Abstract
Objective:
To determine if there is a relationship between pre-wash total motile count and live births in couples undergoing intrauterine insemination (IUI).
Design:
Retrospective review in a single academic center.
Setting:
Not applicable.
Patient(s):
Couples with infertility undergoing ovulation induction with IUI between 2010–2014.
Interventions:
Not applicable.
Main Outcome Measure:
Live births.
Results:
Our cohort included 310 women that underwent 655 IUI cycles with a cumulative live birth rate (LBR) per couple of 20% and LBR per cycle of 10%. Receiver operating characteristic (ROC) analysis yielded no correlation between pre-wash total motile count (TMC) and live births. No live births occurred with TMC under 2 million. Age had a significant negative relationship to LBR (P=0.03). A ROC analysis comparing age and live births indicated a significant decline in live births for women over 37 years (P <0.001, 90% sensitivity, 70% specificity). LBR per couple was decreased to 7% in women over 37 years compared to 25% in women less than 37 years (P=0.002).
Conclusion:
Pre-wash TMC is a poor predictor of live birth. There were no live births with pre-wash TMC under 2 million. The LBR for women over 37 years with IUI was significantly lower than women less than 37 years.
Keywords: Total motile count, intrauterine insemination, live birth rate
Capsule:
Pre-wash total motile count is a poor predictor of live birth in intrauterine insemination cycles. Women greater than 37 years have a decreased live birth rate with intrauterine insemination.
INTRODUCTION
The incidence of infertility in the United States ranges from 12% to 18%, with male factor being solely responsible in approximately 20% of subfertile couples and contributory in another 30–40% (1–5). Intrauterine insemination (IUI) is a common method to achieve pregnancy in couples with varied etiologies, including male factor infertility, anovulation, endometriosis, and unexplained infertility (6). IUI can be performed in conjunction with a natural cycle ovulation or in combination with controlled ovarian hyperstimulation.
Multiple factors influence pregnancy rates following IUI, including duration of infertility, the women’s age, parity, number of follicles present, total motile count (TMC), morphology, and etiology of infertility (7–9). Discrepancies exist in the literature regarding specific semen parameters that should be utilized when deciding treatment protocols. The World Health Organization (WHO) criteria that are used for semen analysis can determine “normal” values and TMC is calculated from these values by multiplying volume, concentration, and percent motility. However, these values are not defined to be clinically applicable for fertility treatment. For example, if a couple has a semen analysis analyzed by WHO 2010 with all normal values (volume 1.5, concentration at 15 million/ml and percentage of motility at 40%) the TMC is calculated to be 9 million. By some, in vitro fertilization (IVF) would be recommended for a couple with a TMC of 9 million, even though all the WHO values are at the “normal” cut-offs (10). In addition, having a TMC value that is clinically applicable would allow different WHO criteria to be used because the “normal” values have changed over the years.
How do we use the semen analysis and TMC to guide our patients through fertility treatments? The TMC is assessed prior to (pre-wash TMC) and after (post-wash TMC) the wash in the IUI process. There are currently no consensus guidelines on a specific value for pre-wash TMC at which to recommend IUI to maximize the likelihood of live birth. This information would be especially useful for patients with limited resources trying to decide the most efficacious and cost-effective next step for fertility treatment. Here, we perform a retrospective study to determine if there is a correlation between pre-wash TMC and LBR in couples undergoing IUI.
MATERIALS AND METHODS
This is a retrospective review of all couples undergoing IUI at the University of Texas Health Science Center at San Antonio from 2010–2014 and was approved by the Institutional Review Board. A physician within the division of Reproductive Endocrinology performed a standard infertility evaluation, which included a semen analysis, assessment of tubal status by hysterosalpingogram and ovulation confirmed by regular menses or ovulation predictor kits. Patients were considered to be a candidate for IUI based on presence of motile sperm with morphology greater than 2 percent in the ejaculate and at least one patent fallopian tube. Semen analysis parameters included in the analysis were from the day of insemination. The primary outcome was live births, calculated as live birth rate (LBR). The secondary outcome was clinical pregnancies.
