Abstract
Introduction:
Genital mycotic infections are common among patients with poorly controlled diabetes. Sodium-glucose co-transporter 2 inhibitors (SGLT2i) induced pharmacological glycosuria increases the risk of these infections (2–3 fold) among patients with type 2 diabetes (T2D). The data about incidence of these infections in Indian setting is unclear.
Aim:
To study the prevalence of genital mycotic infections caused by SGLT2i among Indian patients with T2D.
Materials and Methods:
We collected data of 205 patients with T2D on SGLT2i for more than 1-month duration. Patients with symptoms and/or signs suggestive of genital mycotic infections and who had positive response to antifungal treatment were considered to have infection. Data were collected for a period of 2 months from July to August 2017.
Results:
Among 205 patients, mean age was 52.4 ± 8.7 years and percentage of females was 52.2%. Among SGLT2i, empagliflozin, canagliflozin and dapagliflozin were prescribed to 50.7%, 30.2% and 19.1% patients, respectively. The mean duration of treatment with SGLT2i was 7.6 ± 5.9 months. At least, one episode of genital mycotic infection occurred in 53 (25.9%) patients and 25 (12.2%) had second episode. Incidence of these infections was marginally higher in females than males with no statistically significant difference (P = ns). There was no significant correlation between age, sex, duration of disease, duration of treatment, glycaemic control, type and dose of SGLT2i used with the incidence of genital mycotic infections (P = ns). The patients who had knowledge of side effects of the drug and observed precautions had significantly lesser incidence of infections (P < 0.001). Majority of the infections were mild in nature and responded well to treatment.
Conclusion:
There is a very high risk of genital mycotic among Indian patients with T2D on SGLT2i. All patients should be educated about the risk of genital mycotic infections when on SGLT2i and precautions needed to minimise the risk.
Keywords: Canagliflozin, dapagliflozin, empagliflozin, India, infection, mycotic, SGLT2 inhibitors, type 2 diabetes
INTRODUCTION
Genital mycotic infections like balanoposthitis in males and vulvovaginal infections in females are common among patients with poorly controlled diabetes.[1] Glycosuria due to hyperglycaemia, bacterial adherence to uroepithelium and immune dysfunction predispose to such infections.[2,3,4] Candida albicans is the most common pathogen responsible.[5] These infections are more common among uncircumcised males as warm space underneath the foreskin may promote yeast growth, especially when genital hygiene is poor.[6] Sugar-rich urine that dribbles on to the glans and under the foreskin provides a friendly environment for yeast and bacterial growth. The patients present with symptoms like itching, redness of external genitals, irritation, yellowish-white discharge, dysuria, dyspareunia and so on. Along with tight blood glucose control, treatment involves local application of antifungal ointments and/or oral medications.[7]
Sodium-glucose co-transporter 2 inhibitors (SGLT2i), a new class of oral antidiabetic agents, are approved to treat type 2 diabetes (T2D). They act through an insulin-independent mechanism by inhibiting glucose reabsorption in proximal tubules of kidneys, increasing urinary glucose excretion and reducing amount of circulating glucose.[8,9] These agents are generally well tolerated but pharmacologically induced glycosuria increases the risk of genital mycotic infections and urinary tract infections (UTIs), as it provides a favourable growth environment for otherwise commensal genital microorganisms.[10,11] The incidence of genital mycotic infections is increased approximately 2–3-fold in subjects receiving SGLT2i (∼8–10%) vs. 3–5% in subjects receiving placebo.[12,13,14] These infections either subside spontaneously or respond well to local antifungal treatment. History and clinical presentation are adequate for most diagnoses.[1] In India, three SGLT2i are available, which include empagliflozin, canagliflozin and dapagliflozin. But the data about the incidence of genital mycotic infections caused by SGLT2i among Indian patients with T2D are lacking. Thus, we performed this cross-sectional study to assess the prevalence of genital mycotic infection in our Indian patient population.
