Abstract
Vitamin K (VK), in both its phylloquinone and menaquinone forms, has been hypothesized to undergo ω- and β-oxidation on its hydrophobic side chain in order to generate the observed urinary metabolites, K acid I and K acid II, which are excreted primarily as glucuronide conjugates. Synthetic standards of K acid I, K acid II, and a putative intermediate metabolite, menaquinone (MK)1 ω-COOH, were used to develop and optimize a new atmospheric pressure negative chemical ionization LC-MS/MS assay for the quantitation of these compounds in urine from untreated individuals and subjects treated with a high dose VK supplement. VK catabolites were extracted from urine, deconjugated, and converted to their methyl ester derivatives using previously reported methodology. The assay showed a high degree of sensitivity, with limits of detection below 10–50 fmol of metabolite per milliliter of urine, as well as an inter-assay precision of 8–12%. Metabolite standards provided unambiguous evidence for MK1 ω-COOH as a new human urinary metabolite of VK. This assay provides a minimally invasive, highly sensitive, and specific alternative for monitoring VK status in humans.
Keywords: beta-oxidation, omega-oxidation, liquid chromatography-mass spectrometry, menaquinone, phylloquinone
The designation vitamin K (VK) is given to a number of structurally similar compounds that contain a common 2-methyl-1,4-naphthoquinone head group substituted with a variable alkyl chain at the 3-position of the quinone. Vitamin K1, or phylloquinone (PK), has a phytyl group attached to the 3-carbon, while vitamin K2 encompasses a subset of molecules that contain one or more 5-carbon isoprenyl moieties linked in a linear fashion to this position. These latter compounds are collectively known as menaquinones (MKs), or MKn, where n denotes the number of isoprenyl units in the alkyl side chain. Vitamin K3, or menadione (MN), is unsubstituted at the 3-position.
The two most biologically relevant K vitamers are PK and MK4, which both contain a C20 alkyl side chain that varies only in degree of unsaturation (Fig. 1). PK is biosynthesized in plants and bacteria, but not in animals, thus humans must acquire it by consuming green vegetables (1). By contrast, humans and animals are capable of converting PK into MK4. The mechanism by which this is believed to occur involves first, cleavage of the phytyl side chain of PK in gut endothelium to form MN as an intermediate. MN is then coupled to geranyl geraniol, a terminal allylic C20-isoprenyl alcohol, through the action of UBIAD1, producing MK4 (2–4). Unlike PK, MK4 can be acquired by eating animal products such as meat and liver. Longer chain menaquinones in humans (MK5–MK13) are believed to be the product of either bacterial synthesis by human intestinal flora or dietary intake of foods such as cheese and fermented soybeans (5).
Fig. 1.
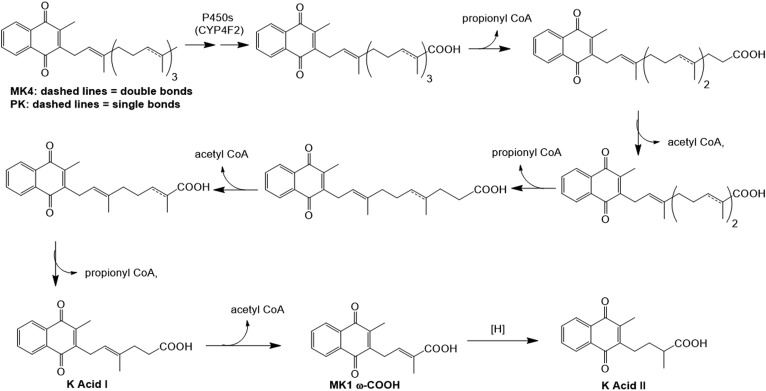
Proposed VK (PK and MK4) catabolic pathway. Both K vitamers are known to undergo successive ω-oxidations, catalyzed primarily by CYP4F2, which result in the production of their respective ω-carboxylic acid metabolites (13, 14). It is believed that these VK acid metabolites then enter the fatty acid β-oxidation pathway, going through several rounds of oxidative side-chain cleavage, which eventually leads to the observed urinary VK metabolites, K acid I and K acid II. MK1 ω-COOH is a putative metabolic intermediate between K acids I and II.
Physiologically, PK and MK4, in their reduced dihydroquinone forms, are utilized as essential cofactors by the enzyme, γ-glutamyl carboxylase (GGCX), in the carboxylation of poly-Glu residues in a number of vital proteins, including several involved in blood coagulation, bone mineralization, and the inhibition of vascular calcification (6, 7). The resulting γ-carboxy glutamate residues produced in these proteins show an increased affinity for calcium ions, the binding of which induces a conformational change that results in a more biologically active form of the VK-dependent protein (8). Correspondingly, low physiological levels of VK have been associated with increased patient risk for both osteoporosis and vascular calcification, the latter of which has been linked to a decrease in the activated form of matrix Gla protein (9–11). As a result of the GGCX carboxylation mechanism, the naphthoquinone moiety of VK is epoxidized at its 2- and 3-position and must be cycled back to its dihydroquinone form, which occurs through successive two electron reductions catalyzed by the enzyme, VK oxidoreductase, before the cofactor can again be utilized by GGCX (12).
