Abstract
Angiopoietin-like (ANGPTL) 8 is a secreted inhibitor of LPL, a key enzyme in plasma triglyceride metabolism. It was previously reported that ANGPTL8 requires another member of the ANGPTL family, ANGPTL3, to act on LPL. ANGPTL3, much like ANGPTL4, is a physiologically relevant regulator of LPL activity, which causes irreversible inactivation of the enzyme. Here, we show that ANGPTL8 can form complexes with either ANGPTL3 or ANGPTL4 when the proteins are refolded together from their denatured states. In contrast to the augmented inhibitory effect of the ANGPTL3/ANGPTL8 complex on LPL activity, the ANGPTL4/ANGPTL8 complex is less active compared with ANGPTL4 alone. In our experiments, all three members of the ANGPTL family use the same mechanism to inactivate LPL, which involves dissociation of active dimeric LPL to monomers. This inactivation can be counteracted by the presence of glycosylphosphatidylinositol-anchored HDL binding protein 1, the endothelial LPL transport protein previously known to protect LPL from spontaneous and ANGPTL4-catalyzed inactivation. Our data demonstrate that ANGPTL8 may function as an important metabolic switch, by forming complexes with ANGPTL3, or with ANGPTL4, in order to direct the flow of energy from triglycerides in blood according to the needs of the body.
Keywords: angiopoietin-like 8, angiopoietin-like 3, angiopoietin-like 4, glycosylphosphatidylinositol-anchored HDL binding protein 1, lipoprotein metabolism, enzymology/enzyme regulation, triglycerides
LPL (EC 3.1.1.34) is a key enzyme for plasma triglyceride (TG) hydrolysis. Its activity is tightly regulated by posttranslational mechanisms, which help to direct the hydrolysis products to tissues for energy production or storage. Three proteins from the angiopoietin-like (ANGPTL) family, ANGPTL3, ANGPTL4, and ANGPTL8, are known to function as negative regulators of LPL activity in white adipose tissue (WAT), brown adipose tissue, and oxidative tissues (1–7). ANGPTL4, the most-studied family member, is expressed at various sites in the body (8). ANGPTL4 consists of an N-terminal coiled-coil domain (ccd) and a C-terminal, fibrinogen-like domain. The N-terminal ccd of ANGPTL4 inactivates LPL by promoting dissociation of the active LPL dimer into inactive monomers (9, 10). In this way, ANGPTL4 acts as a potent energy homeostasis switch causing reduced uptake of FAs from TG-rich lipoproteins in WAT during fasting (11, 12). During physical activity or exercise, ANGPTL4 is upregulated in resting muscles, where it lowers LPL activity and thereby helps to direct FAs toward the contracting muscle tissue for energy production (13). In contrast to ANGPTL4, ANGPTL3 is mainly produced in the liver. It was previously demonstrated that the N-terminal ccd of ANGPTL3 reduces the activity of LPL in oxidative tissues in the fed state, thus directing the flow of lipoprotein-derived FAs to WAT for storage (14). In molar terms, ANGPTL3 is a relatively weak regulator of LPL in comparison to ANGPTL4 (10, 15, 16). Recent studies have shown, however, that the effect of ANGPTL3 on LPL is greatly enhanced by ANGPTL8 (15, 17, 18). ANGPTL8 is mostly produced in the liver and WAT and consists of a ccd, which is similar to those of ANGPTL3 and ANGPTL4, but ANGPTL8 is lacking a C-terminal, fibrinogen-like domain (6, 7). By itself, ANGPTL8 has no detectable effect on the activity of LPL. Reduction of LPL activity by ANGPTL8 occurs only in the presence of ANGPTL3 (15, 18). It was recently demonstrated that ANGPTL8 needs to be coexpressed with ANGPTL3, by the same host cell, in order to have an effect on LPL activity (18).
In this report, we show that recombinant ANGPTL8 produced in Escherichia coli has to be refolded together with ANGPTL3 to inhibit LPL activity under in vitro conditions. Refolded ANGPTL3 alone was able to inactivate LPL, but ANGPTL3 refolded in the presence of ANGPTL8 led up to a 3-fold increase in the molar inhibitory capacity. We also demonstrate that ANGPTL4 and ANGPTL8 form a complex when refolded together and that ANGPTL4 in that complex loses its ability to inactivate LPL. We have observed that the C-terminal helix of ANGPTL8 is important for complex formation with ANGPTL3 or ANGPTL4, rather than for covering the functional site of the protein, as was previously proposed (15).
MATERIALS AND METHODS
Protein expression and purification
The ccds of ANGPTL3 and ANGPTL4 (ccd-ANGPTL3 and ccd-ANGPTL4) and the full-length ANGPTL8, as well as ANGPTL8 with truncated C terminus, were expressed in E. coli BL-21 (DE3) strain. The ccd-ANGPTL4 (sequence 26−184) with a C-terminal 6× His-tag was expressed from a pet29a vector, as described previously (19). The ccd-ANGPTL3 (sequence 17−223) with an N-terminal 6× His-tag, followed by a thrombin cleavage site, was expressed in a pet28a vector; the full-length (sequence 22−198) ANGPTL8 protein with an N-terminal 6x His-tag was expressed in a pet22b vector. All variants, including the truncated ANGPTL8 (sequence 22−171), were produced following the same protocol as for the ANGPTL4 expression. The truncated ANGPTL8 was generated using the QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent), according to the protocol from the manufacturer, using the forward primer ATGGGCTCTTACAGGACATGTAGCATGACAAAGGAGAGAGATGGT and the reverse primer ACCATCTCTCTCCTTTGTCATGCTACATGTCCTGTAAGAGCCCAT. Mutagenesis substituted Arg172 with a stop codon and Gln171 with Ala171 to promote the stability of the C terminus of the protein. These changes were introduced to remove the C-terminal helix of ANGPTL8 (20), which was previously proposed to sterically cover the inhibitory motif in the N-terminal α-helix of ANGPTL8 (15). The mutagenesis was verified by sequencing of the plasmid and by SDS-PAGE of the purified mutant protein. Bovine LPL was purified from milk, as described previously (21). For experiments with heparin-sepharose, LPL was labeled with 125I using the lactoperoxidase method. The protocol for the iodination, as well as properties of the iodinated LPL, has been published previously (22). Glycosylphosphatidylinositol-anchored HDL binding protein 1 (GPIHBP1) without GPI anchor was produced in Drosophila S2 cells as described previously (10). The purity of all proteins was verified using SDS-PAGE, and the protein concentrations were measured using the Pierce™ BCA Protein Assay (Thermo Fisher Scientific), performed according to manufacturer’s protocol, or by measuring OD280.
Formation of ANGPTL complexes
Complexes of full-length or C-terminally truncated ANGPTL8 with ANGPTL3 and ANGPTL4 were formed as follows: the proteins were dialyzed and then stored in PBS buffer containing 5 M guanidine hydrochloride (pH 7.4). For experiments, the stock solutions of ANGPTLs were mixed in the desired molar ratio in 50 µl of the PBS buffer containing 5 M urea (pH 7.4). The mixed sample was then diluted to a total volume of 1 ml with PBS, containing 0.01% (v/v) of Triton X-100, so that the final concentration of protein was 180 nM and the final concentration of urea was 0.25 M. The samples were then incubated at 23°C for at least 10 min to allow folding. For experiments with LPL, the samples with ANGPTLs were further diluted at least 12-fold in the preincubation mixture (see below), so that the final concentration of urea was reduced to less than 21 mM and the concentration of ANGPTL was reduced to 15 nM. To ensure that urea did not interfere with the effects of ANGPTL on LPL activity, experiments with LPL alone were conducted in buffer containing the same concentration of urea as in the experiments with ANGPTL proteins and their complexes.