MATERIALS AND METHODS
This was a multicentre, cross-sectional observational study conducted across northern India. We collected data of patients with T2D receiving SGLT2i for at least 1-month duration from three endocrinology clinics in northern India located in New Delhi, Amritsar (Punjab) and Gurugram (Haryana). SGLT2i were used as second-line or third-line agents in patients who did not achieve target HbA1c level of <7% with metformin monotherapy and optimal lifestyle managemen. Patients with history of symptoms and/or signs suggestive of genital mycotic infections and who had positive response to antifungal treatment were considered to have genital mycotic infections. All the patients were asked about the symptoms of genital fungal infections like itching, redness, white discharge and so on after starting SGLT2i. The past history of any genital mycotic infection before the initiation of SGLT2i was recorded. Further, their knowledge of these symptoms at the time of starting the medicine and precautions taken to avoid them were assessed. Positive history was labelled as episode 1. Episode 2 was defined as having similar symptoms of genital mycotic infections after restarting the medicine. The data were collected for a period of 2 months (July–August 2017). Informed consent was obtained from all participants for collecting data.
Statistical analysis
Descriptive statistics were analysed with the SPSS version 17.0 software. Continuous variables are presented as mean ± SD. Categorical variables are expressed as frequencies and percentages. The Pearson's Chi-square test or the Chi-square test of association was used to determine if there is a relationship between two categorical variables. P < 0.05 was considered statistically significant.
RESULTS
The baseline characteristics of the study population are shown in Table 1. Mean age of the patients was 52.4 ± 8.7 years. Majority of them [154 (75%)] were in the middle-age group (40–60 years) (data not shown in Table). About 52.2% were females. Glycosylated haemoglobin (HbA1C) was >7% in 183 (89%) patients. Half of the patients (50.7%) were on empagliflozin [Table 2]. In most of the patients, SGLT2i was used as a second line to metformin. Most of the patients had duration of diabetes <1 year. The proportion of males and females receiving different SGLT2i were nearly similar as shown in Table 3.
Table 1.
Baseline characteristics
| Characteristics | Total (n=205) |
|---|---|
| Age (years) | 52.4±8.7 |
| Age range (years) | 28-75 |
| Sex | |
| Male (%) | 98 (47.8) |
| Female (%) | 107 (52.2) |
| Mean duration of diabetes (months) | 10.5±5.6 |
| HbA1c (%) | |
| Mean±SD | 8.6±1.4 |
| >7 | 183 (89.0) |
Table 2.
Details of SGLT2 inhibitors
| SGLT2i prescribed | Observation |
|---|---|
| Empagliflozin (%) | 104 (50.7) |
| 10 mg | 74 (71.1) |
| 25 mg | 30 (28.9) |
| Canagliflozin (%) | 62 (30.2) |
| 100 mg | 60 (96.8) |
| 300 mg | 2 (3.2) |
| Dapagliflozin (%) | 39 (19.1) |
| 5 mg | 8 (20.5) |
| 10 mg | 31 (79.5) |
| Duration of treatment with SGLT2i (months) | |
| Mean±SD | 7.6±5.9 |
| Range | 1-36 |
Table 3.
Gender-wise prescription of SGLT2i
| SGLT2i prescribed | Gender | |
|---|---|---|
| Male (n=98) | Female (n=107) | |
| Empagliflozin (%) | 48 (49.0) | 56 (52.3) |
| Canagliflozin (%) | 31 (31.6) | 31 (29.0) |
| Dapagliflozin (%) | 19 (19.4) | 20 (18.7) |
At least, one episode of genital mycotic infection occurred in 53 (25.9%) patients, whereas 25 (12.2%) also reported second episode [Figure 1]. Also, there was no gender-wise difference in occurrence of first or second episode of genital mycotic infections [Figure 1]. Past history of genital mycotic infections was significantly present in 11/53 (20.7%) patients who had infection as compared to 14/152 (9.2%) who did not have genital mycotic infection on SGLT2i (P = 0.027). First episode of infection was reported among 36/53 (67.9%) patients within 6 months of starting treatment. Out of 53 patients with one episode, 29 (54.7%), 13 (24.5%) and 11 (20.8%) were on treatment with empagliflozin, canagliflozin and dapagliflozin, respectively (P = ns). Similarly, out of 25 patients with second episode, 12 (48%), 6 (24%) and 7 (28%) were receiving these SGLT2i, respectively (P = ns). There was no correlation between age, sex, duration of disease, level of glycaemic control, type of SGLT2i and duration of treatment with the incidence of genital mycotic infections [Table 4].
