated with CII, for induction of RA) + Normal Saline.
Group III- CIA + Treated with Methotrexate (MTX, 0.25 mg/kg body weight i.p. once weekly) [19].
Group IV- CIA+ Treated orally daily with WSAq at 300 mg/kg body weight (WSAq300).
*-Dose was selected on the basis of a preliminary study in our laboratory.
2.7. Assessment of Clinical Severity
Clinical severity was assessed by macroscopic scoring system of each affected paw on a regular interval from day 0 - 45th [20].
2.8. Collection of Samples
At the end of the treatment, rats from each group were fasted overnight and blood (retro-orbital vein puncture), joint tissues were collected for estimation of cytokines and activities of antioxidants in serum/tissue.
2.9. Estimation of Cytokines
The pro inflammatory cytokines such as TNF-α, IL-1β, IL-6 and anti-inflammatory cytokine IL-10 were measured in serum by using standard Elisa kits according to the manufacturer’s protocol using a micro plate reader (Thermo Scientific, USA).
2.10. Estimation of NF-κB and MMP-8 Level
Transcription factor NF-κB and MMP-8 level were determined in tissue homogenate/serum by a commercially available rat specific Elisa kit as per the manufacturer’s instructions using a micro plate reader (Thermo Scientific, USA).
2.11. Estimation of Antioxidant Enzymes
Superoxide Dismutase (SOD), Glutathione Peroxidase (GPx), and Catalase (CAT) activity in rat serum were measured using standard kits according to the manufacturer’s protocol.
2.12. Expression Analysis by Quantitative Real-Time PCR (qPCR)
2.12.1. RNA Isolation, Quantification and cDNA Synthesis
The quantitative mRNA levels of iNOS, NF-κB, TNF-α and MMP-8 were analyzed in joint tissue using qPCR to compare mRNA expression levels of each group. After completion of experiment, synovial tissues were removed and fixed in the Trizol reagent for the extraction of RNA. Extracted RNA was dissolved in nuclease free water (NFW) and its quality/quantity was determined spectrophotometrically (260/280 nm, 260/230 nm) using a Nano Drop (Thermo Scientific, Wilmington, DE, USA).
A small amount (1μg) of total RNA, obtained from the individual joint of six rats of each treatment group was used as the input for the amplification and generation of cDNA using the Verso cDNA Synthesis Kit. The obtained cDNA was used to determine the mRNA levels of iNOS, NF-κB, TNF-α and MMP-8 by real-time quantitative PCR using Maxima SYBR Green/ROX qPCR Master mix.
2.12.2. Real Time PCR (qPCR)
Real-time PCR was conducted using a Rotor Gene 96 Real-Time Quantitative Thermal Block (Qiagen). The amplificatory reactions were performed containing 12.5μl (2X) Maxima SYBR Green Premix, each 1μl primer (forward and reverse primer), (Table 1) and 1-2 μl of ≤500ng cDNA and the rest of nuclease free water in a final volume of 25μl. The amplification parameters consisted of an initial denaturation at 95°C for 5 min followed by 40 cycles of a 3-step PCR (denaturation at 95°C for 15 sec, annealing for 30s (iNOS, 57.6°C; NF-κB, 55.6°C; TNF-α, 54.8°C; MMP-8, 57.3°C) and extension at 72°C for 30 sec. The fluorescence resulting from the incorporation of SYBR Green dye into the double-stranded DNA produced during the PCR was quantified using the threshold cycle (Ct). The relative fold change in the expression of iNOS, NF-κB, TNF-α, MMP-8 mRNA was calculated by the 2-ΔΔCt method [21]. The results were normalized to the mRNA expression level of GAPDH in each sample.
Table 1.
Sequence of primers used in Quantitative real-time PCR.
| Gene | Sequence | References |
|---|---|---|
| iNOS | Forward5’CTTGGAAGAGGAACAACTACTGCT3’ Reverse5’-GCCAAATACCGCATACCTGAA-3’ |
[22] |
| NF-κB | Forward5’-ACGATCTGTTTCCCCTCATCT-3’ Reverse5’- TGCTTCTCTCCCCAGGAATA-3’ |
[23] |
| TNF-α | Forward5’- GGGCTTGTCACTCGAGTTTT-3’ Reverse5’-TGCCTCAGCCTCTTCTCATT-3’ |
[24] |
| MMP-8 | Forward5’-TGGTCTTCAGGCTGCTTATG-3’ Reverse-5’- CTTGGACACTCCTTGGGAAT-3’ |
[25] |
| GAPDH | Forward5’-AACGACCCCTTCATTGAC-3’ Reverse-5’-TCCACGACATACTCAGCA-3’ |
[26] |
2.13. Histological Studies
At the end of the study, rats were euthanized under CO2 inhalation and sacrificed. Hind limbs were removed and fixed in 10% buffered formalin. The joints were decalcified in 10% EDTA.2Na solution. The decalcified bones were processed, embedded, cut into 4µm thick sections and haematoxylin and eosin (H & E) staining was carried out. Histopathological changes of the joint were evaluated based on histologic parameters (inflammation, pannus formation, and corrosion of cartilage) using a 0 ~ 4-point scale: 0 = normal; 1 = inflammatory cell infiltration; 2 = pannus formation; 3 = cartilage destructions; 4 = severe cartilage and subchondral bone damage.
2.14. Immunohistochemistry Analysis
CD8+, TNF-α, and IL-1β expression in joint tissues was analyzed through immunohistochemical staining by streptavidin binding (LSAB) method. Briefly, before starting immunohistochemical staining, slides were subjected to heat-induced deparaffinization by putting slides on hot plate and subsequently using xylene, followed by rehydration via constant changing in graded alcohols. Masked epitopes can be retrieved by Heat-Induced Epitope Retrieval (HIER) method with Citrate buffer (pH-6.0)/ Tris-EDTA buffer (pH-8.0). After this, endogenous peroxidases were blocked by adding 4% H2O2 in methanol (v/v) and incubating the slides in the dark for 45 min at room temperature. After washing, sections were incubated overnight at 4°C with anti-rat-CD8+, anti-rat-TNF-α and anti-rat-IL-1β primary antibodies at dilution of 1:100 for CD8+, 1: 250 for TNF-α and 1:200 for IL-1β respectively. The slides were then washed with TBS again to remove the unbound antibodies, HRP-conjugated secondary antibody thereafter. After this, the slides were incubated with HRP substrate i.e. 3,3-diaminobenzidine (DAB) in the dark for 5-10 minutes that converted the DAB into a brown product. Finally, the sections were dehydrated in graded alcohol, air dried and mounted in DPX for microscopic examination.
3. Statistical Analysis
All values are expressed as mean ± SD. Data were analysed by one-way ANOVA using SPSS version 16 (SPSS, Chicago, USA) statistical program and the individual comparisons of the treatment were obtained by Tukey’s test for multiple comparisons. All P values were two-sided. A difference of p<0.05 was considered to be statistically significant.
