Abstract
Background
The functionality of LINK-A lncRNA as an oncogenic lncRNA has only been characterized in triple-negative breast cancer. The present study investigated the possible involvement of LINK-A in ovarian carcinoma.
Material/Methods
We enrolled 76 patients with ovarian carcinoma. Quantitative real-time PCR (qRT-PCR) was performed to analyze LINK-A expression in ovarian biopsies. Effects of LINK-A overexpression on HIF1α and cancer cell migration and invasion were assessed.
Results
We found that LINK-A was significantly higher in ovarian carcinoma patients than in healthy controls in ovarian biopsies and in serum. LINK-A expression effectively distinguished patients with metastatic ovarian carcinoma from healthy controls. LINK-A overexpression promoted cancer cell migration and invasion, and also upregulated the expression of HIF1α.
Conclusions
LINK-A lncRNA may be involved in the metastasis of ovarian carcinoma by upregulating HIF1α.
MeSH Keywords: Diagnostic and Statistical Manual of Mental Disorders; Ovarian Neoplasms; RNA, Long Noncoding; Up-Regulation
Background
Ovarian carcinoma, one of the most common types of female malignancies, causes more than 140 000 deaths in women worldwide every year [1]. Patients with ovarian carcinoma usually suffer from distant tumor metastasis, which is a main cause of deaths among those patients [2]. Specific molecular biomarkers to predict the metastasis of ovarian carcinoma remain lacking. In addition, currently available treatment strategies including olaparib maintenance therapy, chemotherapy, and radiation therapy were also proved to be ineffective in the treatment of metastatic ovarian carcinoma [3]. Therefore, the identification of specific biomarkers for metastasis in ovarian carcinoma may improve the treatment of this disease.
More and more studies have shown that changes in the microenvironment, including oxygen availability, which is critical in cancer metabolism regulation and hypoxia, is a key player in the transactivation of oncogenes [4]. Hypoxia-inducible factor 1 (HIF1α) is a central player in hypoxia and also has pivotal functions in the metastasis of different types of human malignancies, including ovarian carcinoma [5,6]. It has been reported that HIF1α under certain conditions participates in disease development through interactions with long non-coding RNAs (lncRNA) [7,8], which is a subgroup of non-coding RNAs composed of more than 200 nucleotides and plays critical roles in cancer biology [9]. LINK-A, a newly identified oncogenic lncRNA, has characterized functionality only in triple-negative breast cancer [10,11]. The involvement of LINK-A lncRNA in other types of malignancies, as well as the interaction between LINK-A lncRNA and HIF1α, has not been reported. Our study observed that LINK-A lncRNA may be involved in the metastasis of ovarian carcinoma by upregulating HIF1α.
Material and Methods
Patients
Our study included 76 patients with ovarian carcinoma. Those patients were diagnosed and treated at Wuhan Central Hospital, Tongji Medical College, Huazhong University of Science and Technology from March 2016 to March 2018. Inclusion criteria were: 1) patients diagnosed through pathological examinations as having ovarian carcinoma; 2) diagnosis and treatment were performed for the first time; 3) complete clinical data; and 4) patients willing to participate. Exclusion criteria were: 1) patients suffering from other malignancies or ovarian diseases; and 2) patients diagnosed and treated before admission. At the same time, 56 healthy females were also included as a control group. There were no significant differences in age between the 2 groups. Table 1 shows basic information on patients and controls. This study was approved by the Ethics Committee of Wuhan Central Hospital.
Table 1.
Basic information of patients and controls.
| Patients | Controls | |
|---|---|---|
| Cases | 76 | 56 |
| Median age (years) | 49.1±6.1 | 48.2±5.97 |
| Non-distant metastasis | 34 | NA |
| Distant metastasis | 42 | NA |
| Bone metastasis | 14 | NA |
| Celiac metastasis | 20 | NA |
| Lung metastasis | 8 | NA |
NA – not applicable.
Specimen collection
Ovarian biopsies (100–200 mg) and whole blood (5 ml) were obtained from each participant. Healthy controls in this study received ovarian biopsies to detect suspended ovarian lesions, while ovarian lesions were eventually excluded. Blood was kept at room temperature for 2 h, followed by centrifugation at 1200 g for 15 min to collect serum. Ovarian biopsies and serum samples were stored in liquid nitrogen before use.