Measurement of LPL activity
Inhibition of LPL activity by ANGPTL proteins was measured in 96-well flat-bottom plates containing a total volume of 60 µl of 15 nM LPL in PBS buffer (pH 7.4) with 0.01% (v/v) of Triton X-100. LPL was preincubated for 10 min at 23°C on an orbital shaker (at 600 rpm) in the presence or absence of different amounts of ANGPTL proteins. The remaining LPL activity was then determined, as described before, using 20% Intralipid® (Fresenius Kabi, Germany) as a substrate and by measuring the amount of FFAs produced by using the NEFA kit from Wako Diagnostics (10). To study the protection of LPL activity by the presence of GPIHBP1, this protein was added to LPL prior to the addition of the ANGPTL proteins (10). The final concentration of GPIHBP1 was 300 nM. All measurements of LPL activity were performed in triplicate.
Heparin-sepharose chromatography
Heparin-sepharose chromatography was performed using the ÄKTA Purifier system (GE Healthcare, UK). A HiTrap Heparin HP 1 ml column (GE Healthcare) was equilibrated with 50 mM PBS (pH 7.4), containing 20% (wt/vol) glycerol and 0.01% (v/v) Triton X-100 at 4°C. For the experiments, 15 nM LPL, containing a trace amount of 125I-labeled LPL corresponding to 10,000–20,000 cpm, was mixed with equimolar concentrations of any of the ANGPTLs in a total volume of 300 µl of PBS buffer (pH 7.4) with 0.01% (v/v) Triton X-100 and incubated for 10 min at 23°C. The inactivation of LPL was stopped after 10 min by the addition of sodium-taurodeoxycholate to a final concentration of 25 mM, and the reaction mix was rapidly cooled down to 4°C by placing it on ice. The sample was then diluted with 2 vol (660 µl) of ice-cold equilibration buffer and was spun at 12,000 g for 5 min at 4°C in a benchtop centrifuge. The 700 µl of sample was then loaded on the Heparin HP column at a flow rate of 0.5 ml/min via an injection loop. The column was washed with equilibration buffer and was then eluted with a linear gradient of NaCl from 0.15 M to 1.5 M in the same buffer at a flow rate of 1 ml/min. Fractions were analyzed for γ radiation on a Wallac 1480 Wizard 3″. The recovery of radioactivity was more than 70% of the loaded sample. Chromatography on heparin-sepharose was carried out in triplicate for each combination of ANGPTL and LPL.
Coimmunoprecipitation of ANGPTL complexes
Coimmunoprecipitation of the ANGPTLs was carried out using protein G-coupled Dynabeads (Invitrogen), according to the manufacturer’s protocol. Individual ANGPTL proteins and their respective complexes, each at a concentration of 36.4 nM, were diluted in 900 µl PBS (pH 7.4) with 0.02% Tween 20. The proteins and complexes were then captured using 2 µg of the anti-human ANGPTL4 capture Ab from the human ANGPTL4 ELISA kit (catalog no. DY3485, R&D Systems) or 2 µg of the anti-human ANGPTL8 monoclonal mouse Ab (catalog no. MAB8548, R&D Systems). To avoid interference from the capture Abs with detection of the ANGPTL proteins on Western blots, the Abs were first cross-linked to protein G-coupled Dynabeads using BS3 (Sulfo-DSS) bis(sulfosuccinimidyl)suberate (Thermo Fisher Scientific), according to the manufacturer’s protocol. After binding of the protein samples, the beads were washed three times with 200 µl of PBS (pH 7.4) with 0.02% Tween 20. The proteins were then eluted from the Ab beads by the addition of 20 µl of 50 mM glycine (pH 2.8) and 10 µl of 4× reducing loading buffer (Bio-Rad Laboratories) and by heating to 80°C for 10 min. The eluted samples were neutralized by the addition of 10 µl of 1 M Tris-HCl (pH 8.0) after the heat treatment.
Western blot
Samples obtained from coimmunoprecipitation were run under reducing conditions on Mini-PROTEAN TGX stain-free gels (Bio-Rad Laboratories), a 20 sample per well, and were then transferred overnight at 4°C onto a BioTrace™ NT Nitrocellulose Transfer Membrane (Pall Corporation). After transfer, the membranes were washed once for 15 min and twice for 5 min with TTBS buffer [20 mM Tris-HCl, 0.5 M NaCl, and 0.05% (v/v) Tween 20, pH 7.5]. Membranes containing samples of ANGPTL3 and ANGPTL4 were blocked with 2% Amersham ECL Prime Blocking Reagent (GE Healthcare Life Sciences) in TTBS at room temperature. Membranes containing full-length and truncated ANGPTL8 were blocked with 10% FCS (Gibco, Thermo Fisher Scientific) in TTBS for at least 1 h at room temperature. Then, the membranes were incubated for 1 h at room temperature with the primary Abs for the respective target protein, diluted in blocking solution. For ANGPTL3, a 1:1,000 dilution of anti-human ANGPTL3 capture Ab from the human ANGPTL3 ELISA kit (catalog no. DY3829, R&D Systems) was used. For ANGPTL4, a 1:1,000 dilution of anti-human ANGPTL4 capture Ab from the human ANGPTL4 ELISA kit (catalog no. DY3485, R&D Systems) was used. ANGPTL8 was detected with a 1:2,500 dilution of an anti-human ANGPTL8 monoclonal mouse Ab (catalog no. MAB8548, R&D Systems). After incubation with the Abs for 1 h at room temperature, the membranes were washed once for 15 min and twice for 5 min with TTBS buffer. After wash, the membranes were incubated with secondary Abs: for ANGPTL3 with a 1:30,000 dilution of HRP-conjugated goat-anti-rat Abs (catalog no. AS09618, Agisera, Sweden), for ANGPTL4 with a 1:50,000 dilution of HRP-conjugated rabbit-anti-goat Abs (catalog no. P0449, Dako, Agilent), and for ANGPTL8 with a 1:50,000 dilution of HRP-conjugated goat-anti-mouse Abs (catalog no. PK-AB718-9720, PromoKine, Germany). The membranes were washed once for 15 min and twice for 5 min with TTBS buffer and were then visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific), according to manufacturer’s protocols on a Bio-Rad Gel Doc system and analyzed with Bio-Rad Image Lab software (Bio-Rad Laboratories).
Statistics
Data were analyzed using an unpaired t-test in GraphPad Prism 7 (GraphPad Software). Values used in figures are as follows: ns, P > 0.05; * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001.