VK (in both PK and MK forms) is apparently removed from this homeostatic cycle primarily through initial cytochrome P450 (CYP)4F2-mediated metabolism (13, 14). CYP4F2 oxidizes the terminus of the side chain, converting the ω-methyl group first to a primary alcohol, which can then be further oxidized to a carboxylic acid. The resultant VK acid metabolites contain a long hydrocarbon chain that is similar in structure to a fatty acid and is presumed to similarly undergo degradation via the β-oxidation pathway. K acids I and II, which were found to be the major VK metabolites isolated from human and rat urine (subsequent to deconjugation), are the apparent end products of VK catabolism, produced after several rounds of β-oxidative side chain truncation (15–17) (Fig. 1).
K acids I and II, therefore, have obvious potential for use as biomarkers for osteoporosis as well as chronic kidney disease (CKD), because CKD is strongly associated with increased vascular calcification in patients. In fact, quantitation of the urinary K acids can give a more complete analysis of a patient’s overall VK levels than can be obtained from direct measurement of their VK plasma concentrations, due to the physiological compartmentalization that exists between the major K vitamers (18, 19). PK is the more prominent form of VK in plasma, evidenced by the relative importance of PK in blood coagulation, while MK4 is more concentrated in tissues, such as kidneys, brain, and lungs, which likely contributes to its relative importance in other key biological processes like bone mineralization and the inhibition of vascular calcification (10, 20, 21). Thus, direct plasma analysis can only give an accurate read of a patient’s PK levels, whereas the quantitation of urinary K acid concentrations would reflect the patient’s total systemic exposure to VK.
A published method exists for the quantitation of K acids I and II in urine, but the assay relies upon electrochemical detection (ECD) of these metabolites and is therefore of limited utility to most laboratories, which lack the capability to run such an assay (22). Therefore, in this study, we describe the first LC-MS/MS assay for the accurate and precise quantitation of K acids I and II in human urine and report the identification of a new VK metabolite, MK1 ω-COOH (Fig. 1).
MATERIALS AND METHODS
General reagents
The VK supplement, Koncentrated K, was generously gifted to us by Dr. Patrick Theut, President of Red Foot Associates (Manistique, MI). The MK1 internal standard as well as K acid I, K acid II, and MK1 ω-COOH were previously synthesized (23, 24). Organic solvents were obtained from Fisher Scientific (Pittsburgh, PA), and all other chemicals (including unlabeled MK4 and PK) were purchased from Sigma-Aldrich (St. Louis, MO). Urine creatinine content was measured using a creatinine colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocol.
Subject protocol for VK supplementation and urine collection
Six healthy adult male subjects were recruited for the VK supplementation study. Each subject provided a single time-point baseline urine sample immediately prior to the oral ingestion of two capsules of the VK supplement, Koncentrated K (reported to contain 25 mg MK4, 5 mg PK, 0.5 mg MK7, and 2 mg of astaxanthine per capsule). After swallowing the Koncentrated K capsules, the subjects immediately consumed one half pint of 2% milk, to aid in absorption of vitamin, and then collected their urine for the next 24 h postsupplementation. Urine samples were subsequently aliquoted and kept frozen at −80°C until analysis. All subjects provided written informed consent for the study, which was approved by the University of Washington Institutional Review Board. This study abides by the Declaration of Helsinki principles.
Partial purification and deconjugation of urinary VK metabolites
Urine processing and deconjugation was based closely on the methodology of Harrington, et al. (22) with minor modifications. Menaquinone-1 (MK1; internal standard, 100 pmol) was premixed into 2.5 ml of urine, which were then passed through a Bond Elut 3 ml C18 SPE cartridge (Agilent Technologies). The column was washed with 2.5 ml of water and the VK metabolites were eluted with 2 ml of methanol. Concentrated HCl (500 μl) was added to the methanol eluate and the samples were shaken in a water bath at room temperature overnight in the dark. The deconjugated VK metabolites were next diluted with water and extracted into dichloromethane, which was then washed with water to remove traces of inorganic acid. The carboxylic acid moieties of these aglycone catabolites were then converted to their methyl ester derivatives through the addition of excess etheric diazomethane, and solvent was removed under a nitrogen gas stream. Sample residues, taken up in 2 ml hexane, were further purified by loading onto a normal phase Bond Elut LRC-Si 500 mg SPE column (Agilent) and eluting with 10 ml of 15% ether in hexane subsequent to an 8 ml hexane column wash. After solvent evaporation via nitrogen gas stream, sample residues were dissolved in 100 μl of isopropyl alcohol for LC-MS analysis.
Quantitative calibration curves (supplemental Figs. S6–S8) were prepared by spiking concentrated standard mixtures, containing variable amounts of K acid I, K acid II, and MK1 ω-COOH, into 2.5 ml aliquots of a baseline human urine sample (0.05 to 500 pmol/ml final concentrations above endogenous urine levels). The standard urine solutions, prepared in duplicate, were worked-up and analyzed in identical fashion to the samples described above. Because the presence of endogenous catabolites in these urine samples blocked our ability to determine limits of detection for the assay using this type of curve, limits of detection were instead estimated from a different standard curve, which was prepared by spiking the metabolites into buffer rather than urine (at variable concentrations between 0.001 and 100 pmol/ml). Methanolic stock solutions of the K acid catabolites were stored at −80°C and, along with the endogenous K acid conjugates present in urine, proved to be stable at this temperature for at least 12 months, as judged by the strong consistency found between numerous standard curves performed on various aliquots of the same baseline human urine sample within this time frame.
