Abstract
As vascular disease is complex and the various manifestations are influenced by differences in vascular bed architecture, exposure to shear and mechanical forces, cell types involved, and inflammatory responses, in vivo models are necessary to recapitulate the complex physiology and dynamic cellular interactions during pathogenesis. Murine knockout models are commonly used tools for investigators to study the role of a specific gene or pathway in multifaceted disease traits. While valuable, these models are not perfect and this is particularly true in regard to CD73, the extracellular enzyme that generates adenosine from AMP. At baseline, CD73-deficient mice do not present with an overt phenotype, while CD73-deficient humans present with the complex phenotype of vascular calcification, arteriomegaly and tortuosity, and calcification in small joints. Below we highlight differences between the mouse and human systems and discuss the potential to leverage findings in mice to inform us on the human conditions.
Keywords: CD73, adenosine signaling, rare disease, vascular calcification, tortuosity, murine models, cardiovascular disease, VASCULAR DISEASE
The genetic origins and study of the molecular mechanisms driving rare diseases provide a unique window through which we can more fully understand human pathobiology. Rare genetic diseases may uncover novel mechanisms that regulate homeostasis or drive detrimental pathology, and disease discovery can highlight true gaps in our knowledge and, perhaps more importantly, the limitations of the model systems we use. This latter situation is precisely what we and our colleagues encountered with our discovery of the genetic cause of a disease termed Arterial Calcification due to Deficiency of CD73 (ACDC or CALJA, OMIM 211800) that presents with extensive vascular calcification, vessel tortuosity, and joint-capsule calcification.1
Vascular calcification is the most striking phenotype in ACDC patients. Ectopic calcification in the vasculature is associated with a wide range of cardiovascular disease states and strongly correlates with increased cardiovascular risk. It was first identified in advanced atherosclerotic plaques and assumed to be an unregulated and non-biological consequence of degeneration, until more sophisticated imaging techniques identified small calcifications in early-stage plaques, and intriguingly, in the medial layer of otherwise healthy adults.2–4 It is now appreciated that both atherosclerotic (intimal) and vessel wall (medial) vascular calcification are active biological processes that alter vascular homeostasis and exacerbate disease pathologies, and vascular calcification is used as a risk-predicting biomarker.5–9 Currently there is no clinical treatment that halts or reverses pathologic calcification.
The genetic cause of ACDC phenotypes are mutations in the gene ecto-5-prime-nucleotidase (NT5E, NM_002526).1 NT5E encodes for the protein CD73 (EC 3.1.3.5), an extracellular enzyme responsible for the conversion of AMP into adenosine and inorganic phosphate. The discovery of the genetic cause of ACDC was the first to link extracellular CD73 activity and its downstream adenosine receptor signaling to vascular calcification and tortuosity pathogenesis in humans. At the time of this discovery, several CD73 knockout mouse lines were available, yet these models do not present with a baseline phenotype that resembles what is seen in CD73-deficient patients.10–12 Interestingly, much of the current research on CD73 is in the inflammation and cancer fields, and several clinical trials involving anti-CD73 monoclonal antibodies are currently being conducted for the treatment of solid tumors. As immunotherapy and pharmacotherapy focused on CD73-mediated signaling is gaining popularity, it is important to understand the implications of systemic effects of CD73 blockade. In this review, we aim to cover the role of CD73 in various organ systems to highlight how studies from the inflammation and cancer fields may inform our cardiovascular studies, and vice versa.
Adenosine Signaling
ATP is released from cells under conditions of stress (e.g. flow and mechanical stress, inflammation, hypoxia) or cellular death and is rapidly broken down. CD39 takes ATP to ADP and ADP to AMP in a two-step reaction yielding two inorganic phosphate molecules (Pi); ENPP1 breaks down ATP to AMP and pyrophosphate (PPi); and CD73 converts AMP to adenosine and Pi.13–16 Adenosine is referred to as a “retaliatory metabolite” and functions as a signaling molecule that allows cells to adapt to the initial ATP-releasing stress, however, overabundance of adenosine can induce damage; thus, concentrations of extracellular nucleosides must be tightly regulated.17, 18 Further fine-tuning of extracellular nucleoside concentration is regulated via equilibrative nucleoside transporters (ENTs) and pannexin transporters.19, 20 Adenosine signals by binding one of four G-protein coupled adenosine receptors (ARs) which are expressed on a wide range of cells and upregulated under various conditions; the density and combination of ARs on a particular cell will dictate the downstream pathways activated as their individual affinity to adenosine varies.21 The A2a and A2b ARs are classified as Gs-type receptors while A1 and A3 ARs are classified as Gi/o receptors, however it is now understood that AR signaling can be mediated through a variety of pathways.22
ACDC Phenotype
Periarticular calcification
Case reports as far back as 1914 describe patients with ACDC-like phenotypes in the lower-extremity vessels.23–25 Secondary phenotypes associated with these cases of vascular calcification are early-onset arthritis and non-rheumatologic and intermittent joint pains caused by calcifications of the metacarpal phalangeal and interphalangeal joint capsules.1, 25, 26 Joints in the hands and feet of ACDC patients typically have bulky periarticular calcifications with mild joint space narrowing that is worse proximally and without intra-articular calcifications. The joint pain in ACDC patients is dynamic, waxing and waning throughout adulthood.1, 26 One patient was observed to have cyclical changes in mineralization, with exacerbations in pain occurring every 2–3 months. While still under observation this patient was enrolled in a clinical trial testing whether the bisphosphonate etidronate is effective in attenuating the progression of lower extremity arterial calcification and improving vascular blood flow (NCT01585402); the intermittent cyclic pain continued, and interestingly some bulky calcifications resolved while new bulky calcification developed. It is unclear whether these dynamic changes are characteristic of the normal disease pathogenesis, thus other patients with ACDC should be monitored to characterize disease progression.26
Vascular calcification
The most extraordinary phenotype observed in ACDC patients is the vascular calcification. It is localized in the peripheral arteries and is exacerbated in vessels near joints of the lower extremities (e.g. iliac, popliteal, anterior tibial).1, 27 Since the initial discovery of ACDC in 2011, additional patients have been identified and the phenotype has expanded to include calcifications in the brachial artery near the elbow (see Table 1).28, 29 Symptoms include generalized lower extremity pain resulting from vascular incompetence and calculated ankle-brachial indices of less than 0.8. The calcification is non-atherosclerotic, found in the medial layer of affected vessels, and is accompanied by medial dysplasia consisting of an abundance of disorganized vascular smooth muscle cells, an infiltration of immune cells in the vessel wall, and significant deposition of extracellular matrix proteins. Together, this remodeling leads to vessel occlusion and vascular incompetence. More common forms of medial calcification are often non-obstructive, as with Mönckeberg arterial sclerosis,30 but the severe medial calcification in ACDC extends into the vessel lumen and patients develop collateral vasculature as a compensatory mechanism.27
Table 1. NT5E Mutations in Humans and Mice.
