Abstract
OBJECTIVE:
To estimate the association between perceived fertility potential and contraception use and to characterize factors important in contraceptive decision-making in reproductive-aged, female cancer survivors
DESIGN:
Cross-sectional study
SETTING:
Participants were from two state cancer registries, physician referrals, and cancer survivor advocacy groups in the United States
PATIENTS:
483 female survivors aged 18–40
INTERVENTIONS:
Online questionnaire
MAIN OUTCOME MEASURES:
Contraception use
RESULTS:
Eighty-four percent of participants used contraception; 49.7% utilized highly effective, World Health Organization Tier I and II methods (surgical sterilization, intrauterine devices, contraceptive implant, combined hormonal contraceptives, medroxyprogesterone acetate, progestin only pills, contraceptive diaphragm). Contraception non-use was more common among survivors who perceived themselves to be infertile, compared to survivors who perceived themselves to be as or more fertile than similarly aged peers (PR 4.0, 95% CI 2.5–7.4). In mediation analysis that adjusted for clinical infertility, 59% of the association between prior chemotherapy and contraception non-use was explained by perceived infertility. Contraception efficacy (n=62, 25.8%) and ease of use (n=50, 20.8%) were the most cited reasons for using Tier I/II methods; compared with lack of hormones (n=81, 49.7%) as the predominant reason for using less effective, Tier III/IV methods (P<0.001).
CONCLUSION:
While female, reproductive-age cancer survivors had high uptake of contraception, those who perceived themselves to be infertile were less likely to use contraception. Throughout survivorship, clinicians should counsel survivors on fertility potential in the context of their prior cancer treatments and on factors including contraceptive efficacy and hormone-free contraception that inform reproductive decision-making in this population.
Keywords: cancer survivors, contraception, fertility perception, oncofertility
Introduction
Reproductive-age cancer survivors have complex contraception considerations. While some cancer treatments, including alkylating chemotherapy and pelvic radiation, are gonadotoxic, many young cancer survivors retain their fertility potential following cancer therapy (1–6). Hence, the absolute risk of infertility in female childhood cancer survivors of the large Childhood Cancer Survivor Study was modest at 13%, but higher compared to 10% of sibling controls (6). Post-treatment, currently available laboratory and imaging assessments of ovarian reserve, such as antimüllerian hormone levels and antral follicle count, have not been adequately studied in this population and do not appear to predict fecundity in the non-cancer population (7–9); as such, reproductive-age cancer survivors and their healthcare providers have limited resources with which to determine fertility. Yet, these individuals have to make sound decisions on using contraception and preventing unintended pregnancy, because this population faces increased rates of pregnancy-related complications including miscarriage, preterm birth, and low birth weight neonates (10–15). As pregnancy-related risks differ by cancer type and prior treatment exposures, planned pregnancies allow for tailored preconception care such as cardiac screening to optimize maternal and offspring health.
Despite clinical guidelines recommending adequate contraception for survivors of cancers occurring in adolescence and young adulthood, contraception rates are lower and use of emergency contraception is higher in this population than in the general U.S. population of reproductive-age women (16–20). Moreover, studies have shown that cancer survivors also have lower utilization rates of highly effective contraceptive methods categorized as Tier I (surgical sterilization, contraceptive implant, IUD) or II (combined hormonal contraceptive, injectable progestin, progestin only pills, lactation amenorrhea) by the World Health Organization (18).
While existing data have demonstrated suboptimal contraception practices among young, female cancer survivors, factors important in contraceptive decision-making in this population are not well described (18, 19). Specifically, it is unknown whether survivors’ perception of their own fertility potential affects their contraceptive choice. Studies of female childhood cancer survivors indicate that a majority perceive themselves to be at risk of infertility following cancer therapy, however, when their actual cancer treatments are taken into account, only a minority of those with such concerns were actually at risk for infertility (21, 22). If perceived fertility potential is not accurate and adversely affects contraceptive behavior, then this perception would increase the risk for unintended pregnancy.
The objective of this study was to identify factors associated with contraceptive decision-making among female, reproductive-age cancer survivors. We hypothesized that perceived infertility among female cancer survivors is associated with contraception non-use and use of less effective contraceptive methods. We further tested if perceived fertility contributes to, or mediates the association between cancer treatment characteristics and use of contraception.
Methods
Reproductive Window Study
We performed a cross-sectional study using survey data collected at enrollment to the parent Reproductive Window Study, an ongoing study estimating the trajectory of ovarian function over cancer survivorship. Eligibility criteria for the Reproductive Window Study included: cancer diagnoses between ages 15–35, ages 18–40 at study enrollment, completion of primary cancer treatment, and presence of at least one ovary. The following cancer types were included: breast, blood and leukemia, lymphoma, gynecologic (cervix, uterus, ovary), intestines, gall bladder, pancreas, bone, soft tissue tumor of bone/fat, skin, and thyroid. For the current analysis, we included participants who were at risk of unintended pregnancy, excluding those actively attempting conception, reporting no heterosexual intercourse in the year prior to study enrollment, or with history of sterilization with hysterectomy or bilateral salpingo-oophrectomy. Attempting conception was determined by the answer to the survey question, “Are you trying to become pregnant now?” Participants were recruited between March 2015 and May 2017 from the California and Texas Cancer Registries (34.8%), University of California, San Diego Health System (28.0%), referrals from physicians (4.3%) and referrals from cancer advocacy organizations (11.2%). The State of California Committee for the Protection of Human Subjects and the institutional review boards at the University of California, San Diego and the Texas Department of State Health Services approved this study.