Figure 1.
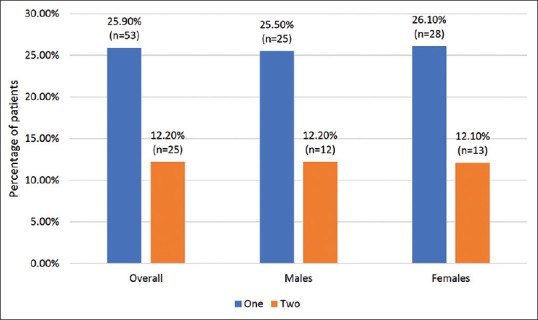
Prevalence of one and two episodes of mycotic infections in study population
Table 4.
Correlation of different parameters with genital mycotic infection
| Parameters | First episode | P | |
|---|---|---|---|
| Yes (n=53) | No (n=152) | ||
| Age group | |||
| <40 | 5 (9.43) | 8 (5.26) | 0.730 |
| 41-50 | 22 (41.51) | 61 (40.13) | |
| 51-60 | 17 (32.08) | 54 (35.53) | |
| >60 | 9 (16.98) | 29 (19.08) | |
| Sex | |||
| Male | 28 (52.83) | 79 (51.97) | 0.916 |
| Female | 25 (47.17) | 73 (48.03) | |
| Duration of diabetes | |||
| ≤1 year | 35 (66.04) | 108 (71.0) | 0.550 |
| >1 year | 18 (33.96) | 44 (28.9) | |
| Glycaemic control | |||
| ≤7% | 3 (5.66) | 9 (5.9) | 0.521 |
| >7% | 50 (94.34) | 133 (87.5) | |
| SGLT2i | |||
| Empagliflozin | 29 (54.72) | 75 (49.34) | 0.575 |
| Dapagliflozin | 13 (24.53) | 49 (32.24) | |
| Canagliflozin | 11 (20.75) | 28 (18.42) | |
| Duration of treatment | |||
| <6 months | 44 (83) | 109 (71.7) | 0.351 |
| >6 months | 9 (17) | 43 (28.2) | |
| Past history of genital mycotic infections | 11 (20.7) | 14 (9.2) | 0.027 |
We need to add figures where yellow is highlighted
The patients who had knowledge of the side effect of the drug and observed precautionary measures had significantly lesser incidence of genital mycotic infections (P < 0.001) as shown in Table 5. The precautions included excess fluid intake, improved genital hygiene and prophylactic application of antifungal ointments and so on. Majority of the genital mycotic infections were mild in nature and almost all the patients responded well to oral and topical antifungal ointments.
Table 5.
Effect of knowledge on the incidence of infections
| Knowledge on infections with SGLT2i | Infection (episode 1) (n=53) | P |
|---|---|---|
| Yes | 14 (26.4) | <0.001 |
| No | 39 (73.6) |
DISCUSSION
Patients with diabetes are at a higher risk for genital mycotic infections. Vaginitis is twice more common among women with diabetes than those without diabetes and balanitis is three times as likely in men with diabetes compared with those without diabetes.[11] SGLT2i increase the risk of genital mycotic infections in both the males and females with diabetes. Based on their mechanism of action, increased rate of genital mycotic infections is expected. Very-high incidence (25.9%) of genital mycotic infections was observed in our patient population, whereas other studies have reported moderate risk of such infections.
