Abstract
Background:
In homeothermic animals, approximately 50% of daily energy expenditure (EE) is spent to maintain a consistent core body temperature (CBT). In humans, little is known about CBT responses to feeding and overfeeding and their relationship to diet-related changes in EE.
Objective:
To study the effects of feeding and overfeeding on CBT and its association with diet-induced thermogenesis (DIT).
Design:
Fifty-three healthy men with normal glucose regulation and a wide range of body composition (mean±SD, body fat: 25±8%, range: 7–43%) had 24-h EE assessed during fasting in a whole-room indirect calorimeter with concomitant CBT measurement by ingestible capsules and 24-h urinary collection for catecholamine measurements. Changes in 24-h EE (DIT) and CBT compared to fasting were assessed during three normal-protein (20%) diets using a crossover design: one eucaloric diet (EBL, 50% carbohydrate, n=37) and two overfeeding diets with 200% energy requirements: a high-fat (FNP, 60% fat, n=25) and a high-carbohydrate (CNP; 75% carbohydrate, n=24) diet.
Results:
The average 24-h CBT (avgCBT) during fasting was 36.81±0.14°C (inter-individual CV=0.4%) and positively correlated with 24-h urinary epinephrine (r=0.61, p<0.001), but not with body composition measures (p>0.05). AvgCBT increased during EBL (Δ=0.06±0.11°C, p=0.002), FNP (Δ=0.13±0.14°C, p<0.001), and CNP (Δ=0.19±0.13°C, p<0.001) and associated with increased DIT during EBL (r=0.43, p=0.01, β=31 kcal/day/0.1°C) and FNP (r=0.60, p=0.002, β=43 kcal/day/0.1°C), but not CNP (p=0.47). A ceiling effect for the increase in CBT, but not in DIT, was observed during feeding and, particularly, overfeeding.
Conclusions:
CBT increases with feeding and is moderately associated with DIT to a different degree depending on the macronutrient composition of the overfeeding diet. There is a ceiling effect such that individuals with a higher CBT during fasting have limited capacity to increase CBT with feeding. Because of body thermoregulatory mechanisms that maintain a constant CBT, these results indicate that CBT has a limited role in the inter-individual variability in DIT.
Keywords: Core body temperature, diet-induced thermogenesis, overfeeding, fasting, energy balance
1. Introduction
The energy expenditure (EE) response to short-term alterations in food intake such as overfeeding or fasting may identify individuals prone to weight gain [1]. We have previously shown in two independent studies that changes in 24-h EE from energy balance either during fasting or overfeeding characterize individuals who have a “thrifty” vs. “spendthrift” metabolic phenotype, where “thrifty” individuals are more resistant to weight loss during 6-week caloric restriction [2] and are more prone to weight gain in free-living conditions [3]. To understand the propensity to weight gain quantified by the diet-related changes in 24-h EE, it is important to study the inter-individual variability in diet-induced thermogenesis (DIT), the component of daily EE that is mostly influenced by substantial changes in food intake which on average contributes to approximately 10% of 24-h EE in humans [4, 5], has a large inter-subject variability [3, 6], and may further define the individual metabolic phenotype. The DIT, also termed thermic effect of food (TEF), is the energy required to absorb, metabolize, and store ingested food (obligatory cost), but also conceptually includes a subject-specific facultative cost that varies by meal size and dietary macronutrient composition [3, 6, 7], which may contribute to the inter-individual variability in EE responses to overfeeding and fasting that ultimately predict weight change.
Approximately half of the basal metabolic rate in humans contributes to maintaining a stable core body temperature (CBT) [8, 9]. Previous studies have reported a positive relationship between EE and CBT when measured orally [10] or rectally [11] during energy balance. During underfeeding, CBT decreases after semi-starvation for 24 weeks [10] and increases during 24-h overfeeding with a diet with balanced proportion of macronutrients (50% carbohydrates and 20% protein) [12, 13]. Importantly, metabolically “thrifty” individuals, defined by greater decrease in EE during fasting, have a lower CBT [12]. Changes in CBT with feeding and overfeeding should reflect the ability to increase DIT and dissipate heat, but the relationships between CBT and DIT in response to overfeeding diets with unbalanced proportion of macronutrients have not been ascertained or quantified. In mice, the sympathetic nervous system (SNS) regulates the body temperature in response to overfeeding by increasing brown adipose tissue activation, thereby contributing to DIT [14–16]. However, whether catecholamines (as markers of SNS tone) influence CBT in humans remains to be determined, especially in a setting of acute overfeeding or fasting. Epinephrine secretion is associated with metabolic adaptation to fasting both in rodents [17] and in humans [7, 18]. Specifically, compared to energy balance conditions urinary epinephrine increases during 24-h fasting and is elevated to a greater extent in metabolically “spendthrift” subjects who can maintain higher EE during fasting as compared to those who are more metabolically “thrifty” who instead show less increases in epinephrine during fasting [7]. The associations between changes in epinephrine and CBT with overfeeding and fasting have not been evaluated.
The aims of the present study were: 1) to continuously measure CBT over 24 hours of fasting, eucaloric feeding, and two different overfeeding diets (high-carbohydrate vs. high-fat) in healthy adult men; 2) to evaluate whether urinary catecholamines (particularly, epinephrine) are determinants of CBT during these dietary interventions; and, 3) to evaluate whether CBT during feeding and overfeeding explains inter-individual differences in DIT which may determine the propensity to weight gain.
2. Subjects and methods
2.1. Participants
This is a cross-sectional analysis of data from an ongoing clinical trial investigating the effects of short-term (24 hours) fasting and overfeeding on energy metabolism in healthy subjects with normal glucose regulation (clinicaltrials.gov identifier: NCT00523627). A total of 170 adults living around Phoenix, Arizona were screened between 2008 and 2016 (Supplemental Figure 1). One-hundred eleven subjects met initial inclusion criteria and were admitted to the local clinical research unit of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Upon admission to the unit, participants were placed on a standard weight-maintaining diet (WMD) consisting of 50% carbohydrate, 30% fat, and 20% protein content, with total energy intake calculated using unit-specific equations [6]. The caloric content of the WMD was adjusted daily by the research dietician by 200 kcal, if necessary, to maintain a stable fasting body weight (measured daily upon awakening on a calibrated scale) within 1% of the weight at the admission. Body composition was assessed by dual-energy X-ray absorptiometry (DXA) scan (DPX-1, Lunar Corp, Madison, Wisconsin, USA) on second day of admission, with fat mass (FM) and fat-free mass (FFM) calculated from percentage body fat (PFAT) and body weight.
All participants underwent an oral glucose tolerance test (OGTT) after three days on the WMD and overnight fast. Only 84 volunteers with normal glucose regulation [19], a major inclusion criterion, continued with the study. Female subjects were excluded from the current analyses due to the known gender differences in CBT and body composition [20–22], and the inability to match dietary interventions to the follicular phase of the menstrual cycle which may alter both CBT and DIT independently. Therefore, only males with valid CBT measurements during 24-h fasting (see below) were included in this study (n=53). Measurements described below were all conducted in the carefully controlled setting of our inpatient clinical research unit to ensure uniform conditions. All described diets were prepared under the same conditions in our metabolic kitchen. All participants provided written, informed consent. The institutional review board of NIDDK approved this study.