4. Results
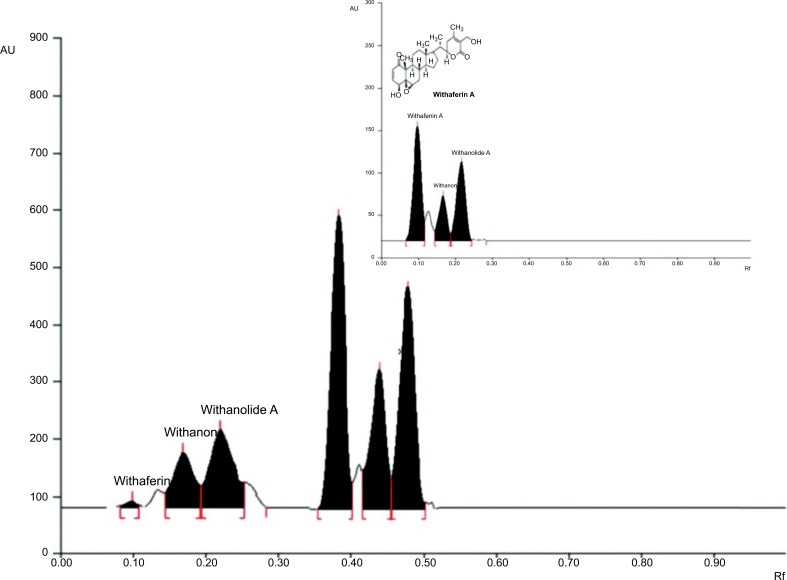
4.1. HPTLC Analysis of Aqueous Extract of Withania somnifera Root
HPTLC chromatograms of standards of Withaferine A, Withanone and Wthanolide in WS and WSAq are shown in Fig. (2). The peak purities of individual Withaferine A, Withanone and Wthanolide were identified by comparing in situ UV spectra. The Limit of Detection (LOD) and Limit of Quantification (LOQ) for Withaferine A and Withanone were 2.0 and 25.00 ng per band and 2.0 and 25.00 ng per band, respectively; whereas for Wthanolide, these were 10.00 and 100.00 ng per band (Fig. 2).
Fig. (2).
HPTLC analysis of aqueous extract of Withania somnifera root.
4.2. Effect of Aqueous Extract of Withania somnifera Root on Clinical Severity of Arthritis
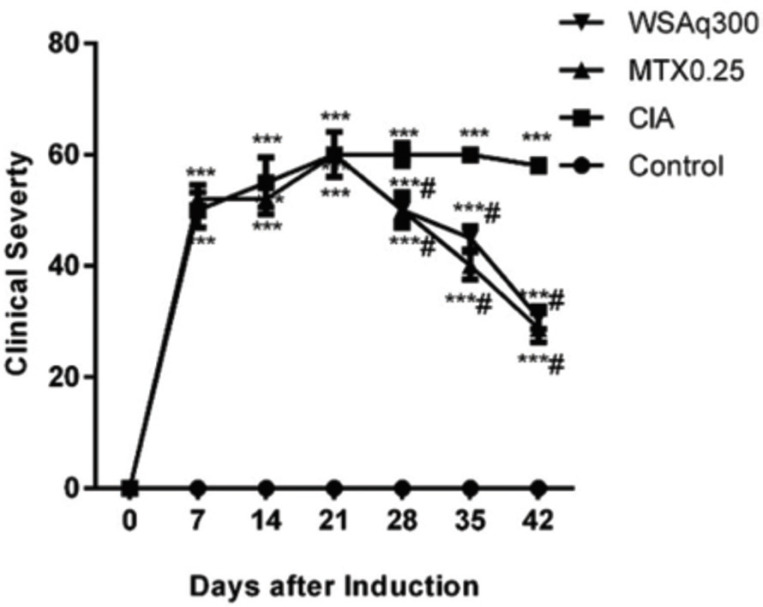
Arthritic rats showed an increase in thickness in paws and ankles as evidenced by increased inflammation (Fig. 3). The extent of inflammation and edema of paw and ankle was decreased in WSAq300 treated rats as compared to the CIA rats (p<0.05).
Fig. (3).
Effects of aqueous extract of Withania somnifera root on Progression and Severity of CIA. ***p<0.0001 vs. control rats, # p<0.0001 vs. CIA rats.
4.3. Effect of Aqueous Extract of Withania somnifera Root on Expression and Synthesis of TNF-α
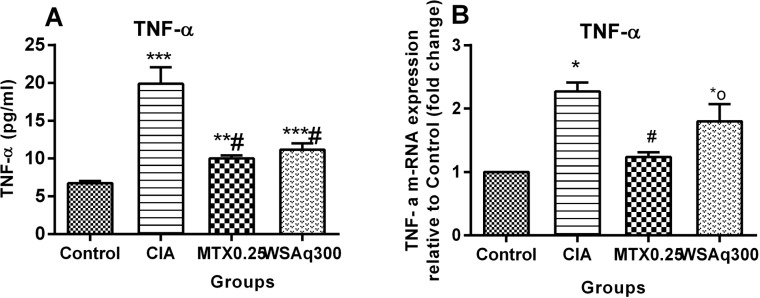
Levels of inflammatory cytokine, TNF-α were measured as a marker of inflammation. As shown in Fig. (4A), the level of TNF-α was elevated in CIA rats (1.95 fold) as compared to control group (p < 0.0001). However, after treatment with WSAq300, the level of TNF-α was decreased significantly (0.44 fold) as compared to CIA rats (p < 0.0001). In compliance with this, transcription levels of TNF-α were also up regulated significantly in CIA rats as compared to control rats (2.27 fold; P<0.01). This increase in mRNA levels was repressed by treatment with WSAq300 (1.80 fold vs. Control rats; p<0.05; Fig. 4B) and the results were comparable to that of MTX-treated rats.
Fig. (4).
Effects of aqueous extract of Withania somnifera root on level of TNF-α in CIA rats. Data are expressed as Means ± SD. of 6 rats per group.*o p<0.05, *p<0.01, **p<0.001, ***p<0.0001 vs. control rats, # p<0.0001vs. CIA rats.