Participants answered questions assessing demographics, cancer history, reproductive characteristics, and family planning, including their perceived fertility, via online questionnaires. Age, type of cancer, and exposure to chemotherapy, radiation, bone marrow transplant, and surgery were assessed by survey, as prior studies show high agreement between patient recall and medical record or cancer registry data regarding diagnosis and general treatment categories (23–25). Reproductive and family planning behavior, including sexual orientation and activity, contraception use, pregnancy history, plans for future family building, history of fertility preservation or family planning consultation, and reasons for contraception non-use, were assessed using questions derived from the 2006–2010 cycle of National Survey for Family Growth (NSFG) and the 2012 Behavioral Risk Factor Surveillance System (BRFSS) (26, 27). Concerns about personal health and child health were measured using the respective subscales of the Reproductive Concerns After Cancer Scale. For each subscale, mean score > 3 indicated moderate to high concern (28). Participants’ perceived fertility was ascertained using a single question with four possible responses: I think I am more fertile, as fertile, less fertile, or infertile compared to women of the same age. Responses were categorized as more/as fertile, less fertile, and infertile. History of clinical infertility in this study was defined by an affirmative answer to the question, “did you ever try to become pregnant for at least a year without becoming pregnant?”
Statistical Analyses
Descriptive data were summarized using frequencies for categorical variables and means and standard deviation for continuous variables that were normally distributed and median (range) for continuous variables that were not normally distributed. Chi-square or Fisher’s exact tests were used to compare contraception users with non-users in regard to perceived fertility and demographic, cancer, and reproductive characteristics. A Freeman-Halton extension on Fisher’s test allowed rows and/or columns with greater than two levels. Characteristics that were significant (P < 0.05) in the bivariable analyses were included in the multivariable log-binomial model that estimated prevalence ratios (PR) of factors related to contraception non-use.
Perceived infertility was then evaluated as a potential mediator, or causal intermediate, of the relationship between chemotherapy (independent variable) and contraception non-use (dependent variable). Among different approaches described for mediation analysis, simple adjustment is a commonly used approach to partition the “total effect” of an exposure (here, chemotherapy) and an outcome (here, contraception use) into the proportion acting through an intermediate (here, perceived infertility). First, a model was constructed to estimate the total effect, or the overall association between chemotherapy and contraception, adjusting for confounding. Addition of perceived infertility to this model then generated PR estimates for a mediation model used to estimate the direct effect. The proportion mediated was derived by comparing the total and direct effect estimates with the equation:
Among participants using contraception, the primary reason for choice of contraceptive and participant characteristics were compared by use of highly effective (WHO Tiers I/II methods including vasectomy, tubal ligation, IUD, the contraceptive implant, combined hormonal contraceptives, medroxyprogesterone acetate, progestin only pills, and contraceptive diaphragm) versus less effective (WHO Tiers III/IV methods including male and female condoms, fertility awareness, withdrawal, and spermicide) methods. Statistical significance was set at P < 0.05. All statistical analyses were performed using SAS v9.3 (Cary, NC).
Results
For the parent study, 1,112 individuals were screened, and 830 (75%) were eligible. Among eligible women, 766 (92%) were enrolled. Among 670 women who completed the enrollment survey at the time of this analysis, 187 participants were excluded from this analysis (120 without intercourse in the year prior to enrollment, 15 with history of hysterectomy, and 57 who were actively attempting pregnancy). Table 1 summarizes participant characteristics of 483 survivors included in the study. The median age of participants was 34.0 years (range 19.3 – 41.0 years) with 7.6 ± 5.4 years since cancer diagnosis (range 0.3 to 26.4 years). Thirty-one percent of participants received family planning counseling within 12 months of participation and 28.6% reported having ever seen a fertility specialist. Thirteen percent of participants (n = 62) reported history of clinical infertility, defined as at least 12 consecutive months of unsuccessful pregnancy attempts.
Table 1.
