Ethylene and abscisic acid antagonistically modulate ascorbic acid biosynthesis and reactive oxygen species accumulation via an EIN3-ABI4-VTC2 transcriptional cascade.
Abstract
During plant growth and development, ethylene and abscisic acid (ABA) play important roles and exert synergistic or antagonistic effects on various biological processes, but the detailed mechanism underlying the interaction of the two phytohormones, especially in the regulation of the accumulation of reactive oxygen species (ROS), is largely unclear. Here, we report that ethylene inhibits but ABA promotes the accumulation of ROS in Arabidopsis (Arabidopsis thaliana) seedlings. Furthermore, changes in the biosynthesis of ascorbic acid (AsA) act as a key factor in integrating the interaction of ethylene and ABA in the regulation of ROS levels. We found that ethylene and ABA antagonistically regulate AsA biosynthesis via ETHYLENE-INSENSITIVE3 (EIN3) and ABA INSENSITIVE4 (ABI4), which are key factors in the ethylene and ABA signaling pathways, respectively. In addition, ABI4 is transcriptionally repressed by EIN3 in ethylene-regulated AsA biosynthesis. Via transcriptome analysis and molecular and genetic experiments, we identified VITAMIN C DEFECTIVE2as the direct target of ABI4 in the regulation of AsA biosynthesis and ROS accumulation. Thus, the EIN3-ABI4- VITAMIN C DEFECTIVE2 transcriptional cascade involves a mechanism by which ethylene and ABA antagonistically regulate AsA biosynthesis and ROS accumulation in response to complex environmental stimuli.
It is well known that ethylene and abscisic acid (ABA) are two crucial phytohormones that play important roles in various plant growth and development processes as well as in stress responses (Bleecker and Kende, 2000; Finkelstein et al., 2002; Vishwakarma et al., 2017; Yin et al., 2017). Interestingly, both synergistic and antagonistic functions of these two phytohormones have been displayed in different processes. For example, ethylene promotes ABA biosynthesis to inhibit root elongation in rice (Oryza sativa; Yin et al., 2015). In addition, during root endodermal suberization in response to nutrient stress, ethylene and ABA cause completely opposite effects (Barberon et al., 2016). The antagonism between ethylene and ABA is mediated in part bySWI-INDEPENDENT 3 LIKE1 and SWI-INDEPENDENT 3 LIKE2 via histone deacetylation to affect seed dormancy (Wang et al., 2013c). Increasing numbers of reports have revealed complex cross talk between ethylene and ABA in the regulation of plant growth and development; however, the underlying mechanism and factors involved in this interaction are still unclear.
Reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) and superoxide anion (O2−), are generated from aerobic metabolism and exist extensively in plant cells (Mittler, 2002; Mittler et al., 2004). ROS were initially presumed to damage living cells and to play an important role in programmed cell death (Mittler, 2002; Mittler et al., 2004). However, in recent years, increasing numbers of studies have revealed that ROS control many different plant biological processes, such as cell division, root growth, and stress responses (Jiang et al., 2012; Dietz et al., 2016; Simmons and Bergmann, 2016; Xu et al., 2017; Waszczak et al., 2018). Because of the dual roles of ROS, an accurate and flexible regulatory mechanism to control ROS balance during plant growth and development has been suggested (Mittler et al., 2004; Mittler, 2017). In fact, nearly all aerobic organisms have efficient ROS-scavenging mechanisms, including enzymatic and nonenzymatic antioxidant systems (Mittler, 2002; Mittler et al., 2004). For example, the balance between superoxide dismutase and other H2O2-scavenging enzymes significantly impacts the steady state of ROS (Mittler et al., 2004).
Ascorbic acid (AsA), an efficient nonenzymatic antioxidant, detoxifies ROS generated not only during photosynthesis and development but also during abiotic and biotic stress responses (Davey et al., 2000; Smirnoff and Wheeler, 2000; Gallie, 2013; Akram et al., 2017). With multiple roles in various growth and development processes, AsA and its regulatory mechanism in plants have garnered increasing amounts of attention (De Tullio and Arrigoni, 2004; Gallie, 2013; Bulley and Laing, 2016). Although alternative pathways have been identified, the l-Gal pathway is considered the dominant method by which higher plants synthesize AsA (Linster and Clarke, 2008; Bulley and Laing, 2016). The first committed step, the Guanosin diphosphate (GDP)-l-Gal phosphorylase (GGP)-catalyzed conversion of GDP-l-Gal into l-Gal, is considered a crucial step for AsA biosynthesis (Linster and Clarke, 2008; Bulley and Laing, 2016).
