Abstract
Purpose:
To report the prevalence and risk factors of cataract and its subtypes in older age group.
Methods:
A total of 6617 subjects were recruited from both rural and urban areas. A detailed history including data on demographic, socioeconomic and ocular history was obtained. Lens opacity was graded according to the Lens Opacity Classification System III (LOCS III).
Results:
Cataract was present in 1094 of the rural and 649 subjects in the urban population. Monotype subtype cataracts were found in 32% and 25% in rural and urban population and 12.68% and 18.6% were mixed cataracts in the rural and urban groups. In baseline characteristics history of diabetes, alcohol intake and presence of age-related macular degeneration were the risk factors in urban group. On multivariate analysis, the only significant risk factors for any cataract in subjects ≥60 years were increasing age in both rural [odds ratio (OR), 1.07] and urban (OR, 1.08) population, and HbA1c (OR, 1.14) in rural population. Overweight (OR, 0.6) was found to be a protective factor, and lower social economic status (OR, 1.52) a risk factor for cataract in urban population. A significant urban–rural difference was found in the prevalence of cataract and its subtypes (P ≤ 0.05).
Conclusion:
We found the risk factors for any cataract in older age group to be increasing age and HbA1c in rural group. Age and lower social economic status were found to be the risk factors in urban arm. A statistically significant difference was found on comparison of the prevalence of cataract and its subtypes between the rural and urban population.
Keywords: Prevalence of cataract, risk factors, rural–urban
Cataract is one of the most common causes of visual impairment in the world. According to the World Health Organisation (WHO), cataract is the leading cause of blindness all over the world, responsible for 47.8% of blindness and accounting for 17.7 million blind people.[1,2] In India, 80% of the blindness is due to cataract.[3,4] Various modifiable risk factors associated with cataract include UV exposure, diabetes, hypertension, body mass index (BMI), drug usage, smoking and socioeconomic factors; but advancing age is the single most important risk factor for cataract.[5,6,7,8,9,10,11,12,13]
Nirmalan et al. studied the prevalence of cataract in a rural population (≥40 years) of Southern India and reported the presence of cataract in 47.5% of their study population, prevalence being less in men compared to women.[14] In a recent population, Vashist et al. reported prevalences of 58% in North India and 53% in South India in the older age group (>60 years) with nuclear cataract being the most common type of cataract in both parts of the country.[15] In India, a very few population based studies have been undertaken to explore the risk factors for cataract in older age group, especially since the proportion of the elderly has been significantly increasing in the country; the 60 + population which stood at 56 million in 1991 is now estimated to have doubled in 2016.[16] The aim of the present study was to examine a proportionate sample of both rural and urban population ≥60 years and to report the age- and gender-adjusted prevalence rates of cataract in the population, and examine associated risk factors.
Methods
Study protocol
A population-based cross-sectional study was conducted in Southern India between 2009 and 2011. The study design and research methodology has been described in detail in our previous report.[17] To summarise, multistage random cluster sampling was used, and a cluster was defined as having a population of up to 2000 people, and if it exceeded this number, the population was divided into two or more clusters. For rural areas, the study areas were Kanchipuram and Thiruvallur districts, and for urban area, the Chennai district. A proper mapping and listing of the households were carried out in a systematic manner to avoid omissions or duplications. A door-to-door survey of all the households on both the sides of the street was conducted in the selected division of both rural and urban arms till we achieved the calculated sample size.
The Institutional Review Board approved this study and a written consent was obtained from the subjects as per the Declaration of Helsinki. People aged 60 years and above and who had resided at the target address for a minimum period of 6 months were recruited for the study. People who resided at the target households for less than 6 months, lived there temporarily (with permanent residence elsewhere), had died after the enumeration but before examination or who could not be contacted after five visits by the social worker at the residence were excluded from the study. Individuals who could not be transported to the examination centre because of health reason were also excluded from the study.
A detailed history including data on demographics and ocular history were obtained from all patients at the base hospital. A detailed questionnaire was also administered to all the subjects, the details and the scoring described in a previous paper.[18] Socioeconomic status (SES) was assessed with a multiple-index questionnaire, and the scoring was characterised as low (score, 0–14), middle (15–28) and high (29–42). BMI was calculated by using the formula weight (in kilograms)/height (in meters)2. Blood pressure (BP) was recorded, in the sitting position, in the right arm; two readings were taken 5 min apart, and the mean of the two was taken as the BP. All the subjects underwent a detailed ophthalmic assessment including visual acuity and spectacle refraction using modified Early Treatment Diabetic Retinopathy Study (ETDRS) chart (Light House Low Vision Products, New York, NY, USA), anterior segment examination using a slit-lamp Zeiss SL 130 (Carl Zeiss, Jena, Germany), measurement of intraocular pressure using Goldmann applanation tonometer (Zeiss AT 030 Applanation Tonometer; Carl Zeiss) and fundus examination using binocular indirect ophthalmoscope (Keeler Instruments Inc., Broomall, PA, USA). Retinal photographs were obtained after pupillary dilatation (Carl Zeiss fundus camera; FF-450, Germany). The presence of age-related macular degeneration (AMD) was graded according to the International age-related maculopathy (ARM) Epidemiological Study Group classification based on the grading in the worst eye.[19] The grading agreement, which was done by two independent observers (retina specialists) in a masked fashion, was 0.62 for early ARM and 0.87 for late ARM. Diabetic retinopathy was graded using the International Clinical Diabetic Retinopathy Severity Scale.[20] The grading agreement between the observers was 0.80.