2.2. Dietary Interventions
The experimental protocol for the acute dietary manipulations has been previously described in detail [3]. After three days on a WMD, all participants underwent an initial eucaloric assessment of 24-h EE in a whole-room calorimeter with a 20%-reduction in the WMD to account for the decreased activity within the calorimeter. The calculated 24-h EE from this initial assessment was then used as the energy intake for a second 24-h evaluation inside the calorimeter to obtain a measure of 24-h EE in a condition closer to a perfect energy balance in this confined setting. The measured 24-h EE of the second eucaloric assessment (EBL) was doubled to obtain the 24-h caloric content of two normal-protein (20% protein) overfeeding diets: one with a high-fat content (FNP, 60% fat) and one with a high-carbohydrate content (CNP; 75% carbohydrate). Additionally, subjects spent 24 hours inside the calorimeter during fasting (FST), where only non-caloric non-caffeinated beverages (e.g., water) were permitted. The three 24-h dietary interventions given inside the calorimeter (FST, FNP, and CNP) were done in random order and with a three-day wash out period on the WMD between each other. The metabolic kitchen weighed leftovers and calculated the actual (not eaten) food intake. If less than 95% of the food given was consumed by the volunteer during a chamber session, the CBT and EE data were excluded from the data analysis (Supplemental Figure 1).
2.3. Core Body Temperature Measurements
Core body temperature was measured using ingestible capsules (CorTemp®, HQ Inc., Palmetto, FL) during each 24-h session in the calorimeter. Prior to the administration to the volunteer, the capsule was tested for accuracy in warm water temperature to ensure measurements within 0.1°C against an oral mercury thermometer. The capsule was ingested at 05:00 am, approximately three hours prior to the volunteer entering the metabolic chamber. Temperature values were transmitted every 30 seconds from the capsule to the receiver worn around the participant’s waist while residing in the calorimeter. The 24-h CBT data were downloaded and filtered using a median filter (size=121 samples) in MATLAB (MathWorks, Natick, MA) to remove instrumental noise. For each 24-h session, the average 24-h CBT (avgCBT) was calculated as the mean value of all valid time points after filtering, and the change in CBT from fasting during each dietary intervention was calculated as: avgCBTDiet – avgCBTFST. Two investigators (KLV and BB) independently reviewed each individual 24-h CBT time course (“trajectory”), and only trajectories deemed physiological, i.e., with CBT values between 36–38°C, and with at least 8 hours of valid measurements were included in the data analyses. Due to differences in the transit times, such as the expedited expulsion of the capsule, and instability in the recordings of the capsule, the overfeeding diets had a smaller sample size and each overfeeding diet consisted of different subjects in each group, although there were no differences in their anthropometric measurements (Supplemental Figure 1). After removal of CBT data with less than 8 hours of measurements, the duration of CBT recording was not different across the diets (p=0.36), where the mean transit time during fasting was 20.8±4.1 hours, 21.1±3.7 hours during eucaloric feeding, 21.3±3.5 hours during high-fat overfeeding, and the lowest transit time was observed in the high-carbohydrate overfeeding (19.4±5.5 hours).
2.4. Energy Expenditure Measurements
The assessment of 24-h EE was previously described in detail [6, 23]. Subjects entered the whole-room indirect calorimeter (metabolic chamber) at 08:00 am after consuming breakfast at 07:00 following an overnight fast. The subject remained in the chamber for 23.25 hours, during which additional meals were provided at 11:00 am, 4:00 pm, and 7:00 pm. The average rates of CO2 consumption and O2 production per minute, the respiratory quotient (RQ), and EE during the 23.25 hours in the chamber were extrapolated to 24 hours, and 24-h EE was calculated using the Lusk’s equation [24]. While in the calorimeter, participants were asked to refrain from exercising and spontaneous physical activity (SPA) was measured by radar sensors and expressed as a percentage of time with detected SPA over the 24-h period. Sleeping EE (SLEEP) was calculated as the average EE between 23:30 and 05:00 when the SPA was less than 1.5%. Ambient temperature inside the calorimeter was set to 24°C and remained constant over the 24 hours by a PID temperature controller, with a precision within 0.5°C. Propane burns were performed monthly as quality control checks of the calorimeter, with average VO2 and VCO2 recovery values of 0.99±0.03 and 0.98±0.02 (mean±SD), respectively.
The diet-induced thermogenesis (DIT, kcal/day) was calculated by subtracting the 24-h EE during fasting from the 24-h EE during the three dietary interventions (EBL, FNP, and CNP). The thermic effect of food (TEF) was calculated by dividing the DIT of the specific diet by the actual energy intake consumed over 24 hours in the metabolic chamber (after accounting for uneaten food), and expressed as percentage (i.e., DITdiet÷intake×100).
2.5. Hormone measurements
Urine was collected during each dietary intervention for 24-h inside the metabolic chamber for the measurement of catecholamines (epinephrine, metanephrine, norepinephrine, and normetanephrine). Catecholamines were measured by Mayo Clinic laboratories using high-performance liquid chromatography for epinephrine and norepinephrine, and liquid chromatography-tandem mass spectrometry stable isotope dilution for metanephrine and normetanephrine [7].
At admission, TSH was measured by a homogeneous sandwich chemiluminescent immunoassay based on LOCI® technology from Dimension Vista® 500 System (Siemens). Fasting plasma were collected both at entry and at exit from the calorimeter in EDTA-containing tubes and frozen to −70°C for later measurements, all completed at the NIDDK central laboratory in Bethesda, MD. Plasma free T3 and Free T4 concentrations were measured using the EIA kit from Phoenix Pharmaceuticals (Burlingame, CA). Intra-assay and inter-assay coefficient of variations (CV) were 3.0% and 4.5% for free T3, and 2.8% and 4.0% for free T4, respectively. Plasma insulin was measured using an automated immunoenzymometric assay (Tosoh Bioscience Inc., Tessenderlo, Belgium).
2.6. Statistical Analysis
Statistical analyses were performed using SAS software (version 9.4; Cary, N.C.). Alpha was set at 0.05. Data are presented as mean±SD. Pearson’s correlation coefficients were used to quantify associations among CBT, EE, anthropometric and hormone measurements. Skewed variables (e.g., catecholamines) were log10-transformed before analyses to achieve a Gaussian distribution. ANOVA was used to evaluate differences in avgCBT among ethnicities. The individual change (Δ) in avgCBT from fasting to feeding (EBL) and overfeeding diets (FNP and CNP) was analyzed by mixed model analysis including all four diets and accounting for repeated measurements within a subject using a compound symmetry covariance structure. Pairwise post-hoc p-values between diets were adjusted for multiple comparisons using the Tukey-Kramer method. Differences between subjects with low (10th percentile) vs. high (90th percentile) CBT during fasting were evaluated by unpaired t-test. Linear regression analysis was used to evaluate ceiling effects in CBT changes during dietary interventions by testing the slope of the best-fit line vs. 1 (identify line).
The differences in minute-by-minute CBT trajectories over 24 hours between dietary interventions were assessed by mixed model analysis accounting for repeated measures using a first-order autoregressive covariance structure. Differences in avgCBT and 24-h EE across dietary interventions were assessed by mixed models using a compound symmetry covariance structure to account for the random order of these interventions and to quantify the intraclass correlation coefficient (ICC). Mixed models were also calculated including potential mediators (e.g., epinephrine) as covariates. Pairwise post-hoc comparisons between diets were done using the Tukey-Kramer method.
3. Results
Subject characteristics are shown in Table 1. On average, subjects were 38.7±10.5 years old, slightly overweight but with a wide range of adiposity (BMI=18–44 kg/m2; PFAT = 7–43%). There were no differences in avgCBT or in any anthropometric characteristic between the subgroups of subjects with CBT measurements during overfeeding and the entire study group including 53 subjects (data not shown).