LC-MS/MS analysis of urinary VK metabolites
LC-MS/MS analyses were conducted on a Waters Xevo TQ-S tandem quadrupole mass spectrometer (Waters Co., Milford, MA.) coupled to an ACQUITY Ultra Performance LC™ (UPLC™) system with integral autoinjector (Waters). The Xevo was operated in atmospheric pressure negative chemical ionization (APCI−) MS/MS [selected reaction monitoring (SRM)] mode at a source temperature of 150°C and a probe temperature of 500°C, and the following mass transitions for the methyl ester derivatives of each metabolite were monitored in separate ion channels: m/z 240 > 185 (MK1, internal standard), m/z 312 > 195 (K acid I), m/z 286 > 185 (MK1 ω-COOH), and m/z 284 > 195 (K acid II). The cone voltages were set to 50 V for K acid I, K acid II, and MK1 ω-COOH and to 60 V for MK1. Optimized collision energies were set to 40, 30, 40, and 18 eV, respectively, for the ester derivatives of K acid I, K acid II, MK1 ω-COOH, and MK1. Compounds were separated by injecting 5 μl of sample onto a Shim-pack XR-ODS 2.2 μ, 2.0 × 75 mm UPLC column (Shimadzu Scientific, Columbia, MD), set to a temperature of 50°C, using a binary solvent gradient of 0.1% aqueous formic acid (solvent A) and methanol (solvent B) with a constant flow rate of 0.35 ml/min. The initial solvent B concentration was set to 65% where it was kept for 3 min, then increased linearly to 98% over 4 min, and there maintained for an additional 5 min. The K acid I, K acid II, and MK1 ω-COOH metabolites were quantified by comparing peak area ratios (relative to the internal standard, MK1) to ratios from the appropriate calibration curve, determined with synthesized metabolite standards (23, 24), using linear regression analysis.
Intra- and inter-assay variability studies
The intra- and inter-assay variability studies were performed on two different single time-point urine samples collected from the same healthy adult male subject (unsupplemented with VK) approximately 6 months apart. For the intra-assay variability study, 3 nmol of MK1 standard was spiked into 30 ml of urine, which was mixed thoroughly by vortex, and the urine was then aliquoted into 11 × 2.5 ml samples. These samples were processed and the deconjugated VK catabolites, as their methyl ester derivatives, were quantified by LC-MS/MS as described above. The inter-assay variability study was carried out over a month-long period and involved four identical experiments, each performed roughly 1 week apart. In each experiment, 3.2 nmol of MK1 standard were spiked into 32 ml of urine, which was then aliquoted into 12 × 2.5 ml samples (48 samples total) and the VK catabolites were again processed and quantified as described.
RESULTS
Quantitation of VK metabolites in human urine
Assay development.
Urine, collected from healthy human adult male subjects, was processed for VK metabolite quantitation using the optimized methodology of Harrington et al. (22). Briefly, urine samples were premixed with internal standard, desalted on a C18 SPE column, and the eluents (containing the VK catabolites primarily as their glucuronide conjugates) (16, 17, 22) were hydrolyzed in methanolic HCl. The aglycone metabolites, already partially converted to their methyl ester derivatives during acidic deconjugation, were fully esterified with etheric diazomethane and the evaporated sample residues were further purified on a silica SPE column prior to LC-MS/MS analysis. We were able to confirm the assertions of Harrington et al. (22) that this procedure generates highly predictable and repeatable recoveries of K acids I and II, as their methyl ester derivatives, with no detectable lactonization products (data not shown).
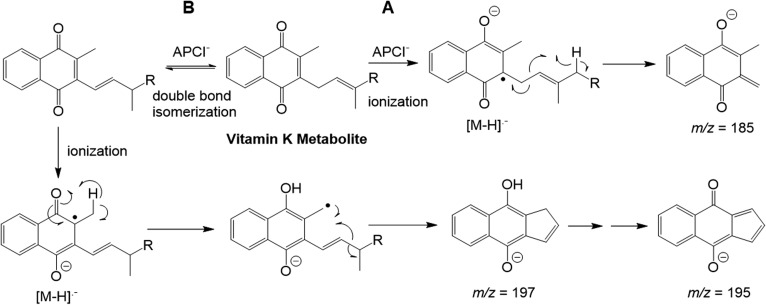
Synthetic standards of K acid I, K acid II, and MK1 ω-COOH were utilized in the development of an optimized LC-MS/MS quantitation assay. Multiple types of MS ionization were tested on both the underivatized and methyl ester versions of these metabolite standards, and it was found that APCI− provided consistently higher sensitivity of detection for all compounds than was observed with electrospray ionization (positive or negative). It has been reported previously that VK compounds lacking a carboxylic acid or even alcohol functionality can still ionize well by APCI− MS because the quinone moiety of these molecules is capable of forming a highly-stabilized radical anion (Fig. 2) (13). Thus, conversion of the K acid metabolites to their methyl ester derivatives does not result in significant loss of sensitivity despite the fact that this technique utilizes negative chemical ionization.
Fig. 2.
Hypothetical APCI− ionization and fragmentation mechanisms for VK analogs. Initial ionization occurs via addition of an electron to the quinone ring, generating a radical anion, as shown. Most VK species then form a major m/z 185 fragment ion via homolytic cleavage between the second and third carbons of the side chain (A). However, the methyl ester of MK1 ω-COOH instead forms major fragment ions at either mass 197 (at relatively low CE) or 195 (high CE) (supplemental Fig. S3). This could occur through a mechanism involving base-catalyzed double bond isomerization followed by cyclization and aromatization as shown in pathway B.