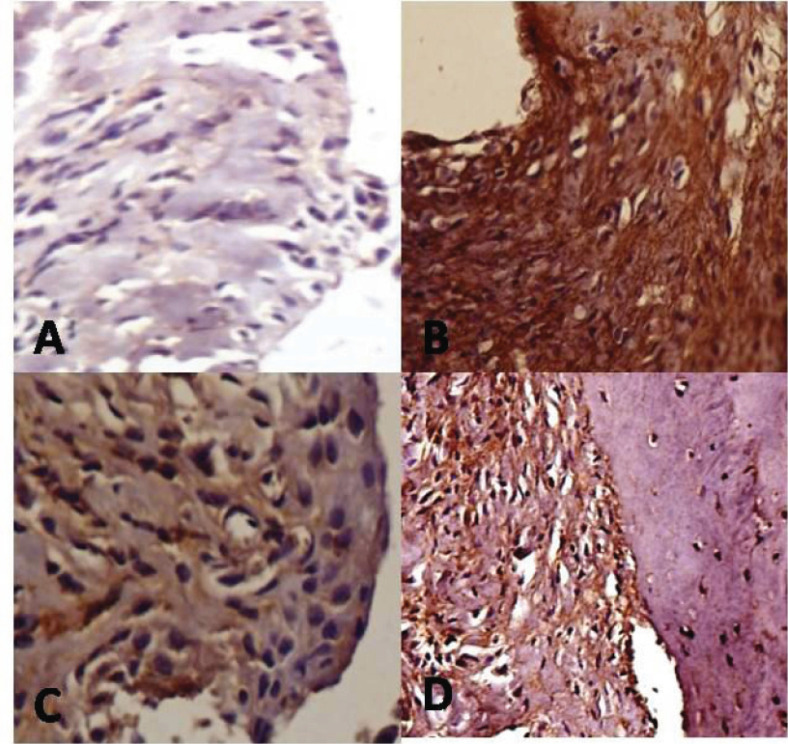
Further, we checked these results by IHC analysis of joint tissue and the results were consistent with that of ELISA and qPCR. Compared to control rats, TNF-α in joint tissue of CIA rats showed an increase in the expression as evidenced by strong cytoplasmic staining in inflammatory cells and synovial fibroblast in the synovial lining of articular cartilage. Treatment with WSAq300 caused a decrease in the expression of TNF-α as evidenced by low number of positively stained cells and the results were very much similar to that of MTX-treated rats (Fig. 5).
Fig. (5).
Effects of Aqueous Extract of Withania somnifera root on expression of TNF-α in knee joints of CIA rats. The positive cells brown cytoplasmic staining. A= Control rat (stained negatively for TNF-α), B= Induced rat, C= MTX treated rat D= WSAq300 treated rats. (Dilution -1:250, 20X). (The color version of the figure is available in the electronic copy of the article).
4.4. Effect of Aqueous Extract of Withania somnifera Root on Expression and Synthesis of Cytokines IL-1β, IL-6, IL-10
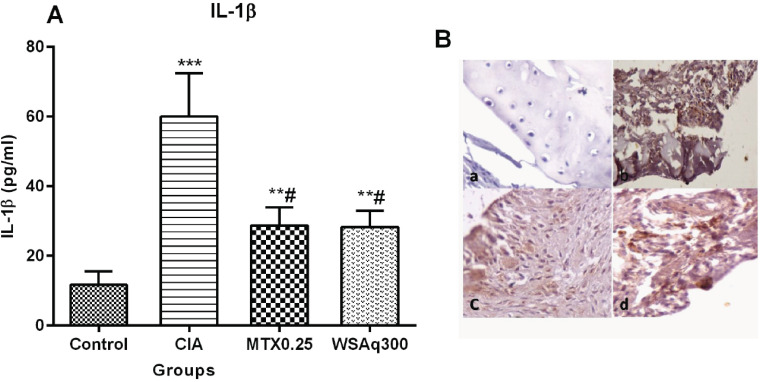
Cytokine IL-1β is reported to play critical roles in the pathogenesis of RA. Therefore, we investigated the therapeutic effects of WSAq treatment on concentration of IL-1β. We found increased level of IL-1β in CIA rats (4.14 fold; p<0.0001) as compared to the healthy control rats. The IL-1β levels were decreased significantly after oral treatment with WSAq300 (0.53 fold; p<0.0001) and the results were comparable to that of MTX-treated rats (Fig. 6). In addition to this, we also investigated whether the ameliorating effects of WSAq300 treatment were associated with decrease in IL-1β protein expression in the arthritic joints by immunohistochemical staining. Strong staining in IL-1β positive cells (macrophages and synovial fibroblast) was observed in articular cartilage of CIA rats as compared to control rats.
Fig. (6).
(A) Effects of aqueous extract of Withania somnifera root on serum level of IL-1β in CIA rats. Data are expressed as Means ± SD. of 6 rats per group. **p<0.001, ***p<0.0001 vs control rats, # p<0.0001vs CIA rats. (B) Effects of WSAq300 on expression of IL-1β in knee joints of CIA rats. The positive cells brown cytoplasmic staining. a= Control rat (stained negatively for IL-1β), b= Induced rat, c= MTX treated rat d= WSAq300 treated rat. (Dilution -1:200,20X). (The color version of the figure is available in the electronic copy of the article).
Treatment with WSAq300 caused a decrease in the expression of IL-1β as evidenced by mild staining.
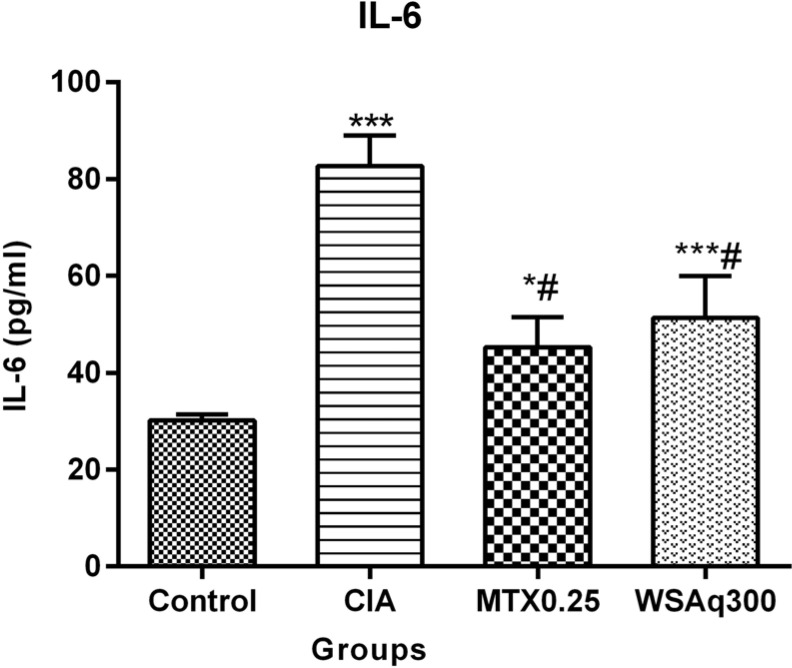
The inflammatory cytokine IL-6 was measured as a marker of inflammation. As shown in Fig. (7), the concentration of IL-6 was found to be increased in CIA rats (1.74 fold) as compared to the control rats and decreased significantly after oral treatment of WSAq300 (0.38 fold; p<0.0001). These results were comparable to that of MTX-treated rats.