Table 1.
Subject Characteristics.
| Males (n=53) | |
|---|---|
| Ethnicity | 12AA/7H/18NA/16W |
| Age (y) | 38.7±10.5 (19.2, 55.8) |
| Weight (kg) | 80.6±13.3 (52.8, 127.1) |
| Height (cm) | 174.2±6.3 (156.5, 188.1) |
| Waist circumference (cm) | 91.6±11.7 (67.3, 137.2) |
| BMI (kg/m2) | 26.5±4.1 (18.3, 44.0) |
| Body fat (%) | 25.3±8.2 (6.9, 42.6) |
| Fat Mass (kg) | 21.1±9.7 (5.9, 54.2) |
| Fat Free Mass (kg) | 59.5±7.1 (43.4, 79.4) |
| Fasting glucose (mg/dL) | 90.3±5.6 (77.0, 99.5) |
| 2-h glucose (mg/dL) | 103.0±22.3 (55.0, 138.0) |
| Fasting insulin (mU/L) | 8.4±5.6 (2.0, 32.0) |
| 2-h insulin (mU/L) | 47.3±37.9 (6.0, 185.0) |
| TSH (μIU/mL) | 1.31±0.80 (0.32, 3.59) |
| Free T4 (ng/dL) | 1.3±0.2 (0.9, 1.7) |
| Free T3 (pg/mL) | 3.0±0.4 (1.9, 3.9) |
Data presented as mean±SD with minimum and maximum values in parenthesis.
Abbreviations: AA, African American; H, Hispanic; NA, Native American; W, White.
The avgCBT during eucaloric feeding was 36.86±0.13°C and was not a determinant of 24-h EE (p=0.83 adjusted for age, ethnicity, FM, FFM, and SPA) (Supplemental Figure 2) or sleeping EE (p=0.79). The minute-by-minute CBT trajectories during all dietary interventions are shown in Figure 1A. In each dietary condition, CBT was higher during the awake and fed state during the day (range: 36.8–37.3°C), while it decreased at night during sleeping (range: 36.5–36.7°C). The average ambient temperature for all the 24-h sessions inside the chamber was 24.1±1.2°C, with no difference across dietary interventions (p=0.77). Ambient temperature was not associated with CBT during any dietary intervention (all p>0.80).
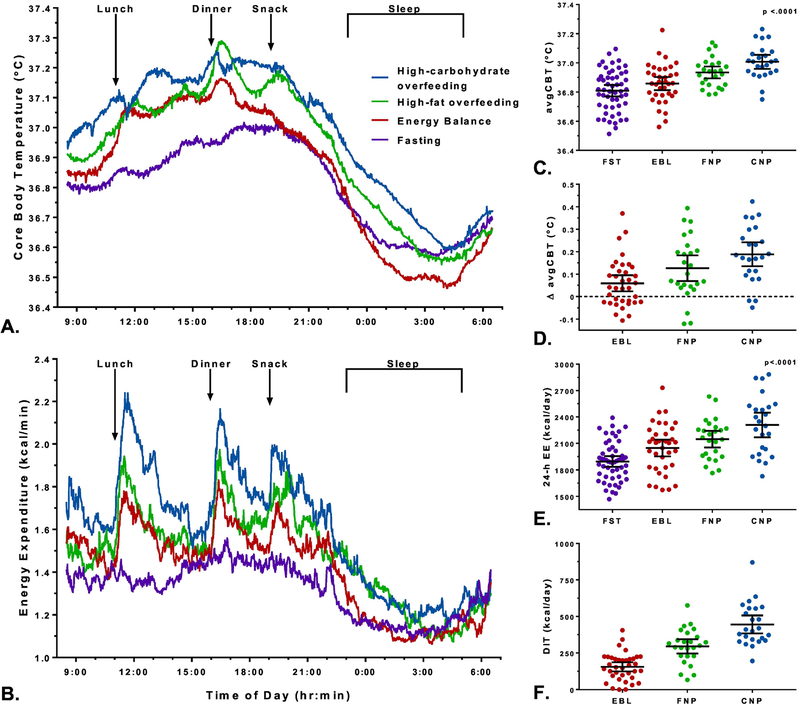
Figure 1. Twenty-four-hour time courses of CBT and EE during fasting, eucaloric feeding, and two different overfeeding diets.
Averaged 24-hour time courses of the minute-by-minute trajectory of core body temperature (CBT) (A) and energy expenditure (EE) (B) measured during the four dietary interventions. All the diets have different trajectories for CBT (p<0.0001) and EE (p<0.0001) by mixed model analysis accounting for repeated measurements.
Eucaloric diet (EBL) with 50% carbohydrate, 30% fat, and 20% protein in red, high-carbohydrate overfeeding diet (CNP) with 75% carbohydrate, 5% fat, and 20% protein in blue, high-fat overfeeding diet (FNP) with 20% carbohydrate, 60% fat, and 20% protein in green, and fasting (FST) in in purple. The energy intake of both overfeeding diets was set to 200% of the 24-h EE during eucaloric conditions, and then corrected for let-overs. Arrows represent the meals given inside the whole-room indirect colorimeter through an air lock, where lunch was given at 11:00, dinner at 16:00, and a snack at 19:00. Sleep time was considered the time periodbetween 23:30 and 5:00 when the activity levels was less than 1.5% by radar system.
Averaged 24-h core body temperature (avgCBT) (Panel C) and energy expenditure (EE) (Panel E) by dietary intervention with 95% CI error bars. 24-h fasting (FST) represented in purple, eucaloric diet (EBL) in red, high-carbohydrate overfeeding diet (CNP) shown in blue, and high-fat overfeeding diet (FNP) represented in green. The individual change (Δ) in avgCBT (Panel D) and DIT (Panel F) during each dietary condition (EBL, FNP, and CNP) was calculated by subtracting the fasting value. * P-values were calculated using mixed models to account for repeated measurements and Tukey-Kramer adjustment. In panel E, 24-h EE was adjusted for age, ethnicity, FM and FFM. Each Δ avgCBT in panel D was different from zero by Student’s one-sample t-test (p<0.05).
Diet composition: Eucaloric diet (EBL) with 50% carbohydrate, 30% fat, and 20% protein; high-carbohydrate overfeeding diet (CNP) with 75% carbohydrate, 5% fat, and 20% protein; and high-fat overfeeding diet (FNP) with 20% carbohydrate, 60% fat, and 20% protein. Overfeeding diets intake was the equivalent of double the 24-h EE of the eucaloric diet.