Both PK and MK4 fragment, by APCI− MS/MS, primarily via homolytic bond cleavage between the first and second carbons of the side chain attached to position 3 of the quinone, resulting in a very prominent 3-methyl-menadione fragment anion, at m/z = 185 (Fig. 2A) (13). We found that the methyl ester derivatives of both K acid I and K acid II generate the same major fragment ion as the parent vitamins (supplemental Figs. S1, S2). Interestingly, MK1 ω-COOH shows a different APCI− MS/MS fragmentation profile, generating a major fragment ion at either m/z = 197 at relatively low collision energy (CE) (CE = 20 V) or m/z = 195 at higher CE (CE = 40 V) with only a negligible ion at mass 185 (supplemental Fig. S3). This is perhaps due to the fact that the side chain double bond is conjugated to the carboxy ester moiety of MK1 ω-COOH, but is isolated (or absent) in the other VK species. We propose that the m/z 197/195 fragment ions may result from a mechanism involving an initial side-chain double bond isomerization followed by radical ionization, hydrogen atom transfer, and then chain truncation/cyclization as shown in Fig. 2B. Conjugation with the carboxy ester moiety of the MK1 ω-COOH derivative should enable base-catalyzed double bond isomerization to occur more readily for this compound compared with VK analogs possessing an isolated double bond at this position, perhaps explaining the difference in the MK1 ω-COOH derivative’s fragmentation profile.
We used this fragmentation data to develop a highly sensitive APCI− MS/MS (SRM) assay where we analyzed for the three VK catabolite derivatives using transitions from their respective molecular ions to either their m/z 185 or 195 fragments. While we initially monitored the K acid I ester in the m/z 312 > 185 SRM channel, we found that human urine contains two additional compounds (perhaps double bond regio-isomers of K acid I) that show up in this same channel, bracketing the K acid I ester, and which proved difficult to fully separate from the target metabolite by LC-MS/MS (supplemental Fig. S4). Because the K acid I ester also produces a minor m/z 195 fragment ion that is absent in the more prominent of the two bracketing compounds (in terms of the 312 > 185 mass transition), we chose to instead analyze the K acid I ester in the m/z 312 > 195 SRM channel, resulting in some loss of sensitivity, but allowing for better overall precision of metabolite quantitation.
Assay calibration and reproducibility.
As outlined in the Materials and Methods section, VK catabolites within unknown urine samples were identified and quantified, after processing, by fitting their LC-MS/MS measured peak area ratios (relative to the internal standard, MK1) to standard curves using linear regression analysis. The assay showed a high degree of sensitivity for all three VK catabolites, with limits of detection below 10 fmol/ml urine for K acid II and MK1 ω-COOH and a limit of detection below 50 fmol/ml urine for K acid I.
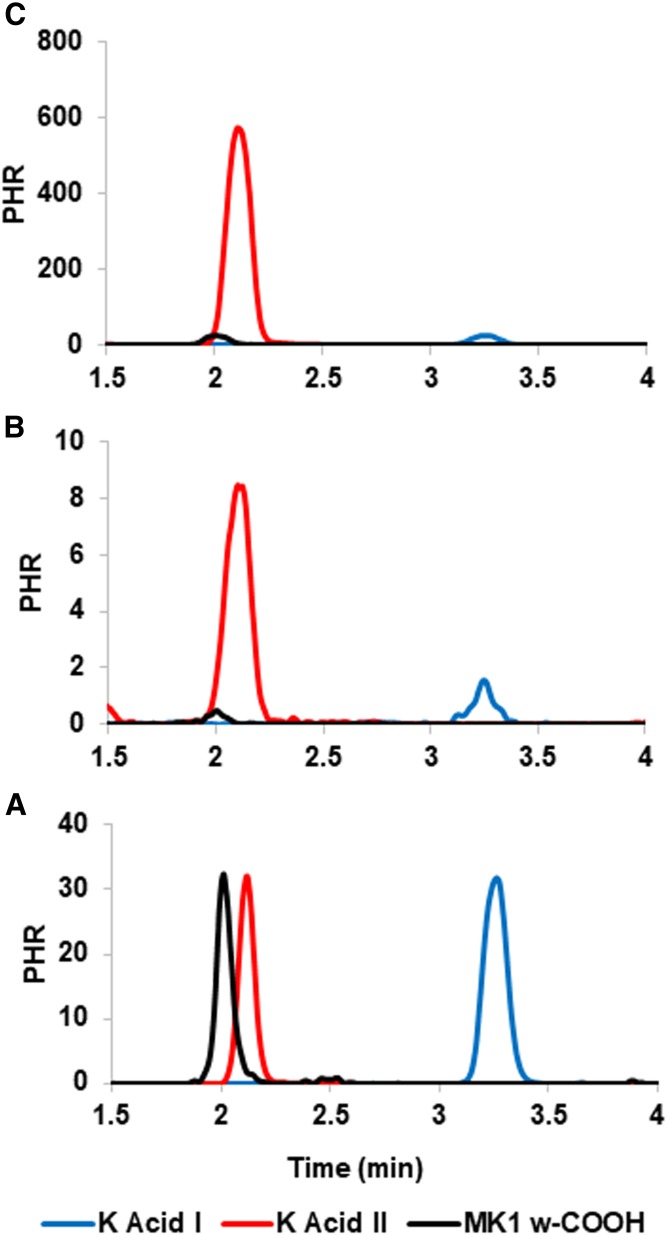
The intra- and inter-assay precision of quantitation for the urinary VK catabolites was tested, respectively, on two single-time point urine samples collected from the same human adult male subject roughly six months apart. Comparison of sample urine LC-MS/MS chromatograms to chromatograms obtained from urine spiked with synthetic catabolite standards allowed for the unambiguous identification of MK1 ω-COOH as a new VK metabolite (Fig. 3). The urinary concentrations determined for the three catabolites in both of these experiments, along with their precisions of measurement, are listed in Table 1.