Fig. (7).
Effects of aqueous extract of Withania somnifera root on serum level of IL-6 in CIA rats. Data are expressed as Means ± SD. of 6 rats per group. *p<0.01, ***p<0.0001 vs. control rats, # p<0.0001 vs. CIA rats.
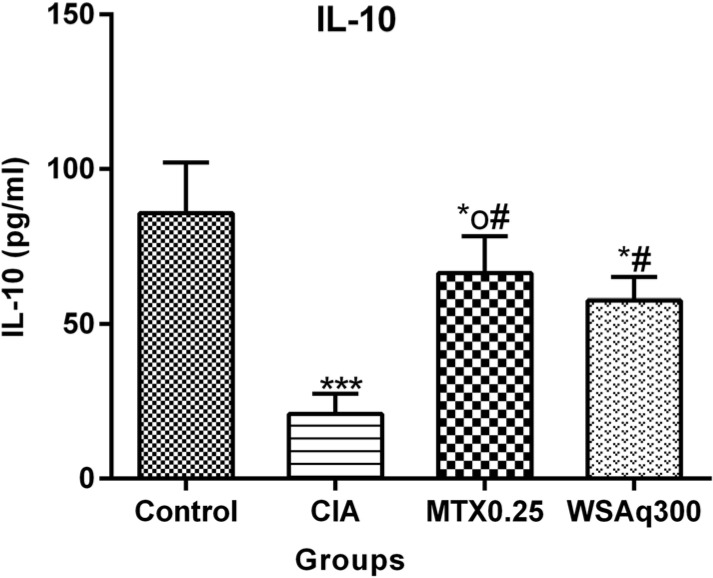
The concentration of IL-10 was evaluated to estimate endogenous defenses against inflammation. As illustrated in Fig. (8), the concentration of IL-10 was significantly decreased in CIA rats when compared to the control rats (p<0.0001). Treatment with WSAq300 caused abrupt increment in the level of IL-10 (1.75 fold) as compared to CIA rats (p<0.0001) and the result was comparable to that of MTX treated group.
Fig. (8).
Effects of aqueous extract of Withania somnifera root on IL-10 in CIA rats. Data are expressed as Means ± SD. of 6 rats per group. *op<0.05, *p<0.01, ***p<0.0001 vs. control rats, # p<0.0001 vs. CIA rats.
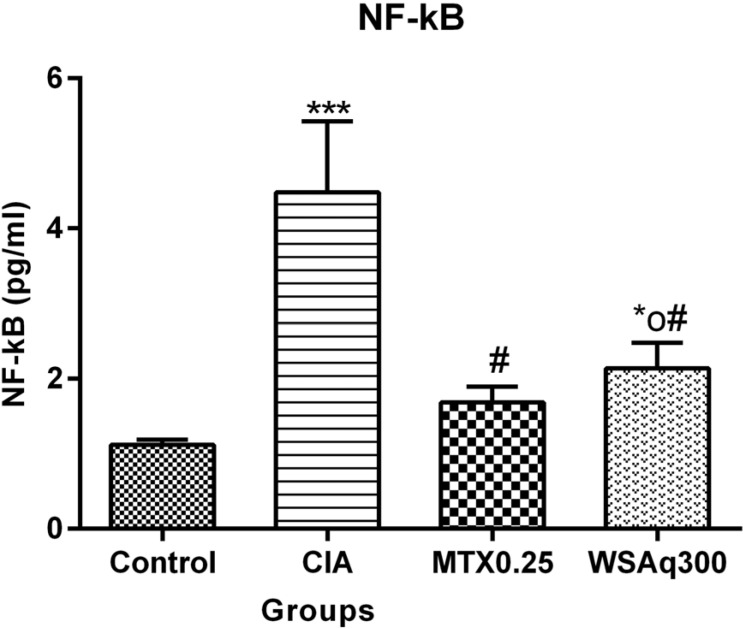
4.5. Effect of Aqueous Extract of Withania somnifera Root on Level of NF-κB
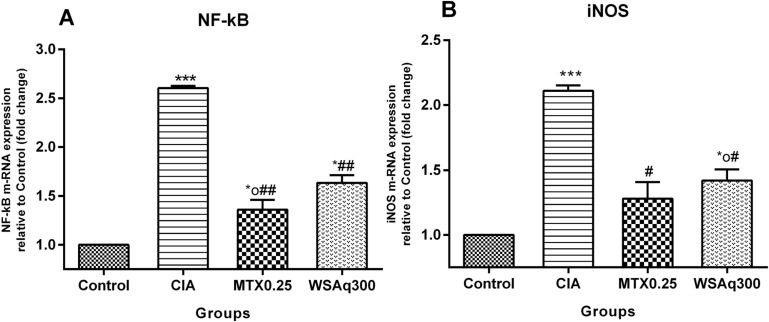
Transcription factor NF-κB was found to be increased in CIA rats as compared to control rats (p<0.0001). After treatment with WSAq300 there was a significant reduction (52.23%) in NF-κB levels as compared to CIA rats (p<0.0001) and the results were similar to that of MTX treated rats (Fig. 9), which were consistent with the mRNA expression pattern (Fig. 11A).
Fig. (9).
Effects of aqueous extract of Withania somnifera root on NF-κB in CIA rats. Data are expressed as Means ± SD. of 6 rats per group. *op<0.05, ***p<0.0001 vs. control rats, # p<0.0001 vs. CIA rats.
Fig. (11).
Effects of WSAq on m-RNA expression of (A) NF-κB (B) iNOS. Data are expressed as Means ± SD. of 6 rats per group. *op<0.05, *p<0.01, ***p<0.0001 vs. control rats, # p<0.0001, ## p<0.01 vs. CIA rats.
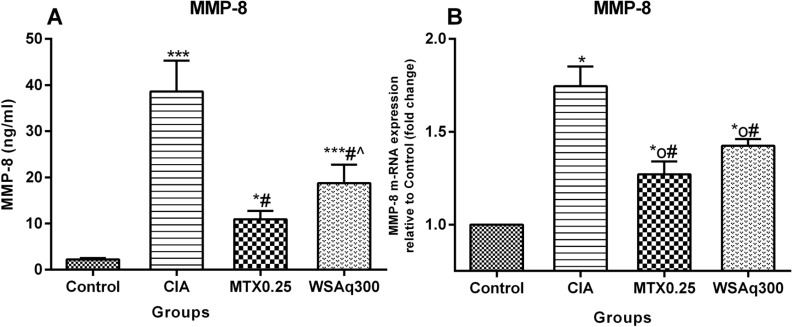
4.6. Effect of Aqueous Extract of Withania somnifera Root Treatment on Level of MMP-8
Metalloproteinase MMP-8 is a marker of cartilage destruction. In our study, we found that MMP-8 level in the serum of induced rats was significantly increased as compared to control rats (p<0.0001). Treatment with WSAq300 caused a significant reduction in MMP-8 as CIA rats, which was comparable to that of MTX treated rats (Fig. 10A). Further this result was confirmed by qPCR analysis. As predictable, mRNA level of MMP-8 was high in CIA rats (1.74 fold vs. Control rats; P<0.01). This increase in mRNA level was very much subdued by WSAq300 treatment (1.43 fold vs. Control rats; P<0.05; Fig. 10B).