A total of twelve patients from the reviewed literature have disease-causing mutations in NT5E/CD73 resulting in ACDC. The patient of family 6 harbors four mutations including c.1086A>G p.T376A, however, his brother is homozygous for this same mutation yet is asymptomatic, thus this mutation unlikely to be causative. CD73-deficient murine models are also represented above. Models utilizing cell-specific knockdown of CD73 crossed cre-drivers to the CD73flox/flox model.
| Human Mutations | ||
|---|---|---|
| Family | Mutation | Consequence |
| Family 1 (5 patients)1 | c.662C>A (exon 3) | p.S221X; nonsense |
| Family 2 (3 patients)1 | c.1073G>A (exon 5) | p.C358Y; missense |
| Family 3 (1 patient)1 | c.662C>A (exon 3) c.1609dupA (exon 9) |
p.S221X; nonsense p.V537fsX7; stop |
| Family 4 (1 patient)63 | c.3G>C (exon 1) c.141C>G (exon 1) c.373G>C (exon 2) |
p.M1l p.D47E p.E125X |
| Family 5 (1 patient)28 | c.751+2T>C (intron 3) | Skips exon 3 |
| Family 6 (1 patient)29 | c.3G>C (exon 1) c.1096T>C (exon 5) c.1086A>G* c. 488C>T |
p.M1l, start codon substitution p.M379T p.T376A silent |
| Murine Models | ||
| Background | Mutation Location | Genetic Modification |
| C57BL/6J10 | Exons 2 and 3 | Neo-Cassette |
| C57BL/612 | Exon 3 | Neo-cassette |
| NMRI11 | Exon 2 | CD73flox/flox (constitutive) |
| C57BL/643 | Exon 2 | Tie2Cre+/CD73flox/flox Endothelial cell specific |
| C57BL/644 | Exon 2 | VillinCre+/CD73flox/flox Intestinal epithelium cell specific |
| C57BL/645 | Exon 2 | CD4Cre+/CD73flox/flox T-cell specific |
As mentioned above, there is an ongoing clinical trial that is attempting to ameliorate ACDC calcification with the bisphosphonate, etidronate (NCT01585402). The use of a bisphosphonate to treat ACDC patients emerged from its ability to halt and in some cases reverse arterial calcifications in young patients suffering from a related inheritable disease, Generalized Arterial Calcification of Infancy (GACI, OMIM # 208000).31–34 Like, ACDC, GACI is an autosomal recessive disorder that presents with extensive medial layer vascular calcification, however, the vascular calcification in GACI patients is global and patients often die in infancy or early childhood due to cardiac failure. ENPP1 is an extracellular enzyme that is upstream of CD73 and catalyzes the breakdown of ATP into AMP and pyrophosphate. Mutations in ENPP1 are the cause of GACI.34 When given bisphosphonates, 65% of GACI patients enrolled survived past infancy, compared to a survival rate 31% when not treated with bisphosphonates.35–37 Etidronate decreased the formation of calcifications in an in vitro ACDC calcification model and reduced existing calcifications in an in vivo ACDC-iPSC teratoma model; with the completion of NCT01585402 we will see whether what was modeled in this hybrid in vivo system can be mirrored in humans.38
Arteriomegaly and Tortuosity
Arteriomegaly and tortuosity are characteristic of ACDC and phenocopies what is seen in aneurysmal remodeling.1, 39 In addition to medial thickening and calcification in the peripheral arteries, ACDC patient vessels exhibit duplication, fragmentation, and loss of curvature of elastin fibers.27 This is most notable along the internal elastic lamina where small calcifications seem to nucleate at these break points. In ACDC we do not know what is happening first: does the calcification induce breakage of the internal elastic lamina, or does breakdown of this structure allow for nucleation of minerals? Looking to other connective tissue disorders provides strong evidence that matrix modulation and calcific deposition are intimately connected. Microcalcifications have been observed along elastin breakpoints in a dilated aorta from a Marfan Syndrome patient,40 in mid-sized arteries and veins of patients with pseudoxanthoma elasticum (PXE),41 and large and mid-sized vessels in GACI.42
CD73 in Mice and Humans
How does a lack of CD73 and its downstream adenosine signaling promote calcification and tortuosity? Are these phenotypes co-dependent or progress independently? To address these questions, in vivo disease modeling would be ideal. CD73-deficient mice were first created in 2004, and these early studies explored diverse organ systems and were found to have alterations in renal homeostasis10, decreased time to thromboocclusion11, and pathologic responses to hypoxia (see Table 1).12 Several conditional knockout models have since been created to explore the role of cell-specific CD73 function in heart failure, tumor growth and metastasis, and intestinal colonization and infection.43–45 Regarding calcification pathophysiology, CD73-deficient mice have pro-mineralization serum based on elevated Pi/PPi ratios but phenotypically present only with calcifications in the cartilaginous tissues of the sternocostal junction, juxta-articular tissues in the lower extremities and the knee, and the Achilles tendon.46 However, CD73-deficient mice do not phenotypically mirror CD73-deficient humans in terms of medial vessel wall calcification, arteriomegaly, and vessel tortuosity. This not only complicates the ability to model this disease in vivo, but also emphasizes the point that mice are not little humans.
Possibly contributing to this particular human-murine disparity is the kinetics of adenosine. In human blood, the half-life of adenosine is less than 15 seconds, while in mice the half-life is approximately 2 minutes.47 This difference may be due to the lack of the gene CECR1, which encodes for adenosine deaminase 2 (ADA2), in the murine genome. ADA2 serves a crucial role in human vascular homeostasis as those with ADA2-deficiency develop widespread vasculitis that presents with systemic necrotizing inflammation of blood vessels as well as aneurysms and stenosis of abdominal arteries.48, 49 This pathobiology is driven by defects in endothelial cell integrity which induces in early-onset stroke and intracranial hemorrhage.49 Ensembl gene tree ENSGT00530000063775 shows that NT5E, the gene encoding of CD73 protein, is present in 170 species, while Ensembl gene tree ENSGT00390000012118 shows CECR1/ADA2 is present in 140 species. Noticeably absent from CECR1/ADA2 gene tree are several model organism species, including the mouse, rat, Chinese hamster, Guinea pig, and rabbit. The lack of CECR1/ADA2 may be contributing to the longer half-life of adenosine in mice compared to human counterparts. To date, there is no murine model that overexpresses human ADA2 to test this hypothesis, which renders studying adenosine formation, processing, and signaling in mice for the purposes of understanding human pathobiology, more challenging. CECR1/ADA2 mutations are hypothesized to result in excessive amounts of adenosine, and mutations that induce ACDC inactivate enzymatic activity and result in too little adenosine. Comparing these two diseases highlights the importance of having the perfect balance of adenosine signaling in different vascular beds.