Semen preparation included either a wash or gradient technique at the discretion of the physician. Both preparations are offered at our clinic as prior studies failed to show superiority of one method of sperm preparation over another (20). Semen analysis was completed utilizing the WHO 2010 guidelines (11). The TMC was calculated by multiplying the total sperm concentration and volume by the motility percentage determined prior to preparation (11). Morphology was not used for the calculations.
Type of ovarian stimulation and medication dosages were chosen at the discretion of the provider based on patient characteristics and length of infertility to achieve a treatment goal of 1–3 dominant follicles. Ovarian stimulation protocols included clomiphene citrate (CC) (n=424), CC in combination with gonadotropins (n=30), letrozole (n=90), letrozole in combination with gonadotropins (n=11), gonadotropins (n=58), and natural cycle (n=42). Patients receiving CC or letrozole took the medication cycle day 3–7. Gonadotropins alone were started on cycle day 3 and continued until desired follicular response. Gonadotropins in combination with CC or letrozole were started after completion of the oral agents and continued until desired follicular response. An ultrasound was performed around cycle day 12 to document follicular growth. Insemination was performed 12 to 36 hours after urine LH surge (n=468) or 12 to 36 hours after subcutaneous administration of 250 mcg of recombinant human chorionic gonadotropin (n=187). A soft catheter was used for insemination and the patient remained supine for 10 minutes after procedure. Clinical pregnancy was defined as fetal pole with cardiac motion on ultrasound. Live birth was defined as a viable delivery beyond twenty-four weeks gestation.
Receiver Operating Characteristic (ROC) curves were calculated determining the relationship of pre-wash TMC and age to live births. Categorical variables were assessed by Chi-Square test or Fischer’s exact test as appropriate. An unpaired t-test was used to compare age and TMC between patients that had a live birth and those that did not. Multivariate logistic regression was used to control covariates (i.e. etiology, duration of infertility, parity, or cycle number) and test for correlations between variables and live births. A P <0.05 was considered statistically significant. IBM SPSS Statistics (Version 23) was used to analyze results.
RESULTS
Our cohort included 310 women that underwent 655 IUI cycles. The overall cumulative LBR per couple was 20% and LBR per cycle was 10%. The mean maternal age was 34 years (± 4 years) with a mean duration of infertility was 26 months, ranging from 3 months to 12 years. The average age of women by stimulation protocol was: 33.8 years with CC, 34.6 years with CC in combination with gonadotropins, 33.6 years with letrozole, 34.1 years with letrozole in combination with gonadotropins, 34.1 years with gonadotropins, and 34.5 years with natural cycle. The mean number of IUI cycles was 2, ranging from 1 to 11 cycles. There were a variety of infertility etiologies, with the most common being male factor, ovulatory dysfunction and unexplained (Table 1). The majority of women in our study underwent controlled ovarian hyperstimulation with CC alone accounting for 65% of cycles, with a LBR of 9% per cycle. The highest LBR was demonstrated in the CC in combination with gonadotropins group with a LBR of 23%. Overall gonadotropins alone, or in combination with CC or letrozole, produced a LBR of 16% per cycle (Table 1).
Table 1.