Fig. (10).
Effects of aqueous extract of Withania somnifera root on MMP-8 in CIA rats. Data are expressed as Means ± SD. of 6 rats per group. *op<0.05, *p<0.01, ***p<0.0001 vs. control rats, # p<0.0001 vs. CIA rats, ^ p<0.0001 vs. MTX treated rats.
4.7. Effect of Aqueous Extract of Withania somnifera Root Treatment on iNOS Expression
To confirm the cellular defense of WSAq against CIA induced oxidative toxicity, iNOS mRNA level was examined by qPCR. It was observed that in CIA rats, iNOS mRNA level was considerably increased (2.11 fold vs. Control rats; P<0.001). After treatment with WSAq300, the levels were appreciably subdued (1.42 fold vs. Control rats; P<0.0001), which probably resulted in a decreased synthesis and production of nitric oxide, a reactive nitrogen species (Fig. 11B).
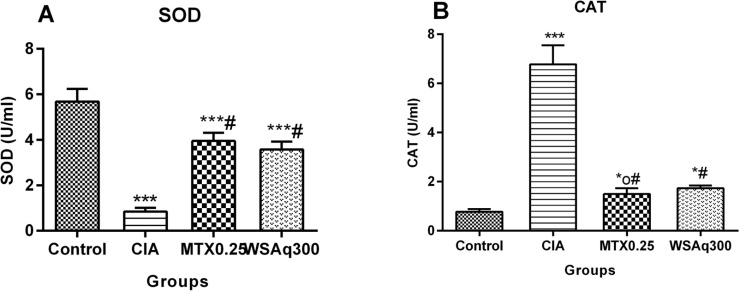
4.8. Effect of Aqueous Extract of Withania somnifera Root on Antioxidant Enzyme Activity
The concentration of SOD was evaluated to estimate endogenous defenses against superoxide anions. As illustrated in Fig. (12A), SOD activity was significantly decreased in arthritic untreated rats when compared to control rats (p < 0.0001). Treatment with WSAq300 appreciably increased the SOD activity (3.65 fold) as CIA rats (p < 0.0001) and the results were comparable to that of MTX treated rats.
Fig. (12).
Effects of aqueous extract of Withania somnifera root on (A) SOD (B) CAT activity. Data are expressed as Means ± SD. of 6 rats per group. *op<0.05, *p<0.01, ***p<0.0001 vs. control rats, #p<0.0001 vs. CIA rats.
In the present study, we also observed a significant increase in the activities of antioxidant enzyme (CAT) in CIA rats when compared to normal rats (Fig. 12B). Treatment with WSAq300 extracts significantly reversed these conditions to near normal levels (p < 0.0001) and the results were comparable to that of MTX treated rats.
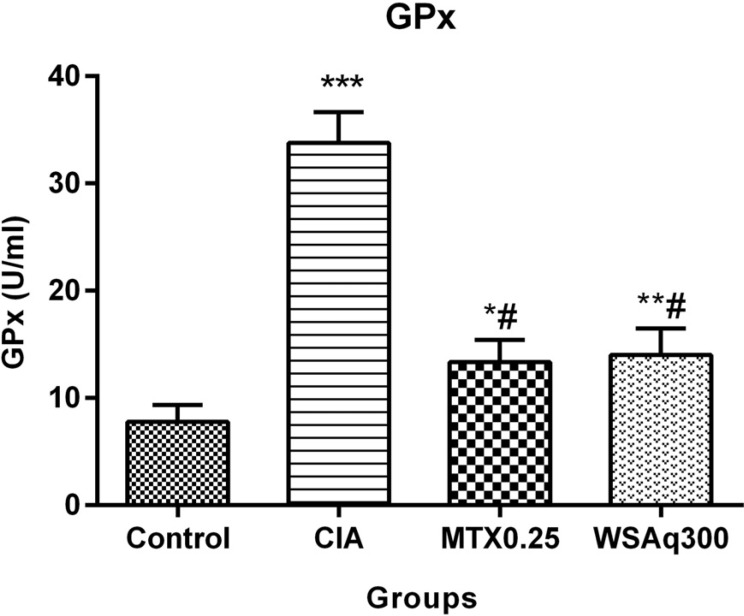
Further serum GPx activities were also assessed to check H2O2 mediated damage. A significant increase in the activity of GPx was observed in the CIA animals as compared to control (Fig. 13). Administration of WSAq300 notably decreased the GPx activity (0.59 fold) in comparison to CIA rats (p < 0.0001) and the results were comparable to that of MTX treated rats.
Fig. (13).
Effects of aqueous extract of Withania somnifera root on GPx activity. Data are expressed as Means ± SD. of 6 rats per group. *p<0.01, **p<0.001, ***p<0.0001 vs. control rats, #p<0.0001 vs. CIA rats.
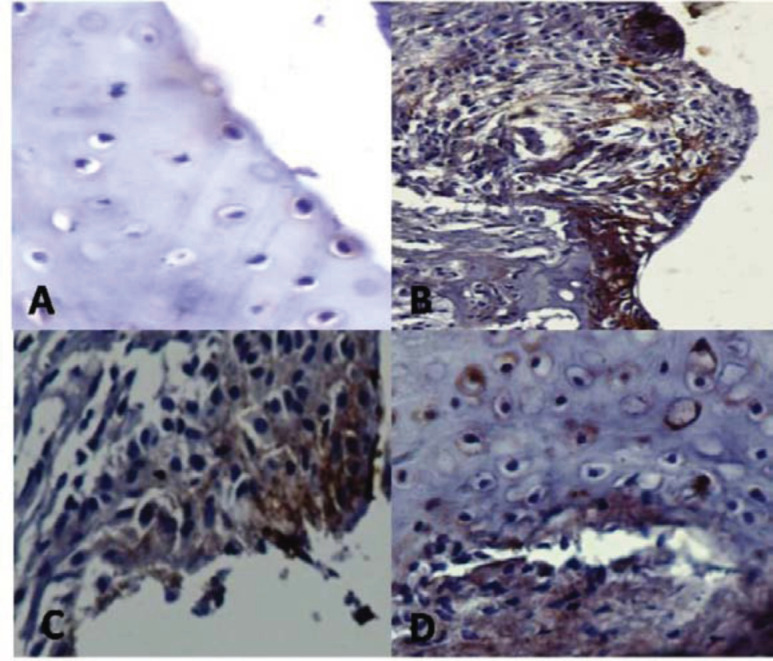
4.9. Effect of Aqueous Extract of Withania somnifera Root on Expression of CD8+ in Joint Tissue
Immunohistochemical analysis for the expression of CD8+was carried out to know about the
immunomodulation activity of WSAq in CIA rats. Result shows the intense positivity of CD8+ cells in joint tissue of CIA rats, which was characterized by brown colored cells compared to control rats. However, WSAq300 treatment downregulated the expression of CD8+ cells in treated rats (Fig. 14).
