Abstract
Purpose:
To compare the efficacy and safety profile of oral azithromycin with that of doxycycline over 9 months in patients experiencing failure with conservative and topical treatment for Meibomian gland dysfunction (MGD), to assess recurrence of MGD, and to determine the number of treatments required.
Methods:
This is a randomized controlled trial with a cross-over design at a tertiary care center. In all, 115 consecutive patients underwent a complete ophthalmological examination before being randomly assigned to oral treatment with doxycline (4 g for 30 days) or azithromycin (1.25 g for 5 days). Patients were evaluated at 3, 6, and 9 months. Therapy was switched or conservative management maintained according to signs and symptoms.
Results:
In the azithromycin group, 83.25% of the patients were stable after one treatment, 16.5% needed a further one or two treatments (some had previously been switched to doxycycline), and 5.77% did not improve despite treatment. In the doxycycline group, 33.79% of patients were stable after one treatment, 66.21% needed a further one or two treatments (some had previously switched to azithromycin), and 29.41% did not improve despite treatment (P < 0.05). Minimal gastrointestinal adverse effects (nausea, diarrhea, abdominal cramp, and decreased appetite) were reported, mostly unchanged at the follow-up visits. At the first visit, more adverse effects were reported in the doxycycline group (14/51, 24%) than in the azithromycin group (3/52, 6%; P < 0.005).
Conclusion:
Both antibiotics were effective and safe for treating patients with persistent MGD, although azithromycin was superior when the reduced dose and the shorter course of therapy (5 days vs. 4 weeks) were taken into consideration. Given the chronic nature of the disease and the improvement in some signs with minimal adverse effects, a shorter therapy seems a safer and more logical alternative to longer regimens.
Keywords: Azithromycin, blepharitis, doxycycline, Meibomian gland disease, tetracyclines
Ocular surface disease (OSD) is a very common and multifactorial condition. According to a recent report from the American Academy of Ophthalmology (AAO),[1] it is one of the most common reasons for visiting an ophthalmologist[2] and affects 15% of Americans older than 65 years.[3] OSD is caused by unstable or insufficient tear film, which results in irritation, pain, inflammation, blurred vision, photophobia, and loss of vision.[4]
Many treatment options have been proposed (topical compresses and cleansers, topical lubricants, immunomodulation, nutritional supplements, oral and/or topical antibiotics, laser and light-based treatments, and surgery), although the refractory nature of the disease makes it largely incurable, thus necessitating expensive and long treatments.[5,6,7]
Meibomian gland dysfunction (MGD) is one of the leading causes of OSD. Inflammatory mediators such as interleukin 1, matrix metalloproteinases, collagen production, nitric oxide, and activated B cells seem to play a key role in the development of this condition, which leads to hyperkeratinization of the ductal epithelium and, therefore, obstruction of the MGs. The subsequent accumulation of meibum is responsible for inflammation and subsequent increased bacterial colonization of the lid margins, as seen in posterior blepharitis.[1,8]
While conservative options such as eyelid warming, massage, and cleansing combined with artificial tears are considered first-line therapy,[9,10] severe and refractory cases require a more aggressive approach.
Tetracyclines (oral or topical) have been found to be effective,[11] owing to their ability to modulate the expression of inflammatory mediators in vivo and in vitro and thus reduce the severity of the signs and symptoms of MGD.[12,13,14] Topical administration of tetracyclines, frequently in combination with local corticosteroids and other drugs, is considered as the second-line therapy, as are oral tetracyclines,[15,16,17,18,19,20,21,22,23] with the caveat that adverse effects can lead to dermatologic and gastrointestinal complications, as well as hypersensitivity.[1]
Lack of consensus on oral dosage is also an important issue that needs to be addressed, as advocated by the AAO.[1] Since azithromycin was recently found to be very effective for treating recurrent blepharitis[8] and considering that a head-to-head randomized comparison study has not been performed, even though individual drugs have been shown to be effective in MGD, the objective of this study was to compare the efficacy and safety profile of a long course of orally administered azithromycin (9 months) with that of doxycycline in patients in whom conservative and topical treatment for MGD with posterior blepharitis failed. We also assessed disease recurrence and determined the number of treatments required.
Methods
We performed a randomized controlled trial with a cross-over design at a tertiary care center. In accordance with the guidelines of the International Workshop on MGD Diagnosis Subcommittee classification and in compliance with the recommendations made by the AAO for clinical trials on antibiotics in MGD,[1,23] we implemented and adapted a 4-point categorical scale (0–3) that had been applied elsewhere.[8] We also applied Schirmer I test, recorded visual acuity (VA), and assessed other parameters, namely, five main symptoms (burning, itching, foreign body sensation, dryness, and eyelid edema) and seven main signs [type of MG secretion, number of occluded gland orifices, conjunctival hyperemia, lid margin redness, ocular surface staining with fluorescein, tear break-up time (TBUT, as seen on slit lamp biomicroscopy), and Schirmer I test result].