In Arabidopsis (Arabidopsis thaliana), there are two genes that encode GGP: VITAMIN C DEFECTIVE2 (VTC2) and VTC5 (Dowdle et al., 2007). The double mutant vtc2 vtc5 is unable to grow unless supplemented with exogenous AsA (Dowdle et al., 2007; Lim et al., 2016). VTC5 presents a lower affinity for GDP-l-Gal and 100- to 1,000-fold lower expression than does VTC2 (Dowdle et al., 2007). Moreover, compared with that of the wild type, the AsA content of the vtc5 mutant is not markedly different, indicating that VTC2 is much more important than VTC5 in AsA biosynthesis (Dowdle et al., 2007). Thus, an increasing number of studies have focused on the regulation of VTC2 in AsA production (Linster and Clarke, 2008; Bulley and Laing, 2016). For instance, VTC2 expression and GGP activity were rapidly increased under high-light illumination, resulting in higher VTC2 activity than that of other enzymes in the pathway (Dowdle et al., 2007). An upstream open reading frame in the 5′ untranslated region of VTC2 affects the translation of VTC2 and plays an essential role in the feedback regulation of AsA biosynthesis (Laing et al., 2015). These findings indicate that VTC2 is a key target for the regulation of AsA biosynthesis.
Recent studies have reported that ROS accumulate to mediate ABA-affected stomatal closure, salicylic acid-mediated root meristem activity, and ethylene-regulated shoot Na homeostasis (Pei et al., 2000; Jiang et al., 2012, 2013; Xu et al., 2017). Therefore, there is an intimate association between ROS levels and phytohormones in plant growth and development (Xia et al., 2015). In the case of ethylene and ABA, diverse effects have been reported. For instance, ROS production is induced by ABA to promote cytosolic Ca2+ in guard cells to mediate stomatal closure (Pei et al., 2000; Wang and Song, 2008), whereas, during rice seed germination, the ROS level is decreased in imbibed seeds with ABA treatment, especially in the embryo region (Ye et al., 2012). Ethylene is increased and required for ROS accumulation during ozone-induced programmed cell death and pathogen response (Overmyer et al., 2000, 2003; Mersmann et al., 2010; Tintor et al., 2013). Conversely, ethylene reduced ROS accumulation to protect seedlings against salt stress and photooxidative damage (Zhong et al., 2009; Peng et al., 2014). Thus, revelation of the mechanism by which ethylene and ABA coregulate ROS accumulation is pivotal to understanding the cross talk between phytohormones and the regulatory pattern of ROS levels in plants.
In this report, we clarify the essential regulation of ethylene and ABA with respect to AsA accumulation in Arabidopsis seedlings, which further inhibits ROS accumulation. The key transcription factors involved in ethylene and ABA signaling, ETHYLENE-INSENSITIVE3 (EIN3) and ABA-INSENSITIVE4 (ABI4; Finkelstein et al., 1998, 2002; Bleecker and Kende, 2000; Hauser et al., 2011; Ju and Chang, 2015), are required to transcriptionally activate the AsA biosynthesis gene VTC2. Therefore, our study provides new insights into the function of the EIN3-ABI4-VTC2 cascade in coordinating the cross talk between ethylene and ABA in AsA biosynthesis and ROS accumulation at the early seedling stage.
RESULTS
AsA Plays an Essential Role in the Antagonistic Regulation of ROS Accumulation by Ethylene and ABA
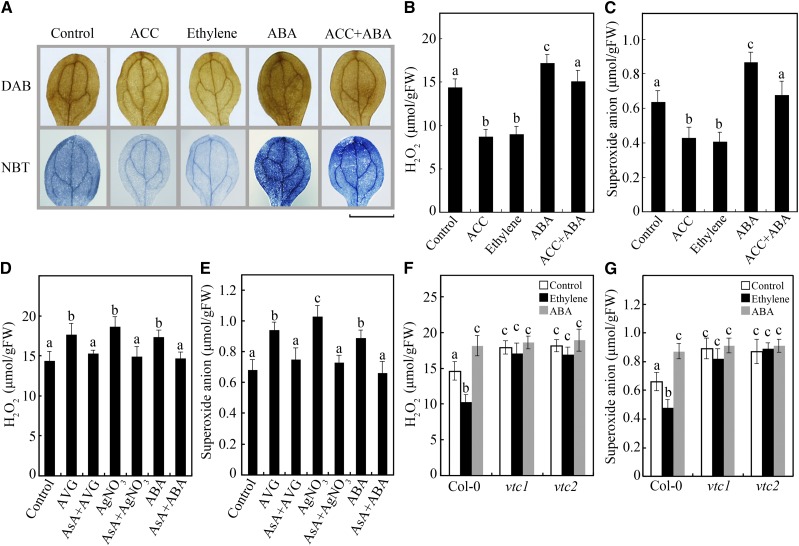