Demographics of Couples Undergoing IUI
| Age (yrs) | Total | Total (%) | Live birth | Cumulative LBR (%) |
|---|---|---|---|---|
| <30 | 68 | 21.9% | 18 | 26.5% |
| 30 – <35 | 112 | 36.1% | 26 | 23.2% |
| 35 – <38 | 49 | 15.8% | 13 | 26.5% |
| 38 – <40 | 37 | 11.9% | 5 | 13.5% |
| >= 40 | 44 | 14.2% | 1 | 2.3% |
| Cumulative Total | 310 | 63 | 20.3% | |
| TMC (mil) | Per Cycle LBR (%) | |||
| 0 – <2 | 28 | 4.3% | 0 | 0.0% |
| 2 – <6 | 59 | 9.0% | 3 | 5.1% |
| 6 – <ll | 92 | 14.0% | 8 | 8.7% |
| 11 – <16 | 71 | 10.8% | 5 | 7.0% |
| 16 – <21 | 64 | 9.8% | 8 | 12.5% |
| >= 21 | 341 | 52.1% | 39 | 11.4% |
| Total by cycle | 655 | 63 | 9.6% | |
| Etiologya | Per Cycle LBR (%) | |||
| Male factor | 108 | 30.1% | 26 | 24.1% |
| Ovulatory dysfunction | 94 | 26.2% | 27 | 28.7% |
| Unexplained | 74 | 20.6% | 8 | 10.8% |
| Tubal factor | 21 | 5.8% | 1 | 4.8% |
| Endometriosis | 20 | 5.6% | 3 | 15.0% |
| Uterine anomaly | 19 | 5.3% | 1 | 5.3% |
| Recurrent pregnancy loss | 19 | 5.3% | 2 | 10.5% |
| Other | 4 | 1.1% | 0 | 0.0% |
| a50 of the 310 couples with more than one etiology | ||||
| Treatment | Per Cycle LBR (%) | |||
| Clomiphene Citrate (CC) | 424 | 64.7% | 37 | 8.7% |
| CC + Gonadotropins | 30 | 4.6% | 7 | 23.3% |
| Letrozole | 90 | 13.7% | 7 | 7.8% |
| Letrozole + Gonadotropins | 11 | 1.7% | 1 | 9.1% |
| Gonadotropins | 58 | 8.9% | 8 | 13.8% |
| Natural cycle | 42 | 6.4% | 3 | 7.1% |
| Ovulatory monitoring | Per Cycle LBR (%) | |||
| Ovulation predictor kit | 468 | 71.5% | 45 | 9.6% |
| Recombinant bHCG | 187 | 28.5% | 18 | 9.6% |
| Sperm preparation | Per Cycle LBR (%) | |||
| Fresh | 529 | 80.8% | 49 | 9.3% |
| Frozen | 126 | 19.2% | 14 | 11.1% |
Infertility secondary to male factor alone accounted for 21% of couples, with a LBR of 27% per couple. In combination with female factors it accounted for an additional 9% of couples, with a LBR of 19%. Overall male factor infertility, alone and in combination with female factors, accounted for 30% of couples with a LBR of 25% (Table 1). Infertility secondary to ovulatory dysfunction accounted for 26% of couples with the highest overall LBR of 29% (Table 1).
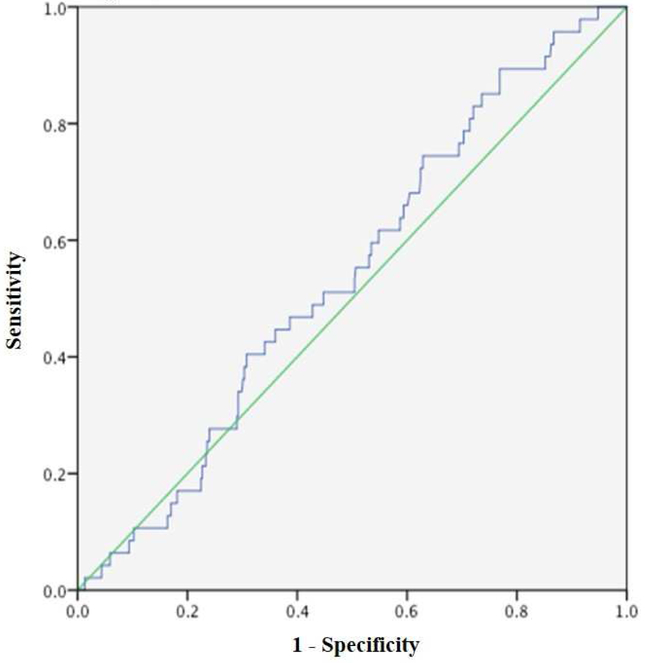
The ROC analysis performed comparing pre-wash TMC and live births indicated there was no significant correlation between variables (area under the curve (AUC) of 0.541 and P=0.4, Figure 1). No live births resulted from 28 IUIs with pre-wash TMC of <2 million. The highest percentages of live births occurred in groups with pre-wash TMC of 16 to <21 million (n=64, 12.5%) and ≥21 million (n=341, 11.4%) (Table 1), but there was no statistical difference.