Grading of lens images
Lens opacities were graded according to the Lens Opacities Classification System III (LOCS III) was performed by experienced ophthalmologist.[21] After the pupils were dilated with tropicamide (1%) and phenylephrine hydrochloride (2.5%) drops, cataract grading was done on a slit-lamp while comparing it with LOCS III standard photographs. The examiner identified the specific lens opacity and assigned a severity grade. The severity of the lens opacities, according to the photographic standards, was separated into four major groups: nuclear opalescence (NO), nuclear cataract (NC), cortical (CC) and posterior subcapsular (PSC). In patients who had undergone unilateral cataract surgery or had a non-gradable lens, the LOCS III score of the fellow eye was used. Those who had undergone bilateral cataract surgery were excluded from the analysis. For assessing the grading agreement, 50 patients with various grades of cataract were recruited from the pilot study and were assessed independently by both the graders. The grading agreements were: NO (k = 0.84), NC (k = 0.88), CC (k = 0.89) and PSC (k = 0.89). Overall, the average grading agreement was high (k = 0.85).
Definitions
Hypertension: Patients with a systolic BP ≥140 mmHg or a diastolic BP ≥90 mmHg or undergoing antihypertensive therapy were regarded as having hypertension.[18]
Smokers: Those who had any history of smoking were classified as smokers.
Past smokes: Were defined as individuals who had smoked previously but did not smoke at least 1 month before the time of interview.
Significant cataracts: A significant NC was identified by the presence of an LOCS III score of >4 for NO or >4 for NC. Similarly, a significant CC was identified by an LOCS III score of >2 for CC, and a significant PSC was identified by an LOCS III score of >2.[22,23]
Refractive errors: Emmetropia was defined as a spherical equivalent between −0.50 and +0.50 diopter sphere (DS). Myopia was defined as a spherical equivalent greater than −0.50 DS. Hyperopia was defined as a spherical equivalent greater than +0.50 DS. Astigmatic correction was measured in minus cylinder format, and was defined as a cylindrical error greater than −0.50 diopter cylinder (DC) at any axis.[24]
Statistical analysis
The age- and gender-specific prevalence rates of cataract and subtypes were assessed. For AMD, the eye with a diagnosis of AMD was first chosen. In case of bilateral diagnosis of AMD, the eye with the ‘worse’ stage of AMD was chosen. Therefore, one eye of each (eligible) subject was included. For cataract analysis, in patients who had undergone unilateral cataract surgery or had a non-gradable lens, the LOCS III score of the fellow eye was considered for the analysis. If both eyes had cataract, the eye with the worse stage of cataract was included for the analysis. Refractive error status was assessed in same eye, which was considered for cataract analysis. The association of the variables with cataract was assessed using the Student's t test for the continuous variables and the Pearson's χ2 test for the categorical variables. Logistic regression analysis was performed to determine risk factors using odds ratio (OR) estimates with 95% confidence intervals. A multivariate regression analysis was performed with P value <0.05 being required for entering the model. The SPSS software (version 13.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
A total of 6617 people [rural (n = 3904) and urban (n = 2713)] were recruited. Of which, 5495 (83%) participated in the study for eye examination; after excluding subjects who did not have cataract evaluation (26) and those with pseudophakia or aphakia (1138), 4331 subjects were included in this study [Fig. 1].
Figure 1.
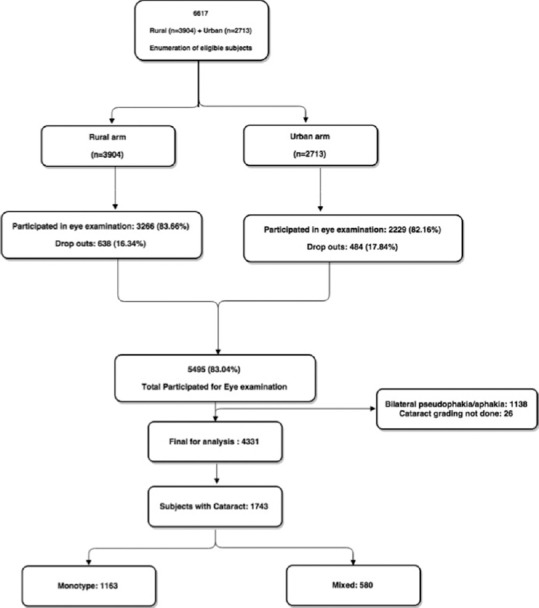
Flowchart showing participation of subjects for cataract evaluation in SNRAM study
Table 1 shows the prevalence of cataract and its subtypes in the rural and urban study population. Cataract was present in 1094 of the rural and 649 subjects in the urban population. Monotype subtype cataracts were found in 32% and 25% in the rural and urban population, respectively, and 12.68% and 18.6% were mixed cataracts in the rural and urban groups. In the monotype group, nuclear cataracts were the most common type of cataract (10.88%) in the rural group and CC in the urban group (11.36%). CC with the presence of PSC was the most common type of mixed cataract in the rural group (5.3%) and nuclear cataract with the presence of CC was the most common type of cataract in the urban group (7.3%).