RESULTS
ANGPTL3 folded together with ANGPTL8 yields increased capacity for LPL inhibition
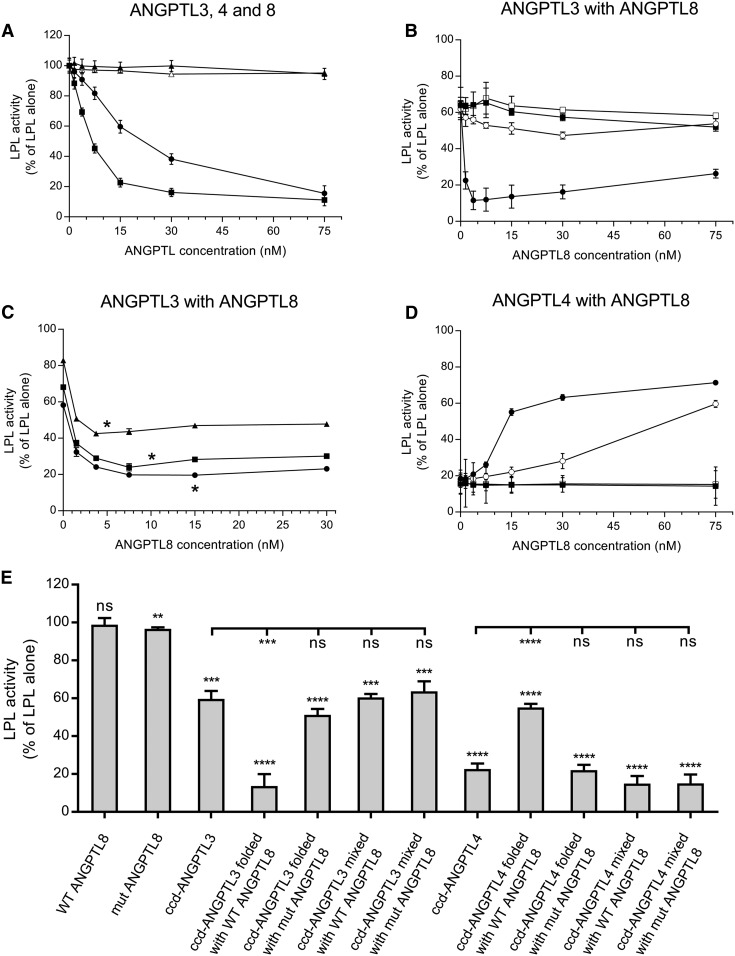
Both ANGPTL3 and ANGPTL4 demonstrated a concentration-dependent inhibition of LPL activity (Fig. 1A). Under our experimental conditions, roughly twice as high protein concentration of ANGPTL3 as of ANGPTL4 was required to reach the same degree of inhibition of LPL activity. ANGPTL8, on the other hand, had no effect on LPL activity on its own. This was in line with previously reported observations (15). We decided to remove the C-terminal helix of ANGPTL8 that was previously proposed to cover and inactivate the inhibitory motif of the ANGPTL8 protein (15). Like the full-length ANGPTL8, the truncated form showed no effect on LPL activity, even at high concentrations (Fig. 1A). To demonstrate that ANGPTL8 requires ANGPTL3 to inhibit LPL, we mixed ANGPTL3 with full-length ANGPTL8, as well as with the truncated ANGPTL8 mutant, in PBS. We observed no additional effect of any of the forms of ANGPTL8 on LPL activity over that already present due to ANGPTL3 (Fig. 1B, E). To investigate whether the proteins needed to be folded together to form a functional complex, we mixed them in their fully denatured states in 5 M urea and then folded them under mild refolding conditions. We observed a dramatic effect on the inhibition of LPL activity from cofolding ANGPTL3 with ANGPTL8 (Fig. 1B, E). The titration profile for LPL inhibition by 15 nM ANGPTL3, refolded with increasing concentrations of ANGPTL8, reached maximal inhibition at substoichiometric ANGTPL8 levels (3.7 nM). This led us to suspect that ANGPTL3/ANGPTL8 complexes are very efficient inhibitors of LPL and that a complex concentration of 5 nM would be sufficient to cause complete depletion of LPL activity. To investigate this, we repeated the experiments with 5, 10, and 15 nM ANGPTL3. Lowering the amount of ANGPTL3 revealed that the maximal inhibition of LPL activity was indeed obtained at close to 1:1 stoichiometry between ANGPTL8 and ANGPTL3 in the refolded complex (Fig. 1C). This is in line with previous observations, which suggest that ANGPTL8 is a potent regulator of LPL and that it plays a key role in the ANGPTL3/ANGPTL8-mediated effect on LPL (15, 18). In contrast to the full-length ANGPTL8, the truncated ANGPTL8 failed to have any additional effect on LPL activity over that of ANGPTL3 alone, even when the two proteins were refolded together (Fig. 1B, E).
Fig. 1.
Effect of ANGPTL proteins on LPL activity in vitro. A: Effects of single ANGPTL proteins on LPL activity. LPL (final concentration 15 nM) was mixed with the indicated concentrations of ccd-ANGPTL3 (filled circles), ccd-ANGPTL4 (filled squares), full-length ANGPTL8 (filled triangles), or ANGPTL8 with truncated C terminus (open triangles) and was incubated for 10 min at 23°C. B: Effect of ANGPTL8 in a complex with ANGPTL3 on LPL activity. LPL (final concentration 15 nM) was mixed with an equimolar concentration of prefolded ANGPTL3, which was mixed with prefolded full-length ANGPTL8 (filled squares) or with prefolded truncated ANGPTL8 (open squares). Results are also shown for the experiments in which LPL was mixed with refolded complexes between ANGPTL3 and full-length ANGPTL8 (filled circles) or between ANGPTL3 and truncated ANGPTL8 (open circles). C: Effect of ANGPTL8 in complex with different concentrations of ANGPTL3 on LPL activity. LPL (final concentration 15 nM) was mixed with refolded complexes between ANGPTL3 and full-length ANGPTL8 with ANGPTL3 at concentrations of 5 nM (triangles), 10 nM (squares), or 15 nM (circles). Asterisks denote the equimolar levels of ANGPTL3 and ANGPTL8. D: Effect of ANGPTL8 in complex with ANGPTL4 on LPL activity. LPL (final concentration 15 nM) was incubated for 10 min with an equimolar concentration of prefolded ANGPTL4, either premixed with prefolded full-length ANGPTL8 (filled squares) or prefolded truncated ANGPTL8 (open squares). Results are also shown for the experiment in which LPL was incubated with refolded complexes between ANGPTL4 and full-length ANGPTL8 (filled circles) or between ANGPTL4 and truncated ANGPTL8 (open circles). E: Summary of the effects of different ANGPTL proteins and their complexes on LPL activity. Data shown correspond to ANGPTL concentrations that were equal to the concentration of LPL. Asterisks above bars represent statistical significance in comparison with the activity of LPL alone, which was set to 100% and is not shown on the graph. Asterisks under the lines represent statistical significance in comparison to ccd-ANGPTL3 and ccd-ANGPTL4. All activity experiments were run in triplicate. ns, P > 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001.
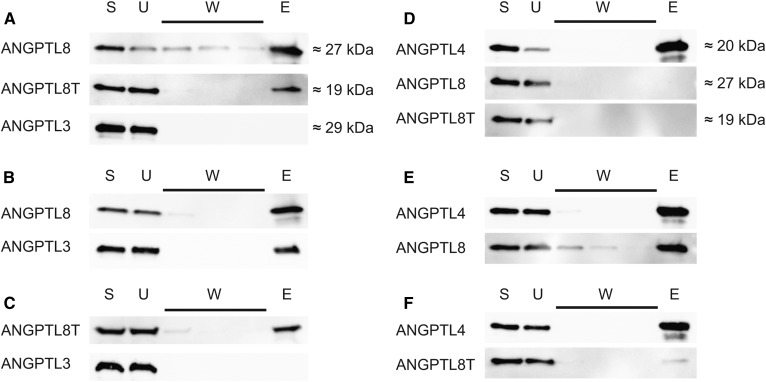
To investigate the role of the C-terminal helix of ANGPTL8 for inhibition of LPL activity, we ran coimmunoprecipitation experiments with ANGPTL3 and either full-length or truncated ANGPTL8. On its own, ANGPTL3 did not bind to the monoclonal anti-ANGPTL8 Ab-coated protein G beads (Fig. 2A). When folded together with ANGPTL8, ANGPTL3 coeluted with ANGPTL8 from the beads, similar to what was shown before (18) (Fig. 2B). This indicates that the proteins form a relatively stable heterooligomer. Truncated ANGPTL8 was, however, incapable of forming complexes with ANGPTL3 (Fig. 2C). This suggests that the C-terminal helix of ANGPTL8 is important for complex formation with ANGPTL3.
Fig. 2.
Coimmunoprecipitation of ANGPTL complexes. Western blot of fractions obtained from coimmunoprecipitation of refolded ANGPTL complexes by protein G beads coated with an anti-ANGPTL8 Ab for ANGPTL3/ANGPTL8 complexes or with anti-ANGPT4 Ab for ANGPTL4/ANGPTL8 complexes. ANGPTL8T stands for the truncated ANGPTL8 protein with residues 172–198 removed. Fractions were as follows: E, elution; S, starting material; U, unbound material; W, washes 1–3. A: A control experiment with the individual proteins and anti-ANGPTL8 protein G beads. B: Complexes between ANGPTL3 and full-length ANGPTL8 captured on anti-ANGPTL8 protein G beads. C: Complexes between ANGPTL3 and truncated ANGPTL8 captured on anti-ANGPTL8 protein G beads. D: Control experiment with the individual proteins and anti-ANGPTL4 protein G beads. E: Complexes between ANGPTL4 and full-length ANGPTL8 on anti-ANGPTL4 protein G beads. F: Complexes between ANGPTL4 and truncated ANGPTL8 on anti-ANGPTL4 protein G beads.