Meibum was generated by applying digital pressure on the lower eyelid at its central third. The secretion was graded as clear, cloudy, turbid, or solid depending on the worst secretion. TBUT was recorded and graded as 0 (over 10 s), 1 (8–10 s), 2 (5–7 s), and 3 (less than 5 s). A single standardized fluorescein strip was used to make the measurement more repeatable.[24] Schirmer I test result was recorded and graded as 0 (>15 mm), 1 (10–15 mm), 2 (9–5 mm), and 3 (<5 mm).
The ocular surface staining score was adapted as a modification of panels in the Oxford scale and performed soon after assessment of TBUT.[7,8,25] The panel most similar to the pattern and the number of dots on the cornea and conjunctiva were chosen, and the corresponding grade was applied [Fig. 1].
Figure 1.
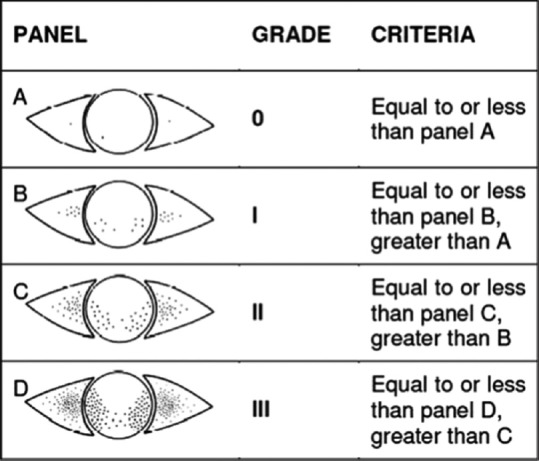
Corneal and conjunctival staining patterns according to Oxford scale (Courtesy of Dr. Kashkouli)
VA was recorded using standard ETDRS optotypes, measured in Logmar, and evaluated separately from the categorical scale. Sample size calculation was adapted to detect a minimum of 1.7 between the scores of the two groups between the first and last follow-up visits, as in previous studies.[8,11] The calculation was based on a type 1 error of 0.05 with a power of 80%. Therefore, 45 patients was the recommended number for each of the initial groups. Considering loss to follow-up and subsequent subdivisions into four groups, we decided to include a total of 115 patients (27% more than the initial calculated sample size).
The inclusion criteria were as follows: age 18–82 years, posterior blepharitis that did not respond to conservative or topical management, and two signs and two symptoms with a score >2 (one of which was MG involvement), according to the scales mentioned above.
The exclusion criteria were as follows: therapy with systemic or topical antibiotics within 1 month before selection, contact lens wearing, liver disease, pregnancy and breast feeding, allergy to azithromycin or cyclins, allergic keratoconjunctivitis, ocular and orbital surgery of any kind, altered lid anatomy, and nonadherence to follow-up.
Block randomization
At the beginning of the study, five patients formed a block, which was assigned to one treatment or another by writing numbers (1–20) on sealed papers, which were randomly selected soon afterward. One masked observer (ASV) secured the papers and another (GDB) was responsible for scoring and examination. Each participant was informed about the purposes of the study and then read and signed an informed consent document. The local ethics committee approved the study, which was performed at Quironsalud Hospital Donostia. Our research adhered to the tenets of the Declaration of Helsinki.
A single observer (GDB), who was masked to treatment type, selected the patients and performed the posttreatment evaluation. Each block of patients was randomly assigned to either a 5-day course of oral azithromycin (Teva Pharma S.L., Alcobendas, Madrid, Spain) (500 mg on the first day and then 250 mg/day for a further 4 days)[8] (A group, 52 patients) or a 1-month course of oral doxycycline (Laboratorios Normon SA, Madrid, Spain) (100 mg twice a day for 7 days and then 100 mg/day for a further 21 days) (D group, 51 patients), which was our standard dosage.
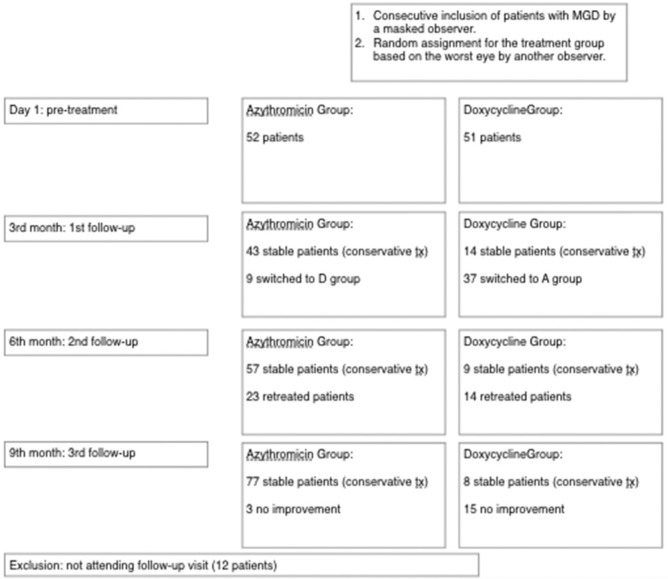
Conservative management was recommended to all patients throughout the study. Between January and September 2016, the sign and symptom scores were recorded prior to treatment and four times after treatment: first visit (1 month), second visit (3 months), third visit (6 months), and fourth visit (9 months) [see flow diagram in Fig. 2].
Figure 2.