3.1. Determinants of CBT
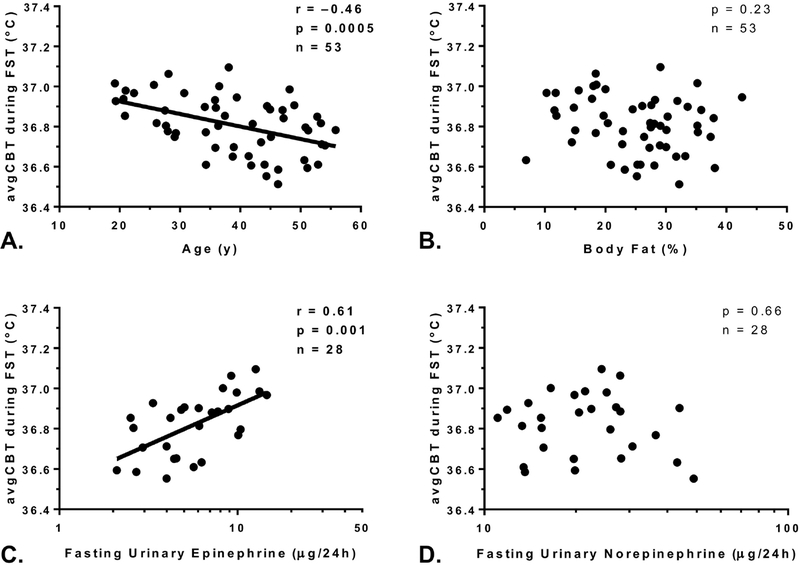
The avgCBT during fasting, as a measure of CBT without any dietary influence, was 36.81±0.14°C (Figure 1C). Despite a small inter-subject variability (CV=0.4%), the avgCBT during fasting was inversely associated with age (r = ‒0.46, p <0.001, β= −0.06°C per 10-yr difference, Figure 2A) with no difference according to ethnicity (p=0.29). There were no associations between avgCBT during fasting (or eucaloric feeding or overfeeding) and SPA (p=0.54), anthropometric measures such as PFAT (p=0.23, Figure 2B), BMI (p=0.31), FFM (p=0.35), or waist circumference (p=0.21).
Figure 2. Determinants of CBT.
The mean 24-h core body temperature (avgCBT) was negatively associated with age (Panel A) but did not correlate with percentage body fat (Panel B). The avgCBT during fasting (FST) was positively correlated with urinary epinephrine, shown in log10-scale on the x-axis (Panel C), but not with urinary norepinephrine (Panel D).
3.2. Changes in CBT during eucaloric feeding and overfeeding
The avgCBT was 36.93±0.10°C during high-fat overfeeding (FNP) and 37.01±0.12°C during high-carbohydrate overfeeding (CNP) (Table 2, Figure 1C), again with very small inter-subject variability (all CVs ≈0.3%) but with a moderate intra-subject consistency across these diets (ICC=0.43, p=0.002, Supplemental Figure 3).
Table 2.
Core Body Temperature, Energy Expenditure, and Urinary Catecholamines Measurements by Dietary Intervention.
| Fasting FST (n=53) |
Energy Balance EBL (n=36) |
High-fat OF FNP (n=25) |
High-carbohydrate OF CNP (n=24) |
|
|---|---|---|---|---|
| avgCBT (°C) | 36.81±0.14 (36.51, 37.10) |
36.86±0.13 (36.56, 37.22) |
36.93±0.10 (36.78, 37.14)a,b |
37.01±0.12 (36.75, 37.23)a,b |
| ΔavgCBT (°C) | N/A | 0.06±0.11 (−0.11, 0.37) |
0.13±0.14b (−0.12, 0.39) |
0.19±0.13b (−0.05, 0.42) |
| Daytime CBT (°C)e | 36.81±0.21 (36.44, 37.41) |
36.88±0.21 (36.49, 37.36) |
36.92±0.17 (36.63, 37.23) |
37.02±0.12a (36.75, 37.23) |
| Night CBT(°C)f | 36.64±0.19 (36.23, 37.26) |
36.55±0.16 (36.16, 36.84) |
36.65±0.14 (36.26, 36.92) |
36.71±0.17a (36.38, 37.07) |
| 24-h EE (kcal/day) | 1895±219 (1469, 2390) |
2059±274 (1573, 2732) |
2148±227a
(1764, 2631) |
2308±329a,b (1728, 2884) |
| SLEEP (kcal/24h) | 1607±192 (1231, 2373) |
1629±210 (1211, 2303) |
1739±166a (1425, 2064) |
1772±237a,b
(1411, 2273) |
| Energy Intake (kcal/24h) | N/A | 2125±287 (1622, 2800) |
3982±465 (3148, 4920) |
3961±538 (3022, 4905) |
| Energy Balance (kcal/day) | −1895±219 (−2390, −1469) |
65±145a (−91, 651) |
1835±166a,b,c (1425, 2064) |
1653±360a,b (331, 2063) |
| DIT (kcal/day) | N/A | 160±93a
(−1.00, 405.00) |
296±119a,b
(65.00, 575.00) |
444±146a,b (195.00, 870.00) |
| TEF (% of Energy Intake) | N/A | 7.3±3.8 (−0.06, 15.73) |
7.40±2.72c (1.54, 11.93) |
11.3±3.6b (6.2, 21.0) |
| RQ (ratio) | 0.79±0.03 (0.74, 0.90) |
0.87±0.03a (0.83, 0.94) |
0.85±0.03a,c (0.80, 0.91) |
0.94±0.03a,b (0.88, 1.00) |
| 24-h SPA (%) | 6.1±3.5 (0.5, 18.3) |
5.6±3.1 (0.6, 16.1) |
6.1±2.7 (0.6, 12.8) |
6.5±3.3 (0.9, 15.5) |
| Ambient temperature (°C) | 24.06±1.17 (20.90, 26.20) |
24.18±1.05 (22.00, 26.19) |
23.94±1.15 (21.40, 26.31) |
24.27±1.30 (22.00, 26.40) |
| Norepinephrine (μg/24h)d | 21.0 (15.4, 28.0) | 25.4 (20.8, 33.8)a | 26.0 (15.6, 34.6) | 21.4 (17.6, 32.4) |
| Normetanephrine (μg/24h)d | 183.4 (142.9, 243.0) | 227.2 (180.2, 340.6)a | 267.0 (199.8, 337.6)a,c | 188.2 (144.4, 270.7) |
| Epinephrine (μg/24h)d | 5.9 (4.0, 9.2) | 4.0 (2.6, 6.0)a | 3.9 (3.0, 5.2)a | 3.3 (3.1, 5.5)a |
| Metanephrine (μg/24h)d | 121.2 (76.2, 177.8) | 129.3 (86.9, 188.1) | 130.8 (86.8, 177.6) | 93.3 (81.6, 174.3) |
| Plasma insulin (mU/L) | −3.3 (−4.2, −2.4)g | 0.7 (−0.7, 2.0) | 0.1 (−1.2, 1.3) | 3.3 (1.3, 5.2)g |
Data presented as mean±SD with minimum and maximum values in parenthesis, except for urinary catecholamines that are presented as median with interquartile range in parenthesis and insulin that is presented as mean with 95% CI in parenthesis.
p < 0.05 versus FST,
p < 0.05 versus EBL,
p < 0.05 versus CNP,
n=29 for FST, n=18 for EBL, n=14 for CNP, n=15 for FNP.
Daytime CBT calculated between 08:00 and 23:00.
Night-time CBT calculated from 23:30 through 05:00, the same time period used to calculate SLEEP.
p < 0.05 versus zero by Student’s t test for parametric data or Wilcoxon test for non-parametric data.
Abbreviations: OF, overfeeding; avgCBT, mean 24-h core body temperature; ΔavgCBT, change in CBT from fasting; 24h EE, 24-hour energy expenditure; SLEEP, sleeping EE; RQ, respiratory quotient; 24h SPA, 24-hours spontaneous physical activity; TEF, thermic effect of food; DIT, diet-induced thermogenesis
Compared to fasting, avgCBT slightly increased during eucaloric feeding (Δ=0.06±0.11°C, p=0.037), and more so during both overfeeding diets (FNP: Δ=0.13±0.14°C, p<0.001; CNP: Δ=0.19±0.13°C, p<0.001) (Figure 1D), with no difference in ΔavgCBT between the two overfeeding diets (p=0.06). Some subjects (n=16) had lower avgCBT during eucaloric feeding compared to fasting, but these 16 subjects did not differ by age, body composition, or ethnicity from others with increased avgCBT during eucaloric feeding.