Fig. 3.
APCI− LC-MS/MS (SRM) chromatograms of VK catabolites K acid II (m/z 286 > 185, red lines), K acid I (m/z 312 > 195, blue lines) and MK1 ω-COOH (m/z 284 > 195, black lines), analyzed as their respective methyl ester derivatives. A: Chromatogram obtained from a human urine sample spiked with synthetic standards of the three derivatized VK catabolites. B: Chromatogram obtained from a human urine sample showing basal (i.e., presupplement) levels of VK catabolites. C: Chromatogram of the urinary extract, obtained from the same subject as in chromatogram B, collected 0–24 h after the subject ingested two capsules of Koncentrated K. All data in the figure were normalized to the height of the internal standard peak, MK1 (peak height ratio).
TABLE 1.
Intra- and inter-assay reproducibility for the quantitation of VK catabolite concentrations in human urine by LC-MS/MS
| Intra-Assay Reproducibility (Sample A, n = 11) | Inter-Assay Reproducibility (Sample B, n = 48) | |||||
| K Acid I | K Acid II | MK1 ω-COOH | K Acid I | K Acid II | MK1 ω-COOH | |
| Mean (pmol/ml) | 1.40 | 4.37 | 0.065 | 1.35 | 2.59 | 0.045 |
| SEM (pmol/ml) | 0.04 | 0.08 | 0.003 | 0.02 | 0.03 | 0.001 |
| Coefficient of. variation (%) | 8.4 | 6.4 | 16.2 | 11.0 | 7.8 | 12.0 |
Samples A and B represent two separate urine samples, collected roughly 6 months apart from the same healthy adult male subject. Concentrations are listed in picomoles of metabolite per milliliter of urine. Inter-assay reproducibility was assessed through four identical experiments conducted over a month-long period.
VK catabolite concentrations in human urine collected from subjects before and after supplementation with Koncentrated K
Six human adult male subjects were each given two capsules of the VK supplement, Koncentrated K, and urine was collected prior and subsequent to supplementation as described in the Materials and Methods section. LC-MS/MS analysis showed a minimum 50-fold increase in concentration, postsupplementation, for all three VK catabolites in each of the six subjects. In order to account for differences in urine dilution levels between subjects, creatinine concentrations were determined for each sample and these were used to normalize the VK catabolite concentrations, as shown in Table 2. The large increase in postsupplementation size for the assigned MK1 ω-COOH peak provides additional confirmation of its status as a newly identified VK metabolite (Fig. 3).
TABLE 2.
Urinary VK catabolite concentrations determined in six healthy adult male subjects before and after taking two capsules of the VK supplement, Koncentrated K
| Subject | Presupplement | 0-24 Hours Postsupplement (0–24 h) | ||||
| K Acid I | K Acid II | MK1 ω-COOH | K Acid I | K Acid II | MK1 ω-COOH | |
| (nmol/mmol creatinine) | (nmol/mmol creatinine) | |||||
| 1 | 0.242 ± 0.014 | 0.987 ± 0.057 | BLQ | 100.1 ± 11.8 | 127.9 ± 17.1 | 2.06 ± 0.29 |
| 2 | 0.102 ± 0.013 | 0.465 ± 0.031 | 0.0031 ± 0.0006 | 32.5 ± 5.2 | 64.7 ± 4.1 | 1.31 ± 0.09 |
| 3 | 0.082 ± 0.005 | 0.641 ± 0.037 | 0.0026 ± 0.0001 | 3.92 ± 0.35 | 44.1 ± 4.4 | 0.47 ± 0.03 |
| 4 | 0.060 ± 0.004 | 0.420 ± 0.017 | 0.0027 ± 0.0002 | 8.53 ± 0.64 | 42.6 ± 4.5 | 0.82 ± 0.12 |
| 5 | 0.172 ± 0.008 | 0.208 ± 0.008 | 0.0026 ± 0.0002 | 16.96 ± 0.87 | 15.6 ± 0.4 | 0.55 ± 0.03 |
| 6 | 0.157 ± 0.025 | 0.476 ± 0.039 | 0.0034 ± 0.0009 | 11.14 ± 0.48 | 45.5 ± 3.2 | 0.80 ± 0.05 |
Catabolite concentrations are normalized to individual urine creatinine levels and show the mean with standard deviation determined from three replicates. BLQ, below limit of quantitation.
DISCUSSION
This work represents the first reported LC-MS assay for the quantitation of VK catabolites in human urine. The assay is both highly sensitive, with limits of detection below 10–50 fmol of catabolite per milliliter of urine, and reproducible, showing a precision of measurement for K acids I and II of 6% to 11% in intra- and inter-assay reproducibility studies (Table 1). Interindividual K acid levels in urine (normalized to creatinine content) collected from six healthy adult male human subjects prior to supplementation with VK, varied by as much as 4- to 5-fold, while the K acid II to K acid I ratio ranged from 1.2 for subject 5 up to 7.8 for subject 3 (Table 2). Urine collected from these same six subjects over a 24 h period after they had each consumed two capsules of the VK supplement, Koncentrated K, showed a 50- to 500-fold increase from presupplement urinary K acid I and K acid II levels.