Real-time quantitative PCR (qRT-PCR)
Trizol reagent (Invitrogen, USA) was used to extract total RNA in strict accordance with the manufacturers’ instructions. A NanoDrop™ 2000 Spectrophotometer (Thermo Fisher Scientific, USA) was used to assess the concentration of each RNA sample, and 100 ng total RNA was subjected to reverse transcription to synthesize cDNA. PCR reactions were prepared using SYBR Green PCR Master Mix (Thermo Fisher Scientific). Primers used were: 5′-TTCCCCCATTTTTCCTTTTC-3′ (upstream) and 5′-CTCTGGTTGGGTGACTGGTT-3′ (downstream) for human LINK-A; 5′-GACCTCTATGCCAACACAGT3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA3′ (reverse) for β-actin. An ABI PRISM 7500 qRT-PCR machine (Applied Biosystems, Rockford, IL) was used to carry out all PCR reactions following those conditions: 1 min at 95°C, followed by 40 cycles of 18 s at 95°C and 32 s at 55°C. Data processing was performed using 2−ΔΔCT method. LINK-A expression was normalized to β-actin endogenous control. All PCR products were subjected to agarose gel electrophoresis and some products were sequenced to make sure the correct PCR products were obtained.
Cell line, cell culture and transfection
Human ovarian carcinoma UWB1.289 (ATCC® CRL-2945™) was purchased from ATCC. Cells were cultured in 50% MEGM medium and 50% ATCC-formulated RPMI-1640 Medium containing 3% fetal bovine serum at 37°C with 5% CO2. Full-length LINK-A cDNA was amplified through PCR using primers containing EcoRI cutting site at 5′ end. LINK-A cDNA was inserted into EcoRI linearized pIRSE2-EGFP vector (Clontech, Palo Alto, CA) and this vector was transfected into 5×106 cells at a concentration of 10 nM using Lipofectamine 2000 reagent (cat. no. 11668-019; Invitrogen, Thermo Fisher Scientific, Inc.). Transfection with empty pIRSE2-EGFP vector was performed to serve as a negative control. Overexpression of LINK-A was confirmed by qRT-CPR. Subsequent experiments were performed if the LINK-A overexpression rate was above 200% compared with control cells.
In vitro cell migration and invasion assay
After transfection, Transwell cell migration and invasion assays were performed to evaluate cell migration and invasion ability. Briefly, cell suspension was prepared using serum-free medium with a cell density of 3×104 cells/ml, and 0.1 ml cell suspension was added into the upper chamber. The lower chamber was filled with RPMI-1640 medium (Thermo Fisher Scientific, USA) containing 20% FBS (Sigma-Aldrich, USA). Cell migration was allowed for 24 h, and membranes were stained with 0.5% crystal violet (Sigma-Aldrich, USA) for 30 min at room temperature. Before the invasion assay, the upper chamber was pre-coated with Matrigel (356234, Millipore, USA) and all other steps were essentially the same as for the migration assay.
Western-blot
RIPA solution (Thermo Fisher Scientific) was used to extract total protein from cells cultured in vitro. BSA assay was performed to measure protein concentration. SDS-PAGE gel (10%) electrophoresis was then performed with 35 μg protein per lane, followed by gel transfer to PVDF membranes (Bio-Rad, USA). Blocking was then performed by incubating membranes with skimmed milk (5%) for 2 h at room temperature. Membranes were then incubated with primary antibodies, including rabbit anti-human HIF1α (ab2185, 1: 1200; Abcam) and GAPDH (ab9485, 1: 1400, Abcam) primary antibodies overnight at 4°C. On the next day, IgG-HRP secondary antibody (1: 1000, MBS435036, MyBioSource) was incubated with the membranes for 3 h at room temperature. Finally, signal development was performed using ECL (Sigma-Aldrich, USA), and HIF1α expression was normalized to GAPDH using Image J software.
Statistical analysis
GraphPad Prism 6 software was used for all statistical analyses. Cell migration and invasion data and gene expression data were compared among multiple groups using one-way analysis of variance and between 2 groups using the unpaired t test. p<0.05 indicates a difference with statistical significance.