Figure 1 – ROC curve for Pre-wash TMC in Predicting Live Births.
ROC curve for pre-wash TMC in predicting live births. The y-axis represents sensitivity, and the x-axis represents 1-specificity. No correlation was identified.
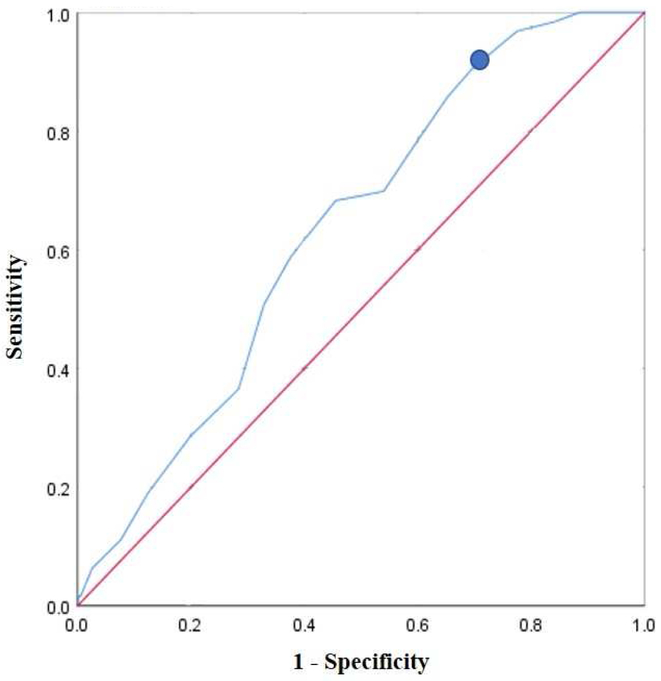
Analysis of the co-variants showed that only age had an effect on LBR. Age had a significant negative relationship to the LBR per cycle (P=0.03). A ROC analysis comparing age and live births (Figure 2) indicated a significant decline in live births for women over 37 years (AUC of 0.635, P <0.001, 90% sensitivity, 70% specificity). LBR per couple was decreased to 7% in women over 37 years compared to 25% in women less than 37 years (P=0.002). Overall, 90% of live births occurred in women 37 years and younger.
Figure 2 – ROC Curve for Age in Predicting Live Births.
ROC curve for age in predicting live births. The y-axis represents sensitivity, and the x-axis represents 1-specificity. The circle denotes an age of 37 years.
The patients that had a live birth compared to those that did not were younger (32.4 years vs 34.3 years, respectively, P=0.024) but there was not statistically significant difference in pre-wash TMC (36.7M vs 49.2M, respectively, P=0.22). Patients undergoing their first IUI cycle compared to all future cycles were younger (33.6 years vs 34.4 years, respectively, P= 0.039) but there was no statistically significant difference in pre-wash TMC (50.4M vs 48.4M, respectively, P=0.8) or LBR (12.3% vs 8.1%, respectively, P=0.1).