Flow diagram of participants in the trial of 5-day oral azithromycin versus 1-month oral doxycycline for treatment of Meibomian gland dysfunction at different stages: pretreatment and first (third month), second (sixth month), and third (ninth month) posttreatment visits
The symptom score was obtained by adding the score (0–3) of five symptoms (range of 0–15). The sign score was also obtained by adding the score (0–3) of seven signs (range of 0–21). The sum of each separate score (total score, range 0–36) was calculated by summing the scores of signs (0–21) and symptoms (0–15) at each follow-up visit.
To avoid bias, this score took into account only the worst eye: since systemic treatment was to be administered, it did not seem useful to further divide patients by eye treated. Based on the reduction in total score (as a percentage), clinical response was divided into four groups: poor (1%–25%), fair (26%–44%), good (45%–75%), and excellent (76%–100%). Adverse effects were also registered at each follow-up visit.
At the beginning of treatment and every 3 months, VA was recorded separately. After the first month, we also assessed adverse effects and VA, although this follow-up was not taken into consideration when deciding on whether to change therapy.
After the first end-point (third month), patients with a fair or poor response were switched to the other medication. If the response was excellent or good at any end-point, the patient was considered stable and no medication was administered; therefore, treatment was conservative. If the patient's condition worsened at the following end-points, they once again received the last successful medication, until the next end-point, and so on. Switches were based on the individual patient's signs and symptoms, although the researcher was blind to the treatment.
Statistics
Chi-square test and Fisher's exact test were used to compare demographic characteristics and the main complaints; t-test was used to compare symptoms, signs, and total mean scores. Chi-square test was applied to analyze the total clinical response and adverse effects. Statistical significance was set at P < 0.05; 95% confidence interval was applied as a measure of precision.
To compare symptoms, signs, and total mean scores, a repeated-measures analysis of variance was used following the Statistical Analysis System (SAS) Institute procedure.[26,27,28] Four different groups were established, depending on the treatment administered, as follows:
Patients treated with azithromycin only (Only A)
Patients treated with doxycycline only (Only D)
Patients initially treated with azithromycin and then switched to doxycycline (A and D)
Patients initially treated with doxycycline and then switched to azithromycin (D and A).
Results
Of the 115 patients, 12 did not complete the study: 1 had to discontinue treatment because of adverse effects and 11 were lost to follow-up. Therefore, 103 consecutive patients (103 eyes) were assessed between January and September 2016. Demographic data and major complaints for both groups are shown in Table 1. There were no statistically significant differences between the groups.
Table 1.
Demographics and main complaints of patient groups, according to drug administration
| Demographics | AZT | DOXY | P |
|---|---|---|---|
| Male/female | 26/26 | 25/26 | 0.75 |
| Mean age (years) (SD) | 53 (15.9) | 51 (15.3) | 0.65 |
| Mean duration of disease (weeks) (SD) | 15 (8.2) | 13 (7.4) | 0.37 |
| Main complaints | |||
| Burning | 15% | 12% | 0.97 |
| Itching | 17% | 18% | 0.61 |
| Foreign body sensation | 15% | 14% | 0.79 |
| Dryness | 10% | 12% | 0.55 |
| Eyelid edema | 8% | 9% | 0.74 |
P did not show significant differences between the two groups at the beginning of the study. AZT: Azythromicin; DOXY: Doxycycline; SD: Standard deviation
In the Only A group, 83.25% of patients were stable after one treatment, 16.5% needed one or two additional treatments (some had previously been switched to doxycycline), and 5.77% did not improve despite treatment. In the Only D group, 33.79% of patients were stable after one treatment, 66.21% needed one or two additional treatments (some had previously been switched to azithromycin), and 29.41% did not improve despite treatment.
Fisher's exact test confirmed that the overall value of the signs and symptoms differed significantly between the four groups (P < 0.001), and the within-subject main effect test indicated that there was a significant effect over time, that is, the total value of signs and symptoms varied over time (decreased between the first visit and the last one) depending on the type of treatment (P < 0.001).
The effects’ test for the within-subjects × between-subjects interaction showed that differences for the time interaction with the treatment regimens was significant, indicating that the value of the signs and symptoms decreased over time differently, depending on the type of treatment applied (P < 0.001).
At the beginning of the treatment, only one significant difference was observed between the Only A group and the A and D group (P < 0.005), although at the end of treatment significant differences were observed for the Only A group compared with the Only D group, with a lower total score of <3 points (P < 0.005), as shown in Tables 2 and 3.
Table 2.
Statistical differences at the beginning of the treatment
| Final group - comparison | Difference between means | Simultaneous 95% confidence limits | ||
|---|---|---|---|---|
| Only A - D and A | 0.52 | −0.72 | 1.77 | |
| Only A - Only D | 1.30 | −0.70 | 3.29 | |
| Only A - A and D | 2.40 | 0.40 | 4.39 | *** |
| Only D - D and A | −0.77 | −2.78 | 1.23 | |
| Only D - A and D | 1.10 | −1.44 | 3.64 | |
| A and D - D and A | −1.87 | −3.88 | 0.13 | |
***Significant comparisons at the 0.05 level
Table 3.