The only other validated assay for K acid I and K acid II in biological fluids is the LC-ECD method described by Harrington et al. (22). While the ECD method offers comparable sensitivity and precision to LC-MS/MS, the latter provides the notable advantage of selectivity that eliminates most background peaks from the analysis by selecting for only mass transitions specific to the K acid catabolites of interest. This results in much cleaner chromatograms and improved signal-to-noise relative to LC-ECD analysis (22) and mostly eliminates the possibility, present with LC-ECD, that overlapping peaks from other urinary constituents with similar redox properties might contribute to the measured catabolite or internal standard peak areas. An additional benefit of our assay methodology is that MS/MS more easily allows us to search for and identify new urinary catabolites of VK.
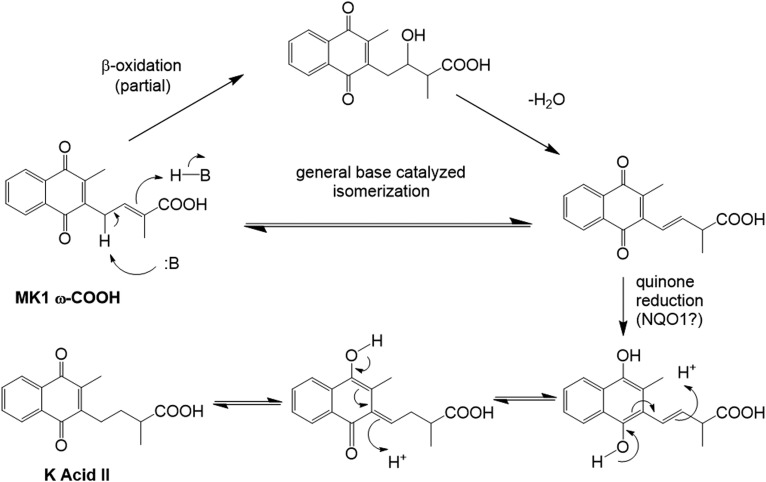
To the best of our knowledge, K acids I and II are the only downstream urinary catabolites of VK that have been previously isolated and identified, generally subsequent to either chemical or enzymatic deconjugation (16, 17, 22). These compounds are believed to be formed metabolically from either PK or MK4 via P450-mediated ω-oxidation reactions that initially generate the terminal carboxylic acids, followed by five rounds of β-oxidative side-chain truncation that ultimately produce K acid I (Fig. 1). A further round of β-oxidative cleavage on K acid I would produce MK1 ω-COOH as a putative intermediate metabolite that then must undergo side-chain double bond reduction in order to generate K acid II. Although it is possible that this reduction could occur directly on the MK1 ω-COOH double bond, a more energetically feasible mechanism is outlined in Fig. 4. Isomerization of the side-chain double bond would put it into direct conjugation with the benzoquinone portion of the molecule. A more favorable quinone reduction, perhaps catalyzed by NADPH quinone oxidoreductase 1, an enzyme that is known to accept MN as a substrate (25), followed by successive proton migrations could then generate K acid II. The initial double bond isomerization in this hypothetical mechanism might result from MK1 ω-COOH undergoing another partial round of β-oxidation to give an alcohol, which, rather than undergo further oxidation to the carbonyl and subsequent carbon-carbon bond cleavage, would instead dehydrate to form the migrated double bond. Alternatively, and perhaps more likely, the isomerization could occur spontaneously via general base catalysis. The protons α to the side-chain double bond of MK1 ω-COOH are particularly acidic inasmuch as deprotonation would produce an allylic anion that is delocalized not only into the quinone on one side of the molecule, but also through the double bond and into the carboxylic acid function on the other side. This reversible deprotonation favors migration of the initial double bond to the more highly conjugated, and thus more thermodynamically stable, position.
Fig. 4.
Hypothetical alternative mechanisms for the conversion of MK1 ω-COOH into the observed urinary metabolite, K acid II.
Through our studies, we were able to confirm that MK1 ω-COOH is indeed a real, albeit low abundance, catabolite of VK. The processed and derivatized urine samples used in our LC-MS/MS assays consistently produce a peak that matches the chromatographic and MS profile for our synthetic standard of the putative metabolite, isolated as its methyl ester derivative (Fig. 3). MK1 ω-COOH is present at very low levels in unsupplemented urine; however, similar to K acids I and II, the MK1 ω-COOH peak area increases by as much as several hundred-fold in urine taken from subjects immediately after VK supplementation (Table 2). These data provide further confirmation that the LC-MS/MS peak identified as MK1 ω-COOH represents a bona fide urinary metabolite of VK.
Interestingly, a very strong correlation exists between the mechanisms of VK and vitamin E (VE) catabolism in humans. Like VK, the VE moniker describes a family of compounds all of which contain a bicyclic head group (similar in size to the VK naphthoquinone functionality) attached to a fatty chain made up of multiple conjoined isoprenyl moieties of variable saturation. Again, like PK and MK4, the side chains of these E vitamers undergo CYP4F2-mediated ω-oxidation and then β-oxidation to generate urinary catabolic end products that are analogous in structure to K acids I and II (15). Sontag and Parker were able to demonstrate that normal HepG2 cells also generate substantial quantities of these two catabolites when tasked with various VE conformers (26). Unfortunately, our attempts to replicate their experiment using either PK or MK4 in place of VE were less successful. Although we were able to tentatively identify very small amounts of the K acids in lysate recovered from cells incubated with up to 20 μM VK, catabolite levels were below the limit of quantitation (data not shown). It may be that the large difference in catabolite formation between VE and VK in HepG2 cells results from the relative difference in their respective ω-oxidation rates, which is reportedly much higher for the E vitamers in comparison to PK and MK4 (13, 14, 27). It is, therefore, likely that further cellular study of VK metabolism would be enhanced by preparation of a HepG2 cell line stably transfected with CYP4F2.