Fig. (14).
Effects of aqueous extract of Withania somnifera root on expression of CD8+ in knee joints of CIA rats. The positive cells show brown cytoplasmic staining. A= Control rat (stained negatively for CD8+), B= Induced rat, C= MTX treated rat, D= WSAq300 treated rat (Dilution -1:100, 20X). (The color version of the figure is available in the electronic copy of the article).
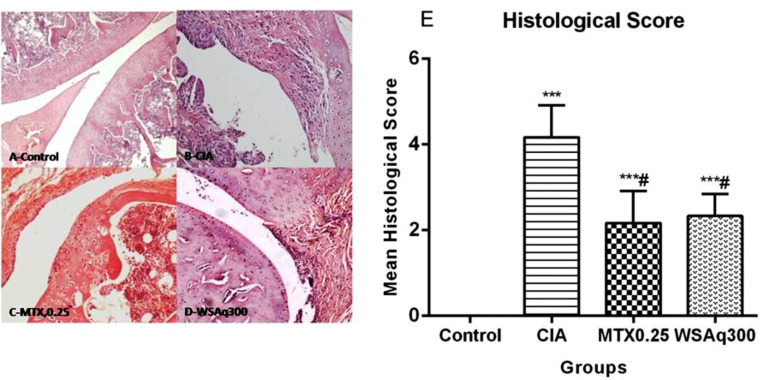
4.10. Effect of Aqueous Extract of Withania somnifera Root on Joint Histology
On day 20th after the primary treatment, the paw joints of the rats were examined. CIA rats show signs of noticeable pathological changes (Fig. 15A), including synovial hyperplasia, increased accumulation of inflammatory cells and extensive pannus formation. Indiscriminant cartilage remodeling and bone erosion were also prominent in CIA rats compared to vehicle control rats, causing an increase in morphological change score of CIA rat (P < 0.0001; Fig. 15E). However, oral administration of WSAq300 restructures these histopathological changes to near normal by significant reduction in bone/cartilage damage and cell influx (P < 0.0001). Thus, treatment of WSAq caused a substantial recovery in the joints of CIA rats (Fig. 15).
Fig. (15).
Effects of aqueous extract of Withania somnifera root, methotrexate therapy on joint tissue. No sign of inflammation and cartilage destruction in control rats (A), large influx of inflammatory cells, pannus formation, cartilage destruction in CIA rats (B), shows significant less inflammation in MTX (C) and WSAq300 (D) treated rats (E) Scoring of histopathological changes. ***p<0.0001 vs. control rats, #p<0.0001 vs. CIA rats.
5. Discussion
The present study is an attempt to evaluate the anti-arthritic effect of WSAq300 in CIA model. RA was assessed by observing architectural alterations of synovial tissue and the serological properties of disease condition such as oxidative stress markers and pro-inflammatory cytokines. In this study, we have demonstrated that treatment with WSAq reduces the symptoms of arthritis in rats by selectively inhibiting NF-κB pathway and cytokines critical to the development and maintenance of inflammation in RA.
Although animal models of RA may not produce exactly all the characteristics of human RA, they can assist in understanding the normal mechanism during RA and are hence used to assess therapeutic efficacy of newly introduced pharmacological agents [27]. The CIA model of rat we used in this study shows similarity in various aspects with human RA [28].
Nuclear factor NF-κB is a pleiotropic transcription factor and works as one of the key supervisors for triggering and amplifying inflammation in RA [29, 30]. In the basal state, NF-κB exists in the cytoplasm complex with the inhibitory-kB (IκB) proteins. On stimulation, phosphorylation of IκB by inhibitory-κB kinase (IKK) triggers its degradation and the activation of NF-κB. On activation, NF-κB translocates to the nucleus and starts the synthesis of various inflammatory mediators such as TNF-α and iNOS. Therefore, modulation of NF-κB activity either through inhibition of NF-κB translocation to the nucleus or through inhibition of DNA binding activity of NF-κB, could be an important strategy to reduce inflammation and cellular injury. Several studies reported that Withaferin A, a vital constituent of WS reduces NF-κB activation with a consequent reduction in cell damage [31]. Thus, we examined the attenuating effect of WSAq300 on NF-κB activity in CIA rats. In line with the above discussion, we found increased levels of NF-κB activity in CIA rats, which decreased after treatment with WSAq300 (Figs. 9-11A). This supports the hypothesis that the probable mechanism of action of WSAq300 in reducing arthritic joint inflammation is intimately related with either the in vivo inhibition of DNA binding activity of NF-κB or inhibition of NF-κB translocation to the nucleus.
In RA, T-cells are directly involved in the pathogenesis of RA including the formation of rheumatoid synovitis. CD8+ T cells plays a vital role in the formation and preservation of ectopic Germinal Centers (GCs) in rheumatoid synovitis. GCs are a characteristic finding in RA synovial membrane [32]. Further, studies also show that CD8+ T cells are directly or indirectly involved in the production of higher amounts of the chemokines and cytokines such as TNF-α in inflamed synovium [33]. In our study, we found an increased expression of CD8+ T cells in the joint tissue of CIA rats, however after treatment with WSAq300, the expression was decreased and this decrease may ultimately cause a decrease in the synthesis and production of inflammatory cytokines.
Tumor Necrosis Factor alpha (TNF-α) is a chief inflammatory mediator that can stimulate the secretion of numerous other inflammatory cytokines such as IL-1β and IL-6. In addition, TNF-α mediated activation of the NF-κB, establishes a positive feedback loop of this destructive cycle [34]. TNF-α along with other inflammatory cytokines has been shown to induce pannus formation, thereby promoting synovitis by recruiting inflammatory cells which lead to cartilage destruction and bone erosion in RA by promoting the expression of various collagenases, particularly collagenase-8 (MMP-8) [35, 36]. This is associated with increased type II collagen denaturation. Systemic levels of MMP-8 are increased in clinically and serologically active RA and are correlated with markers of the systemic inflammation. Hence, reduction in disease severity and bone resorption by inhibiting these molecules could have beneficial effects.