Results
LINK-A expression in ovarian biopsies was higher in metastatic ovarian carcinoma patients than in non-metastatic ovarian carcinoma patients and healthy controls
Differential expression in lesions and normal tissues is a marker of involvement of a certain gene in diseases. In this study, we found expression of LINK-A in ovarian tissues of 42 patients with metastatic ovarian carcinoma, 34 patients with non-metastatic ovarian carcinoma, and 56 healthy controls. As shown in Figure 1, compared with the control group, LINK-A was significantly higher in patients with the 3 types of metastatic ovarian carcinoma (p<0.05), while no significant difference in LINK-A expression was found between non-metastatic ovarian carcinoma and healthy controls (p>0.05). These data suggest that upregulation of LINK-A in ovarian tissues participates in the distant metastasis of ovarian carcinoma.
Figure 1.
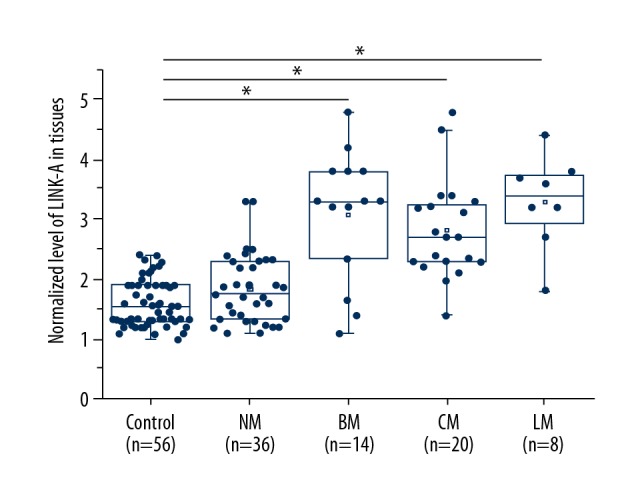
LINK-A expression in ovarian biopsies is higher in metastatic ovarian carcinoma patients than in non-metastatic ovarian carcinoma patients and healthy controls. * p<0.05; NM – non-metastatic ovarian carcinoma; BM – bone metastasis; CM – celiac metastasis; LM – lung metastasis.
Serum levels of LINK-A were higher in metastatic ovarian carcinoma patients than in non-metastatic ovarian carcinoma patients and healthy controls
Changes in circulating substances may reflect the pathological changes in the human body. In this study, LINK-A was detected in serum of all participants. Comparison analysis in Figure 2 showed that, compared with the control group, serum levels of LINK-A were significantly increased in metastatic ovarian carcinoma patients (p<0.05), but not in non-metastatic ovarian carcinoma patients (p>0.05).
Figure 2.
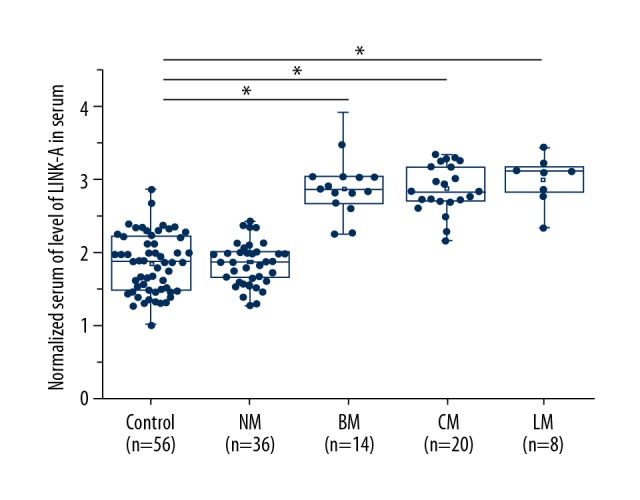
Serum levels of LINK-A were higher in metastatic ovarian carcinoma patients than in non-metastatic ovarian carcinoma patients and healthy controls. * p<0.05; NM – non-metastatic ovarian carcinoma; BM – bone metastasis; CM – celiac metastasis; LM – lung metastasis.