Table 1.
Prevalence of cataract and its subtypes in the rural and urban population
| Rural (n=2651) | Prevalence of cataract | Percentage | 95% CI | Age- and sex-adjusted prevalence* | Urban (n=1680) | Prevalence of cataract | Percentage | 95% CI | Age- and sex-adjusted prevalence* | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Percentage | 95% CI | Percentage | 95% CI | |||||||||
| None | 1557 | 58.7 | [56.9-60.6] | 55.32 | [52.81-57.82] | None | 1031 | 61.4 | [59.0-63.8] | 56.38 | [53.40-59.36] | - |
| Any cataract | 1094 | 41.3 | [39.4-43.2] | 44.68 | [41.74-47.62] | Any cataract | 649 | 38.6 | [36.3-41.0] | 43.62 | [39.68-47.56] | - |
| Monotype | Monotype | - | ||||||||||
| Nuclear cataract | 260 | 9.8 | [8.7-11.0] | 10.88 | [9.38-12.39] | Nuclear cataract | 116 | 6.9 | [5.8-8.2] | 9.18 | [6.90-11.44] | 0.004 |
| Cortical cataract | 124 | 4.7 | [3.9-5.6] | 4.74 | [3.91-5.57] | Cortical cataract | 197 | 11.7 | [10.3-13.4] | 11.36 | [9.87-12.84] | <0.0001 |
| Posterior sub capsular cataract | 202 | 7.6 | [6.7-8.7] | 7.75 | [6.59-8.91] | Posterior sub capsular cataract | 58 | 3.5 | [2.6-4.4] | 3.52 | [2.54-4.49] | <0.0001 |
| Hyper-mature cataract | 188 | 7.1 | [6.2-8.1] | 8.62 | [7.07-10.17] | Hyper-mature cataract | 18 | 1.1 | [0.7-1.7] | 0.97 | [0.60-1.32] | <0.0001 |
| Mixed | Mixed | - | ||||||||||
| NC-CC | 41 | 1.5 | [1.1-2.1] | 1.75 | [1.12-3.38] | NC-CC | 97 | 5.8 | [4.8-7.0] | 7.28 | [5.36-9.20] | <0.0001 |
| NC-PSC | 94 | 3.5 | [2.9-4.3] | 3.64 | [2.86-4.41] | NC-PSC | 15 | 0.9 | [0.5-1.5] | 0.94 | [0.50-1.37] | <0.0001 |
| CC-PSC | 139 | 5.2 | [4.5-6.2] | 5.29 | [4.44-6.12] | CC-PSC | 58 | 3.5 | [2.7-4.4] | 3.64 | [2.61-4.64] | 0.016 |
| NC-CC-PSC | 46 | 1.7 | [1.3-2.3] | 2.00 | [1.31-2.67] | NC-CC-PSC | 90 | 5.4 | [4.4-6.5] | 6.74 | [4.86-8.61] | <0.0001 |
CC=Cortical, NC=Nuclear cataract, PSC=Posterior capsular cataract.*Age- and sex-adjusted to population of India based on census of India 2011
Table 2 shows the baseline characteristics of the subjects with any cataract compared with the subjects with no cataract in the rural and urban population groups. The subjects in the cataract group were older compared to the no cataract group in both the rural and urban population. No gender difference was noted between the cataract and no cataract group in both the rural (P = 0.06) and urban population (P = 0.93). On comparison of the SES between cataract and no cataract group in both the rural and urban population, a statistical significant difference was seen: middle-to-high SES had less prevalence of cataract compared to no cataract group in both the rural (34.6% vs. 38.7%) and urban population (56.9% vs. 63.5%), while the percentage of cataract in low SES group was higher compared to those who had no cataract in both the rural (65.4% vs. 61.3%) and urban population (43.1% vs. 36.5%). In the rural group, the prevalence of AMD was seen less in the cataract group when compared to the no cataract group (15.6% vs. 19.9%). In the rural group, intake of alcohol was statistically significant between the cataract and no cataract group (P = 0.008). A comparison of the refractive error between the two groups in both the rural and urban population showed that the cataract group had higher prevalence of myopia in both the rural (76.1% vs. 65.2%) and urban population (40.3% vs. 30.9%) Whereas, the prevalence of hyperopia was less in the cataract group compared to the no cataract group in both the rural (5.1% vs. 14.0%) and urban population (25.6% vs. 40.9%).
Table 2.