To work around these differences between mice and humans with CD73 deficiency such that we could identify the mechanisms contributing to pathologic calcification, we created induced pluripotent stem cells (iPSCs) from control and ACDC patient fibroblasts.38 Tissue nonspecific alkaline phosphatase (TNAP) processes extracellular pyrophosphate (PPi) into phosphate (Pi) and is a key protein necessary for bone formation. We found that in CD73-deficient cells, TNAP compensates for the lack of CD73 by converting small amounts of AMP to adenosine, however, the adenosine produced was not to the same level as that seen in cells from control patients. As TNAP is upregulated, it preferentially converts PPi, an endogenous inhibitor of mineralization, to Pi, creating a pro-mineralization state. These effects were rescued by using inhibitors for PI3 kinase or MEK1/2, by treating cells with rapamycin, or by administration of an agonist specific for the A2bAR.38
While the in vitro and in vivo disease modeling helps to identify the biological mechanisms contributing to the calcific phenotype, it does not inform us on the mechanisms driving tortuosity and changes in extracellular matrix. Instead of mice, should we look to species lower on the evolutionary tree? The use of zebrafish and drosophila (Danio rerio and Drosophila melanogaster, respectively) are heavily leveraged by the Undiagnosed Disease Network for the speed in which knockout or transgenic lines can be made.50, 51 In addition to sharing over 70% of their genome with humans, the vasculature of the zebrafish is easily visualized for real-time imaging.52, 53 Zebrafish were used to model ADA2-deficiency and show that reducing expression of CECR1 paralogues induced intracranial bleeding, thus mirroring the defects in vessel development or integrity seen in the human patients.48 Zebrafish can also develop vascular calcifications and enpp1 mutants develop calcification in soft tissues of the skin, cartilage, heart, intracranial space and notochord sheet.54
Much of what we know about CD73-mediated adenosine signaling is generated from murine models, yet from the discovery of ACDC we should appreciate that these models are imperfect. These CD73-deficient models employed different methods of mutation generation and background strains (see Table 1), and studies have utilized mice of different age, diet, and sex (see Table 2), yet all CD73-deficient models lack a vascular phenotype despite all having an absence of CD73 enzymatic activity. While the blood may not mimic the microenvironment of the vessel wall, it is tempting to speculate that differences in the kinetics of adenosine in the blood and tissue microenvironment contribute to the differences between CD73 deficiency in mice and humans. Is it possible that overexpressing ADA2 in the CD73-knockout mouse would humanize it such that the phenotypes will mirror those seen in ACDC patients? Would a CD73-deficient zebrafish mirror ACDC better than the mouse because ADA2 is conserved in its genome? Until such models are created, knowledge gained from the CD73-deficient murine studies done to date may help to inform the vascular phenotype of ACDC patients. Below, we highlight a few arms of research.
Table 2. Summary of CD73-Deficient Murine Studies.
CD73-deficient murine studies highlighted within this review identify important scientific findings along with differences in model systems, age, sex, and diet. In studies with male and female rodents, no sex effects were observed.
| Findings | Age (weeks) | Sex | Diet |
|---|---|---|---|
| C57BL/6J Neo-cassette (Exons 2 and 3) | |||
| Decreased ability to alter glomerular arteriolar tone10 | 8–16 | M/F | normal |
| Juxta-articular joint-capsule mineralization, elevated serum Pi/PPi ratio46 | 44–64 | M/F | high phosphorus, low magnesium |
| NMRI CD73flox/flox (Exon 2) | |||
| Decreased time to thromboocclusion (Cre; full body)11 | 8–12 | not defined | normal |
| Increased atherogenesis (Cre; full body)84 | 24 | not defined | normal |
| Increased peak aortic valve flow (Cre; full body)88 | 9+15 diet | not defined | high fat |
| Low CD73 expression on tumor melanoma cells inhibits tumor growth (Cre; full body)76 | 4–8 | M | normal |
| CD73 on endothelial host cells does not modulate tumor growth or metastasis of B16-F10 melanoma cells (Tie2-Cre; endothelial cell specific knockout)43 | 14 | M | normal |
| T cell specific loss of CD73 exhibits worse cardiac ejection fraction and an increase in end diastolic and end systolic volume (CD4-Cre; T cell specific knockout)45 | 8–12 | F | normal |
| Increased nontyphoidal Salmonella colonization and virulence as well as reduced severity of colitis (Villin-Cre; intestinal epithelial cell specific knockout)44 | not defined | not defined | normal |
| C57BL/6 Neo-cassette (Exon 3) | |||
| Fulminant vascular leakage in response to hypoxia12 | 52 | M/F | normal |
| CD73 is protective in collagen-induced arthritis65 | 10–12 | M | normal |
| Inhibits tumor progression74 | not defined | M/F | normal |
| Resistance to carcinogenesis75 | 13–25 | M | normal |
| Decrease in atherosclerosis85 | 4+16 diet | M | western |
| Decrease time to thromboocclusion; CD73 ablation does not decrease anti-thrombotic phenotype of CD39 overexpression93 | 8–12 | M | normal |
CD73 in Inflammation
CD73-mediated adenosine signaling is an inflammatory response modulator. The role of CD73 in T cell proliferation and function was first described in mice in 1989 and has since been shown to drive T cell differentiation and is reduced in inherited and acquired immunodeficiencies.55–58 Adenosine generated by CD39 and CD73 on regulatory T cells (Tregs) is immunosuppressive.59 This is corroborated by evidence that the immunosuppressors dexamethasone and methotrexate work via increasing the expression of CD73 activity and promoting the release of adenine nucleotides, respectively.60, 61 Indeed the mechanism for methotrexate efficacy in the treatment of rheumatoid arthritis is by increasing levels of adenosine; though not rheumatoid-like, the absence of local CD73-mediated adenosine signaling is hypothesized to be the cause of the periarticular calcification seen in the joint capsules of ACDC patients.1, 62
Due to early-onset joint pain there is a push to classify CD73 deficiency within the spectrum of rheumatologic disease.63 Supporting this, there is evidence of low CD73 expression on synovial lymphocytes in more severe forms of juvenile idiopathic arthritis, and the presence of CD73 is protective in an established murine model of rheumatoid arthritis, suggesting an interplay between calcification and inflammatory processes in this localized environment.64,65 However, the joint pain in ACDC is nonresponsive to anti-inflammatories and there is no evidence of auto-immune disease.1, 29, 39, 63 Further research indicates that defective adenosine signaling by A3 adenosine receptor ablation results in articular cartilage degeneration by aggrecanase and collagenase activity of chondrocytes.66 Thus, CD73-mediated adenosine signaling may be protective against cartilage degeneration via the A3 receptor through matrix modulators rather than inflammatory mechanisms; this idea of extracellular matrix modulation also links to the role of CD73-deficiency in breakdown of the elastin lamina in the vessel wall. There is little data whether joint involvement in ACDC is immune-mediated or primarily through mineralization abnormalities, however, we cannot ignore the possibility of an immune-mediated mechanism due to the role CD73 has in immunomodulation in murine models. Interestingly, adenosine signaling is also antinociceptive, which introduces the question: is the joint-capsule phenotype of ACDC patients immune driven or does the lack of local adenosine in these locations increase pain sensitivity independent of an immune response.67, 68
A significant amount of research on CD73 is in the cancer field. The immunosuppressive effects of CD73-generated adenosine in murine models suggest a favorable milieu for tumor growth, thus blocking CD73 activity is currently being explored as a therapeutic target.59 Higher CD73 activity is associated with poorer cancer prognosis,69 and the extracellular fluid of solid tumors was found to have immunosuppressive levels of adenosine that mediated its effects via the activation of the A2aARs on T cells.70–72 CD73-deficient mice are resistant to carcinogenesis and exhibit a decrease in tumor invasion and progression.73–75 In a melanoma model, CD73-deficient mice exhibited a decrease in tumor growth when tumor cells themselves were also CD73 deficient, however, these results were not recapitulated when CD73 was specifically deleted in host endothelial and hematopoietic cells using the Tie2Cre+/CD73flox/flox mouse.43, 76 Currently, there are several human clinical trials using anti-CD73 monoclonal antibodies as primary or combination treatments for cancer (Clinical Trials NCT02503774, NCT03381274, NCT02503774, NCT03616886, NCT03611556, NCT03334617). Among the known ACDC population, one patient had a calcified oligodendrioma, but it is unknown if these patients generally have decreased risk of neoplastic disease.1
CD73-mediated inflammatory mechanisms have also been implicated in murine models of heart failure. Humans with heart failure show an increase in inflammation and cytokine production. Given what is known about T cell modulation by CD73, global CD73KO and CD4Cre+/CD73flox/flox mice (T cell specific) underwent transverse aortic constriction to model heart failure. Although neither genetic model had a comparative increase in heart weight compared to wild-type mice, both of these knockouts exhibited worse ejection fraction as well as an increase end diastolic volume and end systolic volume. Functionally, these global and T cell specific CD73 knockout murine models had higher levels of inflammatory cytokines and fibrosis.45 This suggests that the anti-inflammatory mechanisms of CD73-generated adenosine, specifically on T cells, may be protective in heart failure. No human with CD73-deficiency is known to have heart failure, however, it will be interesting to monitor for this in these patients to determine if progression and disease burden is worse.