In a pooled analysis of four placebo-controlled trials of canagliflozin, the rate of genital mycotic infections reported was 11.4%, 10.4% and 3.2% in females treated with canagliflozin 300 mg, 100 mg and placebo, respectively. The risk is also high in males compared to placebo (3.7–4.2% vs. 0.6% with placebo).[15] In a study among older subjects (aged 55–80 years) with uncontrolled T2D, infection rates with canagliflozin 100 mg and 300 mg were 15.4% and 11.2% among females, respectively than 2.1% with placebo and 3.2% and 6.2% in males, respectively than 0% with placebo.[16] In another pooled analysis of clinical studies, Nyirjesy et al.[17] reported genital mycotic infection in 10.4% women and 4.2% men receiving canagliflozin 100 mg, 11.4% of women and 3.7% of men receiving 300 mg vs. 3.2% of women and 0.6% of men in the placebo group. They also reported 2.5 times more risk of genital mycotic infections among women with past history of infection when on canagliflozin treatment than women with no prior history. Majority of the infections occurred within first 3–6 months. In our study population also, past history of genital mycotic infections was present in significantly higher number of patients with infection as compared to those with no infection on SGLT2i and two-third patients had infection within 6 months of starting treatment.
A pooled analysis of dapagliflozin examined data from 12 randomised placebo-controlled trials reported genital infection in 4.1–5.7% of dapagliflozin groups vs. 0.9% of the placebo group.[18] A pooled analysis of empagliflozin studies showed that the frequency of events consistent with genital infections was approximately 5% vs. 1% for empagliflozin groups and placebo, respectively.[19] In one phase 2 study, 12% of 198 women with T2D were positive for vaginal candida species at baseline; after 12 weeks treatment with canagliflozin, 31% who had been culture negative tested positive compared with 14% women who received placebo or the DPP-4 inhibitor sitagliptin and, of these, one-third to one-quarter were symptomatic.[20]
The likely explanation for very high rate of genital mycotic infections among our patient population may be poor genital hygiene, lack of knowledge of side effects, precautions needed and hot and humid atmosphere of the country which increases the risk for mycotic infections. Goswami et al.[21] reported significantly high prevalence rate (46%) of vulvovaginal candidiasis among Indian women patients with diabetes mellitus as compared to healthy subjects (23%). Though, not all studies report higher incidence of side effects among Indians due to hot and humid conditions,[22] Indian patients with T2D might be at a high risk for genital mycotic infections. They should be monitored closely for this side effect when using SGLT2i.
The high infection rate among male patients in our study population may be because almost all of them were from the communities which do not practice circumcision. It is rare among circumcised men.[1] This may be a reason for similar rate of genital mycotic infections rate among both sexes. Another possible explanation of higher incidence of infections may be higher degree of glycosuria due to carbohydrate-rich Indian diet.
Knowledge of infections with SGLT2i treatment was found to be an important contributor to contain the infection rates as we observed significantly lower rates if patient had prior knowledge of infections. This helps them to observe precautions to avoid infection. Therefore, we must educate patients about the possible mycotic infections at the start of treatment with SGLT2i and explain the different measures to lower their occurrence. These measures include adequate hydration, improved genital hygiene by washing genitals after every act of urination, stopping SGLT2i for a brief period of time and restarting after recovery and may be prophylactic use of antifungal ointments.
In our patient population, the incidence of genital mycotic infections caused by all SGLT2i was same. The risks appear to be similar for each agent. Geerlings et al.[11] reported it as a class effect despite lack of direct comparisons and difficulty to compare data.
Limitations
The shortcomings of our study may be a small sample size. Poor glycaemic control (98% patients had HbA1c >7%) may be an independent risk factor for genital mycotic infections, thereby overestimating the event rate, though we did not find any correlation with level of HbA1c. One major limitation of the study was that the diagnosis of genital mycotic infection was based solely on detailed evaluation of clinical signs and symptoms, which may overestimate the prevalence of genital mycotic infections.
CONCLUSION
There is a very high risk of genital mycotic infections among Indian patients with T2D on SGLT2i. It is more common among uncircumcised males. All patients should be educated about the risk of genital mycotic infections when on SGLT2i and advised to observe necessary precautions like adequate hydration, maintaining good genital hygiene and may be prophylactic application of local antifungal ointments to minimise the risk of infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Dr Vijay M Katekhaye, (Quest MedPharma Consultants, Nagpur, India) for his assistance in reviewing the article.