Statistical differences at the end of treatment
| Final group - comparison | Difference between means | Simultaneous 95% confidence limits | ||
|---|---|---|---|---|
| Only A - D and A | −3.07 | −4.14 | −2.01 | *** |
| Only A - Only D | −1.25 | −2.95 | 0.45 | |
| Only A - A and D | −0.65 | -2.35 | 1.05 | |
| Only D - D and A | −1.82 | −3.53 | −0.12 | *** |
| Only D - A and D | 0.60 | −1.56 | 2.76 | |
| A and D - D and A | −2.42 | −4.13 | −0.72 | *** |
***Significant comparisons at the 0.05 level
Therefore, azithromycin is more effective than doxycycline in terms of amelioration of signs and symptoms. The main differences between the two treatments were as follows:
The proportion of patients who had to change treatment during the study period was lower in the Only A group than in the Only D group (17% vs. 67%; P < 0.005)
The values of signs and symptoms were significantly different between different treatments, with a better result for azithromycin than for doxycycline. This is also true for the patients shifted from D to A after the first change (P < 0.005).
VA was better in the Only A group and in the A and D group than in the Only D group and the Only A group, although the difference was not statistically significant when compared with the Only D group. The mean results for both signs and symptom scores are shown in Table 4. Fig. 3 shows the follow-up of the four groups according to the sum of both scores. Fig. 4 shows trends in VA. Table 5 focuses on sign scores plus VA, and Tables 6 and 7 show group stability and trends.
Table 4.
Mean symptom, sign, and total scores (SD) of 103 patients during the study
| AZT | AZT and DOXY | DOXY | DOXY and AZT | P | |
|---|---|---|---|---|---|
| Pretreatment signs | 11.6 (1.6) | N/A | 10.9 (0.9) | N/A | >0.005 |
| Symptoms | 12 (1.4) | N/A | 11,4 (1.7) | N/A | |
| Total | 23.6 (2.1) | N/A | 22.3 (2) | N/A | |
| First control signs | 7.5 (1.4) | N/A | 7.4 (1.2) | N/A | >0.005 |
| Symptoms | 5 (0) | N/A | 4.6 (1.5) | N/A | |
| Total | 12.5 (1.4) | N/A | 12 (1.8) | N/A | |
| Second control signs | 5.3 (1.2) | N/A | 6.8 (0.6) | N/A | >0.005 |
| Symptoms | 5.9 (0.3) | N/A | 4.5 (1.5) | N/A | |
| Total | 11.2 (1.3) | N/A | 11.3 (1.1) | N/A | |
| Third control signs | 5.5 (1.3) | 6.6 (1.3) | 6.3 (0.5) | 7.7 (1.5) | <0.005* |
| Symptoms | 4.8 (0.4) | 5 (0) | 4.5 (1.5) | 5.6 (1.5) | |
| Total | 10.3 (1.5) | 11.6 (1.3) | 10.8 (1.2) | 13.4 (2) | |
| Fourth follow-up signs | 5.6 (1.4) | 6.3 (1.5) | 6.8 (0.8) | 7.5 (1.3) | <0.005* |
| Symptoms | 4.4 (0.8) | 4.4 (0.5) | 4.5 (1.4) | 5.6 (1.4) | |
| Total | 10 (1.5) | 10.7 (1.6) | 11.3 (1.6) | 13.1 (1.9) |
SD: Standard deviation; AZT: Azythromicin; DOXY: Doxycycline; *Statistical significance
Figure 3.
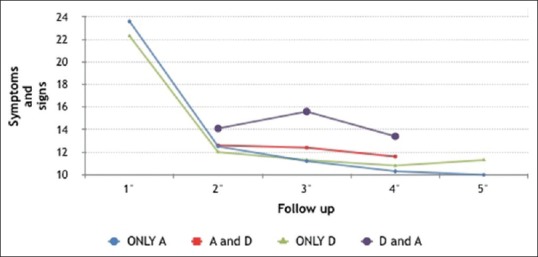
The follow-up of the four groups according to the sum of both scores
Figure 4.
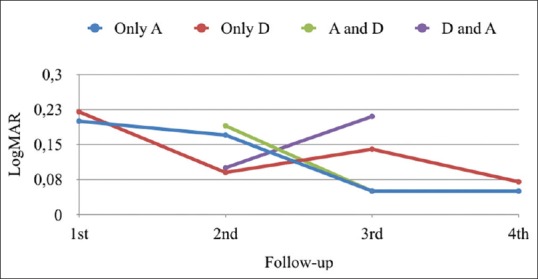
Trends in visual acuity (VA) in logMar units
Table 5.