In light of the differences between CD73-deficiency in the vasculature of mice and humans, it will be interesting to see whether the murine immunomodulating effects of CD73 blockade on cancer will be reproducible in humans. Similarly, as calcified vessels exhibit an infiltration of immune cells, perhaps inflammation is also contributing to the calcific or tortuous phenotype seen in ACDC joints and blood vessels.
CD73 and Vascular Remodeling
Alterations in extracellular matrix homeostasis are a hallmark of aneurysmal disease, and clues regarding the mechanisms driving aneurysm formation have been gleaned from familial rare diseases such as arterial tortuosity syndrome, Marfan Syndrome and Loeys-Dietz syndrome.77 Common to these aneurysmal diseases is the upregulation of TGF-β signaling.78–80 As ACDC tortuosity phenocopies aspects of aneurysmal disease, understanding the pathogenesis of tortuosity in CD73-deficient vessels may provide new insights on a role for CD73 and adenosine signaling in aneurysm formation, and possibly a relationship between TGF-β and CD73/AR signaling pathways. Supporting this, adenosine signaling has been implicated in regulating matrix protein crosslinking via lysyl oxidase (LOX) activity through cAMP-mediated adenosine signaling on vascular smooth muscle cells.81 The vessels of patients with inactivating mutations in LOX exhibit fragmented and disorganized elastic fibers, as LOX is an enzyme responsible for matrix crosslinking of elastin and collagen fibers, predisposing patients to thoracic aneurysms.82, 83 The histopathology found in these LOX-deficient vessels is similar to the peripheral arteries of ACDC patients.27 In light of these similar disease phenotypes our lab is conducting studies on the role of CD73-deficiency in altering the expression of matrix remodeling enzymes and determining whether CD73-mediated adenosine signaling intersects with the TGF-β signaling pathway.
The calcification seen in ACDC patients is non-atherosclerotic and imaging analysis shows that their coronaries and aorta are normal for their age group.1 However, murine models present a complicated view of the function of CD73 in atherosclerosis. Apolipoprotein E (ApoE) knockout and CD73/ApoE double knockout (dKO) mice were fed a normal chow diet for 26 weeks and at this time CD73/ApoE dKOs showed increased atherosclerotic plaque formation compared to ApoE knockout mice. This was most prominent in areas of turbulent flow (e.g. aortic valvular cusp and aortic bifurcation).84 Plaque size was larger in the CD73/ApoE dKOs, but composition of collagen content, extracellular matrix proteins, hyaluronic acid, and lipid content were not different between these strains, suggesting no overt signs of plaque instability. Plasma from the CD73/ApoE dKO mice exhibited higher levels of MCP-1, triglycerides, and endothelin-1. Plaques in the ApoE knockout showed that macrophages and other cells of the mesenchymal lineage upregulated CD73, suggesting that CD73 on these cells helps to attenuate the immune response and reduces atherosclerotic plaque progression; the precise cause of increased number of lesions and lesion size was not defined, but speculated to be due to endothelin-1-induced migration of vascular smooth muscle cells.84
Another study fed male ApoE and CD73/ApoE dKOs a western diet starting at four weeks of age and continuing for 16 weeks. Opposite the findings with normal chow, CD73/ApoE dKOs fed a western diet exhibited less plasma triglycerides, total cholesterol, LDL, and smaller plaque size.85 In vitro studies with human aortic smooth muscle cells showed that knocking down CD73 expression in the presence of fatty acid reduced migration, proliferation, and transformation into foam-like cells.85 Obviously, the dietary differences contributed to the drastic disparities in atherogenesis in the models, but why at baseline is CD73-deficiency atheroprone while under a Western diet CD73-deficiency is anti-atherogenic? Yang et al speculate these mechanistic differences are due to CD73 and adenosine sensitivity to lipids, as previous literature suggests that high-fat diets decrease CD73 expression of adipocytes in muscle,86 while high-fat diets increase CD73 expression of hepatocytes.87 One study looking at aortic valvular disease compared wildtype and CD73-deficient mice fed normal chow or high fat diet for 15 weeks. The authors discovered that CD73-deficient mice on both diets develop aortic valve thickening from melanocyte accumulation that resulted in increased peak flow, which is consistent with early aortic stenosis similar to that induced by high-fat diets.88 Similar to the findings of Yang et al., an A2aAR/ApoE double knockout decreased atherosclerotic lesion size of the aorta and aortic valve, suggesting that a lack of CD73-mediated adenosine signaling is an important regulator in the formation of atherosclerosis in the presence of lipids.89 Complimentary explanations for these different findings may lie in age, sex, and specific strains of the mice (see Tables 1 & 2).
ACDC patients show no overt evidence of atherosclerosis, and they do not have hyperlipidemia or elevated cholesterol levels; from the murine studies of Buchheiser et al., one would hypothesize that ACDC patients would be more prone to atherosclerotic calcification, however that does not appear to be the case. To add complexity, serum CD73 was found to be elevated in human patients with severe peripheral artery disease, and it was also elevated in the vaso vasorum of developing plaques, however it was absent in mature occlusive PAD plaques.90 Thus, in PAD, is the upregulation of CD73 a cause or consequence? Experimental models and tracking disease progression alongside CD73 levels in the serum are needed to determine if downregulation of CD73 contributes to PAD pathogenesis.