REFERENCES
- 1.Nyirjesy P, Sobel JD. Genital mycotic infections in patients with diabetes. Postgrad Med. 2013;125:33–46. doi: 10.3810/pgm.2013.05.2650. [DOI] [PubMed] [Google Scholar]
- 2.Segal E, Soroka A, Schechter A. Correlative relationship between adherence of Candida albicans to human vaginal epithelial cells in vitro and candidal vaginitis. Sabouraudia. 1984;22:191–200. [PubMed] [Google Scholar]
- 3.Wilson RM, Tomlinson DR, Reeves WG. Neutrophil sorbitol production impairs oxidative killing in diabetics. Diabetic Med. 1987;4:37–40. doi: 10.1111/j.1464-5491.1987.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RM, Reeves WG. Neutrophil phagocytosis and killing in insulin-dependent diabetes. Clin Exp Immunol. 1986;63:478–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Reed BD. Risk factors for Candida vulvovaginitis. Obstet Gynecol Surv. 1992;47:551–60. doi: 10.1097/00006254-199208000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Edwards SK, Bunker CB, Ziller F, van der Meijden WI. 2013 European guideline for the management of balanoposthitis. Int J STD AIDS. 2014;25:615–26. doi: 10.1177/0956462414533099. [DOI] [PubMed] [Google Scholar]
- 7.Bohannon NJ. Treatment of vulvovaginal candidiasis in patients with diabetes. Diabetes Care. 1998;21:451–56. doi: 10.2337/diacare.21.3.451. [DOI] [PubMed] [Google Scholar]
- 8.Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2014;104:297–322. doi: 10.1016/j.diabres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: Rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 10.Kalra S, Chawla A. Diabetes and balanoposthitis. J Pak Med Assoc. 2016;66:1039–41. [PubMed] [Google Scholar]
- 11.Geerlings S, Fonseca V, Castro-Diaz D, List J, Parikh S. Genital and urinary tract infections in diabetes: Impact of pharmacologically-induced glucosuria. Diabetes Res Clin Pract. 2014;103:373–81. doi: 10.1016/j.diabres.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 12.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–24. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilding JP, Norwood P, T'joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: Applicability of a novel insulin-independent treatment. Diabetes Care. 2009;32:1656–62. doi: 10.2337/dc09-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussey E, Clark R, Amin M, Kipnes M, Semmes R, O'driscoll E, et al. Early clinical studies to assess safety, tolerability, pharmacokinetics and pharmacodynamics of single dose of sergliflozin, a novel inhibitor of renal glucose reabsorption in healthy volunteers and subjects with type 2 diabetes mellitus. Diabetes. 2007;56:A189. doi: 10.1177/0091270009351879. [DOI] [PubMed] [Google Scholar]
- 15.INVOKANA® (Canagliflozin) [package insert] Titusville, NJ: Janssen Pharmaceuticals Inc; 2013. Available from https://www.invokana.com/prescribing-information.pdf . [Google Scholar]
- 16.Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract (1995) 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 17.Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, et al. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: A pooled analysis of clinical studies. Curr Med Res Opin. 2014;30:1109–19. doi: 10.1185/03007995.2014.890925. [DOI] [PubMed] [Google Scholar]
- 18.Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013;27:479–84. doi: 10.1016/j.jdiacomp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Kohler S, Salsali A, Hantel S, Kaspers S, Woerle HJ, Kim G, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes. Clin Ther. 2016;38:1299–313. doi: 10.1016/j.clinthera.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 20.Hirji I, Andersson SW, Guo Z, Hammar N, Gomez-Caminero A. Incidence of genital infection among patients with type 2 diabetes in the UK General Practice Research Database. J Diabetes Complications. 2012;26:501–5. doi: 10.1016/j.jdiacomp.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Goswami R, Dadhwal V, Tejaswi S, Datta K, Paul A, Haricharan RN, et al. Species-specific prevalence of vaginal candidiasis among patients with diabetes mellitus and its relation to their glycaemic status. J Infect. 2000;41:162–6. doi: 10.1053/jinf.2000.0723. [DOI] [PubMed] [Google Scholar]
- 22.John M, Rafael SC, Deerochanawong VC, Hassanein M, Slee A, Canovatchel W, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus living in hot climates. Int J Clin Pract. 2016;70:775–85. doi: 10.1111/ijcp.12868. [DOI] [PMC free article] [PubMed] [Google Scholar]