Cytokine IL-10, possesses potent anti-inflammatory characteristics and can suppress cartilage and bone pathology in patients with RA by suppressing the activation of IKK and so NF-κB [37]. In the present study, the increased serum levels of inflammatory cytokines TNF-α, IL-1β, IL-6 and MMP-8 were significantly decreased after treatment with WSAq, while, IL-10 levels were increased compared to arthritic rats, suggesting the anti-inflammatory action of WSAq (Figs. 4-8, 10). Further IHC, qPCR analysis of TNF-α, IL-1β, and MMP-8 showed a decrease in the expression of these genes in treated rats (P <0.05), which is consistent with biochemical results.
In the enzymatic antioxidant defence system, Superoxide Dismutase (SOD) scavenges superoxide radicals generated as a by-product of aerobic metabolism by catalyzing the conversion of O2. radicals into hydrogen peroxide. It is the primary intracellular antioxidant enzyme that protects cells and tissues through removing both intracellular and extracellular superoxide radicals and their derivatives. The observed decrease in SOD activity in CIA rats (Fig. 12A) could be due to either via direct oxidative damage of the SOD molecules or via oxidative modification of SOD gene expression, or both (P <0.0001). Our result is in agreement with other studies as well, which show that, increasing ROS causes a decrease in SOD activity [38]. However, after treatment with WSAq300, SOD activity was significantly attenuated to near normal levels (P <0.0001).
Hydrogen peroxide generated as a result of SOD activity is decomposed by ubiquitous protein Catalase (CAT) and Glutathione Peroxidase (GPx) into water and molecular oxygen. Under oxidative stress, the activity of GPx and CAT increases due to an increase of the maximum enzyme reaction rate (Km) or may be due to oxidant-induced transcriptional upregulation of GPx and CAT expression [39, 40]. As observed in the present study and some previously reported studies, oxygen free radicals are responsible for the increase in GPx and CAT activity (Fig. 12B and 13) in CIA rats, as compared to controls (P <0.0001; P <0.01 respectively). These results suggest an adaptive response in CIA rats to the increase in oxidative stress, and could be a consequence of a process of enzyme induction. These imbalances in the activity of enzymatic antioxidant are ameliorated by treatment with WSAq300 (P <0.0001). This result suggests that WSAq has an antioxidant activity and is responsible for the decrease in the production of ROS, which finally may inhibit the tissue damage caused due to oxidative stress.
Finally, all the results are positively correlated with histological analysis of the joint tissue of CIA rats that show a reduction in oxidative stress, inflammation and joint damage in WSAq300 treated rats, suggesting that WSAq300 is effective in suppressing the development of RA [41] (Fig. 15).
Conclusion
In conclusion, anti-inflammatory and cartilage protective effects of WSAq support the use of WS for the treatment of arthritis. However, further study is required for isolation and identification of these components of WSAq that will open new avenues for the development of effective preventive therapies against arthritis.
Acknowledgements
The authors are thankful to the Department of Biotechnology (DBT), Ministry of Science & Technology (Govt. of India) for providing financial support (SAN No.102/IFD/SAN/5388/2011-2012).
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The experimental protocol on animal was approved by the Institutional Animal Ethics Committee (IAEC Approval No. - IAEC/2014-104, dated 6th March, 2014), University College of Medical Sciences and GTB Hospital, University of Delhi, Dilshad Garden, Delhi, India.
HUMAN AND ANIMAL RIGHTS
No humans were used in this study. All the reported experiments on animals in the study were in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA).
Consent for Publication
Not applicable.
conflict of interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Scott D.L., Wolfe F., Huizinga T.W. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Carson D.A., Chen P.P., Kipps T.J. New roles for rheumatoid factor. J. Clin. Invest. 1991;87(2):379–383. doi: 10.1172/JCI115007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komatsu N., Takayanagi H. Inflammation and bone destruction in arthritis: Synergistic activity of immune and mesenchymal cells in joints. Front. Immunol. 2012;3:77. doi: 10.3389/fimmu.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuno H., Yudoh K., Katayama R., Nakazawa F., Uzuki M., Sawai T., Yonezawa T., Saeki Y., Panayi G.S., Pitzalis C., Kimura T. The role of TNF-alpha in the pathogenesis of inflammation and joint destruction in Rheumatoid Arthritis (RA): A study using a human RA/SCID mouse chimera. Rheumatology (Oxford) 2002;41(3):329–337. doi: 10.1093/rheumatology/41.3.329. [DOI] [PubMed] [Google Scholar]
- 5.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 6.Kamanli A., Naziroğlu M., Aydilek N., Hacievliyagil C. Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem. Funct. 2004;22(1):53–57. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 7.Seven A., Güzel S., Aslan M., Hamuryudan V. Lipid, protein, DNA oxidation and antioxidant status in rheumatoid arthritis. Clin. Biochem. 2008;41(7-8):538–543. doi: 10.1016/j.clinbiochem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths H. Is the generation of neo-antigenic determinants by free radicals central to the development of autoimmune rheumatoid disease? Autoimmun. Rev. 2008;7(7):544–549. doi: 10.1016/j.autrev.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Luo X., Zuo X., Mo X., Zhou Y., Xiao X. Treatment with recombinant Hsp72 suppresses collagen-induced arthritis in mice. Inflammation. 2011;34(5):432–439. doi: 10.1007/s10753-010-9250-z. [DOI] [PubMed] [Google Scholar]
- 10.Gerlag D.M., Ransone L., Tak P.P., Han Z., Palanki M., Barbosa M.S., Boyle D., Manning A.M., Firestein G.S. The effect of a T cell-specific NF-kappa B inhibitor on in vitro cytokine production and collagen-induced arthritis. J. Immunol. 2000;165(3):1652–1658. doi: 10.4049/jimmunol.165.3.1652. [DOI] [PubMed] [Google Scholar]
- 11.Roth S.H. Coming to terms with nonsteroidal anti-inflammatory drug gastropathy. Drugs. 2012;72(7):873–879. doi: 10.2165/11633740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Schiff M., Keiserman M., Codding C., Songcharoen S., Berman A., Nayiager S., Saldate C., Aranda R., Becker J.C., Nys M., Bars M.L., Reed D.M., Poncet C., Dougados M. Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: Open label extension of the ATTEST Study. Ann. Rheum. Dis. 2011;70(11):2003–2007. doi: 10.1136/annrheumdis-2011-200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdel-Tawab M., Werz O., Schubert-Zsilavecz M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011;50(6):349–369. doi: 10.2165/11586800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Cronstein B.N. The mechanism of action of methotrexate. Rheum. Dis. Clin. North Am. 1997;23(4):739–755. doi: 10.1016/s0889-857x(05)70358-6. [DOI] [PubMed] [Google Scholar]