CD73 and thrombus formation
Platelet function and recruitment heavily rely on internal reserves of ATP and ADP that are released into the extracellular compartment after initial platelet activation; ADP is a platelet agonist while adenosine is a known inhibitor of platelet function.91, 92 It is then reasonable to assume that CD73 activity may impact platelet function by adenosine generation and thus, initial thrombus formation. This could have profound implications on other vascular pathology such as acute myocardial infarction or peripheral thrombus formation. In two murine studies, time to thromboocclusion after low doses of oxidative stress was significantly decreased, although minimally, in carotid arteries of CD73 knockout mice.11, 93, 94 Pharmacologic inhibition of CD73 via α-β-methylene-ADP (APCP) negated the anti-thrombotic phenotype of overexpressing CD39 (CD39OE), an enzyme that converts ATP to AMP in a two-step removal of the phosphate groups, but genetic ablation of CD73 had no effect. The authors hypothesize that this discrepancy is due to APCP’s unknown off-target effects and ability to inhibit ENPP1, which is upstream of CD39, though they speculate that the latter seems less likely due to low endothelial and monocyte expression. In relation to humans, Netsch et al. found that mesenchymal stem cells inhibited platelet function from CD73-generated adenosine.93, 94 Comparing this to ACDC, one patient underwent an embolectomy of the popliteal artery,38 and studies are currently being conducted to determine whether ACDC patients have premature thromboocclusive coagulation phenotypes similar to the murine model.
Discussion and Gaps in Knowledge
The differences seen between the phenotypes of CD73-deficient mice and humans highlight the limitations of using the murine model systems for the study of human disease (see Figure 1). The creation of an adequate model is crucial as dissecting the mechanisms driving ACDC phenotypes may provide powerful insights for vascular calcification, matrix dysregulation, aneurysmal disease, arthritis, and pain modulation. In vitro studies should be conducted with medial layer cells, as this is the primary location of ACDC pathology; however, in light of the role of CD73 and adenosine signaling on modulating the extracellular matrix and inflammatory cell differentiation and signaling, it is important to develop co-culture or 3D organoid models to study the interplay between different cells and matrix components. In vivo studies to generate double knockout mouse models or to induce calcification by other means should also be pursued as perhaps a “second hit” may induce an ACDC-like phenotype in CD73-deficient mice. Lastly, maybe by taking a step down the evolutionary tree we could identify a more useful model system.
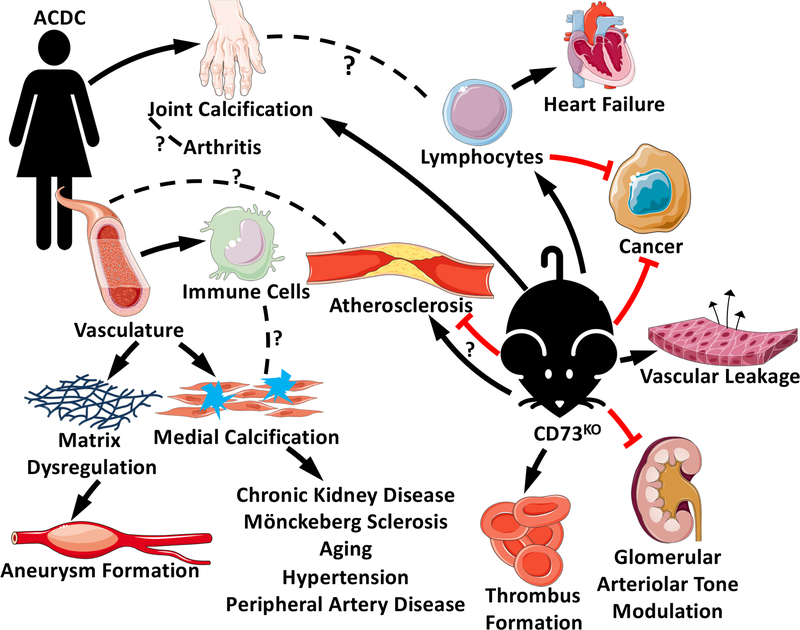
Figure 1. Summary of the Phenotypic Differences between CD73-deficient Mice and Humans.
At the 11 o’clock position, we represent a patient with ACDC and her associated phenotypes. Symptoms such as joint pain are due to extensive calcifications surrounding joint capsules, perhaps presenting an opportunity to study CD73 in distinct forms of arthritis. Vascular pathology is the predominant phenotype in ACDC with immune cell infiltration, elastic lamina degradation, and medial calcification, all resulting in symptoms and pathologies seen in more common vascular diseases. These phenotypes are in contrast with the murine CD73-deficient models represented by the image at the 4 o’clock position. Unlike their human counterpart, CD73-deficient models do not present with the same vascular pathology. Instead, studies have shown both a decrease and increase in atherosclerosis, and a decrease in time to thrombooclusion. These mice are less able to modulate glomerular arteriolar tone and exhibit more vascular leakage in response to hypoxia. Perhaps the most thoroughly studied, cancer progression is decreased in most CD73-deficient murine studies in part by lymphocyte activity. Lymphocytes have also been shown to worsen heart failure in these models. Finally, these models can develop joint calcification, the only known phenotype that resembles human disease.
As CD73 becomes an important target in cancer therapy, studying the disease mechanisms and effects of patients with inherited CD73 deficiency can better inform us of what patients receiving anti-CD73 monoclonal antibodies, and thus partially acquiring CD73 deficiency, may experience. Long term monitoring of consequences of receiving these antibodies is crucial as it may increase risk of vessel matrix dysregulation, and joint calcifications and pain, heart failure, and ectopic medial calcification, with particular attention being given to those with co-morbidities (e.g. diabetes, chronic kidney disease, osteoporosis, hypertension) who are more prone to this type of calcification. This new and interesting population undergoing experimental therapies could themselves be another model system.
HIGHLIGHTS.
CD73 deficiency in humans leads to the development of medial calcification and matrix remodeling of the lower-extremity arteries and joint calcifications, yet CD73 deficient murine models do not recapitulate the human vascular phenotype
In spite of these drastic differences between mice and humans several clinical trials are currently testing anti-CD73 antibodies for cancer therapy.
This review highlights the known differences between the mouse and human systems and discusses the potential to leverage findings in mice to inform us on the human conditions.
ACKNOWLEDGEMENTS
We would like to thank Jason Dobbins for carefully reading this manuscript and providing editorial support.
SOURCES OF FUNDING
CSH is supported by the National Institutes of Health (K22HL117917 and R01HL142932) and the University of Pittsburgh School of Medicine.