Baseline characteristics of the rural and urban study population
| Rural population | Urban population | ||||||
|---|---|---|---|---|---|---|---|
| Risk factors | No cataract (n=1557) | Any cataract (n=1094) | P | Risk factors | No cataract (n=1031) | Any cataract (n=649) | P |
| n (%) or mean±SD | n (%) or mean±SD | n (%) or mean±SD | n (%) or mean±SD | ||||
| Age | 64.3±5.1 | 66.4±6.4 | <0.0001 | Age | 64.5±5.2 | 67.8±7.0 | <0.0001 |
| BMI | BMI | ||||||
| Normal | 21.6±1.8 | 21.4±1.7 | 0.0040 | Normal | 22.3±1.7 | 22.0±1.6 | 0.0003 |
| Under weight | 16.7±1.50 | 16.5±1.2 | 0.0003 | Under weight | 16.9±1.3 | 17.1±1.3 | 0.0022 |
| Over weight | 26.8±1.3 | 26.6±1.2 | 0.0007 | Over weight | 27.0±1.3 | 26.9±1.3 | 0.1249 |
| Obese | 34.1±4.3 | 34.8±4.4 | <0.0001 | Obese | 32.7±2.8 | 33.2±3.1 | 0.0001 |
| HbA1c | 6.3±1.7 | 6.5±1.8 | 0.146 | HbA1c | 5.8±1.5 | 6.0±1.7 | 0.260 |
| Sex | Sex | ||||||
| Men | 756 (48.6) | 492 (45.0) | 0.069 | Men | 419 (40.6) | 265 (40.8) | 0.938 |
| Women | 801 (51.4) | 602 (55.0) | Women | 612 (59.4) | 384 (59.2) | ||
| Social economic status | Social economic status | ||||||
| Middle to high | 603 (38.7) | 378 (34.6) | 0.028 | Middle and high | 655 (63.5) | 369 (56.9) | 0.006 |
| Low | 954 (61.3) | 716 (65.4) | Low | 376 (36.5) | 280 (43.1) | ||
| HTN | 136 (8.7) | 76 (6.9) | 0.095 | HTN | 343 (33.3) | 201 (31.0) | 0.327 |
| Diabetes | 352 (22.6) | 204 (18.6) | 0.014 | Diabetes | 301 (29.2) | 211 (32.5) | 0.150 |
| Use of tobacco | 638 (41.0) | 478 (43.7) | 0.163 | Use of tobacco | 228 (22.1) | 163 (25.1) | 0.156 |
| Smoking | Smoking | ||||||
| Non-smoker | 1326 (85.2) | 954 (87.2) | 0.136 | Non-smoker | 923 (89.5) | 586 (90.3) | 0.612 |
| Past smoker | 30 (1.9) | 19 (1.7) | 0.720 | Past smoker | 28 (2.7) | 14 (2.2) | 0.475 |
| Present smoker | 201 (12.9) | 121 (11.1) | 0.151 | Present smoker | 80 (7.8) | 49 (7.6) | 0.875 |
| Alcohol | 251 (16.1) | 136 (12.4) | 0.008 | Alcohol | 103 (10.0) | 68 (10.5) | 0.748 |
| DR | 24 (2.0) | 13 (2.0) | 0.995 | DR | 44 (4.6) | 26 (6.0) | 0.266 |
| ARMD | 247 (19.9) | 86 (15.6) | 0.028 | ARMD | 155 (16.1) | 47 (12.8) | 0.135 |
| Refractive error | Refractive error | ||||||
| Emmetropia | 254 (20.8) | 92 (18.8) | 0.354 | Emmetropia | 284 (28.1) | 176 (34.1) | 0.016 |
| Myopia | 796 (65.2) | 373 (76.1) | <0.0001 | Myopia | 312 (30.9) | 208 (40.3) | <0.0001 |
| Hyperopia | 171 (14.0) | 25 (5.1) | <0.0001 | Hyperopia | 413 (40.9) | 132 (25.6) | <0.0001 |
HTN=History of hypertension, DR=Diabetic retinopathy, ARMD=Age-related macular degeneration, BMI=Body mass index, HbA1C=Glycogenated haemoglobin
Table 3 shows the results of univariate and multivariate analyses identifying the risk factors for the presence of any cataract in the rural and urban population. The goodness of model fit was also assessed, and the Nagelkereke pseudo-R-squared values for the rural and urban arms were 0.07 and 0.06. On univariate analysis, increasing age (rural: OR, 1.07, urban: OR, 1.10) was found to be a risk factor for cataract in both the rural and urban groups. Prevalence of diabetes (P = 0.014) was found to be a significant protective factor in the rural population. However, on multivariate analysis, the only significant risk factors for any cataract in subjects ≥60 years were increasing age in both the rural (OR, 1.07) and urban (OR, 1.08) population. Higher HbA1c was found to be a risk factor for cataract in the rural population (OR, 1.14). In the urban population, overweight (OR, 0.6) was found to be a protective factor for cataract and lower SES (OR, 1.52) a risk factor.
Table 3.