- 15.Dar N.J., Hamid A., Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Cell. Mol. Life Sci. 2015;72(23):4445–4460. doi: 10.1007/s00018-015-2012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao R., Shah N., Lee J.S., Katiyar S.P., Li L., Oh E., Sundar D., Yun C.O., Wadhwa R., Kaul S.C. Withanone-rich combination of Ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol. Cancer Ther. 2014;13(12):2930–2940. doi: 10.1158/1535-7163.MCT-14-0324. [DOI] [PubMed] [Google Scholar]
- 17.Ichikawa H., Takada Y., Shishodia S., Jayaprakasam B., Nair M.G., Aggarwal B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol. Cancer Ther. 2006;5(6):1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 18.Campo G.M., Avenoso A., Campo S., Ferlazzo A.M., Altavilla D., Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res. Ther. 2003;5(3):R122–R131. doi: 10.1186/ar748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hultqvist M., Olofsson P., Gelderman K.A., Holmberg J., Holmdahl R. A new arthritis therapy with oxidative burst inducers. PLoS Med. 2006;3(9):e348. doi: 10.1371/journal.pmed.0030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmdahl R., Carlsen S., Mikulowska A., Vestberg M., Brunsberg U., Hansson A., Sunduall M., Jansson L., Pettersson U. Genetic analysis of murine models for rheumatoid arthritis. In: Adolpho K.W., editor. Human Genome Methods. New York: CRC Press; 1998. pp. 215–238. [Google Scholar]
- 21.Schmittgen T.D., Zakrajsek B.A., Mills A.G., Gorn V., Singer M.J., Reed M.W. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: Comparison of endpoint and real-time methods. Anal. Biochem. 2000;285(2):194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L., Sun T., Yu E., Yu L., Luo J., Li H., Fu Z. TNF -α expression, not iNOS expression, is correlated with NF-B activation in the spinal cord of rats following peripheral nerve injury. Afr. J. Biotechnol. 2011;10(34):6372–6380. [Google Scholar]
- 23.Khan M.S., Halagowder D., Devaraj S.N. Methylat-ed chrysin induces co-ordinated attenuation of the canonical Wnt and NF-κB signaling pathway and upregulates apoptotic gene expression in the early hepatocarcinogenesis rat model. Chem. Biol. Interact. 2011;193(1):12–21. doi: 10.1016/j.cbi.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Adán N., Guzmán-Morales J., Ledesma-Colunga M.G., Perales-Canales S.I., Quintanar-Stéphano A., López-Barrera F., Méndez I., Moreno-Carranza B., Triebel J., Binart N. Martínez, de. La.; Escalera. G.; Thebault, S.; Clapp, C. Prolactin promotes carti-lage survival and attenuates inflammation in in-flammatory arthritis. J. Clin. Invest. 2013;123(9):3902–3913. doi: 10.1172/JCI69485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschner J., Rath-Deschner B., Agarwal S. Regulation of matrix metalloproteinase expression by dynamic tensile strain in rat fibrochondrocytes. Osteoarthritis Cartilage. 2006;14(3):264–272. doi: 10.1016/j.joca.2005.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azizian M., Bathaie S.Z., Ashrafi M., Hoshyar R. Investigation of p53 and p27 expressions in the N-nitroso-N-methylureainduced breast cancer in female Wistar Albino rats. Physiol. Pharmacol. 2014;18(3):337–346. [Google Scholar]
- 27.Shimozuru Y., Yamane S., Fujimoto K., Terao K., Honjo S., Nagai Y., Sawitzke A.D., Terato K. Collagen-induced arthritis in nonhuman primates: multiple epitopes of type II collagen can induce autoimmune-mediated arthritis in outbred cynomolgus monkeys. Arthritis Rheum. 1998;41(3):507–514. [Google Scholar]
- 28.Horsfall A.C., Butler D.M., Marinova L., Warden P.J., Williams R.O., Maini R.N., Feldmann M. Suppression of collagen-induced arthritis by continuous administration of IL-4. J. Immunol. 1997;159(11):5687–5696. [PubMed] [Google Scholar]
- 29.Morel J., Berenbaum F. Signal transduction pathways: New targets for treating rheumatoid arthritis. Joint Bone Spine. 2004;71(6):503–510. doi: 10.1016/j.jbspin.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Tu S., Hu Y., Zeng K., Zhang M., Lai X., Weichen Z. Effects of triptolide on the expression and activity of NF-kappaB in synovium of collagen-induced arthritis rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2005;25(5):543–545. doi: 10.1007/BF02896012. [DOI] [PubMed] [Google Scholar]
- 31.Maitra R., Porter M.A., Huang S., Gilmour B.P. Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation. J. Inflamm. (Lond.) 2009;6:15. doi: 10.1186/1476-9255-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young C.L., Adamson T.C., Vaughan J.H., Fox R.I. Immunohistologic characterization of synovial membrane lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1984;27(1):32–39. doi: 10.1002/art.1780270106. [DOI] [PubMed] [Google Scholar]
- 33.Kang Y.M., Zhang X., Wagner U.G., Yang H., Beckenbaugh R.D., Kurtin P.J., Goronzy J.J., Weyand C.M. CD8 T cells are required for the formation of ectopic germinal centers in rheumatoid synovitis. J. Exp. Med. 2002;195(10):1325–1336. doi: 10.1084/jem.20011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Filippin L.I., Vercelino R., Marroni N.P., Xavier R.M. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin. Exp. Immunol. 2008;152(3):415–422. doi: 10.1111/j.1365-2249.2008.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morel J., Berenbaum F. Signal transduction pathways: New targets for treating rheumatoid arthritis. Joint Bone Spine. 2004;71(6):503–510. doi: 10.1016/j.jbspin.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Hashizume M., Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis (Egypt) 2011;2011:765624. doi: 10.1155/2011/765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schottelius A.J., Mayo M.W., Sartor R.B., Baldwin A.S., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J. Biol. Chem. 1999;274(45):31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 38.Mazzetti I., Grigolo B., Borzì R.M., Meliconi R., Facchini A. Serum copper/zinc superoxide dismutase levels in patients with rheumatoid arthritis. Int. J. Clin. Lab. Res. 1996;26(4):245–249. doi: 10.1007/BF02602957. [DOI] [PubMed] [Google Scholar]
- 39.Pandey K.B., Rizvi S.I. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid. Med. Cell. Longev. 2010;3(1):2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dieterich S., Bieligk U., Beulich K., Hasenfuss G., Prestle J. Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation. 2000;101(1):33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Khan M.A., Subramaneyaan M., Arora V.K., Banerjee B.D., Ahmed R.S. Effect of Withania somnifera (Ashwagandha) root extract on amelioration of oxidative stress and autoantibodies production in collagen-induced arthritic rats. J. Complement. Integr. Med. 2015;12(2):117–125. doi: 10.1515/jcim-2014-0075. [DOI] [PubMed] [Google Scholar]