Participant characteristics and contraception use versus non-use
| Total Cohort n (%) n = 483 | Contraception Non-users n (%) n = 74 | Contraception Users n (%) n = 406 | p-value | |
|---|---|---|---|---|
| Current age (y) | ||||
| 18 – 24 | 32 (6.7) | 3 (4.0) | 29 (7.3) | |
| 25 – 30 | 108 (22.7) | 11 (14.5) | 97 (24.3) | 0.14 |
| 31 – 35 | 174 (36.6) | 31 (40.8) | 143 (35.8) | |
| 36 – 41 | 161 (33.9) | 31 (40.8) | 130 (32.6) | |
| Race | ||||
| White | 360 (74.5) | 53 (68.8) | 307 (75.6) | |
| Black | 10 (2.1) | 2 (2.6) | 8 (2.0) | 0.24 |
| Asian/Pacific Islander | 29 (6.0) | 3 (3.9) | 26 (6.4) | |
| Mixed/Other | 84 (17.4) | 19 (24.7) | 65 (16.0) | |
| Hispanic ethnicity | 109 (22.6) | 20 (26.0) | 89 (21.9) | 0.44 |
| BMI (kg/m2) | ||||
| < 18.5 | 117 (24.8) | 17 (22.7) | 100 (25.3) | |
| 18.5 – 24.9 | 212 (45.0) | 34 (45.3) | 178 (45.0) | 0.58 |
| 25 – 29.9 | 70 (14.9) | 9 (12.0) | 61 (15.4) | |
| ≥ 30 | 72 (15.3) | 15 (20.0) | 57 (14.4) | |
| Education: college graduate Income | 365 (75.6) | 54 (70.1) | 311 (76.6) | 0.23 |
| < $51,000 | 113 (23.4) | 16 (20.8) | 97 (23.9) | 0.83 |
| ≥ $51,000 | 341 (70.6) | 56 (72.7) | 285 (70.2) | |
| Current health insurance | 460 (95.2) | 72 (93.5) | 388 (95.6) | 0.44 |
| Cancer Characteristics | ||||
| Cancer Category | ||||
| Breast | 113 (23.4) | 20 (26.0) | 93 (22.9) | |
| Leukemia/Lymphoma | 153 (31.7) | 28 (36.4) | 125 (30.8) | |
| Gynecologic†† | 28 (5.8) | 6 (7.8) | 22 (5.4) | 0.41 |
| Gastrointestinal††† | 14 (2.9) | 3 (3.9) | 11 (2.7) | |
| Bone/Soft tissue | 33 (6.8) | 4 (5.2) | 29 (7.1) | |
| Thyroid/Skin | 142 (29.4) | 16 (20.8) | 126 (31.0) | |
| Chemotherapy | 304 (62.9) | 57 (74.0) | 247 (60.8) | 0.03 |
| Bone Marrow Transplant | 15 (3.1) | 5 (6.5) | 10 (2.5) | 0.06 |
| Surgical cancer therapy | 332 (68.7) | 49 (63.6) | 283 (69.7) | 0.29 |
| Radiation | 235 (48.7) | 43 (55.8) | 192 (47.3) | 0.17 |
| Age at cancer diagnosis | ||||
| Less than 18 | 53 (11.2) | 8 (10.5) | 45 (11.3) | |
| 18 – 24 | 163 (34.3) | 22 (29.0) | 141 (35.3) | 0.14 |
| 25 – 35 | 258 (54.3) | 46 (60.5) | 213 (53.4) | |
| Years since cancer diagnosis | ||||
| < 2 | 64 (13.3) | 13 (16.9) | 51 (12.6) | |
| 2 – 5 | 123 (25.5) | 21 (27.3) | 102 (25.1) | 0.48 |
| > 5 | 296 (61.3) | 43 (55.8) | 253 (62.3) | |
| Reproductive Characteristics | ||||
| History of infertility | 62 (12.8) | 23 (29.9) | 39 (9.6) | <0.001 |
| Infertility before cancer | 33 (6.8) | 11 (14.3) | 22 (5.4) | <0.01 |
| Infertility after cancer | 39 (8.1) | 18 (23.4) | 21 (5.2) | <0.001 |
| Menses within the last year | ||||
| 0 – 3 | 41 (9.9) | 11 (19.0) | 30 (8.4) | |
| 4 – 9 | 82 (19.7) | 10 (17.2) | 72 (20.1) | <0.001 |
| 10 – 12 | 293 (70.4) | 37 (63.8) | 256 (71.5) | |
| Menses within the last year* | ||||
| 0 – 3 | 62 (20.8) | 30 (39.0) | 32 (14.5) | |
| 4 – 9 | 40 (13.4) | 10 (13.0) | 30 (13.6) | <0.001 |
| 10 – 12 | 196 (65.8) | 37 (48.0) | 159 (71.9) | |
| Ever pregnant | 257 (53.2) | 46 (59.7) | 211 (52.0) | 0.21 |
| Times pregnant | ||||
| 1 | 70 (27.2) | 13 (28.3) | 57 (27.0) | |
| 2 | 102 (39.7) | 20 (43.5) | 82 (38.9) | 0.73 |
| ≥ 3 | 85 (33.1) | 13 (28.3) | 72 (34.1) | |
| Ever live birth | 225 (46.6) | 42 (54.6) | 183 (45.1) | 0.13 |
| Recent intercourse** | 426 (88.2) | 67 (87.0) | 359 (88.4) | 0.73 |
| Reproductive concerns | ||||
| Personal health (> 3) | 178 (36.9) | 28 (36.4) | 150 (37.0) | 0.92 |
| Child health (> 3) | 292 (60.5) | 45 (58.4) | 247 (60.8) | 0.69 |
Data missing for some variables
Gynecologic cancer = cervix, uterus, ovary
Gastrointestinal cancer = pancreas, gallbladder, stomach, small intestine, colon, appendix, rectum
Excluding participants on birth control pills, depot medroxyprogesterone, implant, IUD, contraceptive patch and contraceptive ring
Recent intercourse indicates heterosexual intercourse within 3 months of completing the enrollment questionnaire
Forty-three percent of participants felt more or as fertile as other similarly aged women, while 37% perceived themselves to be less fertile, and 19% perceived themselves to be infertile compared to women of the same age. More fertile (n=22) and as fertile (n=187) were collapsed into one category because contraceptive choices were similar between the two groups (data not shown). Eighty-four percent of participants used contraception; among these, specific methods of contraception included barrier/withdrawal methods (49.8%), combined hormonal contraceptives (22.7%), IUD (20.7%), vasectomy (8.4%), tubal sterilization (6.4%), rhythm method (1.5%), Depo-Provera (1.5%), and contraceptive implant (1.2%). We did not ascertain hormonal versus copper intrauterine device (IUD).