3.3. Ceiling Effect on CBT changes during feeding and overfeeding
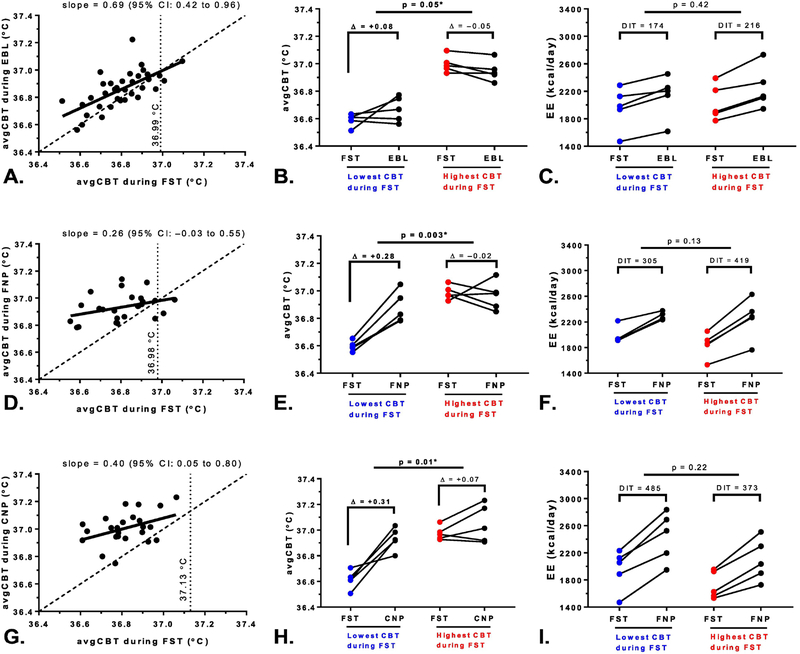
To determine whether the changes in CBT during feeding or overfeeding were dependent on the baseline (i.e., fasting) CBT value, the avgCBT during fasting and the avgCBT during each dietary intervention were plotted against each other (Figure 3; panels A, D, and G) where the slope of the regression line was statistically compared to the slope of the identity line. During eucaloric feeding, the slope of the regression line was 0.69 (95% CI: 0.42–0.96), where the upper 95% limit of slope remains below the identity line (slope=1) thus indicating a ceiling effect. Similarly, a stronger ceiling effect was observed during high-fat overfeeding (slope=0.26, CI: ‒0.03 to 0.55) and high-carbohydrate overfeeding (slope=0.40, CI: 0.01 to 0.80). The intersection point between the regression and identity lines was mathematically calculated and indicated the upper CBT value where there is no increase in CBT from fasting despite feeding or overfeeding. The upper CBT limit was 37°C both during eucaloric feeding and high-fat overfeeding, while it was the highest during high-carbohydrate overfeeding at 37.1°C.
Figure 3. Ceiling effect of CBT changes during feeding and overfeeding.
The graphs in the first column depict the mean 24-h core body temperature (avgCBT) during fasting (FST) on the x-axis versus the avgCBT during eucaloric feeding (EBL, panel A), high-fat overfeeding (FNP, panel D), and high-carbohydrate overfeeding (CNP, panel G) on the y-axis. In each graph, the solid regression line between avgCBT during fasting and the avgCBT during each diet was statistically compared to the dashed identity line to test the ceiling effect on CBT during eucaloric feeding and overfeeding. The vertical dashed lines represent the upper CBT value during each diet above which CBT did not increase with feeding or overfeeding, which was mathematically calculated as the point where the regression line intersected the identity line. During EBL, the upper CBT limit was 36.99 °C, 36.98 °C during FNP, and was the highest during the CNP diet at 37.13 °C, where there was no increase in CBT despite feeding and overfeeding.
To further demonstrate the ceiling effects, the five subjects with the highest avgCBT during 24-h fasting (in red) had smaller changes in CBT from fasting compared to the subjects with the lowest CBT during 24-h FST (in blue) during EBL (panel B), FNP (panel E), and CNP (panel H), despite no differences in the concomitant change in EE during these diets (panels C, F, and I). Analyses between groups (lowest vs. highest mean CBT during 24-h fasting) were carried out using Student’s unpaired t-test.
To further investigate this ceiling effect of CBT during feeding and overfeeding, we performed a post-hoc analysis comparing the five subjects with the highest avgCBT versus the five subjects with the lowest avgCBT during fasting. While these two groups had similar anthropometric characteristics (data not shown) and showed comparable increases in EE during feeding and overfeeding (panels C, F, and I), the five subjects with the lowest avgCBT during fasting had greater increases in CBT during eucaloric feeding (ΔavgCBT=0.08±0.12°C vs. −0.05±0.04°C, p=0.05, Figure 3B) and both high-fat (0.28±0.09°C vs. ‒0.02±0.13°C, p=0.003, Figure 3D) and high-carbohydrate (0.31±0.13°C vs. 0.07±0.11°C, p=0.01, Figure 3F) overfeeding when compared to the five subjects with the highest avgCBT during fasting who instead showed no change in avgCBT during all dietary interventions.
3.4. Relationships between CBT and DIT during feeding and overfeeding
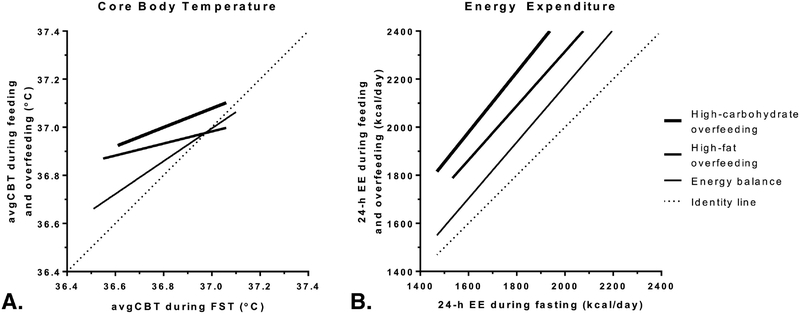
On average, 24-h EE increased from fasting during eucaloric feeding (DIT=160±93 kcal/day, p<0.001), high-fat (296±119 kcal/day, p<0.001) and high-carbohydrate (444±146 kcal/day, p<0.001) overfeeding (Table 2, Figure 2C), with the DIT during high-carbohydrate overfeeding being higher than that during high-fat overfeeding (p<0.001). Unlike diet-related changes in CBT (Figure 4A), the increase in 24-h EE from fasting (i.e., DIT) did not show a ceiling effect during any diet and approximately constant over the entire range of 24-h EE values (Figure 4B).
Figure 4. Relationships between CBT and 24-h EE with feeding and overfeeding with respect to their fasting values.
Positive relationships between the mean 24-h core body temperature (avgCBT, Panel A) and 24-h energy expenditure (EE, Panel B) during fasting (FST, x-axis) vs. eucaloric feeding (EBL), 200% high-fat (FNP) and high-carbohydrate (CNP) overfeeding. Solid lines represent best-fit lines in each condition. Strong ceiling effects for the diet-related increases from fasting were observed for avgCBT but not for 24-h EE.