Inhibition of LPL by ANGPTL4 is diminished when it forms a complex with ANGPTL8
There are many structural and functional similarities between ANGPTL3 and ANGPTL4. ANGPTL4 is produced in WAT, where ANGPTL8 is also expressed (6, 7). This raised the hypothesis that ANGPTL8 might be able to form heterooligomers with ANGPTL4, similar to what was found with ANGPTL3. We wanted to examine whether such complexes formed and, if so, what their impact was on LPL activity in vitro. When mixed with prefolded ANGPTL4, neither full-length ANGPTL8 nor truncated ANGPTL8 had any effect on LPL activity (Fig. 1D, E). As in the case with ANGPTL3, full-length ANGPTL8 or truncated ANGPTL8 were active only when they were mixed with ANGPTL4 in 5 M urea and then diluted in PBS to allow refolding under mild conditions. To our surprise, full-length ANGPTL8 diminished the inhibitory effect of ANGPTL4 on LPL (Fig. 1D, E). The activity of LPL increased with increasing concentrations of ANGPTL8. The truncated form of ANGPTL8 had a similar effect on the inhibition with ANGPTL4, but this effect occurred at 5-fold higher concentrations of ANGPTL8 than with full-length ANGPTL8 (Fig. 1D, E). We then used coimmunoprecipitation to verify complex formation between ANGPTL4 and ANGPTL8. Protein G beads with anti-ANGPTL4 Ab were unable to bind full-length or truncated ANGPTL8 on their own (Fig. 2D). As in the case with ANGPTL3, full-length ANGPTL8 formed a stable complex with ANGPTL4 (Fig. 2E). Truncated ANGPTL8 was also able to bind to ANGPTL4, but this interaction was diminished in comparison to that of the full-length ANGPTL8 protein (Fig. 2F). This observation was in line with results from measurements of LPL activity in the presence of ANGPTL4, demonstrating that truncated ANGPTL8 reduced the inhibition of LPL, but that much higher concentrations were required of the truncated ANGPTL8 than of the full-length ANGPTL8 protein (Fig. 1D, E).
GPIHBP1 protects LPL from the inhibitory effects of ANGPTL proteins
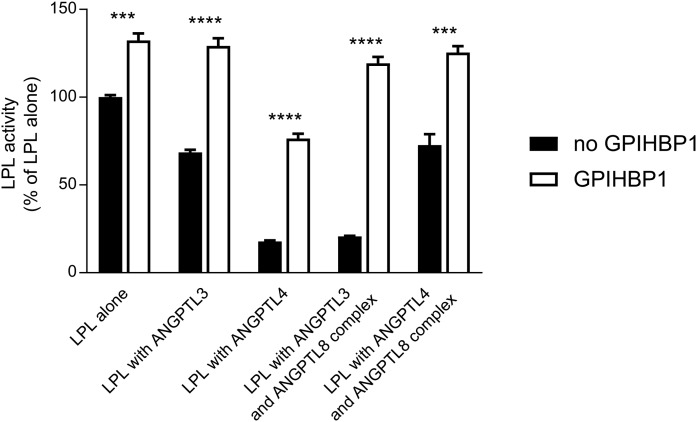
It is well established that GPIHBP1 preserves LPL activity from decay by spontaneous, irreversible inactivation due to the unfolding of the catalytic domain and that GPIHBP1 is also capable of counteracting the inhibitory effects of ANGPTL4 and ANGPTL3 on LPL activity (10, 16, 23, 24). The proteins from the ANGPTL family share a similar LPL inhibitory motif in the N-terminal α-helix (15). We wanted to test whether the effects of the refolded complexes of ANGPTL8 with ANGPTL3 or ANGPTL4 were prevented by GPIHBP1. To test this, a 20-fold molar excess of purified GPIHBP1 was preincubated with LPL prior to the addition of equimolar concentrations (compared with LPL) of the different combinations of the ANGPTL proteins. LPL activity was measured after a 10 min incubation with the ANGPTL proteins at room temperature. Already at this time, 25% of the activity of LPL alone was spontaneously lost in comparison with LPL incubated with GPIHBP1, recapitulating the intrinsically unstable nature of the LPL protein (23). ANGPTL3 inhibited 31% of the LPL activity when compared with LPL alone, but had no effect on LPL activity in the presence of GPIHBP1. ANGPTL4 alone and the refolded ANGPTL3/ANGPTL8 complex caused roughly 80% loss of LPL activity compared with the control level. GPIHBP1 completely counteracted the inhibitory effect of the ANGPTL3/ANGPTL8 complex on LPL. In the case of ANGPTL4, the presence of GPIHBP1 reduced the loss of LPL activity to 42%. Also shown is that the ANGPTL4/ANGPTL8 complex was less potent than ANGPTL4 alone. The complex inactivated only one-third of the LPL. In this case, the presence of GPIHBP1 preserved all of the LPL activity (Fig. 3).
Fig. 3.
GPIHBP1 protects LPL from inactivation by ANGPTL proteins. LPL (final concentration 15 nM) was incubated for 10 min at 23°C with equimolar concentrations of ANGPTL3, ANGPTL4, or ANGPTL3 refolded with ANGPTL8 or ANGPTL4 refolded with ANGPTL8 (filled bars). In parallel, experiments were performed in the presence of 300 nM GPIHBP1 (open bars). Activity experiments were performed in triplicate. *** P ≤ 0.001; **** P ≤ 0.0001.