Diagnostic values of LINK-A for metastatic ovarian carcinoma patients and non-metastatic ovarian carcinoma
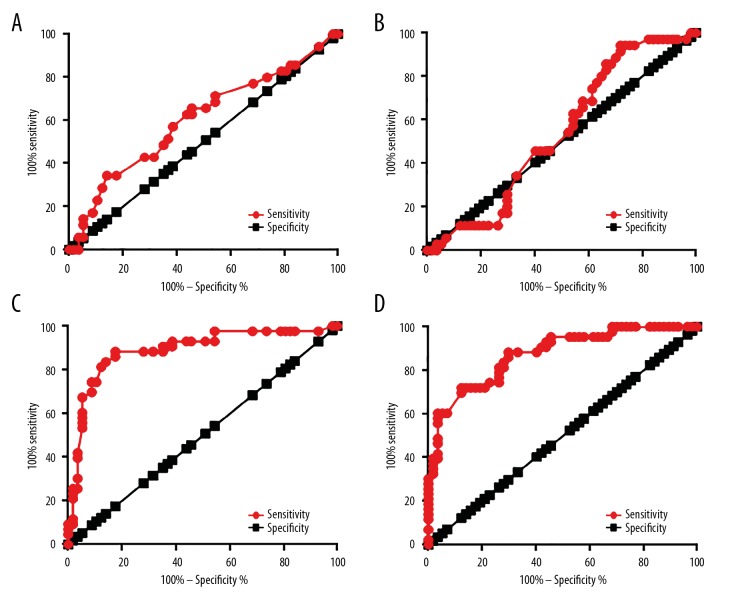
ROC curve analysis was performed to evaluate the diagnostic value of LINK-A expression in ovarian tissues and serum for metastatic ovarian carcinoma patients and non-metastatic ovarian carcinoma. For non-metastatic ovarian carcinoma, the area under the curve (AUC) of LINK-A expression in ovarian tissues was 0.6033, with a standard error of 0.06212 and 95% confidence interval of 0.4815 to 0.7250 (p>0.05, Figure 3A). The AUC of LINK-A expression in serum was 0.5283, with standard error of 0.06670 and 95% confidence interval of 0.3976 to 0.6591 (p>0.05, Figure 3B). For metastatic ovarian carcinoma, the AUC of LINK-A expression in serum was 0.8898 with standard error of 0.03558 and 95% confidence interval of 0.8201 to 0.9596 (p<0.0001, Figure 3C), and the AUC of LINK-A expression in serum was 0.8696, with standard error of 0.04142 and 95% confidence interval of 0.7864 to 0.9488 (p<0.0001, Figure 3D).
Figure 3.
Diagnostic values of LINK-A for metastatic ovarian carcinoma patients and non-metastatic ovarian carcinoma. The ROC curve of the use of LINK-A expression in ovarian tissues (A) and serum (B) for non-metastatic ovarian carcinoma and the use of LINK-A expression in ovarian tissues (C) and serum (D) for metastatic ovarian carcinoma.