The main purpose of this work was to develop a more widely useful, minimally invasive assay for VK quantitation that can be used to probe interindividual variation in VK status. Due to the compartmentalization of the various forms of VK in humans, i.e., PK is the far more prevalent K vitamer in plasma, while MK4 is the more dominant vitamer in certain tissues (6, 18, 19), merely measuring plasma concentrations of the vitamers gives an incomplete picture of an individual’s VK disposition. However, because MK4 and PK are both primarily metabolized to the same end products, K acids I and II, the sensitive, precise, and specific quantitation of these catabolites in urine should provide a better measure of an individual’s VK status at any given time. Because VK homeostasis has a significant impact on a large number of different biological processes beyond coagulation (7, 28, 29), there are numerous potential applications for this assay. Urinary K acid levels could be used as a potential biomarker for patients who are at risk for a variety of VK-related physical ailments, including CKD and osteoporosis, among others, as these conditions are strongly correlated with depleted VK levels in patients (9, 11, 30). The assay may also be useful for studying potential gene and environment effects on VK homeostasis.
It should be noted that there are several potential limitations in using this assay as a biomarker for individual VK status. The fact that PK, MK4, and other menaquinones generate the same catabolic end products renders the assay useful as a more “global” indicator of VK status, but could be seen as a detriment if the researcher’s goal is to differentiate between metabolic contributions from the two vitamers. Perhaps more importantly, inasmuch as a major purpose of the assay is to assess relative VK status between individuals, interindividual differences in VK metabolism may play a significant role in the interpretation of the assay results. We have demonstrated previously that (hepatic) CYP4F2 and CYP4F11 catalyze the catabolism of both PK and MK4 (13), so it would be informative to know what effect genetic variation in these CYP4F enzymes might have on initial VK ω-hydroxylation and subsequent catabolite levels, and whether differences in CYP4F enzyme tissue distribution affect PK versus MK4 metabolism. Additional functional studies with the common alleles of CYP4F2 and CYP4F11 and supplementation studies with the individual forms of PK and MK4 may help to address some of these questions in the future. Finally, due to the lack of availability of synthetic standards for the conjugated VK urinary catabolites and the fact that the glucuronide/sulfate status of these metabolites has not yet been fully assessed in human urine, we were unable to obtain proper extraction rates for the metabolites in this matrix. However, because of the high reproducibility and low variability in our assay results, we believe it is very likely that the metabolite extraction rates are acceptably high using this methodology and that our results therefore give an accurate assessment of individual urinary VK catabolite concentrations.
Supplementary Material
Footnotes
Abbreviations:
- APCI−
- atmospheric pressure negative chemical ionization
- CE
- collision energy
- CKD
- chronic kidney disease
- CYP
- cytochrome P450
- ECD
- electrochemical detection
- GGCX
- γ-glutamyl carboxylase
- MK
- menaquinone
- MN
- menadione
- PK
- phylloquinone
- SRM
- selected reaction monitoring
- VE
- vitamin E
- VK
- vitamin K
This work was supported in part by National Institutes of Health Grants GM109743 and GM116691. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Booth S. L., and Suttie J. W.. 1998. Dietary intake and adequacy of vitamin K. J. Nutr. 128: 785–788. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa K., Hirota Y., Sawada N., Yuge N., Watanabe M., Uchino Y., Okuda N., Shimomura Y., Suhara Y., and Okano T.. 2010. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 468: 117–121. [DOI] [PubMed] [Google Scholar]
- 3.Okano T., Shimomura Y., Yamane M., Suhara Y., Kamao M., Sugiura M., and Nakagawa K.. 2008. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 283: 11270–11279. [DOI] [PubMed] [Google Scholar]
- 4.Thijssen H. H., Vervoort L. M., Schurgers L. J., and Shearer M. J.. 2006. Menadione is a metabolite of oral vitamin K. Br. J. Nutr. 95: 260–266. [DOI] [PubMed] [Google Scholar]
- 5.Elder S. J., Haytowitz D. B., Howe J., Peterson J. W., and Booth S. L.. 2006. Vitamin K contents of meat, dairy, and fast food in the U.S. diet. J. Agric. Food Chem. 54: 463–467. [DOI] [PubMed] [Google Scholar]
- 6.Fusaro M., Mereu M. C., Aghi A., Iervasi G., and Gallieni M.. 2017. Vitamin K and bone. Clin. Cases Miner. Bone Metab. 14: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vermeer C. 2012. Vitamin K: the effect on health beyond coagulation - an overview. Food Nutr. Res. 56: doi:10.3402/fnr.v56i0.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallin R., Stanton C., and Hutson S. M.. 1993. Intracellular maturation of the gamma-carboxyglutamic acid (Gla) region in prothrombin coincides with release of the propeptide. Biochem. J. 291: 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallieni M., and Fusaro M.. 2014. Vitamin K and cardiovascular calcification in CKD: is patient supplementation on the horizon? Kidney Int. 86: 232–234. [DOI] [PubMed] [Google Scholar]