Mean score (±SD) of seven signs before and during the follow-up visits in 103 patients with Meibomian gland dysfunction, treated with either oral doxycycline or azithromycin, plus VA evaluations
| MG secretion | MG plugging | Bulbar conjuntival redness | Em redness | Schirmer I | TBUT | Staining | VA | P | |
|---|---|---|---|---|---|---|---|---|---|
| Pretreatment signs AZT | 1.8 (0.7) | 2 (0.7) | 1.6 (0.6) | 2 (0.6) | 1.2 (0.4) | 1.3 (0.5) | 1.7 (0.7) | 0.6 (0.1) | >0.005 |
| Pretreatment signs DOXY | 1.5 (0.7) | 1.6 (0.5) | 2 (0.8) | 1.5 (0.7) | 1 (0) | 1.7 (0.7) | 1.6 (0.5) | 0.6 (0.1) | |
| First control signs AZT | 1 (0.4) | 1.3 (0.5) | 0.9 (0.4) | 1.2 (0.6) | 1.1 (0.4) | 1.1 (0.4) | 0.9 (0.4) | 0.7 (0.1) | >0.005 |
| First control signs DOXY | 1.1 (0.6) | 1.1 (0.3) | 1 (0) | 1.4 (0.5) | 1 (0) | 1.1 (0.3) | 0.7 (0.5) | 0.8 (0.1) | |
| Second control signs AZT | 0.6 (0.7)* | 0.9 (0.5) | 0.5 (0.5)* | 1 (0.4) | 1 (0.4) | 0.9 (0.4) | 0.4 (0.5)* | 0.9 (0.1)* | >0.005 |
| Second control signs DOXY | 0.9 (0.7)* | 1.1 (0.7) | 0.8 (0.4)* | 1 (0) | 0.9 (0.3) | 1 (0) | 1.1 (0.3)* | 0.75 (0.1)* | <0.005* |
| Third control signs AZT | 0.7 (0.6)* | 0.9 (0.5) | 0.6 (0.5)* | 0.7 (0.5) | 1 (0.3) | 1 (0.2) | 0.6 (0.5) | 0.9 (0.1)* | >0.005 |
| Third control signs DOXY | 1 (0)* | 0.8 (0.6) | 1 (0)* | 0.8 (0.4) | 1 (0) | 1 (0) | 0.7 (0.5) | 0.7 (0.2)* | <0.005* |
| Third control signs AZT and DOXY | 1.2 (0.4) | 0.9 (0.3) | 0.7 (0.5)* | 0.8 (0.4) | 1.1 (0.3) | 1.2 (0.4) | 0.7 (0.5*) | 0.9 (0.2)* | >0.005 |
| Third control signs DOXY and AZT | 0.9 (0.6) | 1.1 (0.6) | 1.3 (0.5)* | 0.8 (0.4) | 1.2 (0.4) | 1.2 (0.4) | 1.2 (0.6)* | 0.7 (0.1)* | <0.005* |
| Fourth control signs AZT | 0.8 (0.5)* | 0.7 (0.5) | 0.6 (0.5)* | 0.6 (0.5) | 1.2 (0.4) | 1 (0)* | 0.7 (0.5) | 0.9 (0.1) | >0.005 |
| Fourth control signs AZT and DOXY | 1 (0.5)* | 0.7 (0.5) | 0.8 (0.4)* | 0.5 (0.5) | 1.3 (0.5) | 1.3 (0.5)* | 0.7 (0.5) | 0.9 (0.1) | <0.005* |
| Fourth control signs DOXY | 0.9 (0.3)* | 0.9 (0.3) | 1 (0) | 0.9 (0.3) | 1 (0)* | 1 (0) | 1.1 (0.3) | 0.85 (0.1) | >0.005 |
| Fourth control signs DOXY and AZT | 1.2 (0.4)* | 0.9 (0.4) | 1.1 (0.4) | 0.9 (0.4) | 1.2 (0.4)* | 1.1 (0.4) | 1.1 (0.3) | 0.8 (0.1) | <0.005* |
Statistical significance in signs during follow-ups are colored in grey. VA: Visual acuity; Mg: Meibomian gland; TBUT: Tear break-up time; SD: Standard deviation; AZT: Azythromicin; DOXY: Doxycycline; *Statistical significance
Table 6.
Group survivals
| AZT | DOXY | |
|---|---|---|
| 1st follow-up (no treat./tot) | 43/52 | 14/51 |
| Switched patients | 9 | 37 |
| 2nd follow-up (no treat./tot) | 57/80 | 9/23 |
| Retreated patients | 23 | 14 |
| 3rd follow-up (no treat./tot) | 77/80 | 8/23 |
| No improvement | 3 | 15 |
The table shows group survival rate for each group since the beginning of the study, considering the actual number of patients at any follow-up, and how many of any group were stable or were switched to any other one AZT: Azythromicin; DOXY: Doxycycline
Table 7.
Group performances at follow-ups according to treatment shifts
| Group trends | Excellent | Good | Fair | Poor | P |
|---|---|---|---|---|---|
| Second control signs AZT | 0.0% | 77.1% | 22.9% | 0.0% | P<0.005* |
| Second control signs DOXY | 0.0% | 26.8% | 47.7% | 25.5% | |
| Third control signs AZT | 3.7% | 61.3% | 35.1% | 0.0% | P>0.005 |
| Third control signs DOXY | 0.0% | 60.5% | 39.5% | 0.0% | |
| Third control signs AZT and DOXY | 0.0% | 36.3% | 52.8% | 10.9% | P<0.005* |
| Third control signs DOXY and AZT | 0.0% | 9.9% | 51.3% | 38.8% | |
| Fourth control signs AZT | 8.7% | 56.2% | 28.9% | 6.1% | P<0.005* |
| Fourth control signs DOXY | 0.0% | 15.4% | 72.4% | 12.2% | |
| Fourth control signs AZT and DOXY | 0.0% | 69.6% | 30.4% | 0.0% | P<0.005* |
| Fourth control signs DOXY and AZT | 0.0% | 8.0% | 61.1% | 30.9% |
AZT: Azythromicin; DOXY: Doxycycline; *Statistical significance
Safety profile
Both groups reported gastrointestinal adverse effects (described as nausea, diarrhea, abdominal cramp, and decreased appetite), which remained largely unchanged at the follow-up visits. At the first visit (immediately after ending the doxycycline course), more adverse effects were reported in the Only D group (14/51, 24%) than in the Only A group (3/52, 6%) (P < 0.005) [Table 8].[8] These effects were transitory, although one led treatment to be stopped; therefore, the patient was excluded from the subsequent follow-up visits. Of note, a further 11 patients were lost to follow-up; therefore, we were unable to establish any cause–effect relationship.