Effects of LINK-A overexpression on HIF1α and cancer cell migration and invasion
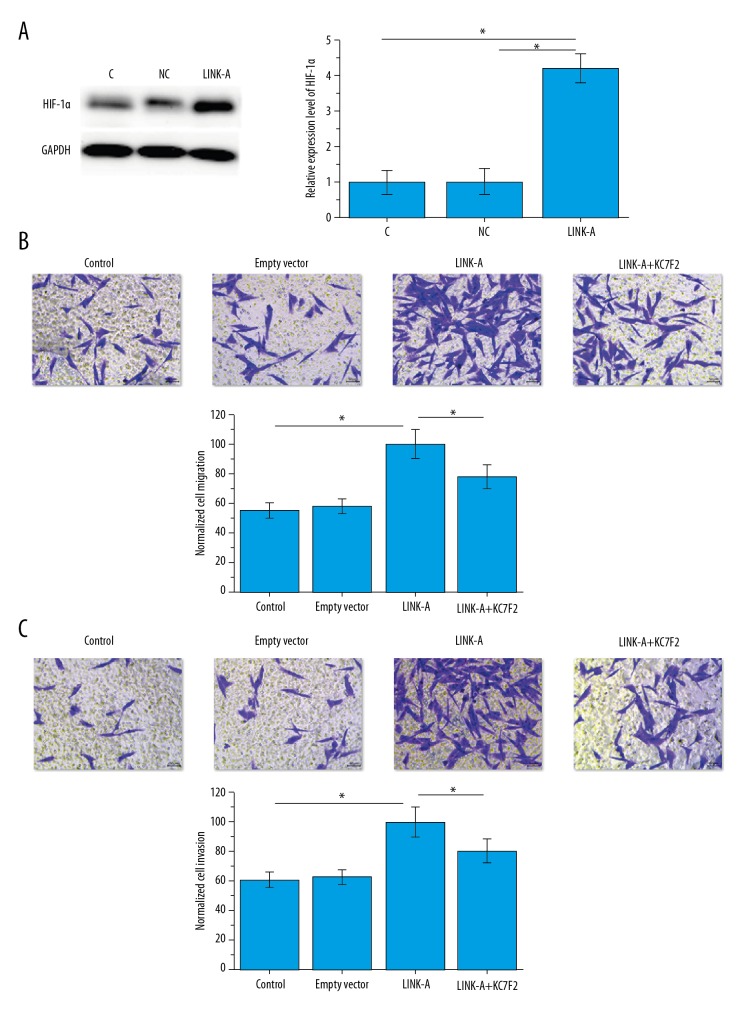
The above data suggest that LINK-A is involved in the metastasis of ovarian carcinoma. HIF1α plays pivotal roles in the metastasis of different types of malignancies, including ovarian carcinoma [6]. Therefore, LINK-A expression vector was transfected into human ovarian carcinoma cells and the effects on HIF1α expression were observed. Our results showed that LINK-A overexpression significantly upregulated the level of HIF1α in ovarian carcinoma cells (p<0.05, Figure 4A). In addition, LINK-A overexpression also significantly promoted migration (p<0.05, Figure 4B) and invasion (p<0.05, Figure 4C) of cancer cells. In addition, treatment with KC7F2 (HIF1α transcription inhibitor, 10 nM, Catalog No. S7946, Selleck Chemicals) significantly reduced the expression of LINK-A (data not shown) and reduced the effects of LINK-A overexpression on cell migration (p<0.05, Figure 4B) and invasion (p<0.05, Figure 4C). Therefore, LINK-A appears to promote ovarian cancer metastasis by enhancing HIF1α expression or stabilizing HIF1α protein.
Figure 4.
Effects of LINK-A overexpression on HIF1α and cancer cell migration and invasion. HIF1α expression (A), and cancer cell migration (B) and invasion (C) after LINK-A overexpression. * p<0.05.
Discussion
Our study shows that LINK-A, a newly identified oncogenic lncRNA in breast cancer [10,11], may also be an oncogene in ovarian carcinoma. LINK-A is likely involved in the metastasis of ovarian carcinoma and this function is probably achieved through the upregulation of HIF1α. Genetic factors play pivotal roles in the development of ovarian carcinoma [12,13], and several of those factors, such as BET bromodomain protein BRD4, have been proved to be promising therapeutic targets for these diseases [12]. A recent study has shown that the progression of ovarian cancer is also accompanied by changes in expression pattern of a large set of lncRNAs, and the differential expression of those lncRNAs determines nonequivalent outcomes [14]. In another study, Liu et al. showed that differentially expressed lncRNAs may have different metastatic potentials [15]. However, to the best of our knowledge, there has been no report on metastasis-specific lncRNA in ovarian carcinoma. In our study, no significant differences in expression level of LINK-A were found between patients with non-metastatic ovarian carcinoma and healthy controls, while significantly higher expression of LINK-A was observed in patients with metastatic ovarian carcinoma. Therefore, LINK-A may only participate in the metastasis of this disease.