Risk factors for any cataract in the rural and urban population
| Rural population | Urban population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk factors | Univariate analysis | Multivariate analysis | Risk factors | Univariate analysis | Multivariate analysis | ||||
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Age | 1.07 [1.05-1.08] | <0.0001 | 1.07 [1.03-1.1] | <0.0001 | Age | 1.10 [1.08-1.11] | <0.0001 | 1.08 [1.04-1.12] | <0.0001 |
| BMI | BMI | ||||||||
| Normal | 1 | 1 | Normal | 1 | 1 | ||||
| Under weight | 1.32 [1.11-1.58] | 0.002 | 1.26 [0.84-1.9] | 0.266 | Under weight | 1.60 [1.14-2.24] | 0.007 | 1.72 [0.9-3.29] | 0.104 |
| Over weight | 0.75 [0.58-0.97] | 0.026 | 0.94 [0.59-1.5] | 0.799 | Over weight | 0.70 [0.55-0.89] | 0.004 | 0.6 [0.37-0.95] | 0.031 |
| Obese | 1.06 [0.74-1.53] | 0.739 | 0.93 [0.42-2.03] | 0.854 | Obese | 0.71 [0.50-0.99] | 0.047 | 0.76 [0.39-1.47] | 0.417 |
| HbA1c | 1.07 [0.99-1.16] | 0.094 | 1.14 [1.03-1.26] | 0.011 | HbA1c | 1.07 [0.97-1.19] | 0.169 | 1.09 [0.94-1.27] | 0.228 |
| Sex | Sex | ||||||||
| Male | 1 | 1 | Male | 1 | 1 | ||||
| Female | 1.16 [0.99-1.35] | 0.069 | 1.12 [0.76-1.66] | 0.555 | Female | 0.99 [0.81-1.21] | 0.938 | 1.05 [0.61-1.81] | 0.849 |
| SES | SES | ||||||||
| Middle to high | 1 | 1 | Middle and high | 1 | 1 | ||||
| Low | 1.20 [1.02-1.41] | 0.028 | 1.17 [0.83-1.64] | 0.368 | Low | 1.32 [1.08-1.61] | 0.006 | 1.57 [1.04-2.37] | 0.033 |
| History of hypertension | 0.78 [0.58-1.05] | 0.095 | 1.27 [0.8-2.01] | 0.314 | History of hypertension | 0.90 [0.73-1.11] | 0.327 | 1.09 [0.7-1.71] | 0.702 |
| Diabetes | 0.79 [0.65-0.95] | 0.014 | 0.71 [0.49-1.04] | 0.080 | Diabetes | 1.17 [0.95-1.44] | 0.151 | 1.25 [0.76-2.08] | 0.378 |
| Use of tobacco | 1.12 [0.96-1.31] | 0.163 | 1.25 [0.84-1.85] | 0.274 | Use of tobacco | 1.18 [0.94-1.49] | 0.157 | 0.93 [0.57-1.52] | 0.763 |
| Smoking | Smoking | ||||||||
| Non-smoker | 1 | 1 | Non-smoker | 1 | 1 | ||||
| Past smoker | 0.88 [0.49-1.57] | 0.667 | 1.36 [0.54-3.45] | 0.512 | Past smoker | 0.79 [0.41-1.51] | 0.471 | 0.45 [0.13-1.55] | 0.203 |
| Current smoker | 0.84 [0.66-1.06] | 0.146 | 0.95 [0.48-1.89] | 0.894 | Current smoker | 0.97 [0.67-1.40] | 0.849 | 0.56 [0.23-1.38] | 0.206 |
| Alcohol | 0.74 [0.59-0.93] | 0.008 | 0.77 [0.44-1.37] | 0.374 | Alcohol | 1.05 [0.76-1.46] | 0.748 | 1.03 [0.48-2.21] | 0.947 |
| DR | 1.00 [0.51-1.97] | 0.995 | 1.04 [0.47-2.27] | 0.930 | DR | 1.33 [0.81-2.18] | 0.267 | 0.94 [0.4-2.21] | 0.881 |
BMI=Body mass index, HbA1C=Glycogenated haemoglobin, SES=Socioeconomic status, DR=Diabetic retinopathy
Discussion
We report the prevalence and risk factors for cataract in population ≥60 years in South India. The prevalence of cataract was 44.6% in the rural and 43.6% in the urban population, the prevalence of monotype cataract was higher compared to the mixed type in both the rural and urban population. In the monotype group, the most common type was NC (10.9%) in the rural and CC in the urban population (11.3%). Of the mixed ones, the most common cataract was a combination of CC and PSC (5.29%) in the rural population and nuclear cataract combined with CC in the urban population (7.28%). Table 4 shows the comparison of prevalence of cataract and its subtypes in studies which have used LOCS III to grade the cataract. The prevalence of cataract ranges from 23% to 59.2%.[14,15,25,26,27,29] Our prevalence data fall somewhere in between. The varying difference in the prevalence of a cataract could be due to various reasons including differences in ethnicity, age group of the population and also the variability in the cut-off point adopted within the LOCS III system to define the presence of cataract. The studies from Indian subcontinent have reported a higher prevalence of cataract; Aravind Comprehensive Eye Study (ACES) in 2003 reported the prevalence of cataract among people >40 years to be 47.5%, and the INDEYE study in 2011 reported the prevalence to be 58% in North India and 53% in South India, respectively.[14,15]
Table 4.