Participants who perceived that they were infertile were less likely to use contraception (60.6%), compared to participants who perceived that they were less fertile (88.8%) or more or as fertile (90.5%) (p<0.0001). Compared to women who did not report perceiving that they were infertile, participants who perceived that they were infertile were four-fold more likely to not use contraception (PR 4.0, 95% CI 2.5–7.4). Perception of fertility was also related to several participant characteristics, including actual history of infertility, age, gravidity, parity, menstrual pattern, cancer type, and cancer therapy (Table 2). However, only 24.5% of survivors who perceived themselves to be infertile reported history of clinical infertility.
Table 2.
Participant characteristics and self-reported perceived fertility potential
| Participant Characteristics† | More/As fertile n (%) n=210 | Less fertile n (%) n=179 | Infertile n (%) n=94 | P-value |
|---|---|---|---|---|
| Current age (y) | ||||
| 18 – 24 | 8 (3.9) | 21 (11.9) | 3 (3.3) | |
| 25 – 30 | 44 (21.2) | 41 (23.3) | 23 (25.3) | 0.001 |
| 31 – 35 | 82 (39.4) | 68 (38.6) | 24 (26.4) | |
| 36 – 41 | 74 (35.6) | 46 (26.1) | 41 (45.1) | |
| Race | ||||
| White | 158 (75.2) | 132 (73.7) | 70 (74.7) | |
| Black | 6 (2.9) | 1 (0.6) | 3 (3.2) | 0.39 |
| Asian/Pacific Islander | 14 (6.7) | 13 (7.3) | 2 (2.1) | |
| Mixed/Other | 32 (15.2) | 33 (18.4) | 19 (20.2) | |
| Hispanic ethnicity | 47 (22.3) | 41 (22.9) | 21 (22.3) | 0.96 |
| BMI (kg/m2) | ||||
| < 18.5 | 42 (20.9) | 50 (28.3) | 25 (26.9) | |
| 18.5 – 24.9 | 98 (48.8) | 73 (41.2) | 41 (44.1) | 0.75 |
| 25 – 29.9 | 31 (15.4) | 26 (14.7) | 13 (14.0) | |
| ≥ 30 | 30 (14.9) | 28 (15.8) | 14 (15.1) | |
| Education: college graduate | 164 (78.1) | 135 (75.4) | 66 (70.2) | 0.20 |
| Income | ||||
| Less than $51,000 | 43 (20.5) | 48 (26.8) | 22 (23.4) | 0.48 |
| Greater than $51,000 | 156 (74.3) | 122 (68.2) | 63 (67.0) | |
| Current health insurance | 201 (95.7) | 169 (94.4) | 90 (95.7) | 0.84 |
| Cancer Characteristics | ||||
| Cancer category | ||||
| Breast | 40 (19.1) | 48 (26.8) | 25 (26.6) | |
| Leukemia/Lymphoma | 63 (30.0) | 59 (33.0) | 31 (33.0) | |
| Gynecologic†† | 10 (4.8) | 7 (3.9) | 11 (11.7) | 0.02 |
| Gastrointestinal††† | 5 (2.4) | 6 (3.4) | 3 (3.2) | |
| Bone/soft tissue | 14 (6.7) | 12 (6.7) | 7 (7.5) | |
| Thyroid, Skin | 78 (37.1) | 47 (26.3) | 17 (18.1) | |
| Chemotherapy | 109 (51.9) | 121 (67.6) | 74 (78.7) | <0.001 |
| Bone Marrow Transplant | 1 (0.5) | 4 (2.2) | 10 (10.6) | <0.001 |
| Surgical cancer therapy | 150 (71.4) | 124 (69.3) | 58 (61.7) | 0.16 |
| Radiation | 99 (47.1) | 87 (48.6) | 49 (52.1) | 0.65 |
| Age at cancer diagnosis | ||||
| Less than 18 | 25 (12.0) | 20 (11.4) | 8 (8.8) | |
| 18 – 24 | 74 (35.6) | 61 (34.7) | 28 (30.8) | 0.34 |
| 25 – 35 | 109 (52.4) | 95 (53.9) | 55 (60.4) | |
| Years since cancer diagnosis | ||||
| < 2 | 19 (9.1) | 30 (16.8) | 15 (16.0) | |
| 2 – 5 | 41 (19.5) | 55 (30.7) | 27 (28.7) | 0.002 |
| ≥ 5 | 150 (71.4) | 94 (52.5) | 52 (55.3) | |
| Reproductive Characteristics | ||||
| History of infertility | 11 (5.2) | 28 (15.6) | 23 (24.5) | <0.001 |
| Prior to cancer diagnosis | 6 (2.9) | 14 (7.8) | 13 (13.8) | <0.001 |
| After cancer diagnosis | 5 (2.4) | 19 (10.6) | 15 (15.9) | |
| Menses within the last year | ||||
| 0 – 3 | 38 (18.1) | 33 (18.4) | 37 (39.4) | |
| 4 – 9 | 32 (15.2) | 32 (17.9) | 18 (19.2) | <0.001 |
| 10 – 12 | 140 (66.7) | 114 (63.7) | 39 (41.5) | |
| Menses within the last year* | ||||
| 0 – 3 | 17 (13.7) | 14 (14.4) | 31 (40.3) | |
| 4 – 9 | 15 (12.1) | 14 (14.4) | 11 (14.3) | <0.001 |
| 10 – 12 | 92 (74.2) | 69 (71.2) | 35 (45.4) | |