Table 8.
Treatment side effects in percentage (number of patients between brackets) at each follow-up; its significance, if any, is evidenced with an asterisk (*)
| Nausea | Cramps | Diarrhoea | Decreased appetite | |
|---|---|---|---|---|
| First control signs AZT | 2% (1) | 2% (1) | 0% (0) | 2% (1) |
| First control signs DOXY | 16% (8) | 14% (7) | 2% (1) | 16% (8) |
| P (Fisher’s test) | 0.02* | 0.04* | 0.50 | 0.02* |
| Second control signs AZT | 0% (0) | 0% (0) | 0% (0) | 2% (1) |
| Second control signs DOXY | 2% (1) | 2% (1) | 2% (1) | 4% (2) |
| P (Fisher’s test) | 0.50 | 0.50 | 0.50 | 0.88 |
| Third control signs AZT | 0% (0) | 2% (1) | 0% (0) | 2% (1) |
| Third control signs DOXY | 14% (7) | 10% (5) | 4% (2) | 6% (3) |
| P (Fisher’s test) | 0.01* | 0.15 | 0.25 | 0.50 |
| Fourth control signs AZT | 2% (1) | 2% (1) | 2% (1) | 0% (0) |
| Fourth control signs DOXY | 4% (2) | 2% (1) | 6% (3) | 2% (1) |
| P (Fisher’s test) | 0.88 | 1.00 | 0.50 | 0.50 |
AZT: Azythromicin; DOXY: Doxycycline; *Statistical significance
Patients from the switched group (previously treated with azithromycin) complained of symptoms they had experienced before the third follow-up visit, although these disappeared after the first month, in a similar fashion to the previous follow-up visit (22% vs. 4%; P < 0.005).
Discussion
Tetracyclines have long been used for the treatment of blepharitis; however, their efficacy has been questioned because of the lack of randomized studies evaluating their performance at fixed doses over specific time periods.[1]
Other than the clinical trial comparing oral azithromycin and doxycycline by Kashkouli et al.,[8] to the best of our knowledge, our study is the first to show the effect and behavior of these drugs over 9 months. During this relatively long period, both medications proved to be effective and safe and succeeded in prolonging survival, although to different extents.
In general, azithromycin performed better throughout the study, showing a significant improvement in most patients (65%). This improvement appeared more quickly and was maintained throughout the period. In particular, azithromycin improved VA, conjunctival redness, and corneal staining. Patients treated with doxycycline had similar results, although only in a relatively small percentage of patients (10%), most of whom switched to the Only A group.
Difficult refraction and/or sample characteristics may have biased the results for VA, although the statistical analysis of patient demographics did not reveal significant differences in either group. Therefore, in our opinion, these differences may be related to the improvement in lacrimation and quality of the cornea following treatment.
While conservative options such as eyelid warming, massage, and cleansing combined with artificial tears are generally effective (first-line therapy),[9,10] severe and refractory cases need to be approached differently. Oral and/or topical tetracyclines have been found to be effective owing to their ability to modulate the expression of many of the previously described inflammatory mediators in vivo and in vitro,[11,12,13,14] thus significantly decreasing the frequency and intensity of the signs and symptoms of MGD. Symptoms can be very troublesome, are usually recurrent, and have a severe impact on the quality of life. They also generate a financial burden, since, in many health systems, the medications needed are not covered by the national health system or insurance schemes and can be expensive,[5] especially considering the chronic course of the disease.
Topical use, frequently in combination with topical corticosteroids (second-line therapy), has been proposed, as has oral administration.[15,16,17,18,19,20,21,22,23] Lack of consensus on oral dosage is also an important issue, which we tried to address. Our choice of treatment was based on the rationale for treating patients in whom conservative and topical therapy had failed and who were affected by grade 2 or 3 posterior blepharitis.
Consistent with Kashkouli et al.,[8] azithromycin administered in a short regimen (5 days) proved to be very effective, with minimal adverse effects, and patients remained stable throughout the course of the study. Other articles have used different dosages of azithromycin: Igami et al.[21] administered three cycles of 500 mg/day for 3 days with a 1-week interval, while Bakar et al.[22] opted for the same dosage administered weekly for 4 weeks. In this study, our dosage of azithromycin was chosen according to the doses reported in the literature and to address another important issue such as number and time interval between each dose.