ABBREVIATIONS
- A1AR
A1 Adenosine Receptor
- A2aAR
A2a Adenosine Receptor
- A2bAR
A2b Adenosine Receptor
- A3AR
A3 Adenosine Receptor
- ACDC
Arterial Calcification due to Deficiency of CD73
- ADA2
Adenosine Deaminase 2
- ADP
Adenosine Diphosphate
- AMP
Adenosine Monophosphate
- APCP
a-β-methylene-ADP
- ApoE
Apolipoprotein E
- ARs
Adenosine Receptors
- ATP
Adenosine Triphosphate
- CALJA
Calcification of Joints and Arteries
- CD39
Cluster of Differentiation 39
- CD73
Cluster of Differentiation 73
- CECR1
Cat Eye Syndrome Chromosome Region, Candidate 1
- dKO
Double Knockout
- ENPP1
Ectonucleotide Pyrophosphatase/Phosphodiesterase 1
- GACI
Generalized Arterial Calcification of Infancy
- iPSC
Induced Pluripotent Stem Cells
- KO
Knockout
- LDL
Low-Density Lipoproteins
- LOX
Lysyl Oxidase
- NT5E
Ecto-5-Prime-Nucleotidase
- Pi
Inorganic Phosphate
- PPI
Pyrophosphate
- PXE
Pseudoxanthoma Elasticum
- TGFb
Transforming Growth Factor beta
- TNAP
Tissue Non-specific Alkaline Phosphatase
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.St Hilaire C, Ziegler SG, Markello TC, et al. Nt5e mutations and arterial calcifications. N Engl J Med. 2011;364:432–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loecker TH, Schwartz RS, Cotta CW, Hickman JR Jr. Fluoroscopic coronary artery calcification and associated coronary disease in asymptomatic young men. J Am Coll Cardiol. 1992;19:1167–1172 [DOI] [PubMed] [Google Scholar]
- 3.Cooley BC, Nevado J, Mellad J, et al. Tgf-beta signaling mediates endothelial-to-mesenchymal transition (endmt) during vein graft remodeling. Sci Transl Med. 2014;6:227ra234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright I The microscopical appearances of human peripheral arteries during growth and aging. J Clin Pathol. 1963;16:499–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nature Materials. 2016;15:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaha MJ, Budoff MJ, Rivera JJ, Katz R, O’Leary DH, Polak JF, Takasu J, Blumenthal RS, Nasir K. Relationship of carotid distensibility and thoracic aorta calcification: Multi-ethnic study of atherosclerosis. Hypertension. 2009;54:1408–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vliegenthart R, Oudkerk M, Hofman A, Oei HH, van Dijck W, van Rooij FJ, Witteman JC. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation. 2005;112:572–577 [DOI] [PubMed] [Google Scholar]
- 9.St Hilaire C, Liberman M, Miller JD, Early Career C. Bidirectional translation in cardiovascular calcification. Arterioscler Thromb Vasc Biol. 2016;36:e19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of gfr in ecto-5’-nucleotidase/cd73-deficient mice. J Clin Invest. 2004;114:634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koszalka P, Ozuyaman B, Huo Y, et al. Targeted disruption of cd73/ecto-5’-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95:814–821 [DOI] [PubMed] [Google Scholar]
- 12.Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5’-nucleotidase (cd73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908 [DOI] [PubMed] [Google Scholar]
- 14.Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced ca(2+) waves are propagated via atp release and purinergic receptor activation. Am J Physiol Cell Physiol. 2000;279:C295–307 [DOI] [PubMed] [Google Scholar]
- 15.Ohta A, Sitkovsky M. Role of g-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920 [DOI] [PubMed] [Google Scholar]
- 16.Cohen MV, Walsh RS, Goto M, Downey JM. Hypoxia preconditions rabbit myocardium via adenosine and catecholamine release. J Mol Cell Cardiol. 1995;27:1527–1534 [DOI] [PubMed] [Google Scholar]
- 17.Newby AC. Adenosine and the concept of retaliatory metabolites. Trends Biochem. Sci 1984;9:42–44 [Google Scholar]
- 18.Jacobson KA. Introduction to adenosine receptors as therapeutic targets. Handb Exp Pharmacol. 2009:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boswell-Casteel RC, Hays FA. Equilibrative nucleoside transporters-a review. Nucleosides Nucleotides Nucleic Acids. 2017;36:7–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esseltine JL, Laird DW. Next-generation connexin and pannexin cell biology. Trends Cell Biol. 2016;26:944–955 [DOI] [PubMed] [Google Scholar]
- 21.St Hilaire C, Carroll SH, Chen H, Ravid K. Mechanisms of induction of adenosine receptor genes and its functional significance. J Cell Physiol. 2009;218:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J-F, Eltzschig HK, Fredholm BB. Adenosine receptors as drug targets — what are the challenges? Nature Reviews Drug Discovery. 2013;12:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitin LCJ. A case of arterial and periarticular calcinosis of unknown etiology, radiology. Radiology. 1954;44 [Google Scholar]
- 24.Magnus-Levy VA. Ueber ungewohnliche verkakung der arterien. Deutsche Medizinische Wochenschrift. 1914;40:1305–1309 [Google Scholar]
- 25.Sharp J Heredo-familial vascular and articular calcification. Ann Rheum Dis. 1954;13:15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez LB, Link T, Chaganti K, Motamedi D. Arterial calcification due to cd73 deficiency (acdc): Imaging manifestations of ectopic mineralization. Skeletal Radiol. 2016;45:1583–1587 [DOI] [PubMed] [Google Scholar]
- 27.Markello TC, Pak LK, St Hilaire C, Dorward H, Ziegler SG, Chen MY, Chaganti K, Nussbaum RL, Boehm M, Gahl WA. Vascular pathology of medial arterial calcifications in nt5e deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol Genet Metab. 2011;103:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Nijs T, Albuisson J, Ockeloen CW, Legrand A, Jeunemaitre X, Schultze Kool LJ, Riksen NP. Isolated arterial calcifications of the lower extremities: A clue for nt5e mutation. Int J Cardiol. 2016;212:248–250 [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka K, Kuroda S, Takahashi K, Sasano T, Furukawa T, Matsumura A. Calcification of joints and arteries with novel nt5e mutations with involvement of upper extremity arteries. Vasc Med. 2017;22:541–543 [DOI] [PubMed] [Google Scholar]
- 30.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: Review and perspectives. Eur Heart J. 2014;35:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meradji M, de Villeneuve VH, Huber J, de Bruijn WC, Pearse RG. Idiopathic infantile arterial calcification in siblings: Radiologic diagnosis and successful treatment. J Pediatr. 1978;92:401–405 [DOI] [PubMed] [Google Scholar]
- 32.Thomas P, Chandra M, Kahn E, McVicar M, Naidich J, LaCorte M. Idiopathic arterial calcification of infancy: A case with prolonged survival. Pediatr Nephrol. 1990;4:233–235 [DOI] [PubMed] [Google Scholar]
- 33.Thiaville A, Smets A, Clercx A, Perlmutter N. Idiopathic infantile arterial calcification: A surviving patient with renal artery stenosis. Pediatr Radiol. 1994;24:506–508 [DOI] [PubMed] [Google Scholar]