Prevalence of cataract in the present study with comparison to literature
| Study group | Year | Study region | Cataract grading | Cut-off for cataract grading | n | Age (years) | Prevalence of cataract | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Any cataract | NC | CC | PSC | Mixed | |||||||
| Tanjong Pagar, Singapore | 2002 | Tanjong Pagar district | LOCS III | Nuclear cataract ≥4 Cortical cataract ≥2 Posterior subcapsular cataract ≥2 |
1206 | >40 | 34.7 | 22.6 | 23.9 | 7.0 | - |
| Sumatra Eye Study, Indonesia | 2005 | Rural Sumatra | LOCS III | Nuclear cataract ≥4 Cortical cataract ≥4 Posterior subcapsular cataract ≥2 |
919 | >21 | 23.0 | 35.7 | 30.1 | 15.1 | - |
| Su-Ying Tsai et al. Taiwan | 2003 | Shihpai, Taipei, Taiwan | LOCS III | Nuclear cataract ≥2 Cortical cataract ≥2 Posterior subcapsular cataract ≥2 |
1361 | >65 | 59.2 | 38.9 | 21.9 | 9.2 | - |
| Aravind Comprehensive Eye Study | 2003 | Rural area | LOCS III | Nuclear cataract ≥3 Cortical cataract ≥3 Posterior subcapsular cataract ≥2 |
5150 | >40 | 47.5 | 43.5 | 13.9 | 19.9 | 47.7 |
| Meiktila Eye Study | 2008 | Rural Area | LOCS III | Nuclear cataract ≥4 Cortical cataract ≥2 Posterior subcapsular cataract ≥2 |
2044 | >40 | 40.39 | 27.35 | 20.91 | 11.34 | - |
| INDEYE Study | 2011 | Both rural and urban | LOCS III | Nuclear cataract ≥4 Cortical cataract ≥3 Posterior subcapsular cataract ≥2 |
4946 | >60 | NI 58 | NI 48 | NI 7.6 | NI 21 | - |
| SI 53 | SI 38 | SI 10.2 | SI 17 | ||||||||
| Present study | Both rural and urban | LOCS III | Nuclear cataract ≥4 Cortical cataract ≥2 Posterior subcapsular cataract ≥2 |
Rural: 2651 | >60 | 44.68 | 10.88 | 4.74 | 7.75 | 12.7 | |
| Urban: 1680 | >60 | 43.62 | 9.18 | 11.36 | 3.52 | 18.6 | |||||
LOCS=Lens Opacities Classification System, NI=North India, SI=South India
On re-analysing our data as per the grading criteria used in the INDEYE study.[15] The prevalence of monotype cataract in the rural group was as follows, nuclear cataract (14.5%), CC (1.6%), PSC (15.2%) and hyper-mature cataract (6.3%). And the prevalence of monotype cataract in urban group was as follows, nuclear cataract (15.2%), CC (6.1%), PSC (5.5%) and hyper-mature cataract (0.8%). The prevalence of nuclear cataract was high in both the rural and urban group, as seen in the INDEYE study. However, the prevalence of monotype cataract was still less in the present study compared to the INDEYE study. This reduction in temporal trend may represent the efficacy of the National Programme for Control of Blindness by the Government of India, an initiative to reduce cataract blindness. Previously, we have reported the prevalence of cataract in people with diabetes >40 years and found it to be much higher; 65.7%.[8] As ACES was a study in population >40 years; the prevalence may not be restricted to age-related cataract and might include the diabetic cataracts as well in younger population.
Consistent with the other studies from Indian subcontinent, nuclear cataract is the most common subtype of cataract. Chua et al.[30] have shown that the severity of nuclear, CC and PSC was significantly correlated with genetic ancestry in their South East Asian population. They found people of Malay ancestry had a greater severity for all cataract subtypes than the people of Chinese ancestry. This could explain the ethnic differences in the prevalence of cataract subtypes.
The prevalence of hyper-mature cataract in the present study was found to be 8.62% in the rural and 0.97% in the urban population, which is similar than that reported by Avachat et al.[31] (11.5%) in 2014, and Raizada et al.[32] (7.1%) in 1984. In the present study the difference in prevalence of hyper-mature cataract between the rural and urban population could be due to less availability and utilisation of the healthcare services in rural India.
In this study, both men and women did not show significant difference in the prevalence of cataract. This is in disagreement to published literature as women have been reported to have higher prevalence in other population based studies.[14,15] The possible reason could be due to the increase in women empowerment, positive gender ratios and higher female literacy rates in the study region, urban (Chennai – 86.64%) and rural areas (Kanchipuram – 79.02%, Thiruvallur – 78.32%).
In the present study, we found urban–rural difference in the prevalence of cataract and its subtypes (P ≤ 0.05). Nirmalan et al.[14] studied the prevalence of cataract in a rural population of Southern India and found the prevalence to be 47.5%, this is higher than that compared to the present study. Though the INDEYE study had both rural and urban samples, they did not report any difference in prevalence of cataract among rural and urban population.[15]
In the present study higher HbA1c was not found to be a risk factor for cataract in rural population, whereas the WESDR (Wisconsin Epidemiological Study on Diabetic Retinopathy) and Beaver Dam Eye study found a significant association of cataract with glycosylated haemoglobin.[33,34] Similar to our study, the Blue Mountain Eye Study, Visual Impairment Project and Barbados Eye Study also found a correlation between myopia and cataract.[35,36,37,38] The development of age-related cataract is widely known to be associated with myopic shift in refraction. Although it may be argued that myopia may be a consequence of cataract rather than a risk factor; laboratory-based evidence shows higher levels of malondialdehyde (MDA) in both cataractous lens and vitreous of myopic eyes; contributing to catarctogenesis.[39,40]
The major strengths of the study include its population-based design and standard documentation of cataract by LOCS III. This data are extremely useful for healthcare providers to develop long-term strategies to combat avoidable blindness. It is heartening to see a declining prevalence of cataract as compared to epidemiological studies done in past in India. The study also found lifestyle variable, glycaemic control, as risk factors for cataract. It is possible that modulating this variable may delay the occurrence of cataract, however, this warrants further studies. A limitation of the study is the inability to validate the causal relationship between the significant risk factors and presence of cataract. Other risk factors like sunlight exposure and nutritional history, which may play an important role in catarctogenesis, were also not studied in this study.