| Ever pregnant | 142 (67.6) | 72 (40.2) | 43 (45.7) | <0.001 |
| Times pregnant | ||||
| 1 | 31 (21.8) | 29 (40.3) | 10 (23.2) | 0.03 |
| 2 | 65 (45.8) | 22 (30.5) | 15 (34.9) | |
| ≥ 3 | 46 (32.4) | 21 (29.2) | 18 (41.9) | |
| Ever live birth | 129 (61.4) | 57 (31.8) | 39 (41.5) | <0.001 |
| Recent intercourse** | 192 (91.4) | 153 (85.5) | 81 (83.2) | 0.15 |
Data missing for some variables
Gynecologic cancer = cervix, uterus, ovary
Gastrointestinal cancer = pancreas, gallbladder, stomach, small intestine, colon, appendix, rectum
Excluding participants on birth control pills, depot medroxyprogesterone, implant, IUD, contraceptive patch and contraceptive ring
Recent intercourse indicates heterosexual intercourse within 3 months of completing the enrollment questionnaire
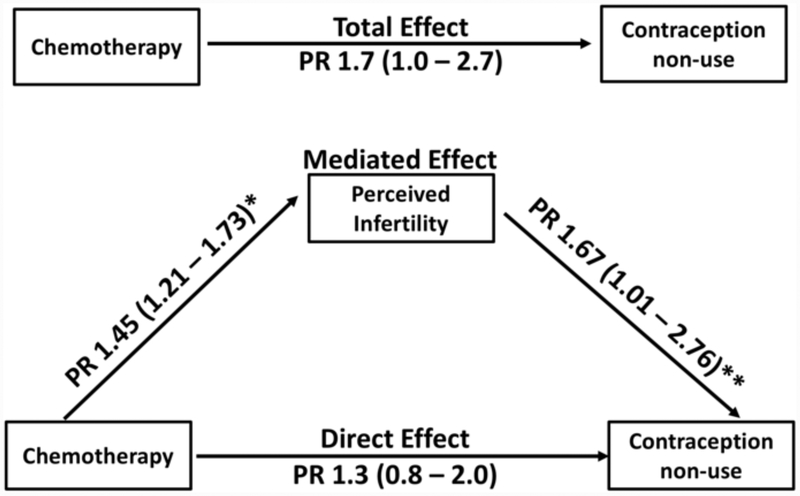
History of chemotherapy, infertility before or after cancer diagnosis, and fewer menses over the past year were also associated with contraception non-use (Table 1). Menstrual pattern remained associated with contraception non-use when analysis was restricted to participants now using hormonal contraception (p<0.001) or to participants who were not amenorrheic in the last year (p=0.04, data not shown). In a model that included chemotherapy (total effect PR 1.7, 95% CI 1.1–2.7) and history of infertility (PR 2.9, 95% CI 1.9–4.3), both factors remained significantly associated with lower prevalence of contraception. Menstrual pattern was not included in the model, because of co-linearity with infertility. In the model that further adjusted for perceived infertility, the direct effect of chemotherapy on contraception non-use that was not mediated by perceived infertility was attenuated (PR 1.3, 95% CI 0.8–2.0) (Figure 2). Comparison of the total and direct effects of chemotherapy on contraception non-use showed that 59% of the association between prior chemotherapy and not using contraception was explained by perceived infertility.
Figure 2. Mediation Analysis.
The Total Effect prevalence ratio (PR) demonstrates the association between chemotherapy and contraception non-use, Direct Effect PR reflects the effect of chemotherapy on contraception non-use, and Mediated Effect describes the proportion of the association between chemotherapy and contraception non-use relationship that is explained by perceived fertility.
*Adjusted for history of history of infertility
** Adjusted for history of infertility and history of chemotherapy
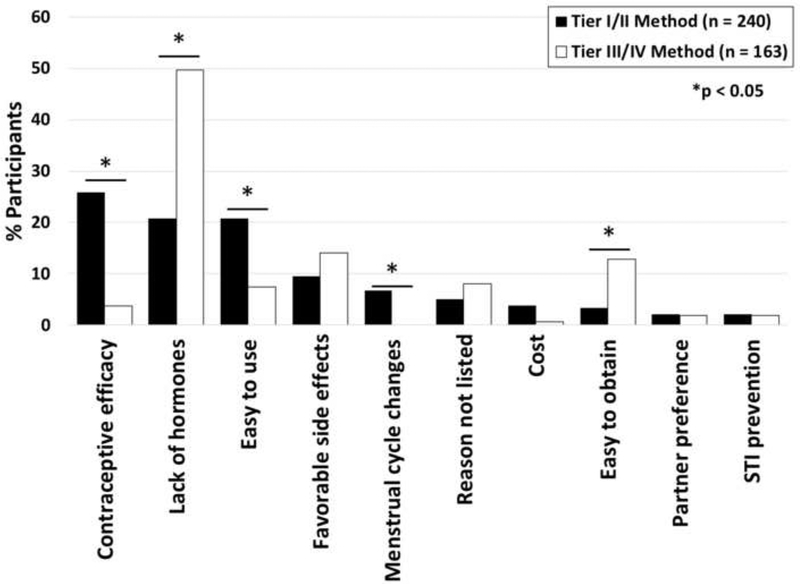
Among participants using contraception, 146 (35.9%), 94 (23.1%), 118 (29.1%), and 44 (10.8%) utilized WHO Tier I, II, III, and IV methods, respectively. Perception of fertility was not associated with use of highly effective methods (Supplemental Table 1). History of radiation, more recent cancer diagnosis, and nulliparity were associated with less use of highly effective methods. The primary reason for birth control choice differed between those using highly effective methods and those using less effective methods (Figure 2). Participants using highly effective methods were more likely to cite contraceptive efficacy (n=62, 25.8%) and ease of use (n=50, 20.8%) as the primary reason for contraceptive choice, compared with women who used less effective methods. Women who used less effective methods were more likely to cite lack of hormones (n=81, 49.7%) as the primary factor influencing their choice. Side effect profile, prevention of sexually transmitted infection, and partner preference were not associated with efficacy of chosen contraceptive method. Among women who did not use contraception, the most common primary reason for non-use was perceived infertility (Supplemental Table 2).