Metastasis is one of the leading causes of death in patients with cancer [16], and accurate prediction and observation of the existing of metastasis will improve the survival of patients. Pathological changes in the human body induce changes in substances in blood, and detecting these changes may provide references for diagnosis of disease [17]. Several circulating lncRNAs have been proved to have potential utility in the diagnosis of ovarian carcinoma [18], and almost all of these lncRNAs showed altered expression in the whole process of cancer development and lack the potential for the specific diagnosis of cancer metastasis. In our study, serum LINK-A was detected in all participants, but upregulation was only observed in patients with metastatic ovarian carcinoma. ROC curve analysis also showed that LINK-A only has diagnostic potential for metastatic ovarian carcinoma. Therefore, LINK-A may serve as a potential metastatic marker for ovarian carcinoma.
It has been reported that LINK-A participates in triple-negative breast cancer by activating normoxic HIF1α signaling [10]. In our study we also observed overexpression of HIF1α in ovarian carcinoma cells after LINK-A overexpression under normal O2 condition. LINK-A overexpression also promoted the migration and invasion of ovarian carcinoma cells, but treatment with HIF1α inhibitor significantly reduced the enhancing effect of LINK-A overexpression on cancer cell migration and invasion. Therefore, LINK-A may promote the metastasis of ovarian carcinoma by upregulating HIF1α signaling. Our study also indicates that triple-negative breast cancer and ovarian carcinoma, which are the 2 most common malignancies, may share similar pathological pathways. However, our study only proved a LINK-A–HIF1α sequential signaling in ovarian carcinoma. Our future studies will try to identify the mediators between LINK-A and HIF1α in ovarian carcinoma.
Conclusions
LINK-A is specifically upregulated in metastatic ovarian carcinoma but not in non-metastatic ovarian carcinoma. However, the sample size of our study was relatively small and not all types of metastasis were included. Our future studies will try to solve those shortcomings and further confirm our conclusions.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet. 2014;384:1376–88. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Ovarian Cancer Research Program of BC, Cheryl Brown Ovarian Cancer Outcomes Unit. OVCARE: Research platforms. 2013. www.ovcare.ca.
- 4.Marchiq I, Pouysségur J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J Mol Med (Berl) 2016;94:155–71. doi: 10.1007/s00109-015-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson RW, Sowder ME, Giaccia AJ. Hypoxia and bone metastatic disease. Curr Osteoporos Rep. 2017;15:231–38. doi: 10.1007/s11914-017-0378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KS, Sengupta S, Berk M, et al. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006;66:7983–90. doi: 10.1158/0008-5472.CAN-05-4381. [DOI] [PubMed] [Google Scholar]
- 7.Xiang S, Gu H, Jin L, et al. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1α via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci USA. 2018;115:E1465–74. doi: 10.1073/pnas.1711257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, Han C, Zhang Y, et al. LncRNA PVT1 regulate expression of HIF1α via functioning as ceRNA for miR-199a-5p in non-small cell lung cancer under hypoxia. Mol Med Rep. 2018;17:1105–10. doi: 10.3892/mmr.2017.7962. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–63. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin A, Li C, Xing Z, et al. The LINK-A lncRNA activates normoxic HIF1α signalling in triple-negative breast cancer. Nat Cell Biol. 2016;18:213–24. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin A, Hu Q, Li C, et al. The LINK-A lncRNA interacts with PtdIns (3, 4, 5) P 3 to hyperactivate AKT and confer resistance to AKT inhibitors. Nat Cell Biol. 2017;19:238–51. doi: 10.1038/ncb3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baratta MG, Schinzel AC, Zwang Y, et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc Natl Acad Sci USA. 2015;112:232–37. doi: 10.1073/pnas.1422165112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryland GL, Hunter SM, Doyle MA, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87. doi: 10.1186/s13073-015-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, Sun Y, Sun Y, et al. Comprehensive analysis of lncRNA expression profiles reveals a novel lncRNA signature to discriminate nonequivalent outcomes in patients with ovarian cancer. Oncotarget. 2016;7:32433–48. doi: 10.18632/oncotarget.8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SP, Yang JX, Cao DY, et al. Identification of differentially expressed long non-coding RNAs in human ovarian cancer cells with different metastatic potentials. Cancer Biol Med. 2013;10:138–41. doi: 10.7497/j.issn.2095-3941.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 17.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 18.Tripathi MK, Doxtater K, Keramatnia F, et al. Role of lncRNAs in ovarian cancer: Defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018:1635–43. doi: 10.1016/j.drudis.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]