- 34.Rutsch F, Ruf N, Vaingankar S, et al. Mutations in enpp1 are associated with 'idiopathic' infantile arterial calcification. Nature Genetics. 2003;34:379. [DOI] [PubMed] [Google Scholar]
- 35.Ramjan KA, Roscioli T, Rutsch F, Sillence D, Munns CF. Generalized arterial calcification of infancy: Treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab. 2009;5:167–172 [DOI] [PubMed] [Google Scholar]
- 36.Rutsch F, Boyer P, Nitschke Y, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edouard T, Chabot G, Miro J, Buhas DC, Nitschke Y, Lapierre C, Rutsch F, Alos N. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur J Pediatr. 2011;170:1585–1590 [DOI] [PubMed] [Google Scholar]
- 38.Jin H, St Hilaire C, Huang Y, et al. Increased activity of tnap compensates for reduced adenosine production and promotes ectopic calcification in the genetic disease acdc. Sci Signal. 2016;9:ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, He JW, Fu WZ, Zhang CQ, Zhang ZL. Calcification of joints and arteries: Second report with novel nt5e mutations and expansion of the phenotype. J Hum Genet. 2015;60:561–564 [DOI] [PubMed] [Google Scholar]
- 40.Wanga S, Hibender S, Ridwan Y, et al. Aortic microcalcification is associated with elastin fragmentation in marfan syndrome. J Pathol. 2017;243:294–306 [DOI] [PubMed] [Google Scholar]
- 41.Gheduzzi D, Sammarco R, Quaglino D, Bercovitch L, Terry S, Taylor W, Ronchetti IP. Extracutaneous ultrastructural alterations in pseudoxanthoma elasticum. Ultrastructural pathology. 2003;27:375–384 [PubMed] [Google Scholar]
- 42.Federici D, Torii S, Ciuffreda M, Galletti L, Lorini L, Bonanomi E, Gianatti A, Iascone M, Park J, Guo L, Romero ME, Kolodgie FD, Guagliumi G, Virmani R. Coronary pathology of inherited generalized arterial calcification of infancy: A case report. Cardiovasc Pathol. 2018;36:15–19 [DOI] [PubMed] [Google Scholar]
- 43.Burghoff S, Gong X, Viethen C, Jacoby C, Flogel U, Bongardt S, Schorr A, Hippe A, Homey B, Schrader J. Growth and metastasis of b16-f10 melanoma cells is not critically dependent on host cd73 expression in mice. BMC cancer. 2014;14:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao DJ, Saeedi BJ, Kitzenberg D, Burney KM, Dobrinskikh E, Battista KD, Vazquez-Torres A, Colgan SP, Kominsky DJ. Intestinal epithelial ecto-5’-nucleotidase (cd73) regulates intestinal colonization and infection by nontyphoidal salmonella. Infect Immun. 2017;85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quast C, Alter C, Ding Z, Borg N, Schrader J. Adenosine formed by cd73 on t cells inhibits cardiac inflammation and fibrosis and preserves contractile function in transverse aortic constriction-induced heart failure. Circulation. Heart failure. 2017;10. [DOI] [PubMed] [Google Scholar]
- 46.Li Q, Price TP, Sundberg JP, Uitto J. Juxta-articular joint-capsule mineralization in cd73 deficient mice: Similarities to patients with nt5e mutations. Cell Cycle. 2014;13:2609–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soderback U, Sollevi A, Fredholm BB. The disappearance of adenosine from blood and platelet suspension in relation to the platelet cyclic amp content. Acta Physiol Scand. 1987;129:189–194 [DOI] [PubMed] [Google Scholar]
- 48.Zhou Q, Yang D, Ombrello AK, et al. Early-onset stroke and vasculopathy associated with mutations in ada2. N Engl J Med. 2014;370:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Navon Elkan P, Pierce SB, Segel R, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N Engl J Med. 2014;370:921–931 [DOI] [PubMed] [Google Scholar]
- 50.Wangler MF, Yamamoto S, Chao HT, Posey JE, Westerfield M, Postlethwait J, Members of the Undiagnosed Diseases N, Hieter P, Boycott KM, Campeau PM, Bellen HJ. Model organisms facilitate rare disease diagnosis and therapeutic research. Genetics. 2017;207:9–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gahl WA, Markello TC, Toro C, et al. The national institutes of health undiagnosed diseases program: Insights into rare diseases. Genet Med. 2012;14:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howe K, Clark MD, Torroja CF, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chavez MN, Aedo G, Fierro FA, Allende ML, Egana JT. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front Physiol. 2016;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Apschner A, Huitema LF, Ponsioen B, Peterson-Maduro J, Schulte-Merker S. Zebrafish enpp1 mutants exhibit pathological mineralization, mimicking features of generalized arterial calcification of infancy (gaci) and pseudoxanthoma elasticum (pxe). Dis Model Mech. 2014;7:811–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massaia M, Perrin L, Bianchi A, Ruedi J, Attisano C, Altieri D, Rijkers GT, Thompson LF. Human t cell activation. Synergy between cd73 (ecto-5’-nucleotidase) and signals delivered through cd3 and cd2 molecules. Journal of immunology (Baltimore, Md. : 1950). 1990;145:1664–1674 [PubMed] [Google Scholar]
- 56.Massaia M, Attisano C, Redoglia V, Bianchi A, Thompson LF, Dianzani U, Pileri A. Amplification of t cell activation induced by cd73 (ecto-5’ nucleotidase) engagement. Advances in experimental medicine and biology. 1991;309b:155–158 [DOI] [PubMed] [Google Scholar]
- 57.Thompson LF, Ruedi JM, Glass A, Low MG, Lucas AH. Antibodies to 5’-nucleotidase (cd73), a glycosyl-phosphatidylinositol-anchored protein, cause human peripheral blood t cells to proliferate. Journal of immunology (Baltimore, Md. : 1950). 1989;143:1815–1821 [PubMed] [Google Scholar]
- 58.Yang L, Kobie JJ, Mosmann TR. Cd73 and ly-6a/e distinguish in vivo primed but uncommitted mouse cd4 t cells from type 1 or type 2 effector cells. Journal of immunology (Baltimore, Md. : 1950). 2005;175:6458–6464 [DOI] [PubMed] [Google Scholar]
- 59.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by cd39 and cd73 expressed on regulatory t cells mediates immune suppression. J Exp Med. 2007;204:1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5’-nucleotidase: Findings in a study of ecto-5’-nucleotidase gene-deficient mice. Arthritis Rheum. 2007;56:1440–1445 [DOI] [PubMed] [Google Scholar]
- 61.Bavaresco L, Bernardi A, Braganhol E, Wink MR, Battastini AM. Dexamethasone inhibits proliferation and stimulates ecto-5’-nucleotidase/cd73 activity in c6 rat glioma cell line. J Neurooncol. 2007;84:1–8 [DOI] [PubMed] [Google Scholar]
- 62.Friedman B, Cronstein B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ichikawa N, Taniguchi A, Kaneko H, Kawamoto M, Sekita C, Nakajima A, Yamanaka H. Arterial calcification due to deficiency of cd73 (acdc) as one of rheumatic diseases associated with periarticular calcification. J Clin Rheumatol. 2015;21:216–220 [DOI] [PubMed] [Google Scholar]