Discussion
While effective contraception is important to prevent unintended pregnancies in female, reproductive-age cancer survivors because of, increased perinatal risks, this population has been shown to contracept less frequently and effectively than other reproductive-age women (18, 19). This cross-sectional study demonstrated that survivors’ perceived infertility was independently associated with not using contraception, even after accounting for clinical infertility. Mediation analysis suggested that perception of infertility explains a large proportion of the relationship between history of chemotherapy and current contraception non-use. Moreover, we identified factors such as contraceptive efficacy and lack of hormones as important to contraception decision-making among survivors. Taken together, these findings highlight the importance of improving family planning counseling to encompass discussion of fertility potential and highlight factors that are important to patients’ contraception decision making, such as hormone-free methods and methods that have high efficacy.
Results of this study identified survivors’ perceived infertility as a strong predictor of contraception non-use. This exposure was of interest because survivors’ perceptions of their fertility may be inconsistent with their true risk (21, 22). While the relative risk of infertility is increased in cancer survivors, the absolute risk remains low even in the setting of gonadotoxic therapy (6). This was evident in our study population, with the majority of women who perceived themselves to be infertile having no medical history of clinical infertility. Unfortunately, this survey did not capture women without pregnancy within 12 months of unprotected intercourse if they did not report trying to conceive during that time. In this way, we may underestimate the proportion of women with infertility in this cohort. Nonetheless it is likely that there are women in our cohort who inaccurately perceive reduced fertility. Further, mediation analysis suggested that the relationship between prior chemotherapy and contraception non-use is largely explained by perceived infertility, suggesting that survivors’ behavior is influenced by their prior cancer therapy. Inaccurate perceived infertility may increase likelihood of unintended, high-risk pregnancies if fertile survivors elect to defer contraception with the belief that they are unable to conceive. Individualized, patient-specific counseling regarding risks to fertility in the context of prior cancer therapies may reduce this risk.
We have previously published age-adjusted comparisons of contraception use between another cohort of reproductive-age cancer survivors and the general population recruited to the NSFG (18). We demonstrated significantly lower rates of female sterilization and higher rates of condom use in cancer survivors compared to the general population. The current cancer survivor population is similar to our prior cohort in high use of barrier/withdrawal methods and low use of female sterilization. In addition, while not age-adjusted, the rate of contraception use in our current cohort of survivors at risk of unintended pregnancy (84%), is similar to that described in women at risk of unintended pregnancy the general population (89%) (29). While we did not directly compare the current cohort to the general U.S. population, this distribution of contraceptive methods in cancer survivors appears different from those without cancer.
Prior to this study, factors contributing to reduced contraception use among young adult cancer survivors were largely unknown, with the exception of exogenous hormone avoidance in breast cancer survivors (18, 19, 30, 31). This study confirmed lack of sex steroids as an important factor in contraceptive decision making, with 49.7% percent of participants who used less effective (Tiers III/IV methods) citing this as their primary reason for contraceptive choice. Alternately, users of highly effective (Tiers I/II) methods most often cited contraceptive efficacy, ease of use, or lack of hormones as their primary reason for contraceptive choice. Together, these factors suggest the importance of contraceptive counseling, particularly regarding non-hormonal, long-acting reversible contraceptives (LARCs) such as the copper IUD. As previously demonstrated by the Contraceptive CHOICE Project, a majority of women may select (68%) and continue (77%) highly-effective methods such LARCs, thereby decreasing risks of unintended pregnancies when provided with comprehensive counseling and no-cost contraception (32–34).
The strengths of our study include large cohort size, diversity of cancer types and treatments represented, and recruitment largely from cancer registries, increasing generalizability. Our study was limited by use of cross-sectional data, which limits assessment of temporal relationships between cancer history and perception of fertility, and perception of fertility with current contraceptive behavior. We were also limited by sample size of participants using specific contraceptive methods, and therefore unable to examine the association between individual methods and participant characteristics. Additionally, while cancer survivors can accurately recall type of cancer and exposure to treatment categories (chemotherapy, radiation, bone marrow transplant, and surgery), patient recall of more specific gonadotoxic treatments is poor (2, 3, 5). Hence, we were unable to measure the association between gonadotoxicity of cancer treatments and contraception. We are also limited in characterizing the overall recruitment rate, because recruitment to the parent study included social media outreach which is difficult to quantify. We do know that study enrollment from the two state cancer registries was0.6%, lower than that observed in other registry-based studies of young female cancer survivors (26 – 51%)(35–39). We speculate this is due to single contact via mail and the parent study requirement for longitudinal self-collected biosamples including dried blood spots. Importantly, generalizability of our study is limited as the population features underrepresentation of black women, and is largely college educated and insured.