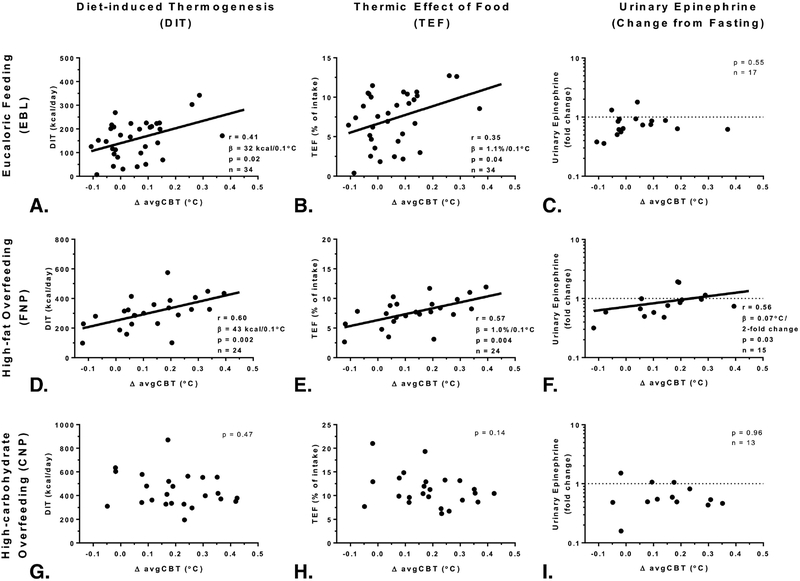
A greater increase in avgCBT from fasting correlated with higher DIT both during eucaloric feeding (r=0.41, p=0.02, β=32 kcal/day per 0.1°C increase in avgCBT, Figure 5A) and high-fat overfeeding (r=0.60, p=0.002, β=43 kcal/day per 0.1°C increase in avgCBT, Figure 5D), but not during high-carbohydrate overfeeding (p=0.47, Figure 5G). Similar results were obtained for TEF (Figure 5, panels B, E, and H). Upon removal of the 14 individuals who did not increase their avgCBT during eucaloric feeding compared to fasting, a greater increase in avgCBT remained associated with a greater DIT (r=0.53, p=0.02, β=54 kcal/day per 0.1°C increase in avgCBT), and similar results were found when removing individuals that did not increase their avgCBT during high-fat overfeeding (n=3) (r=0.50, p=0.02, β=40 kcal/day per 0.1°C increase in avgCBT).
Figure 5. Relationships between changes in CBT, DIT, and urinary epinephrine concentration feeding and overfeeding.
Relationships between the change in mean 24-h core body temperature from fasting (ΔavgCBT) and the diet induced thermogenesis (DIT, graphs in the first column) and the thermic effect of food (TEF, graphs in the middle column) during eucaloric feeding (EBL, Panels A and B), during high-fat overfeeding (FNP, Panels D and E), but not during high-carbohydrate overfeeding (CNP, Panels G and H). Relationships between the change in urinary epinephrine from fasting expressed as fold change on a log10 scale (y-axis) and change in avgCBT during FNP (Panel F) but during eucaloric feeding (Panel C) or high-carbohydrate overfeeding (Panel I). After removal of four volunteers with the highest change in CBT (>0.2°C), the relationship was no longer significant (p=0.28).
Diet composition: Eucaloric diet (EBL) with 50% carbohydrate, 30% fat, and 20% protein; high-carbohydrate overfeeding diet (CNP) with 75% carbohydrate, 5% fat, and 20% protein; and high-fat overfeeding diet (FNP) with 20% carbohydrate, 60% fat, and 20% protein. The total energy intake of overfeeding diets was twice the 24-h EE measured during the eucaloric diet (EBL). The DIT was calculated as: 24hEEDiet −24hEEFST; the TEF was calculated by: DITDiet/Energy IntakeDiet ×100.
3.5. Relationships between CBT and hormone measurements
The median 24-hour urinary catecholamine concentrations during each dietary intervention are presented in Table 2. The avgCBT during fasting was positively associated with fasting epinephrine concentration (r=0.61, p=0.001, Figure 2C), such that a two-fold difference in fasting epinephrine concentration was associated with a 0.07°C higher avgCBT during fasting. This association between CBT and epinephrine in fasting conditions was independent of age (partial r=0.58, p=0.001 adjusted for age). There were no associations between avgCBT during fasting and urinary norepinephrine (p=0.66, Figure 2D), normetanephrine (p=0.89), metanephrine (p=0.15), thyroid hormones or insulin concentrations (all p>0.05).
Next, we evaluated whether the changes in catecholamines during feeding and overfeeding were reflective of the concomitant changes in avgCBT during these dietary interventions. Compared to fasting, urinary epinephrine concentrations were on average lower by 27% during eucaloric feeding (fold change =0.73, CI; 0.59 to 0.89, p=0.006), by 21% during high-fat overfeeding (fold change=0.79, CI; 0.61 to 1.04, p=0.06), and by 42% during high-carbohydrate overfeeding (fold change=0.58, CI; 0.42 to 0.82, p=0.003). A greater decrease in urinary epinephrine from fasting conditions to high-fat overfeeding was associated with a smaller increase in avgCBT (r=0.56, p=0.03, Figure 5F), whereas no associations were found during eucaloric feeding (p=0.55, Figure 5C) or high-carbohydrate overfeeding (p=0.96, Figure 5I). In a mixed model including epinephrine as covariate (p=0.03), the differences in avgCBT across diets were still significant (p<0.001). There were no relationships between the changes in avgCBT and the changes in insulin, free plasma T3, free T4, or TSH concentrations after all dietary interventions (all p>0.05).
4. Discussion
In healthy men with a wide range of body size and adiposity, we found that the CBT during 24-h fasting did not substantially vary among individuals and was not dependent on body size or composition, while it was inversely correlated with age and, independently, positively associated with urinary epinephrine concentration. Overall, CBT increased from fasting during eucaloric feeding and more so during overfeeding diets, and these increases in CBT correlated with diet-induced thermogenesis (DIT) during feeding and high-fat overfeeding but not high-carbohydrate overfeeding. Importantly, a ceiling effect for the diet-induced increases in CBT, but not in 24-h EE (i.e., DIT), was observed with feeding and overfeeding, namely, the increase in CBT was contingent upon the fasting CBT value such that there was no meaningful increase in CBT with feeding/overfeeding when the fasting CBT exceeded approximately 37°C. Lastly, the change in CBT in response to overfeeding was associated with urinary epinephrine concentration only during high-fat overfeeding, but not during high-carbohydrate overfeeding.
Consistent with previous reports in humans [13, 20], measures of body size and composition were not associated with CBT during fasting or eucaloric feeding, while CBT was inversely associated with age as previously shown [25–27]. The insulating properties of adipose tissue in the abdominal wall has been previously linked to decreased DIT, as shown both in individuals with obesity and in lean subjects with artificial thermal insulation of their body [28]. Evidence for a possible role for CBT in human obesity came from a small study showing that subjects with obesity had lower CBT during diurnal hours compared to lean individuals [29]. However, concordant to our negative findings for the relationship between body composition and CBT, two other studies failed to show a difference in CBT between individuals with or without obesity [13, 20], thus the relationship between CBT and body composition appears unlikely. The lack of association with body composition indicates that heat dissipation may occur via physiological means such as vasodilatation rather than structural characteristics of body such as thermal insulation of fat tissue [30]. We also observed a strong positive association between 24-h urinary epinephrine concentration and CBT in fasting conditions, such that individuals who had a higher urinary epinephrine concentration during fasting also had a higher CBT, and this association was independent of age. As fasting is associated with increased epinephrine release by the adrenal medulla presumably stimulated by a reduction in plasma glucose concentration [18], increased CBT may constitute one of the physiological effects together with (or secondary to) increased heart rate and lipolysis [31] exerted by elevated epinephrine concentrations observed during fasting [7]. Further, the capacity to increase CBT during high-fat overfeeding was associated with an increase (or lack of decrease) in epinephrine concentrations compared to fasting levels. Nevertheless, due to the cross-sectional nature of our data, it is difficult to establish the causality of this association between epinephrine and CBT, namely, if the epinephrine response to high-fat diet influences CBT or vice versa, or if they are secondary to the increased DIT observed during high-fat overfeeding.