Conclusion
Increasing age and HbA1c are associated with risk for cataract in the rural group, while age and lower social economic status are the risk factors in the urban population.
Financial support and sponsorship
Jamshetji Tata trust, Mumbai.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rao GN, Khanna R, Payal A. The global burden of cataract. Curr Opin Ophthalmol. 2011;22:4–9. doi: 10.1097/ICU.0b013e3283414fc8. [DOI] [PubMed] [Google Scholar]
- 2.Liu YC, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390:600–12. doi: 10.1016/S0140-6736(17)30544-5. [DOI] [PubMed] [Google Scholar]
- 3.Mohan M. Survey of blindness-India (1986–1989). In: Summary Results: Programme for the Control of Blindness. Ministry of Health and Family Welfare, Government of India: New Delhi. 1992 [Google Scholar]
- 4.Dandona L, Dandona R, Srinivas M Giridhar P, Vilas K, Prasad MN, et al. Blindness in the Indian state of Andhra Pradesh. Invest Ophthalmol Vis Sci. 2001;42:908–16. [PubMed] [Google Scholar]
- 5.Dolin PJ. Ultraviolet radiation and cataract: A review of the epidemiological evidence. Br J Ophthalmol. 1994;78:478–82. doi: 10.1136/bjo.78.6.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnaiah S, Vilas K, Shamanna BR, Rao GN, Thomas R, Balasubramanian D. Smoking and its association with cataract: Results of the Andhra Pradesh eye disease study from India. Invest Ophthalmol Vis Sci. 2005;46:58–65. doi: 10.1167/iovs.04-0089. [DOI] [PubMed] [Google Scholar]
- 7.Nangia V, Jonas JB, Sinha A, Matin A, Kulkarni M. Refractive error in central India: The central India eye and medical study. Ophthalmology. 2010;117:693–9. doi: 10.1016/j.ophtha.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Raman R, Pal SS, Adams JS, Rani PK, Vaitheeswaran K, Sharma T. Prevalence and risk factors for cataract in diabetes: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study, report no.17. Invest Ophthalmol Vis Sci. 2010;51:6253–61. doi: 10.1167/iovs.10-5414. [DOI] [PubMed] [Google Scholar]
- 9.Leske MC, Wu SY, Hennis A, Connell AM, Hyman L, Schachat A. Diabetes, hypertension, and central obesity as cataract risk factors in a black population. The Barbados Eye Study. Ophthalmology. 1999;106:35–41. doi: 10.1016/s0161-6420(99)90003-9. [DOI] [PubMed] [Google Scholar]
- 10.Kuang TM, Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Body mass index and age-related cataract: The Shihpai Eye Study. Arch Ophthalmol. 2005;123:1109–14. doi: 10.1001/archopht.123.8.1109. [DOI] [PubMed] [Google Scholar]
- 11.Klein BE, Klein R, Lee KE, Danforth LG. Drug use and five year incidence of age-related cataracts. The Beaver Dam Eye Study. Ophthalmology. 2001;108:1670–4. doi: 10.1016/s0161-6420(01)00656-x. [DOI] [PubMed] [Google Scholar]
- 12.Solberg Y, Rosner M, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol. 1998;42:535–47. doi: 10.1016/s0039-6257(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 13.Klein BE, Klein R, Lee KE, Meuer SM. Socioeconomic and lifestyle factors and the 10-year incidence of age-related cataracts. Am J Ophthalmol. 2003;136:506–12. doi: 10.1016/s0002-9394(03)00290-3. [DOI] [PubMed] [Google Scholar]
- 14.Nirmalan PK, Robin AL, Katz J, Tielsch JM, Thulasiraj RD, Krishnadas R, et al. Risk factors for age related cataract in a rural population of southern India: The Aravind Comprehensive Eye Study. Br J Ophthalmol. 2004;88:989–94. doi: 10.1136/bjo.2003.038380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vashist P, Talwar B, Gogoi M, Maraini G, Camparini M, Ravindran RD, et al. Prevalence of cataract in an older population in India: The India study of age-related eye disease. Ophthalmology. 2011;118:272–8. doi: 10.1016/j.ophtha.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S. Alarm sounded over Greying of India's population. Lancet. 1997;350:271. [Google Scholar]
- 17.Raman R, Pal SS, Ganesan S, Gella L, Vaitheeswaran K, Sharma T. The prevalence and risk factors for age-related macular degeneration in rural-urban India, Sankara Nethralaya Rural-Urban Age-related Macular degeneration study, Report No.1. Eye (Lond) 2016;30:688–97. doi: 10.1038/eye.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal S, Raman R, Paul PG, Rani PK, Uthra S, Gayathree R, et al. Sankara Nethralaya-diabetic retinopathy epidemiology and molecular genetic study (SN-DREAMS 1): Study design and research methodology. Ophthalmic Epidemiol. 2005;12:143–53. doi: 10.1080/09286580590932734. [DOI] [PubMed] [Google Scholar]