- 10.Palermo A., Tuccinardi D., D’Onofrio L., Watanabe M., Maggi D., Maurizi A. R., Greto V., Buzzetti R., Napoli N., Pozzilli P., et al. 2017. Vitamin K and osteoporosis: myth or reality? Metabolism. 70: 57–71. [DOI] [PubMed] [Google Scholar]
- 11.Wuyts J., and Dhondt A.. 2016. The role of vitamin K in vascular calcification of patients with chronic kidney disease. Acta Clin. Belg. 71: 462–467. [DOI] [PubMed] [Google Scholar]
- 12.Rettie A. E., and Tai G.. 2006. The pharmocogenomics of warfarin: closing in on personalized medicine. Mol. Interv. 6: 223–227. [DOI] [PubMed] [Google Scholar]
- 13.Edson K. Z., Prasad B., Unadkat J. D., Suhara Y., Okano T., Guengerich F. P., and Rettie A. E.. 2013. Cytochrome P450-dependent catabolism of vitamin K: omega-hydroxylation catalyzed by human CYP4F2 and CYP4F11. Biochemistry. 52: 8276–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald M. G., Rieder M. J., Nakano M., Hsia C. K., and Rettie A. E.. 2009. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 75: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landes N., Birringer M., and Brigelius-Flohe R.. 2003. Homologous metabolic and gene activating routes for vitamins E and K. Mol. Aspects Med. 24: 337–344. [DOI] [PubMed] [Google Scholar]
- 16.Shearer M. J., and Barkhan P.. 1973. Studies on the metabolites of phylloquinone (vitamin K 1) in the urine of man. Biochim. Biophys. Acta. 297: 300–312. [DOI] [PubMed] [Google Scholar]
- 17.Tadano K., Yuzuriha T., Sato T., Fujita T., Shimada K., Hashimoto K., and Satoh T.. 1989. Identification of menaquinone-4 metabolites in the rat. J. Pharmacobiodyn. 12: 640–645. [DOI] [PubMed] [Google Scholar]
- 18.Ferland G., Doucet I., and Mainville D.. 2016. Phylloquinone and menaquinone-4 tissue distribution at different life stages in male and female Sprague-Dawley rats fed different VK levels since weaning or subjected to a 40% calorie restriction since adulthood. Nutrients. 8: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentili A., Cafolla A., Gasperi T., Bellante S., Caretti F., Curini R., and Fernandez V. P.. 2014. Rapid, high performance method for the determination of vitamin K(1), menaquinone-4 and vitamin K(1) 2,3-epoxide in human serum and plasma using liquid chromatography-hybrid quadrupole linear ion trap mass spectrometry. J. Chromatogr. A. 1338: 102–110. [DOI] [PubMed] [Google Scholar]
- 20.Groenen-van Dooren M. M., Soute B. A., Jie K. S., Thijssen H. H., and Vermeer C.. 1993. The relative effects of phylloquinone and menaquinone-4 on the blood coagulation factor synthesis in vitamin K-deficient rats. Biochem. Pharmacol. 46: 433–437. [DOI] [PubMed] [Google Scholar]
- 21.Schurgers L. J., Dissel P. E., Spronk H. M., Soute B. A., Dhore C. R., Cleutjens J. P., and Vermeer C.. 2001. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z. Kardiol. 90 (Suppl. 3): 57–63. [DOI] [PubMed] [Google Scholar]
- 22.Harrington D. J., Soper R., Edwards C., Savidge G. F., Hodges S. J., and Shearer M. J.. 2005. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J. Lipid Res. 46: 1053–1060. [DOI] [PubMed] [Google Scholar]
- 23.Fujii S., Shimizu A., Takeda N., Oguchi K., Katsurai T., Shirakawa H., Komai M., and Kagechika H.. 2015. Systematic synthesis and anti-inflammatory activity of omega-carboxylated menaquinone derivatives–Investigations on identified and putative vitamin K(2) metabolites. Bioorg. Med. Chem. 23: 2344–2352. [DOI] [PubMed] [Google Scholar]
- 24.Teitelbaum A. M., Scian M., Nelson W. L., and Rettie A. E.. 2015. Efficient Syntheses of Vitamin K Chain-Shortened Acid Metabolites. Synthesis (Stuttg.). 47: 944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prochaska H. J., and Talalay P.. 1986. Purification and characterization of two isofunctional forms of NAD(P)H: quinone reductase from mouse liver. J. Biol. Chem. 261: 1372–1378. [PubMed] [Google Scholar]
- 26.You C. S., Sontag T. J., Swanson J. E., and Parker R. S.. 2005. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J. Nutr. 135: 227–232. [DOI] [PubMed] [Google Scholar]
- 27.Sontag T. J., and Parker R. S.. 2002. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277: 25290–25296. [DOI] [PubMed] [Google Scholar]
- 28.Kim M., Na W., and Sohn C.. 2013. Vitamin K1 (phylloquinone) and K2 (menaquinone-4) supplementation improves bone formation in a high-fat diet-induced obese mice. J. Clin. Biochem. Nutr. 53: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwabara A., Tanaka K., Tsugawa N., Nakase H., Tsuji H., Shide K., Kamao M., Chiba T., Inagaki N., Okano T., et al. 2009. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos. Int. 20: 935–942. [DOI] [PubMed] [Google Scholar]
- 30.Tsugawa N., Shiraki M., Suhara Y., Kamao M., Ozaki R., Tanaka K., and Okano T.. 2008. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J. Bone Miner. Metab. 26: 79–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.