Our current findings provide further evidence of the role of CBT in human energy metabolism. Although we did not observe an association between CBT and 24-h EE or sleeping EE during energy balance, we found positive associations between diet-related changes in CBT and concomitant changes in EE, i.e., DIT, which we precisely estimated over 24 hours during eucaloric feeding and overfeeding. Interestingly, while we observed nearly constant diet-related increases in 24-h EE (i.e., DIT) over the entire range of 24-h EE (Figure 4B), we found strong evidence for a ceiling effect in the increases in CBT with feeding and overfeeding (Figure 4A), indicating any effect of DIT on CBT (or vice versa) is limited to those subjects with lower CBT. Specifically, individuals with the lowest CBT during fasting had the greatest capability to increase their CBT during feeding whereas those with relatively higher CBT during fasting did not increase their CBT with feeding (Figure 3) despite both groups had equivalent DIT. The ceiling effect observed for diet-induced changes in CBT may signify the body’s requirement to maintain CBT within a narrow range [32, 33] despite the thermogenic effect of overfeeding diets such as those rich in carbohydrates [34, 35], which might be prone to drive CBT above the physiologic range. Although our cross-sectional analysis does not prove causality, the increase in CBT is likely the result of the increase in EE during feeding (e.g., the obligatory energy cost of processing ingested nutrients, which largely determines DIT and leads to heat production in the body) and may signify the intrinsic ability of one individual to dissipate heat when fed, where the ceiling effect represents the maximal ability to achieve this. We observed a nearly stepwise increase in CBT from fasting to 100% feeding (mean increase= +0.06°C) and to 200% overfeeding a high-fat diet (+0.13°C) and more so during the highly thermogenic overfeeding diet with ¾ calories from carbohydrates (+0.19°C), suggesting that heat release from absorption and digestion (e.g. breaking covalent bonds) of nutrients may contribute to the increased heat production and, consequently, to higher increase in CBT. Further, we also demonstrated that the magnitude of DIT both during eucaloric feeding and high-fat overfeeding was proportional to the increase in CBT during these dietary interventions, whereas this relationship was not present during high-carbohydrate overfeeding (Figure 5G–5H). The reason for the lack of association between DIT and CBT during this thermogenic diet may rely on the observed ceiling effect that limited the increase in CBT up to 37.1°C, which might represent the maximal temperature at which enzymatic reactions work optimally. High-carbohydrate overfeeding generated the highest DIT but this substantial increase in EE did not result in a proportional increase in CBT possibly due to the mechanisms to preserve maximal body temperature under physiologic conditions, or changes in other mediators such as activins and follistatins, recently found to have a role in EE regulation [36].
Our study had significant strengths including the study design that provided a very precise measurement of 24-h EE during eucaloric feeding by two consecutive eucaloric assessments to better achieve energy balance, which was then used to formulate the overfeeding interventions by doubling 24-h EE that represents a very accurate measure of body energy requirements. Together with the 24-h assessment during fasting, we were able to precisely calculate DIT during each feeding conditions and to investigate the associations between DIT and CBT in two different overfeeding regimens, whose total energy intake was doubled compared to eucaloric energy needs. Further, we also continuously measured CBT during four different dietary interventions given for 24 hours in the controlled setting of metabolic chamber, allowing for assessments obtained in comparable conditions. Yet, we do have limitations for our current findings. Our current analysis only included men, which limits its generalizability to the whole population; however, we sought to eliminate the strong sex-specific differences both on CBT [13, 20] and body composition which may have influenced these results. In addition, we had a smaller sample size for the two distinct overfeeding interventions due to technical issues such as device malfunction, intestinal transit time, and instability of the capsule recording during these extreme dietary interventions characterized by twice the kcal provided during eucaloric feeding. However, our sensitivity analysis ensured that the subgroup of individuals with overfeeding data were not different in their anthropometric characteristics compared the entire cohort. Further, we did not record skin temperature measurements to investigate differences in vasoconstriction threshold which may provide more insight into the heat dissipation that governs the changes in CBT during feeding. Lastly, our study lacks measurements of plasma glucose, non-esterified fatty acids, growth hormone, IGF-1, and IGFBP-3 concentrations, which could be informative of inter-individual differences in CBT especially during fasting given our findings for epinephrine and the major role of lipolysis in the fasting state.
5. Conclusions
In conclusion, we evaluated CBT during 24-h fasting, eucaloric feeding, and two overfeeding diets and found that CBT increases in feeding and overfeeding conditions and partly mediates the adaptive EE response (DIT) to these acute dietary interventions. A ceiling effect for dietary-induced changes in CBT was observed and quantified, such that individuals can only reach and not exceed a maximal threshold for CBT of approximately 37°C during feeding and overfeeding conditions. This indicates that the relationship between DIT and CBT is limited to only subjects with relatively low CBT. Individuals with baseline CBT of approximately 37°C do not increase their CBT further while DIT still increases, thus suggesting that heat is subsumed in these individuals in alternate ways and that other physiologic factors (e.g., hormones, SNS activity, etc.) are involved in EE responses to overfeeding diets. Although low CBT might identify individuals with greater capacity to increase CBT, change in CBT is secondary to DIT. Given this and the demonstrated ceiling effect for CBT (but not DIT), targeting CBT is not likely to be effective in increasing EE and producing weight loss. Because of human body thermoregulatory mechanisms that tightly regulate and maintain temperature in a narrow physiologic range, CBT has a limited role in explaining the substantial inter-individual variability in DIT which determines the propensity to weight gain.
Supplementary Material
Highlights.
Core body temperature (CBT) is a determinant of human energy expenditure (EE)
CBT increases from fasting during feeding and overfeeding
However, there is a ceiling effect for diet-induced changes in CBT
CBT is modestly associated with diet-induced thermogenesis (DIT)
The relationship between DIT and CBT is limited to only subjects with lower CBT.
Acknowledgements
The authors would like to thank the volunteers for their time and commitment to our study and appreciate the excellent care of the dietary and nursing team in our inpatient unit. The authors wish to thank Susan Bonfiglio, PA, for her hard work on this clinical protocol, particularly in collecting CBT measurements. The authors report no conflict of interest. This research was supported by the Intramural Research Program of the NIH, The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This study was presented in abstract form at The Obesity Society 2017 annual meeting, Washington, DC, October 29-November 2, 2017.