- 19.The International ARM Epidemiological Study Group. An international classification and grading system for age related maculopathy and age related macular degeneration. Surv Ophthalmol. 1995;35:367–74. doi: 10.1016/s0039-6257(05)80092-x. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Global Diabetic Retinopathy Project Group. Global Diabetic Retinopathy Project Group. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 21.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The lens opacities classification system III. The longitudinal study of cataract study group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 22.Delcourt C, Cristol JP, Tessier F, Leger CL, Michel F, Papoz L. Risk factors for cortical nuclear, and posterior subcapsular cataracts: The POLA study: Pathologies Oculaires Liees a l’Age. Am J Epidemiol. 2000;151:497–504. doi: 10.1093/oxfordjournals.aje.a010235. [DOI] [PubMed] [Google Scholar]
- 23.Leske MC, Chylack LT, Jr, He Q, Wu SY, Schoenfeld E, Friend J, et al. Incidence and progression of cortical and posterior subcapsular opacities: The Longitudinal Study of Cataract. The LSC Group. Ophthalmology. 1997;104:1987–93. doi: 10.1016/s0161-6420(97)30043-8. [DOI] [PubMed] [Google Scholar]
- 24.Dandona R, Dandona L, Naduvilath TJ, Srinivas M, McCarty CA, Rao GN. Refractive errors in an urban population in Southern India: The Andhra Pradesh Eye Disease Study. Invest Ophthalmol Vis Sci. 1999;40:2810–8. [PubMed] [Google Scholar]
- 25.Husain R, Tong L, Fong A, Cheng JF, How A, Chua WH, et al. Prevalence of cataract in rural Indonesia. Ophthalmology. 2005;112:1255–62. doi: 10.1016/j.ophtha.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Seah SK, Wong TY, Foster PJ, Ng TP, Johnson GJ. Prevalence of lens opacity in Chinese residents of Singapore: The tanjong pagar survey. Ophthalmology. 2002;109:2058–64. doi: 10.1016/s0161-6420(02)01221-6. [DOI] [PubMed] [Google Scholar]
- 27.Athanasiov PA, Casson RJ, Sullivan, Newland HS, Shein WK, Muecke JS, et al. Cataract in rural Myanmar: Prevalence and risk factors from the Meiktila Eye Study. Br J Ophthalmol. 2008;92:1169–74. doi: 10.1136/bjo.2008.139725. [DOI] [PubMed] [Google Scholar]
- 28.Foster PJ, Wong TY, Machin D, Johnson GJ, Seah SK. Risk factors for nuclear, cortical and posterior subcapsular cataracts in the Chinese population of Singapore: The Tanjong Pagar Survey. Br J Ophthalmol. 2003;87:1112–20. doi: 10.1136/bjo.87.9.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai SY, Hsu WM, Cheng CY, Liu JH, Chou P. Epidemiologic study of age-related cataracts among an elderly Chinese population in Shih-Pai, Taiwan. Ophthalmology. 2003;110:1089–95. doi: 10.1016/S0161-6420(03)00243-4. [DOI] [PubMed] [Google Scholar]
- 30.Chua J, Koh JY, Tan AG, Zhao W, Lamoureux E, Mitchell P, et al. Ancestry, socioeconomic status, and age-related cataract in Asians: The Singapore epidemiology of eye diseases study. Ophthalmology. 2015;122:2169–78. doi: 10.1016/j.ophtha.2015.06.052. [DOI] [PubMed] [Google Scholar]
- 31.Avachat SS, Phalke V, Kambale S. Epidemiological correlates of cataract cases in tertiary health care center in rural area of maharashtra. J Family Med Prim Care. 2014;3:45–7. doi: 10.4103/2249-4863.130273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raizada IN, Mathur A, Narang SK. A study of prevalence and risk factors of senile cataract in rural areas of western U.P. Indian J Ophthalmol. 1984;32:339–42. [PubMed] [Google Scholar]
- 33.Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119:295–300. doi: 10.1016/s0002-9394(14)71170-5. [DOI] [PubMed] [Google Scholar]
- 34.Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: The Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782–90. doi: 10.1016/s0002-9394(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 35.Younan C, Mitchell P, Cumming RG, Rochtchina E, Wang JJ. Myopia and incident cataract and cataract surgery: The blue mountains eye study. Invest Ophthalmol Vis Sci. 2002;43:3625–32. [PubMed] [Google Scholar]
- 36.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–65. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 37.Wensor M, McCarty CA, Taylor HR. Prevalence and risk factors of myopia in Victoria, Australia. Arch Ophthalmol. 1999;117:658–63. doi: 10.1001/archopht.117.5.658. [DOI] [PubMed] [Google Scholar]
- 38.Wu SY, Nemesure B, Leske MC. Refractive errors in a black adult population: The Barbados Eye Study. Invest Ophthalmol Vis Sci. 1999;40:2179–84. [PubMed] [Google Scholar]
- 39.Micelli-Ferrari T, Vendemiale G, Grattagliano, Boscia F, Arnese L, Altomare E, et al. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br J Ophthalmol. 1996;80:840–3. doi: 10.1136/bjo.80.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonelli F, Nesti A, Pensa M, Romano L, Savastano S, Rinaldi E, et al. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp Eye Res. 1989;49:181–7. doi: 10.1016/0014-4835(89)90088-2. [DOI] [PubMed] [Google Scholar]