Doxycycline was administered in a longer regimen (1 month) and according to our previous protocol, which differs from those reported elsewhere in that it was less aggressive. The drug was less well tolerated and resulted in a reduced duration of stability in most patients, even if it was as effective as azithromycin in 10% of initially treated patients, thus suggesting that there is a subgroup of the population in which both antibiotics are equally effective.
Further studies are needed to assess which specific patient characteristics could help the clinician choose between these two antibiotics. Doxycycline has been administered at several doses. For example, Iovieno et al.[16] recommended 200 mg/day for 2 weeks, and then 100 mg/day for a further 2 weeks, whereas Quarterman et al.[17] recommended 100 mg a day for 12 weeks and Sobolewska et al.[18] prescribed 40 mg/day for 8 months. All these regimens were successful with few adverse effects.
In this study, we adhered to our protocol, which had been our previous gold standard before we tried azithromycin, to obtain a more accurate and realistic comparison with our previous method of treating resistant blepharitis.
We decided to switch patients from one treatment to another when treatment was seen to have failed at the scheduled check-ups to test each antibiotic against persistent blepharitis and to have a broad perspective of how each worked in problematic and recurrent cases. While this approach made the evaluation of our results more challenging, we believed it would be very useful in clinical practice, when the physician has to decide what is best for the patient. Following this practical approach, we did not use indirect and more precise TBUT methods as recommended by Nelson et al.,[23] since these were not available in our practice, as is frequently the case in most parts of the world. We used standardized fluorescein strips, which deliver smaller amounts of dye, to make the measurement more repeatable,[24] although we believe this is a limitation of our study. Creating a third group of patients already scheduled to switch medication at some point during follow-up could have been an option, although we believed that such a study design would have biased both the masked treatment and the realistic performance of the study itself, since it was not possible to forecast which patients would have responded positively or negatively to each medication.
Thus, the study was based on real-life situations and empirical experience and, in the present case, scientific hypotheses from the literature. Our study is limited by the absence of a control group. However, since we chose to include only patients whose conservative and topical treatments failed, it would not have been ethical to use a placebo, as previously addressed.[29,30] In any case, further studies are needed to assess whether topical azithromycin used in fixed combinations and/or administered according to different regimens can be as effective as oral azithromycin. Our study is also limited by the fixed dosages of both oral antibiotics administered. However, we tried to learn from previous experience to replicate experimental models based on randomization and masking that can provide practical clinical data for ophthalmologists. Such an approach could provide quantifiable objective measurements broken down into a separate analysis of signs and symptoms, rather than calculating scores, such as a simple mean with no internal differentiation.
Shorter regimens and regimens with longer off-treatment intervals can also help improve adherence and decrease drug administration, thus reducing costs for patients and for health service.[5,31,32]
Conclusion
Oral doxycycline and azithromycin were both effective and safe for the treatment of patients with persistent MGD over a 9-month period. Both drugs had an effect on symptoms, although this was not significant; however, they did have a better and significant effect on signs, especially VA, conjunctival redness, and corneal staining. In addition, azithromycin was more effective more quickly, with fewer side effects. This effectiveness was maintained throughout the study period.
The results were similar for doxycycline, although only in a small percentage of patients. Considering the reduced time of administration of azithromycin (5 days vs. 4 weeks) and the chronic nature of the disease, as well as the positive effect on some signs over a shorter period with minimal side effects and cost-effectiveness, repeating a 5-day treatment for recurrence of blepharitis seems a safer and more logical alternative to longer regimens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wladis EJ, Bradley EA, Bilyk JR, Yen MT, Mawn LA. Oral antibiotics for Meibomian gland-related ocular surface disease. A report by the American Academy of Ophthalmology. Ophthalmology. 2016;123:492–6. doi: 10.1016/j.ophtha.2015.10.062. [DOI] [PubMed] [Google Scholar]
- 2.Lemp MA. Epidemiology and classification of dry eye. Adv Exp Med Biol. 1998;438:791–803. doi: 10.1007/978-1-4615-5359-5_111. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118:1264–8. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–5. doi: 10.1097/IJG.0b013e31815c5f4f. [DOI] [PubMed] [Google Scholar]
- 5.Azithromycin and doxycycline Pricesp. [Last accessed on 2016 Oct 10]. Available from: www.costco.com 2016 .