DISCUSSION
Given the increasing prevalence of Assisted Reproductive Technology, more research is required to establish guidelines for when to recommend IUI versus IVF to infertility patients. The importance of using clinical evidence to guide these recommendations has recently been emphasized (12, 13). The average cost of IUI in the United States ranges from approximately $500 to $2,500 depending on medications utilized, compared to IVF that costs between $6,000 and $15,000 (14). The overall IUI success rate, as measured by clinical pregnancy rate, is reported to be about 9.5% per cycle on average, however several factors including TMC as well as maternal and paternal age can dramatically impact the success rate (14). In our patient population, cost of treatment is a significant barrier for care and balancing financial considerations and desired treatment options is challenging. A randomized noninferiority trial and cost-effectiveness analysis performed by Tjon-Kon-Fat evaluated natural conception in couples with unexplained infertility and a poor prognosis and concluded that IUI with controlled ovarian hyperstimulation should remain the treatment of first choice (15). Our study does provide evidence of efficacy for IUI with a TMC greater than 2 million.
Recent studies have shown a significant increase in clinical pregnancy rates when TMC was >5 million (8, 16, 17). Merviel et al. reported that a TMC ≥5 million offered the best chance of pregnancy in a woman under 30 years with cervical or anovulatory infertility (8). Badawy et al. performed a prospective observational study that concluded there was little chance of clinical pregnancy with IUI treating male factor infertility when the woman is older than 35 years of age, number of motile sperm inseminated is <5 million, or normal sperm morphology is <30% (7). A recent review of IUI results proposed at least three cycles of IUI with ovarian stimulation is the most cost-effective option with TMC >10 million (18). In our study, we did not find a significant pre-wash TMC threshold that was predictive of live birth.
A recent study by Goldman et al. evaluated older women (38 to 42 years) with unexplained infertility comparing IUI versus immediate IVF and demonstrated increased pregnancy rates with fewer cycles in the immediate IVF group (19). In our study, women over 37 years regardless of infertility etiology were shown to have decreased LBR with IUI compared to younger women. Similar to the findings of Merviel et al., our population had an increased occurrence of live birth if they were undergoing fertility treatments for ovulatory dysfunction (8).
The limitation of this study is that it is a retrospective review of a heterogeneous population. In counseling patients whose etiology of infertility may not be known, the heterogeneity of the population can be useful to predict overall chance of success and does increase the external validity of the study. This study did include same sex couples (using donor sperm) with no personal history of infertility. This could have falsely increased our success rate as compared to infertile couples undergoing IUI. Additionally, due to our small sample size, a larger more homogenous sample size should be studied in the future to increase the power and predictability of these results. A larger sample size would also facilitate further analysis based on treatment type.
The intention of our study was to determine if pre-wash TMC values could be used to reliably predict LBR in our patient population, and whether this could be applied as a pre-counseling tool in the decision-making process. Our analysis did not reveal a significant TMC threshold to be predictive of success with a live birth. However, the data does offer some guidance as to which etiologies are more likely to result in a successful outcome in our practice and has suggested other avenues of further investigation.
In conclusion, women younger than 37 years had increased LBR compared to older women in IUI cycles. Couples with a pre-wash TMC of <2 million have very low success rates with IUI. Pre-wash TMC is a poor predictor of live birth.
Acknowledgements:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation: This work was presented at the 2016 American Society for Reproductive Medicine meeting in Salt Lake City, Utah.
The material contained in the manuscript has not been published, has not been submitted or is not being submitted elsewhere for publication. All authors are in agreement to submission of this manuscript.