Funding: This study was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Clinical Trial Registration Number (from clinicaltrials.gov): NCT00523627
Abbreviations:
- 24-h EE
24-hour energy expenditure
- avgCBT
average core body temperature
- CBT
core body temperature
- CNP
high-carbohydrate overfeeding diet
- CRU
clinical research unit
- DIT
diet-induced thermogenesis
- DXA
dual-energy X-ray absorptiometry
- EBL
energy balance
- EE
energy expenditure
- FFM
fat free mass
- FM
fat mass
- FNP
high-fat overfeeding diet
- OGTT
oral glucose tolerance test
- PFAT
percent body fat
- RQ
respiratory quotient
- SPA
spontaneous physical activity
- TEF
thermic effect of food
- WMD
weight-maintaining diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: The authors have nothing to disclose.
References
- [1].Piaggi P, Vinales KL, Basolo A, Santini F, Krakoff J. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. Journal of endocrinological investigation. 2018;41:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Reinhardt M, Thearle MS, Ibrahim M, Hohenadel MG, Bogardus C, Krakoff J, et al. A Human Thrifty Phenotype Associated With Less Weight Loss During Caloric Restriction. Diabetes. 2015;64:2859–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schlogl M, Piaggi P, Pannacciuli N, Bonfiglio SM, Krakoff J, Thearle MS. Energy Expenditure Responses to Fasting and Overfeeding Identify Phenotypes Associated With Weight Change. Diabetes. 2015;64:3680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].D’Alessio DA, Kavle EC, Mozzoli MA, Smalley KJ, Polansky M, Kendrick ZV, et al. Thermic effect of food in lean and obese men. J Clin Invest. 1988;81:1781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Jonge L, Bray GA. The thermic effect of food and obesity: a critical review. Obes Res. 1997;5:622–31. [DOI] [PubMed] [Google Scholar]
- [6].Thearle MS, Pannacciulli N, Bonfiglio S, Pacak K, Krakoff J. Extent and determinants of thermogenic responses to 24 hours of fasting, energy balance, and five different overfeeding diets in humans. J Clin Endocrinol Metab. 2013;98:2791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vinales KL, Schlogl M, Piaggi P, Hohenadel M, Graham A, Bonfiglio S, et al. The Consistency in Macronutrient Oxidation and the Role for Epinephrine in the Response to Fasting and Overfeeding. The Journal of clinical endocrinology and metabolism. 2017;102:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Landsberg L, Young JB, Leonard WR, Linsenmeier RA, Turek FW. Is obesity associated with lower body temperatures? Core temperature: a forgotten variable in energy balance. Metabolism. 2009;58:871–6. [DOI] [PubMed] [Google Scholar]
- [9].Landsberg L Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev. 2012;13 Suppl 2:97–104. [DOI] [PubMed] [Google Scholar]
- [10].Rising R, Keys A, Ravussin E, Bogardus C. Concomitant interindividual variation in body temperature and metabolic rate. Am J Physiol. 1992;263:E730–4. [DOI] [PubMed] [Google Scholar]
- [11].van Marken Lichtenbelt WD, Schrauwen P, van de Kerckhove S, Westerterp-Plantenga MS. Individual variation in body temperature and energy expenditure in response to mild cold. American Journal of Physiology-Endocrinology and Metabolism. 2002;282:E1077–E83. [DOI] [PubMed] [Google Scholar]
- [12].Reinhardt M, Schlogl M, Bonfiglio S, Votruba SB, Krakoff J, Thearle MS. Lower core body temperature and greater body fat are components of a human thrifty phenotype. Int J Obes (Lond). 2016;40:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hoffmann ME, Rodriguez SM, Zeiss DM, Wachsberg KN, Kushner RF, Landsberg L, et al. 24-h core temperature in obese and lean men and women. Obesity (Silver Spring). 2012;20:1585–90. [DOI] [PubMed] [Google Scholar]
- [14].Landsberg L, Saville ME, Young JB. Sympathoadrenal system and regulation of thermogenesis. American Journal of Physiology-Endocrinology And Metabolism. 1984;247:E181–E9. [DOI] [PubMed] [Google Scholar]
- [15].Young JB, Landsberg L. Stimulation of the sympathetic nervous system during sucrose feeding. Nature. 1977;269:615–7. [DOI] [PubMed] [Google Scholar]
- [16].Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–5. [DOI] [PubMed] [Google Scholar]
- [17].Landsberg L Feast or famine: the sympathetic nervous system response to nutrient intake. Cell Mol Neurobiol. 2006;26:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247:E35–40. [DOI] [PubMed] [Google Scholar]
- [19].Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81–90. [DOI] [PubMed] [Google Scholar]
- [20].Heikens MJ, Gorbach AM, Eden HS, Savastano DM, Chen KY, Skarulis MC, et al. Core body temperature in obesity. Am J Clin Nutr. 2011;93:963–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. Jama. 1992;268:1578–80. [PubMed] [Google Scholar]
- [22].Sund-Levander M, Forsberg C, Wahren LK. Normal oral, rectal, tympanic and axillary body temperature in adult men and women: a systematic literature review. Scandinavian journal of caring sciences. 2002;16:122–8. [DOI] [PubMed] [Google Scholar]
- [23].Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. J Clin Invest. 1986;78:1586–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Waalen J, Buxbaum JN. Is older colder or colder older? The association of age with body temperature in 18,630 individuals. The journals of gerontology Series A, Biological sciences and medical sciences. 2011;66:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gomolin IH, Aung MM, Wolf-Klein G, Auerbach C. Older is colder: temperature range and variation in older people. Journal of the American Geriatrics Society. 2005;53:2170–2. [DOI] [PubMed] [Google Scholar]
- [27].Chamberlain JM, Terndrup TE, Alexander DT, Silverstone FA, Wolf-Klein G, O’Donnell R, et al. Determination of normal ear temperature with an infrared emission detection thermometer. Annals of emergency medicine. 1995;25:15–20. [DOI] [PubMed] [Google Scholar]
- [28].Brundin T, Thorne A, Wahren J. Heat leakage across the abdominal wall and meal-induced thermogenesis in normal-weight and obese subjects. Metabolism. 1992;41:49–55. [DOI] [PubMed] [Google Scholar]
- [29].Grimaldi D, Provini F, Pierangeli G, Mazzella N, Zamboni G, Marchesini G, et al. Evidence of a diurnal thermogenic handicap in obesity. Chronobiology international. 2015;32:299–302. [DOI] [PubMed] [Google Scholar]
- [30].Abreu-Vieira G, Xiao C, Gavrilova O, Reitman ML. Integration of body temperature into the analysis of energy expenditure in the mouse. Mol Metab. 2015;4:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jensen MD, Haymond MW, Gerich JE, Cryer PE, Miles JM. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J Clin Invest. 1987;79:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kurz A Physiology of thermoregulation. Best Pract Res Clin Anaesthesiol. 2008;22:627–44. [DOI] [PubMed] [Google Scholar]
- [33].Hammel HT. Regulation of internal body temperature. Annu Rev Physiol. 1968;30:641–710. [DOI] [PubMed] [Google Scholar]
- [34].Mizobe T, Nakajima Y, Ueno H, Sessler DI. Fructose administration increases intraoperative core temperature by augmenting both metabolic rate and the vasoconstriction threshold. Anesthesiology. 2006;104:1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, et al. Comparison of thermogenic effect of fructose and glucose in normal humans. Am J Physiol. 1986;250:E718–24. [DOI] [PubMed] [Google Scholar]
- [36].Perakakis N, Mougios V, Fatouros I, Siopi A, Draganidis D, Peradze N, et al. Physiology of Activins/Follistatins: Associations With Metabolic and Anthropometric Variables and Response to Exercise. J Clin Endocrinol Metab. 2018;103:3890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.