Dissociation of LPL dimers by ANGPTL proteins and their complexes
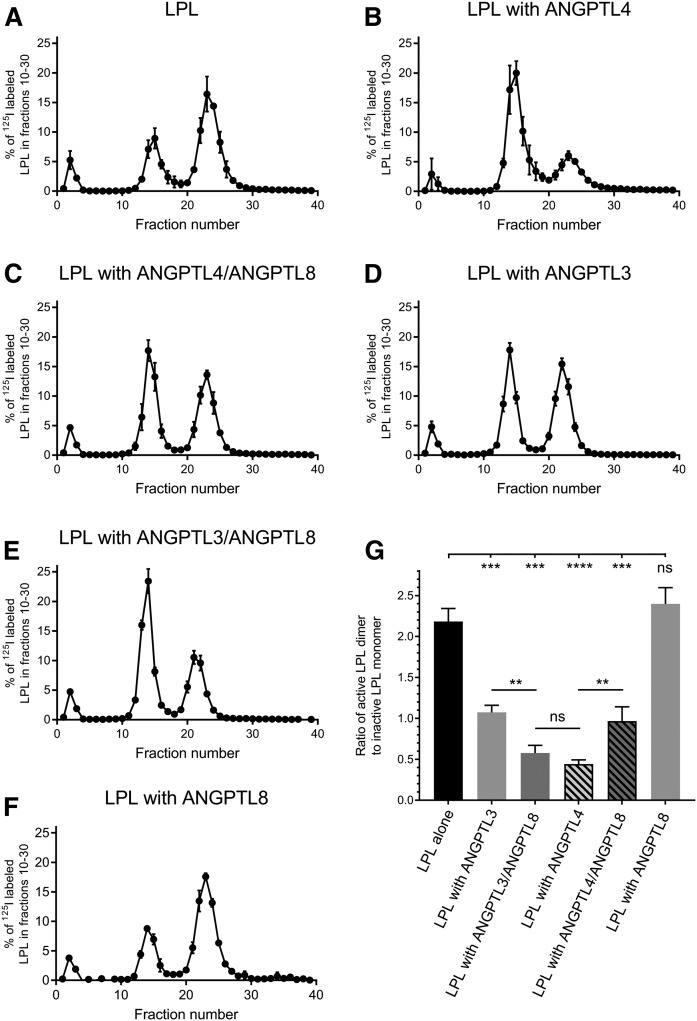
We next studied the potential mechanisms responsible for the inhibition of LPL activity by the ANGPTLs. It was previously concluded that ANGPTL4 acts as an unfolding chaperon for LPL because ANGPTL4 promotes dissociation of the intrinsically unstable LPL dimer to inactive monomers (9, 10). Because all three ANGPTLs share a similar inhibitory motif, we suspected that the effects of complexes with ANGPTL8 on the LPL molecule would be similar to that of ANGPTL4. We, therefore, performed chromatography on heparin-sepharose, as was previously done to study the effect of ANGPTL4 on LPL (9). It is well established that monomers and dimers of LPL interact with heparin with a different affinity (25–27). Therefore, LPL can be separated on a heparin-sepharose column into inactive LPL monomers and active LPL dimers (Fig. 4A). As demonstrated previously, ANGPTL4 promoted dissociation of LPL dimers to monomers. After 10 min of preincubation of 180 nM LPL with an equimolar concentration of ANGPTL4, there was only 30.6 ± 2.5% of active dimers remaining (Fig. 4B) (9). In contrast, when 180 nM LPL alone was incubated for 10 min at room temperature, there was 68.5 ± 1.6% of active dimers remaining (Fig. 4A). With 180 nM ANGPTL3, 51.7 ± 2.0% of LPL maintained its dimeric status (Fig. 4D). This was in line with the differences in effects on LPL activity (Fig. 1A). In the presence of the complexes of 180 nM ANGPTL3 with equimolar amounts of ANGPTL8, 36.4 ± 3.8% of LPL remained in the dimer state. Based on these observations, we conclude that the ANGPTL3/ANGPTL8 complex is more efficient than ANGPTL3 alone in catalyzing the dissociating of LPL dimers. These results are in line with the loss of LPL activity (Fig. 1E), demonstrating that the ANGPTL3/ANGPTL8 complex inactivates LPL as efficiently as ANGPTL4 (Fig. 4G). In the presence of complexes formed between 180 nM ANGPTL4 and equimolar concentrations of ANGPTL8, the dissociation of LPL was significantly attenuated; 48.9 ± 4.5% of LPL remained as dimers, compared with 30.6 ± 2.5% with ANGPTL4 alone (Fig. 4E). This observation is again well aligned with measurements of LPL activity, where the effect of the ANGPTL4/ANGPTL8 complex on LPL was comparable to the effect of ANGPTL3 (Fig. 1E). ANGPTL8 alone had no effect on the amount of LPL dimers (Fig. 4F), a result that was in line with the LPL activity measurements (Fig. 1A).
Fig. 4.
Separation of active and inactive forms of LPL by chromatography on heparin-sepharose after incubation with ANGPTL3 or ANGPTL4 or their refolded complexes with ANGPTL8. Heparin-sepharose chromatograms of LPL after a 10 min incubation with or without ANGPTL proteins. Results are presented as percent of eluted 125I-labeled LPL, which serves as a tracer for the total LPL protein. The gradient started at 0.15 M [NaCl] from fraction 5 and ended at 2.00 M [NaCl] at fraction 35. According to previously published experiments, the early peaks to the left corresponds to unbound LPL, the peak in fractions 10–19 corresponds to inactive LPL monomers, and the peak in fractions 20–30 corresponds to active LPL dimers (9). A: LPL alone. B: LPL incubated with ANGPTL4. C: LPL incubated with the ANGPTL4/ANGPTL8 complex. D: LPL incubated with ANGPTL3. E: LPL incubated with the ANGPTL3/ANGPTL8 complex. F: LPL incubated with ANGPTL8. G: Ratios for areas under the curve from the experiments in A–E. Bars represent the ratio of active dimeric LPL (fractions 20–30) to inactive monomeric LPL (fractions 10–19). All chromatography experiments were performed in triplicate. ns, P > 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001.
DISCUSSION
In this study, we have analyzed the effects of purified human ANGPTL proteins expressed in E. coli on LPL in order to investigate the mode of action of the ANGPTL8 protein. We used only the N-terminal ccds of ANGPTL3 and ANGPTL4, because previous observations had shown that the ccd-ANGPTLs have similar effects on LPL activity as full-length analogs expressed in eukaryotic cells (10, 19). Additionally, ANGPTL3 and ANGPTL4 are found both in full-length and cleaved states in human plasma (28–31). The C-terminal, fibrinogen-like domain of ANGPTL4 stimulates intracellular lipolysis in adipose tissue and is also involved in the regulation of angiogenesis (32). There is, however, no evidence for direct effects of the C-terminal domains of ANGPTL proteins on LPL. We have avoided the full-length proteins in the present study because preliminary experiments showed that they would be even more challenging to keep in solution during the refolding process.
Previous studies proposed that ANGPTL3 has weak effects on LPL on its own and that only the complex of ANGPTL3 with ANGPTL8 acts as a regulator of LPL activity (15, 17, 18). Our present results, together with our previous observations, demonstrate that the N-terminal ccd of ANGPTL3 is capable of inactivating LPL, even at substoichiometric concentrations, by catalyzing the unfolding of large parts of the catalytic domain (10, 33). The levels of ANGPTL3 in human plasma are the highest among all three ANGPTLs studied here, and this level should be high enough to enforce LPL inhibition on its own (33, 34). Nonetheless, no correlation between plasma levels of ANGPTL3 and plasma TG or LPL activity in tissues has yet been established. This may be explained by observations demonstrating that LPL is strongly stabilized by the presence of plasma lipoproteins and by GPIHBP1 (10, 35). The molecular mechanism for the action of ANGPTL3 on LPL remains unclear as well. Some authors concluded that ANGPTL3 reduces LPL activity by reversible inhibition (36), whereas others reported that ANGPTL3 catalyzes inhibition of LPL by inducing a similar irreversible unfolding of the hydrolase domain in LPL, as does ANGPTL4 (10). Our data strongly favor the second model—the effect of ANGPTL3 on LPL activity is weaker, but the mechanism is nonetheless similar to that of ANGPTL4. Heparin-sepharose experiments demonstrated that treatment of LPL with ANGPTL3 resulted in increased amounts of inactive monomers compared with untreated LPL.
Our observations confirm that, on its own, ANGPTL8 has no effect on LPL (15, 18). Only when ANGPTL8 and ANGPTL3 were mixed from their denatured states, and then refolded together under nondenaturing conditions, the complexes of ANGPTL3/ANGPTL8 became more potent than ANGPTL3 alone. Our observations match previously published data, demonstrating that a peptide representing the inhibitory motif of ANGPTL8 was a far more potent inhibitor of LPL activity than was the corresponding peptide representing the inhibitory motif of ANGPTL3 (15). Titration experiments revealed that the highest capacity to inhibit LPL is formed at a nearly 1:1 ratio between ANGPTL8 and ANGPTL3.
Our results are in line with the recently published work by Chi et al. (18), who observed that ANGPTL8 has to be coexpressed with ANGPTL3 to have an effect on LPL activity. In a cellular model using coimmunoprecipitation, they demonstrated that ANGTPL3 and ANGPTL8 form a complex. We performed coimmunoprecipitation of refolded complexes of ANGPTL3 with ANGPTL8 using Abs coupled to protein G beads. When folded together with ANGPTL8, ANGPTL3 coeluted from beads coated with the anti-ANGPTL8 Ab. Neither our data nor data from Chi et al. (18) are sufficient to deduce the molecular mechanism underlying the synergistic effects of ANGPTL8 and ANGPTL3. ANGPTL8 could affect the conformation of ANGPTL3 so that it becomes more competent for interaction with LPL, ANGPTL8 could itself attain a conformation in the complex that is suitable for interaction with LPL, or the effect is dependent on an optimal binding of both proteins to LPL, which only occurs when ANGPTL8 is in complex with ANGPTL3.