- 6.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–65. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 7.Ramamurthi S, Rahman MQ, Dutton GN, Ramaesh K. Pathogenesis, clinical features and management of recurrent corneal erosions. Eye (Lond) 2006;20:635–44. doi: 10.1038/sj.eye.6702005. [DOI] [PubMed] [Google Scholar]
- 8.Kashkouli MB, Fazel AJ, Kiavash V, Nojomi M, Ghiasian L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: A randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99:199–204. doi: 10.1136/bjophthalmol-2014-305410. [DOI] [PubMed] [Google Scholar]
- 9.Romero JM, Biser SA, Perry HD, Levinson DH, Doshi SJ, Terraciano A, et al. Conservative treatment of meibomian gland dysfunction. Eye Contact Lens. 2004;30:14–19. doi: 10.1097/01.ICL.0000095229.01957.89. [DOI] [PubMed] [Google Scholar]
- 10.Guillon M, Maissa C, Wong S. Eyelid margin modification associated with eyelid hygiene in anterior blepharitis and meibomian gland dysfunction. Eye Contact Lens. 2012;38:319–25. doi: 10.1097/ICL.0b013e318268305a. [DOI] [PubMed] [Google Scholar]
- 11.Foulks GN, Borchman D, Yappert M, Kakar S. Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: A comparative clinical and spectroscopic pilot study. Cornea. 2013;32:44–53. doi: 10.1097/ICO.0b013e318254205f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sobrin L, Liu Z, Monroy DC, Solomon A, Selzer MG, Lokeshwar BL, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41:1703–9. [PubMed] [Google Scholar]
- 13.Fernandez-Robredo P, Recalde S, Moreno-Orduna M, García-García L, Zarranz-Ventura J, García-Layana A. Azithromycin reduces inflammation in a rat model of acute conjunctivitis. Mol Vis. 2013;19:153–65. [PMC free article] [PubMed] [Google Scholar]
- 14.Siller SS, Broadie K. Matrix metalloproteinases and minocycline: Therapeutic avenues for fragile X syndrome. Neural Plast. 2012;2012:124548. doi: 10.1155/2012/124548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo SE, Lee DC, Chang MH. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol. 2005;19:258–63. doi: 10.3341/kjo.2005.19.4.258. [DOI] [PubMed] [Google Scholar]
- 16.Iovieno A, Lambiase A, Micera A, Stampachiacchiere B, Sgrulletta R, Bonini S. In vivo characterization of doxycycline effects on tear metalloproteinases in patients with chronic blepharitis. Eur J Ophthalmol. 2009;19:708–16. doi: 10.1177/112067210901900504. [DOI] [PubMed] [Google Scholar]
- 17.Quarterman MJ, Johnson DW, Abele DC, Lesher JL, Jr, Hull DS, Davis LS. Ocular rosacea.Signs, symptoms, and tear studies before and after treatment with doxycycline. Arch Dermatol. 1997;133:49–54. doi: 10.1001/archderm.133.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Sobolewska B, Doycheva D, Deuter C, Pfeffer I, Schaller M, Zierhut M. Treatment of ocular rosacea with once-daily low-dose doxycycline. Cornea. 2014;33:257–60. doi: 10.1097/ICO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Min K, Kim EK, Kim TI. Minocycline controls clinical outcomes and inflammatory cytokines in moderate and severe meibomian gland dysfunction. Am J Ophthalmol. 2012;154:949–57. doi: 10.1016/j.ajo.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Aronowicz JD, Shine WE, Oral D, Vargas JM, McCulley JP. Short term oral minocycline treatment of meibomianitis. Br J Ophthalmol. 2006;90:856–60. doi: 10.1136/bjo.2006.091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igami TZ, Holzchuh R, Osaki TH, Santo RM, Kara-Jose N, Hida RY. Oral azithromycin for treatment of posterior blepharitis. Cornea. 2011;30:145–9. doi: 10.1097/ICO.0b013e318207fc42. [DOI] [PubMed] [Google Scholar]
- 22.Bakar O, Demircay Z, Toker E, Cakir S. Ocular signs, symptoms and tear function tests of papulopustular rosacea patients receiving azithromycin. J Eur Acad Dermatol Venereol. 2009;23:544–9. doi: 10.1111/j.1468-3083.2009.03132.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson JD, Craig JP, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II introduction. Ocul Surf. 2017;15:269–75. doi: 10.1016/j.jtos.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Korb DR, Greiner JV, Herman J. Comparison of fluorescein break-up time measurement reproducibility using standard fluorescein strips versus the dry eye test (DET) method. Cornea. 2001;20:811–5. doi: 10.1097/00003226-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–50. doi: 10.1097/00003226-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 26.SAS/STAT User's Guide, Version 6. 4th ed. Cary, NC: SAS Institute Inc; 1989. [Google Scholar]
- 27.DiIorio FC. Belmont, CA: Duxbury Press; 1991. SAS Applications and Programming: A Gentle Introduction. [Google Scholar]
- 28.Stevens JP. 3rd ed. Mahway, NJ: Lawrence Erlbaum Ass. Inc; 1996. Applied Multivariate Statistics for the Social Sciences. [Google Scholar]
- 29.Ellenberg SS, Temple R. Placebo-controlled trials and active-controlled trials in the evaluation of new treatments. Ann Intern Med. 2000;133:464–70. doi: 10.7326/0003-4819-133-6-200009190-00015. [DOI] [PubMed] [Google Scholar]
- 30.Rid A, Saxena A, Baqui AH, Bhan A, Bines J, Bouesseau MC, et al. Placebo use in vaccine trials: Recommendations of a WHO expert panel. Vaccine. 2014;32:4708–12. doi: 10.1016/j.vaccine.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthes J, Albus C. Improving adherence with medication: A selective literature review based on the example of hypertension treatment. Dtsch Arztebl Int. 2014;111(4):41–7. doi: 10.3238/arztebl.2014.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging. 2011;28:267–82. doi: 10.2165/11588830-000000000-00000. [DOI] [PubMed] [Google Scholar]