Conflicts of Interest: None
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013; 99:1324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. Heavy metals and couple fecundity, the LIFE Study. Chemosphere. 2012; 87:1201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zinaman MJ, Clegg ED, Brown CC, O’Connor J, Selevan SG. Estimates of human fertility and pregnancy loss. Fertil Steril. 1996; 65:503–9. [PubMed] [Google Scholar]
- 4.Hull MG, Glazener CM, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. Br Med J (Clin Res Ed). 1985; 291:1693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod. 1991; 6:811–6. [DOI] [PubMed] [Google Scholar]
- 6.Reindollar RH, Regan MM, Neumann PJ, Levine BS, Thornton KL, Alper MM, et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil Steril. 2010; 94:888–99. [DOI] [PubMed] [Google Scholar]
- 7.Badawy A, Elnashar A, Eltotongy M. Effect of sperm morphology and number on success of intrauterine insemination. Fertil Steril. 2009; 91:777–81. [DOI] [PubMed] [Google Scholar]
- 8.Merviel P, Heraud MH, Grenier N, Lourdel E, Sanguinet P, Copin H. Predictive factors for pregnancy after intrauterine insemination (IUI): an analysis of 1038 cycles and a review of the literature. Fertil Steril. 2010; 93:79–88. [DOI] [PubMed] [Google Scholar]
- 9.Huang HY, Lee CL, Lai YM, Chang MY, Wang HS, Chang SY, et al. The impact of the total motile sperm count on the success of intrauterine insemination with husband’s spermatozoa. J Assist Reprod Genet. 1996; 13:56–63. [DOI] [PubMed] [Google Scholar]
- 10.Van Voorhis BJ, Barnett M, Sparks AE, Syrop CH, Rosenthal G, Dawson J. Effect of the total motile sperm count on the efficacy and cost-effectiveness of intrauterine insemination and in vitro fertilization. Fertil Steril. 2001; 75661–8. [DOI] [PubMed] [Google Scholar]
- 11.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010; 16:231–45. [DOI] [PubMed] [Google Scholar]
- 12.Bahadur G, Ilahibuccus A, Al-Habib A, Okolo S. Intrauterine insemination practice and the UK NICE guidelines. Hum Reprod. 2015; 30:1277–8. [DOI] [PubMed] [Google Scholar]
- 13.Bahadur G, Homburg R, Muneer A, Racich P, Alangaden T, Al-Habib A, et al. First line fertility treatment strategies regarding IUI and IVF require clinical evidence. Hum Reprod. 2016; 31:1141–6. [DOI] [PubMed] [Google Scholar]
- 14.Thijssen A, Creemers A, Van der Elst W, Creemers E, Vandormael E, Dhont N, et al. Predictive value of different covariates influencing pregnancy rate following intrauterine insemination with homologous semen: a prospective cohort study. Reprod Biomed Online. 2017; 34:463–72. [DOI] [PubMed] [Google Scholar]
- 15.Tjon-Kon-Fat RI, Bensdorp AJ, Bossuyt PM, Koks C, Oosterhuis GJ, Hoek A, et al. Is IVF-served two different ways-more cost-effective than IUI with controlled ovarian hyperstimulation? Hum Reprod. 2015; 30:2331–9. [DOI] [PubMed] [Google Scholar]
- 16.Dickey RP, Pyrzak R, Lu PY, Taylor SN, Rye PH. Comparison of the sperm quality necessary for successful intrauterine insemination with World Health Organization threshold values for normal sperm. Fertil Steril. 1999; 71:684–9. [DOI] [PubMed] [Google Scholar]
- 17.Khalil MR, Rasmussen PE, Erb K, Laursen SB, Rex S, Westergaard LG. Homologous intrauterine insemination. An evaluation of prognostic factors based on a review of 2473 cycles. Acta Obstet Gynecol Scand. 2001; 80:74–81. [DOI] [PubMed] [Google Scholar]
- 18.Cohlen B, Bijkerk A, Van der Poel S, Ombelet W. IUI: review and systematic assessment of the evidence that supports global recommendations. Hum Reprod Update. 2018; 24:300–19. [DOI] [PubMed] [Google Scholar]
- 19.Goldman MB, Thornton KL, Ryley D, Alper MM, Fung JL, Hornstein MD, et al. A randomized clinical trial to determine optimal infertility treatment in older couples: the Forty and Over Treatment Trial (FORT-T). Fertil Steril. 2014; 101:1574–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boomsma CM1, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007; CD004507. [DOI] [PubMed] [Google Scholar]