Previous studies had suggested that the C-terminal part of ANGPTL8 forms a short helix that covers the inhibitory site of ANGPTL8 and prevents this protein from having an effect on LPL on its own (15, 20). Haller et al. (15) proposed that the functional site of ANGPTL8 opens up when this protein is in complex with ANGPTL3 and that this allows ANGPTL8 to add to the inactivation action on LPL. A monoclonal Ab against the C-terminal helix of ANGPTL8 counteracted its effect in vivo, which led to the hypothesis that the Ab covered the functional site of ANGPTL8, as well as its site for interaction with ANGPTL3 (15). We generated ANGPTL8 with a deletion corresponding to the proposed third helix (residues 172–198) and found that, on its own, the truncated ANGPTL8 had no effect on LPL. In addition to that, deletion of the third helix of ANGPTL8 resulted in a blunted effect of the ANGPTL3/ANGPTL8 complex on LPL activity. Coimmunoprecipitation showed that this was due to the inability of the truncated ANGPTL8 to form a complex with ANGPTL3. The inhibitory Ab in the work of Haller et al. (15) was able to disrupt the complex between ANGPTL3 and ANGPTL8, and this resulted in the loss of the effect of ANGPTL8 on LPL activity (18).
The structural similarities between all three ANGPTL proteins studied here raised the possibility that ANGPTL8 could also form complexes with ANGPTL4. Both proteins are expressed in the same cells of several tissues (6–8). In adipose tissue, ANGPTL4 is upregulated in the fasted state, resulting in low amounts of active LPL on the capillaries in adipose tissue, and therefore allows TGs to be taken up preferentially in oxidative tissues (9, 37). ANGPTL8, on the other hand, is upregulated in the fed state when there are low amounts of ANGPTL4 present in adipose tissue. In brown adipose tissue, ANGPTL8 expression is increased on cold exposure, whereas ANGPTL4 expression is decreased, resulting in increased LPL activity and TG uptake by the tissue (38). We found that when the recombinant forms of ANGPTL4 and ANGPTL8 were refolded together, complexes were formed. The complex of ANGPTL4 with ANGPTL8 had less effect on LPL activity compared with ANGPTL4 alone. The effect of ANGPTL8 on ANGPTL4 was concentration-dependent, and the titration experiment revealed that a 1:1 stoichiometry of ANGPTL4 to ANGPTL8 mirrored the maximal attainable inhibition of ANGPTL4 activity. This stoichiometry is close to the ratio between estimated levels of ANGPTL4 and ANGPTL8 in human tissues (8). In WAT, the levels of ANGPTL4 and ANGPTL8 differ quite dramatically, depending on the nutritional state of the body, and the changes occur in the opposite directions (39). This suggests that formation of an ANGPTL4/ANGPTL8 heterooligomer could neutralize the effect of ANGPTL4 on LPL activity. Our results shed light on the observation, made by Wang et al. (39), that full-body KO of ANGPTL8 in mice diminishes the uptake of radiolabeled palmitate from VLDL in the fed state in WAT. They speculated that “ANGPTL8 spares LPL in this organ,” (Ref. 39; p. 16113) whereas our data suggest that ANGPTL8 acts as an inhibitor of ANGPTL4.
A slight inhibitory effect of ANGPTL8 on ANGPTL4 was observed when the truncated mutant of ANGPTL8 was folded together with ANGPTL4. However, 5-fold higher concentrations of the mutant were needed in order to achieve effects comparable with the full-length ANGPTL8. Experiments with coimmunoprecipitation demonstrated that the truncated ANGPTL8 interacted with ANGPTL4, but this interaction was not as strong as the interaction with full-length ANGPTL8. These results point to the possibility that the C-terminal helix of ANGPTL8 may be involved in the complex formation with ANGPTL4, but other parts of ANGPTL8 may also play a role in this interaction.
The observation that ANGPTL8 can neutralize the inhibitory effect of ANGPTL4 on LPL is novel. Data from Chi et al. (18) demonstrate that ANGPTL8 is not secreted unless it is in a complex with ANGPTL3. The same might be true for ANGPTL8 in a complex with ANGPTL4. This, together with the observations that ANGPTL8 is expressed in the same tissues as ANGPTL4 and that ANGPTL8 is upregulated under conditions when ANGPTL4 is downregulated, points to the possibility that ANGPTL8 could be a physiologically relevant inhibitor of ANGPTL4 (6, 7). The interaction of ANGPTL8 with ANGPTL4 can modulate the effects of ANGPTL4 on LPL when ANGPTL4 should be depressed, i.e., when the adipose should store fat rather than inhibit LPL and stimulate intracellular lipolysis.
GPIHBP1 can protect LPL both from spontaneous and ANGPTL4- or ANGPTL3-catalyzed inactivation (10, 16, 23). It was previously proposed that ANGPTL4 inhibits LPL mainly in the subendothelial space, close to the site where both proteins are produced (40). Recent evidence suggests, however, that intracellular degradation of LPL may also be promoted by the ANGPTL4 protein (41, 42). This makes ANGPTL4 an autocrine/paracrine regulator of LPL, which is unable to inactivate LPL when the enzyme has reached GPIHBP1 on the basal side of the capillary endothelium (10, 37). What remains unclear is where ANGPTL3 and the ANGPTL3/ANGPTL8 complexes might act on LPL. Both ANGPTL proteins are synthesized in the liver, making them endocrine regulators of LPL activity in extrahepatic tissues. Our present results, together with previous observations, demonstrate that GPIHBP1 protects LPL from inactivation by all three ANGPTL proteins and by their respective complexes (10, 16). The only ANGPTL protein, which had an effect on LPL when bound to GPIHBP1, was ANGPTL4, but the inhibitory effect was still blunted. Our data indicate that if LPL is attached to GPIHBP1 on the luminal side of capillaries, it will be protected from inactivation by ANGPTL3 and ANGPTL8. In addition, interaction with lipoproteins protects LPL both from spontaneous and ANGPTL4- or ANGPTL3-induced inactivation (35). This suggests that ANGPTL3 and its complex with ANGPTL8 may have to enter the subendothelial space, where the concentration of stabilizing lipoproteins is lower than in the circulating blood, and LPL might not yet be bound to GPIHBP1. Another possible window for the action of ANGPTL3/ANGPTL8 on LPL is when the lipoprotein is sufficiently unloaded via TG hydrolysis, causing LPL to be released from the particle (43).
LPL is relatively stable at 4°C and below but is rapidly inactivated at higher temperatures. Previous studies had demonstrated that ANGPTL3 and ANGPTL4 promote the spontaneous inactivation of LPL at 25°C, possibly by lowering the activation energy needed for the dissociation of dimers into inactive monomers (9, 10). Because all three ANGPTLs share sequence homology in their putative functional site, we speculated that ANGPTL8 and its complexes with ANGPTL3 and ANGPTL4 should act on LPL by a similar mechanism. As was shown previously in studies with ANGPTL4 by Sukonina et al. (9), we observed that treatment of LPL with ANGPTL4 shifted the ratio between dimers and monomers toward the inactive monomeric state of LPL. ANGPTL3 alone, or in complex with ANGPTL8, appeared to inactivate LPL by a similar mechanism. The ANGPTL3/ANGPTL8 complex was more potent in converting dimers to monomers than ANGPTL3 alone. In contrast, ANGPTL4 in complex with ANGPTL8 had a reduced effect on the dissociation of LPL dimers to monomers. These data supported and explained the results from experiments on LPL activity in the presence of ANGPTL proteins.