- 64.Botta Gordon-Smith S, Ursu S, Eaton S, Moncrieffe H, Wedderburn LR. Correlation of low cd73 expression on synovial lymphocytes with reduced adenosine generation and higher disease severity in juvenile idiopathic arthritis. Arthritis Rheumatol. 2015;67:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chrobak P, Charlebois R, Rejtar P, El Bikai R, Allard B, Stagg J. Cd73 plays a protective role in collagen-induced arthritis. Journal of immunology (Baltimore, Md. : 1950). 2015;194:2487–2492 [DOI] [PubMed] [Google Scholar]
- 66.Shkhyan R, Lee S, Gullo F, Li L, Peleli M, Carlstrom M, Chagin AS, Banks NW, Limfat S, Liu NQ, Evseenko D. Genetic ablation of adenosine receptor a3 results in articular cartilage degeneration. Journal of molecular medicine (Berlin, Germany). 2018;96:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17:188–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janes K, Symons-Liguori AM, Jacobson KA, Salvemini D. Identification of a3 adenosine receptor agonists as novel non-narcotic analgesics. Br J Pharmacol. 2016;173:1253–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang T, Xu X, Qiao M, Li X, Zhao C, Zhou F, Gao G, Wu F, Chen X, Su C, Ren S, Zhai C, Zhou C. Comprehensive evaluation of nt5e/cd73 expression and its prognostic significance in distinct types of cancers. BMC cancer. 2018;18:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu XR, He XS, Chen YF, Yuan RX, Zeng Y, Lian L, Zou YF, Lan N, Wu XJ, Lan P. High expression of cd73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137 [DOI] [PubMed] [Google Scholar]
- 71.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer research. 1997;57:2602–2605 [PubMed] [Google Scholar]
- 72.Ohta A, Gorelik E, Prasad SJ, et al. A2a adenosine receptor protects tumors from antitumor t cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z, Yin L. Rna interference of ecto-5’-nucleotidase (cd73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448 [DOI] [PubMed] [Google Scholar]
- 74.Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, Jalkanen S, Salmi M. Altered purinergic signaling in cd73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–1241 [DOI] [PubMed] [Google Scholar]
- 75.Stagg J, Beavis PA, Divisekera U, Liu MC, Moller A, Darcy PK, Smyth MJ. Cd73-deficient mice are resistant to carcinogenesis. Cancer research. 2012;72:2190–2196 [DOI] [PubMed] [Google Scholar]
- 76.Koszalka P, Golunska M, Stanislawowski M, Urban A, Stasilojc G, Majewski M, Wierzbicki P, Skladanowski AC, Bigda J. Cd73 on b16f10 melanoma cells in cd73-deficient mice promotes tumor growth, angiogenesis, neovascularization, macrophage infiltration and metastasis. The international journal of biochemistry & cell biology. 2015;69:1–10 [DOI] [PubMed] [Google Scholar]
- 77.Morris SA. Arterial tortuosity in genetic arteriopathies. Current Opinion in Cardiology. 2015;30:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the tgf-β receptor. New England Journal of Medicine. 2006;355:788–798 [DOI] [PubMed] [Google Scholar]
- 79.Coucke PJ, Willaert A, Wessels MW, et al. Mutations in the facilitative glucose transporter glut10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457 [DOI] [PubMed] [Google Scholar]
- 80.Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of tgf-β activation contributes to pathogenesis in marfan syndrome. Nature Genetics. 2003;33:407. [DOI] [PubMed] [Google Scholar]
- 81.Ravid K, Smith-Mungo LI, Zhao Z, Thomas KM, Kagan HM. Upregulation of lysyl oxidase in vascular smooth muscle cells by camp: Role for adenosine receptor activation. J Cell Biochem. 1999;75:177–185 [DOI] [PubMed] [Google Scholar]
- 82.Robins SP. Biochemistry and functional significance of collagen cross-linking. Biochem Soc Trans. 2007;35:849–852 [DOI] [PubMed] [Google Scholar]
- 83.Guo DC, Regalado ES, Gong L, et al. Lox mutations predispose to thoracic aortic aneurysms and dissections. Circ Res. 2016;118:928–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buchheiser A, Ebner A, Burghoff S, Ding Z, Romio M, Viethen C, Lindecke A, Kohrer K, Fischer JW, Schrader J. Inactivation of cd73 promotes atherogenesis in apolipoprotein e-deficient mice. Cardiovascular research. 2011;92:338–347 [DOI] [PubMed] [Google Scholar]
- 85.Yang J, Jian R, Yu J, Zhi X, Liao X, Yu J, Zhou P. Cd73 regulates vascular smooth muscle cell functions and facilitates atherosclerotic plaque formation. IUBMB life. 2015;67:853–860 [DOI] [PubMed] [Google Scholar]
- 86.Burghoff S, Flogel U, Bongardt S, Burkart V, Sell H, Tucci S, Ikels K, Eberhard D, Kern M, Kloting N, Eckel J, Schrader J. Deletion of cd73 promotes dyslipidemia and intramyocellular lipid accumulation in muscle of mice. Arch Physiol Biochem. 2013;119:39–51 [DOI] [PubMed] [Google Scholar]
- 87.Kucukoglu O, Guldiken N, Chen Y, Usachov V, El-Heliebi A, Haybaeck J, Denk H, Trautwein C, Strnad P. High-fat diet triggers mallory-denk body formation through misfolding and crosslinking of excess keratin 8. Hepatology. 2014;60:169–178 [DOI] [PubMed] [Google Scholar]
- 88.Zukowska P, Kutryb-Zajac B, Jasztal A, Toczek M, Zabielska M, Borkowski T, Khalpey Z, Smolenski RT, Slominska EM. Deletion of cd73 in mice leads to aortic valve dysfunction. Biochim Biophys Acta Mol Basis Dis. 2017;1863:1464–1472 [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Zhang W, Zhu C, Bucher C, Blazar BR, Zhang C, Chen JF, Linden J, Wu C, Huo Y. Inactivation of the adenosine a2a receptor protects apolipoprotein e-deficient mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1046–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jalkanen J, Hollmen M, Jalkanen S, Hakovirta H. Regulation of cd73 in the development of lower limb atherosclerosis. Purinergic signalling. 2017;13:127–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stafford NP, Pink AE, White AE, Glenn JR, Heptinstall S. Mechanisms involved in adenosine triphosphate--induced platelet aggregation in whole blood. Arterioscler Thromb Vasc Biol. 2003;23:1928–1933 [DOI] [PubMed] [Google Scholar]
- 92.Fuentes E, Pereira J, Mezzano D, Alarcon M, Caballero J, Palomo I. Inhibition of platelet activation and thrombus formation by adenosine and inosine: Studies on their relative contribution and molecular modeling. PLoS One. 2014;9:e112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Covarrubias R, Chepurko E, Reynolds A, Huttinger ZM, Huttinger R, Stanfill K, Wheeler DG, Novitskaya T, Robson SC, Dwyer KM, Cowan PJ, Gumina RJ. Role of the cd39/cd73 purinergic pathway in modulating arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2016;36:1809–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Netsch P, Elvers-Hornung S, Uhlig S, Kluter H, Huck V, Kirschhofer F, Brenner-Weiss G, Janetzko K, Solz H, Wuchter P, Bugert P, Bieback K. Human mesenchymal stromal cells inhibit platelet activation and aggregation involving cd73-converted adenosine. Stem Cell Res Ther. 2018;9:184. [DOI] [PMC free article] [PubMed] [Google Scholar]