Although use of nationally standardized questions from NSFG and BRFSS improved the generalizability of our survey responses, interpretation of some data was limited by the standardized response options. For example, in response to the BRFSS question querying the primary reason for contraception non-use, 30% of participants who did not use contraception responded, “don’t know why,” “don’t want to,” or “other”, limiting the development of interventions based upon responses to this question. We speculate a proportion of “other” responses may refer to concerns of exposure to sex steroid hormones, particularly in the setting of breast cancer(30). For example, 9/24 (37.5%) of breast cancer participants selected “other” as the primary reason for contraception non-use, compared with 12/70 (17%) of survivors of other cancer types. In response to the same question, we also observed a small proportion (9.5%) that selected “don’t care if I get pregnant,” suggesting ambivalence on pregnancy (40). Interestingly, the proportion of participants who did not contracept because of perceived infertility appears to be higher in this cohort of survivors (40.5%) than in the general population (8.6%), consistent with our findings that cancer survivors’ perceived fertility is related to their contraceptive behavior (40).
This study provides a novel assessment of factors associated with how female, reproductive-age cancer survivors choose to contracept. Interventions to improve contraceptive decision making in this population should address efficacy, ease of use, and safety of contraceptive methods in the context of individualized fertility awareness.
Supplementary Material
Figure 1.
Among female, reproductive-age cancer survivors using contraception (n=406), primary reason for contraceptive choice by use of highly effective (WHO Tiers I/II) vs less effective (Tiers III/IV) methods.
Table 3.
Unadjusted, adjusted, and mediation models of contraception non-use vs use
| Unadjusted Model PR (95% CI) | Model 1: PR (95% CI) | Model 2: PR (95% CI) | |
|---|---|---|---|
| Chemotherapy | |||
| No | Reference | Reference | Reference |
| Yes | 1.7 (1.0 – 2.7) | 1.7 (1.1 – 2.7) | 1.3 (0.8 – 2.0) |
| History of infertility | |||
| No | Reference | Reference | |
| Yes | 2.9 (1.9 – 4.3) | 2.1 (1.4 – 3.15) | |
| Perceived fertility | |||
| More/As fertile | Reference | ||
| Less Fertile | 1.0 (0.3 – 1.8) | ||
| Infertile | 3.1 (1.8 – 5.3) |
PR = prevalence ratio
Funding:
This work was supported by the California Breast Cancer Research Program (20OB-0144), and the National Institutes of Health HD080952–04 and HD0007203. The sponsors had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none
Capsule: Perceived infertility, independent of clinical infertility, is related to contraception nonuse in female cancer survivors. Contraceptive counseling should address cancer treatment-specific fertility risks and factors important to survivors’ contraception decision-making.
References
- 1.Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide-induced ovarian failure. N Engl J Med 1973;289:1159–62. [DOI] [PubMed] [Google Scholar]
- 2.Wallace WH, Thomson AB, Kelsey TW. The radiosensitivity of the human oocyte. Hum Reprod 2003;18:117–21. [DOI] [PubMed] [Google Scholar]
- 3.Koyama H, Wada T, Nishizawa Y, Iwanaga T, Aoki Y. Cyclophosphamide-induced ovarian failure and its therapeutic significance in patients with breast cancer. Cancer 1977;39:1403–9. [DOI] [PubMed] [Google Scholar]
- 4.Hershlag A, Schuster MW. Return of fertility after autologous stem cell transplantation. Fertil Steril 2002;77:419–21. [DOI] [PubMed] [Google Scholar]
- 5.Siris ES, Leventhal BG, Vaitukaitis JL. Effects of childhood leukemia and chemotherapy on puberty and reproductive function in girls. N Engl J Med 1976;294:1143–6. [DOI] [PubMed] [Google Scholar]
- 6.Barton SE, Najita JS, Ginsburg ES, Leisenring WM, Stovall M, Weathers RE et al. Infertility, infertility treatment, and achievement of pregnancy in female survivors of childhood cancer: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 2013;14:873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner AZ, Pritchard D, Stanczyk FZ, Kesner JS, Meadows JW, Herring AH et al. Association Between Biomarkers of Ovarian Reserve and Infertility Among Older Women of Reproductive Age. JAMA 2017;318:1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broer SL, Mol BW, Hendriks D, Broekmans FJ. The role of antimullerian hormone in prediction of outcome after IVF: comparison with the antral follicle count. Fertil Steril 2009;91:705–14. [DOI] [PubMed] [Google Scholar]
- 9.Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P et al. Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 2013;19:26–36. [DOI] [PubMed] [Google Scholar]
- 10.Winther JF, Boice JD Jr., Svendsen AL, Frederiksen K, Stovall M, Olsen JH. Spontaneous abortion in a Danish population-based cohort of childhood cancer survivors. J Clin Oncol 2008;26:4340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green DM, Sklar CA, Boice JD Jr., Mulvihill JJ, Whitton JA, Stovall M et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Signorello LB, Cohen SS, Bosetti C, Stovall M, Kasper CE, Weathers RE et al. Female survivors of childhood cancer: preterm birth and low birth weight among their children. J Natl Cancer Inst 2006;98:1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson C, Engel SM, Mersereau JE, Black KZ, Wood WA, Anders CK et al. Birth Outcomes Among Adolescent and Young Adult Cancer Survivors. JAMA Oncol 2017;3:1078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shliakhtsitsava K, Romero SAD, Dewald SR, Su HI. Pregnancy and child health outcomes in pediatric and young adult leukemia and lymphoma survivors: a systematic review. Leuk Lymphoma 2018;59:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shliakhtsitsava K, Suresh D, Hadnott T, Su HI. Best Practices in Counseling Young Female Cancer Survivors on Reproductive Health. Semin Reprod Med 2017;35:378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee opinion no. 607: Gynecologic concerns in children and adolescents with cancer. Obstet Gynecol 2014;124:403–8. [DOI] [PubMed] [Google Scholar]