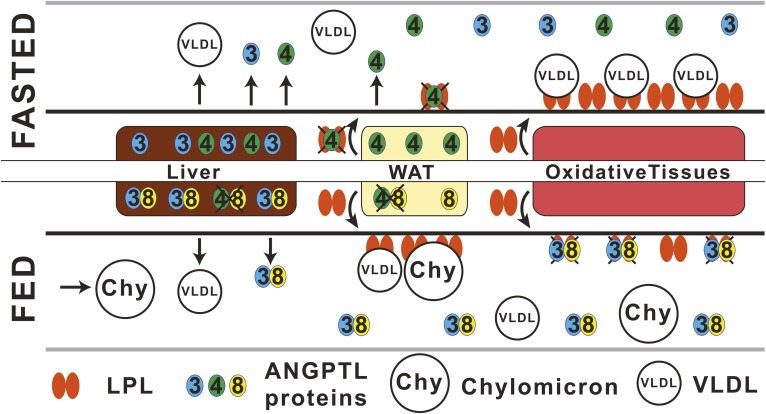
In summary, we propose a revised model for the action for the ANGPTL3-4-8 triad for control of LPL activity (Fig. 5). In the fasted state, ANGPTL4 levels increase in WAT, causing inactivation of LPL locally in an autocrine/paracrine fashion in the subendothelial space and decreasing the amounts of active LPL that reaches the luminal side of capillaries in WAT. As a result, TGs lodged in circulating VLDLs are used in other tissues for oxidation and energy production, instead of for storage in WAT. In addition, the increased levels of the C-terminal, fibrinogen-like domain of ANGPTL4 may stimulate the intracellular lipolysis inside adipocytes, which releases stored TGs into the blood in the form of FAs and thereby further decreases the lipid content in WAT (32). The liver produces soluble ANGPTL3 and ANGPTL4, which can act on LPL wherever the enzyme is exposed to blood. However, the average concentration of ANGPTL4 in human plasma is too low to have any effect on LPL (33, 35). In the fed state, or upon cold exposure, the expression of ANGPTL4 is reduced in WAT or brown adipose tissue, respectively. The low levels of ANGPTL4, still present in the cells, may form inactive complexes with ANGPTL8, whose expression is greatly increased (6, 7, 38). The ANGPTL4/ANGPTL8 complex may be unable to promote the intracellular degradation of LPL and thus allow more LPL to be secreted by WAT, but the precise mechanism for this process remains unclear (41, 42). Because of the decreased levels of ANGPTL4 in the subendothelial space, LPL can reach GPIHBP1 and the luminal side of the capillaries and hydrolyze TGs from VLDLs and chylomicrons. This promotes TG storage in WAT. In the fed state, the hepatic ANGPTL8 expression is increased, and ANGPTL8 forms complexes with ANGPTL3. These complexes are secreted for inactivation of LPL on the surface of capillaries of oxidative tissues, protecting the tissue from overload by TGs. Although LPL is protected by interactions with GPIHBP1 and lipoproteins while on the luminal side of the capillaries, these interactions are transient, and ANGPTL3/ANGPTL8 complexes may act on LPL in the short periods of time when LPL is not bound to either lipoproteins or GPIHBP1 (43).
Fig. 5.
Proposed mechanism for the action of the ANGPTL3-4-8 inhibitory triad. In the fasted state, WAT produces large amounts of ANGPTL4 that inactivate LPL locally, intracellularly, or in the subendothelial space. In this situation, little or no active LPL can reach the luminal surface of capillaries in WAT. Therefore, hydrolysis of VLDLs will preferentially occur in metabolically active tissues instead of in WAT. In the fasted state, liver produces ANGPTL3 and ANGPTL4 that could potentially act on LPL in the bloodstream. However, the average concentration of ANGPTL4 in human plasma is too low to have any effect on the LPL activity in the presence of TG-rich lipoproteins (33, 35). In the fed state, the production of ANGPTL4 in WAT is reduced, and ANGPTL4 may form inactive complexes with ANGPTL8, whose expression is greatly increased in the fed state both in WAT and in the liver. The result is that large amounts of active LPL may reach the luminal surface of the blood capillaries in WAT, so that TGs from VLDLs and chylomicrons (Chy) can be hydrolyzed and TG storage in WAT is promoted. In the fed state, the production of ANGPTL8 is upregulated in the liver. ANGPTL8 forms potent complexes with ANGPTL3, which inactivates LPL on the surface of oxidative tissues. In the fed state, the expression of ANGPTL4 is decreased in the liver, and the newly synthesized ANGPTL4 may form inactive complexes with ANGPTL8. The figure was adapted from the work of Wang et al. (14).
Acknowledgments
The authors thank Valeria Saar (Umeå University) for her technical assistance and advice.
Footnotes
Abbreviations:
- ANGPTL
- angiopoietin-like
- ccd
- coiled-coil domain
- GPIHBP1
- glycosylphosphatidylinositol-anchored HDL binding protein 1
- TG
- triglyceride
- WAT
- white adipose tissue.
This work was supported by Swedish Research Council Grant 2015-0292 and Hjärt Lungfonden Grant 20170465.
REFERENCES
- 1.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (Angptl)4 overexpression and targeted disruption of Angptl4 and Angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950. [DOI] [PubMed] [Google Scholar]
- 2.Romeo S., Yin W., Kozlitina J., Pennacchio L. A., Boerwinkle E., Hobbs H. H., and Cohen J. C.. 2009. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Invest. 119: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dewey F. E., Gusarova V., O’Dushlaine C., Gottesman O., Trejos J., Hunt C., Van Hout C. V., Habegger L., Buckler D., Lai K-M. V., et al. 2016. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 374: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dewey F. E., Gusarova V., Dunbar R. L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C. V., Bruse S., Dansky H. M., et al. 2017. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 377: 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang R. 2012. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 424: 786–792. [DOI] [PubMed] [Google Scholar]
- 6.Quagliarini F., Wang Y., Kozlitina J., Grishin N. V., Hyde R., Boerwinkle E., Valenzuela D. M., Murphy A. J., Cohen J. C., and Hobbs H. H.. 2012. Atypical angiopoietin-like protein that regulates ANGPTL3. Proc. Natl. Acad. Sci. USA. 109: 19751–19756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren G., Kim J. Y., and Smas C. M.. 2012. Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 303: E334–E351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagerberg L., Hallström B. M., Oksvold P., Kampf C., Djureinovic D., Odeberg J., Habuka M., Tahmasebpoor S., Danielsson A., Edlund K., et al. 2014. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics. 13: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sukonina V., Lookene A., Olivecrona T., and Olivecrona G.. 2006. Angiopoietin-like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc. Natl. Acad. Sci. USA. 103: 17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mysling S., Kristensen K. K., Larsson M., Kovrov O., Bensadouen A., Jørgensen T. J., Olivecrona G., Young S. G., and Ploug M.. 2016. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife. 5: e20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H. F. J., Hesselink M. K., Schrauwen P., and Müller M.. 2009. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 29: 969–974. [DOI] [PubMed] [Google Scholar]
- 12.Aryal B., Singh A. K., Zhang X., Varela L., Rotllan N., Goedeke L., Chaube B., Camporez J-P., Vatner D. F., Horvath T. L., et al. 2018. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight. 3: 97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catoire M., Alex S., Paraskevopulos N., Mattijssen F., Evers-van Gogh I., Schaart G., Jeppesen J., Kneppers A., Mensink M., Voshol P. J., et al. 2014. Fatty acid-inducible ANGPTL4 governs lipid metabolic response to exercise. Proc. Natl. Acad. Sci. USA. 111: E1043–E1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., McNutt M. C., Banfi S., Levin M. G., Holland W. L., Gusarova V., Gromada J., Cohen J. C., and Hobbs H. H.. 2015. Hepatic ANGPTL3 regulates adipose tissue energy homeostasis. Proc. Natl. Acad. Sci. USA. 112: 11630–11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haller J. F., Mintah I. J., Shihanian L. M., Stevis P., Buckler D., Alexa-Braun C. A., Kleiner S., Banfi S., Cohen J. C., Hobbs H. H., et al. 2017. ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J. Lipid Res. 58: 1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenburg W. K., Yu D., Lee E-C., Xiong W., Gololobov G., Key B., Gay J., Wilganowski N., Hu Y., Zhao S., et al. 2009. GPIHBP1 stabilizes lipoprotein lipase and prevents its inhibition by angiopoietin-like 3 and angiopoietin-like 4. J. Lipid Res. 50: 2421–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gusarova V., Banfi S., Alexa-Braun C. A., Shihanian L. M., Mintah I. J., Lee J. S., Xin Y., Su Q., Kamat V., Cohen J. C., et al. 2017. ANGPTL8 blockade with a monoclonal antibody promotes triglyceride clearance, energy expenditure, and weight loss in mice. Endocrinology. 158: 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi X., Britt E. C., Shows H. W., Hjelmaas A. J., Shetty S. K., Cushing E. M., Li W., Dou A., Zhang R., and Davies B. S. J.. 2017. ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol. Metab. 6: 1137–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robal T., Larsson M., Martin M., Olivecrona G., and Lookene A.. 2012. Fatty acids bind tightly to the N-terminal domain of angiopoietin-like protein 4 and modulate its interaction with lipoprotein lipase. J. Biol. Chem. 287: 29739–29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqa A., Ahmad J., Ali A., Paracha R. Z., Bibi Z., and Aslam B.. 2016. Structural characterization of ANGPTL8 (betatrophin) with its interacting partner lipoprotein lipase. Comput. Biol. Chem. 61: 210–220. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson-Olivecrona G., and Olivecrona T.. 1991. Phospholipase activity of milk lipoprotein lipase. Methods Enzymol. 197: 345–356. [DOI] [PubMed] [Google Scholar]
- 22.Olivecrona G., and Lookene A.. 1997. Noncatalytic functions of lipoprotein lipase. Methods Enzymol. 286: 102–116. [DOI] [PubMed] [Google Scholar]
- 23.Mysling S., Kristensen K. K., Larsson M., Beigneux A. P., Gårdsvoll H., Fong L. G., Bensadouen A., Jørgensen T. J., Young S. G., and Ploug M.. 2016. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 5: e12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristensen K. K., Midtgaard S. R., Mysling S., Kovrov O., Hansen L. B., Skar-Gislinge N., Beigneux A. P., Kragelund B. B., Olivecrona G., Young S. G., et al. 2018. A disordered acidic domain in GPIHBP1 harboring a sulfated tyrosine regulates lipoprotein lipase. Proc. Natl. Acad. Sci. USA. 115: E6020–E6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lookene A., Chevreuil O., Østergaard P., and Olivecrona G.. 1996. Interaction of lipoprotein lipase with heparin fragments and with heparan sulfate: stoichiometry, stabilization, and kinetics. Biochemistry. 35: 12155–12163. [DOI] [PubMed] [Google Scholar]
- 26.Lookene A., Nielsen M. S., Gliemann J., and Olivecrona G.. 2000. Contribution of the carboxy-terminal domain of lipoprotein lipase to interaction with heparin and lipoproteins. Biochem. Biophys. Res. Commun. 271: 15–21. [DOI] [PubMed] [Google Scholar]
- 27.Spillmann D., Lookene A., and Olivecrona G.. 2006. Isolation and characterization of low sulfated heparan sulfate sequences with affinity for lipoprotein lipase. J. Biol. Chem. 281: 23405–23413. [DOI] [PubMed] [Google Scholar]
- 28.Ono M., Shimizugawa T., Shimamura M., Yoshida K., Noji-Sakikawa C., Ando Y., Koishi R., and Furukawa H.. 2003. Protein region important for regulation of lipid metabolism in angiopoietin-like 3 (ANGPTL3): ANGPTL3 is cleaved and activated in vivo. J. Biol. Chem. 278: 41804–41809. [DOI] [PubMed] [Google Scholar]
- 29.Jin W., Wang X., Millar J. S., Quertermous T., Rothblat G. H., Glick J. M., and Rader D. J.. 2007. Hepatic proprotein convertases modulate HDL metabolism. Cell Metab. 6: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandard S., Zandbergen F., Tan N. S., Escher P., Patsouris D., Koenig W., Kleemann R., Bakker A., Veenman F., Wahli W., et al. 2004. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J. Biol. Chem. 279: 34411–34420. [DOI] [PubMed] [Google Scholar]
- 31.Ge H., Yang G., Yu X., Pourbahrami T., and Li C.. 2004. Oligomerization state-dependent hyperlipidemic effect of angiopoietin-like protein 4. J. Lipid Res. 45: 2071–2079. [DOI] [PubMed] [Google Scholar]
- 32.McQueen A. E., Kanamaluru D., Yan K., Gray N. E., Wu L., Li M. L., Chang A., Hasan A., Stifler D., Koliwad S. K., et al. 2017. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem. 292: 16122–16134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reimund M., Kovrov O., Olivecrona G., and Lookene A.. 2017. Lipoprotein lipase activity and interactions studied in human plasma by isothermal titration calorimetry. J. Lipid Res. 58: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morinaga J., Zhao J., Endo M., Kadomatsu T., Miyata K., Sugizaki T., Okadome Y., Tian Z., Horiguchi H., Miyashita K., et al. 2018. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: a cross-sectional study. PLoS One. 13: e0193731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nilsson S. K., Anderson F., Ericsson M., Larsson M., Makoveichuk E., Lookene A., Heeren J., and Olivecrona G.. 2012. Triacylglycerol-rich lipoproteins protect lipoprotein lipase from inactivation by ANGPTL3 and ANGPTL4. Biochim. Biophys. Acta. 1821: 1370–1378. [DOI] [PubMed] [Google Scholar]
- 36.Shan L., Yu X-C., Liu Z., Hu Y., Sturgis L. T., Miranda M. L., and Liu Q.. 2009. The angiopoietin-like proteins ANGPTL3 and ANGPTL4 inhibit lipoprotein lipase activity through distinct mechanisms. J. Biol. Chem. 284: 1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivecrona G. 2016. Role of lipoprotein lipase in lipid metabolism. Curr. Opin. Lipidol. 27: 233–241. [DOI] [PubMed] [Google Scholar]
- 38.Fu Z., Yao F., Abou-Samra A. B., and Zhang R.. 2013. Lipasin, thermoregulated in brown fat, is a novel but atypical member of the angiopoietin-like protein family. Biochem. Biophys. Res. Commun. 430: 1126–1131. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Quagliarini F., Gusarova V., Gromada J., Valenzuela D. M., Cohen J. C., and Hobbs H. H.. 2013. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA. 110: 16109–16114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makoveichuk E., Sukonina V., Kroupa O., Thulin P., Ehrenborg E., Olivecrona T., and Olivecrona G.. 2012. Inactivation of lipoprotein lipase occurs on the surface of THP-1 macrophages where oligomers of angiopoietin-like protein 4 are formed. Biochem. Biophys. Res. Commun. 425: 138–143. [DOI] [PubMed] [Google Scholar]
- 41.Dijk W., Beigneux A. P., Larsson M., Bensadoun A., Young S. G., and Kersten S.. 2016. Angiopoietin-like 4 promotes intracellular degradation of lipoprotein lipase in adipocytes. J. Lipid Res. 57: 1670–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dijk W., Ruppert P. M. M., Oost L. J., and Kersten S.. 2018. Angiopoietin-like 4 promotes the intracellular cleavage of lipoprotein lipase by PCSK3/furin in adipocytes. J. Biol. Chem. 293: 14134–14145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson M., Vorrsjö E., Talmud P., Lookene A., and Olivecrona G.. 2013. Apolipoproteins C–I and C–III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J. Biol. Chem. 288: 33997–34008. [DOI] [PMC free article] [PubMed] [Google Scholar]