- 17.Coccia PF, Pappo AS, Altman J, Bhatia S, Borinstein SC, Flynn J et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw 2014;12:21–32; quiz [DOI] [PubMed] [Google Scholar]
- 18.Dominick SA, McLean MR, Whitcomb BW, Gorman JR, Mersereau JE, Bouknight JM et al. Contraceptive Practices Among Female Cancer Survivors of Reproductive Age. Obstet Gynecol 2015;126:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn MM, Letourneau JM, Rosen MP. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception 2014;89:466–71. [DOI] [PubMed] [Google Scholar]
- 20.Medica ACO, Stark SS, Hadnott TN, Dietz AC, Romero SAD, Natarajan L et al. Use of emergency contraception among female young adult cancer survivors. Fertil Steril 2018;109:1114–20 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilleland Marchak J, Elchuri SV, Vangile K, Wasilewski-Masker K, Mertens AC, Meacham LR. Perceptions of Infertility Risks Among Female Pediatric Cancer Survivors Following Gonadotoxic Therapy. J Pediatr Hematol Oncol 2015;37:368–72. [DOI] [PubMed] [Google Scholar]
- 22.Cherven B, Mertens A, Meacham LR, Williamson R, Boring C, Wasilewski-Masker K. Knowledge and risk perception of late effects among childhood cancer survivors and parents before and after visiting a childhood cancer survivor clinic. J Pediatr Oncol Nurs 2014;31:339–49. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Diamant AL, Thind A, Maly RC. Validity of self-reports of breast cancer treatment in low-income, medically underserved women with breast cancer. Breast Cancer Res Treat 2010;119:745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips KA, Milne RL, Buys S, Friedlander ML, Ward JH, McCredie MR et al. Agreement between self-reported breast cancer treatment and medical records in a population-based Breast Cancer Family Registry. J Clin Oncol 2005;23:4679–86. [DOI] [PubMed] [Google Scholar]
- 25.Kadan-Lottick NS, Robison LL, Gurney JG, Neglia JP, Yasui Y, Hayashi R et al. Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 2002;287:1832–9. [DOI] [PubMed] [Google Scholar]
- 26.Lepkowski JM, Mosher WD, Davis KE, Groves RM, Van Hoewyk J. The 2006–2010 National Survey of Family Growth: sample design and analysis of a continuous survey. Vital Health Stat 2 2010:1–36. [PubMed] [Google Scholar]
- 27.Prevention CfDCa. Behavioral Risk Factor Surveillance System In. Vol. 2017. Atlanta, Georgia: National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health, 2017. [Google Scholar]
- 28.Gorman JR, Su HI, Roberts SC, Dominick SA, Malcarne VL. Experiencing reproductive concerns as a female cancer survivor is associated with depression. Cancer 2015;121:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 2018;97:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young Australian women with breast cancer. Health Care Women Int 2006;27:94–110. [DOI] [PubMed] [Google Scholar]
- 31.Karaoz B, Aksu H, Kucuk M. A qualitative study of the information needs of premenopausal women with breast cancer in terms of contraception, sexuality, early menopause, and fertility. Int J Gynaecol Obstet 2010;109:118–20. [DOI] [PubMed] [Google Scholar]
- 32.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol 2010;203:115 e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Neil-Callahan M, Peipert JF, Zhao Q, Madden T, Secura G. Twenty-four-month continuation of reversible contraception. Obstet Gynecol 2013;122:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE et al. Effectiveness of long-acting reversible contraception. N Engl J Med 2012;366:1998–2007. [DOI] [PubMed] [Google Scholar]
- 35.Schover LR, Rybicki LA, Martin BA, Bringelsen KA. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999;86:697–709. [DOI] [PubMed] [Google Scholar]
- 36.Nakayama K, Liu P, Detry M, Schover LR, Milbourne A, Neumann J et al. Receiving information on fertility- and menopause-related treatment effects among women who undergo hematopoietic stem cell transplantation: changes in perceived importance over time. Biol Blood Marrow Transplant 2009;15:1465–74. [DOI] [PubMed] [Google Scholar]
- 37.Letourneau JM, Smith JF, Ebbel EE, Craig A, Katz PP, Cedars MI et al. Racial, socioeconomic, and demographic disparities in access to fertility preservation in young women diagnosed with cancer. Cancer 2012;118:4579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Partridge AH, Gelber S, Peppercorn J, Sampson E, Knudsen K, Laufer M et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol 2004;22:4174–83. [DOI] [PubMed] [Google Scholar]
- 39.Huyghe E, Sui D, Odensky E, Schover LR. Needs assessment survey to justify establishing a reproductive health clinic at a comprehensive cancer center. J Sex Med 2009;6:149–63. [DOI] [PubMed] [Google Scholar]
- 40.Bensyl DM, Iuliano DA, Carter M, Santelli J, Gilbert BC. Contraceptive use--United States and territories, Behavioral Risk Factor Surveillance System, 2002. MMWR Surveill Summ 2005;54:1–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.