To verify the function of ethylene and ABA in the accumulation of ROS, we performed assays to measure two key kinds of ROS, H2O2 and O2−, in 7-d-old Arabidopsis seedlings pretreated with 10 μm 1-aminocyclopropane-1-carboxylate (ACC; an ethylene precursor), 10 μL L−1 ethylene gas, or 1 μm ABA. Based on the degree of staining with diaminobenzidine (DAB) or nitroblue tetrazolium (NBT), the accumulation of H2O2 and O2− in Columbia-0 (Col-0) concomitantly decreased in response to treatment with ACC or ethylene, whereas it increased in response to treatment with ABA (Fig. 1A), confirming that ethylene inhibits ROS accumulation while ABA promotes it. When the seedlings were treated with ACC and ABA simultaneously, the staining degree was between that resulting from the two treatments (Fig. 1A), indicating that the effects of ethylene and ABA on ROS accumulation could counteract each other. These results were further demonstrated by measurements of the H2O2 and O2− contents in the seedlings above in response to the indicated treatments (Fig. 1, B and C). In addition, in response to treatment with the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) and the ethylene perception inhibitor silver nitrate (AgNO3), the H2O2 and O2− contents in Col-0 increased (Fig. 1, D and E), demonstrating the negative role of ethylene in the accumulation of ROS. These results indicate that ethylene and ABA antagonistically regulate ROS accumulation in Arabidopsis at the early seedling stage.
Figure 1.
Ethylene and ABA antagonistically regulate ROS accumulation via AsA. A, DAB and NBT staining of ROS in Col-0 seedlings treated with 10 μm ACC, 10 μL L−1 ethylene gas, 1 μm ABA, or both ACC and ABA. Bar = 0.2 cm. B and C, Measurements of H2O2 (B) and O2− (C) in Col-0 in response to treatment with ACC, ethylene, ABA, or both ACC and ABA. D and E, Measurements of H2O2 (D) and O2− (E) in Col-0 in response to treatment with 5 μm AVG, 5 μm AgNO3, 1 μm ABA, or supplemented with 10 μm AsA. F and G, Measurements of H2O2 (F) and O2− (G) in Col-0, vtc1, and vtc2 in response to treatment with ethylene or ABA. Seven-day-old seedlings were treated with the indicated conditions for 12 h. Values in B to G are means ± sd (n = 3). Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test). FW, Fresh weight.
ROS levels in plants are controlled by biosynthesis processes and antioxidant systems, including both enzymatic and nonenzymatic pathways (Mittler, 2002; Mittler et al., 2004). Although studies have reported that several enzymes involved in ROS production and scavenging are regulated by ethylene or ABA (Wang and Song, 2008; Jiang et al., 2013; Peng et al., 2014), the function of AsA, a crucial nonenzymatic antioxidant, in the regulation of ROS accumulation in response to ethylene and ABA is still unclear. Thus, we investigated whether the addition of AsA affected ROS scavenging. As expected, the effects of AVG, AgNO3, and ABA on ROS levels were suppressed in response to AsA application (Fig. 1, D and E), implying that AsA is required in the regulation of ROS accumulation in response to ethylene and ABA. To further verify these observations, we measured the H2O2 and O2− contents in two AsA biosynthesis mutants, vtc1 and vtc2, in response to treatment with ethylene or ABA. Consistent with the results of previous reports (Gao and Zhang, 2008; Pető et al., 2013), vtc1 and vtc2 generated high levels of ROS because of reduced AsA production (Fig. 1, F and G). More importantly, both the inhibitory effects of ethylene and the promotional effects of ABA on ROS accumulation were inhibited in these mutants (Fig. 1, F and G), demonstrating that AsA is essentially required for ethylene- and ABA-regulated ROS accumulation.
Ethylene and ABA Antagonistically Modulate AsA Biosynthesis and ROS Accumulation via EIN3 and ABI4
Since AsA is a downstream nonenzymatic antioxidant in ethylene- and ABA-regulated ROS accumulation, we investigated how AsA biosynthesis is controlled by the interaction of ethylene and ABA. First, we measured the AsA content in Col-0 seedlings in response to treatment with ACC, ethylene, or ABA. Our data showed that the levels of AsA were increased by treatment with ethylene and its precursor ACC but were reduced by treatment with ABA (Fig. 2A). Consistent with the functions of ethylene and ABA in ROS accumulation, the AsA level in Col-0 in response to treatment with both ACC and ABA is similar to that in the control (Fig. 2A), indicating that ethylene and ABA antagonistically regulate AsA biosynthesis.
Figure 2.
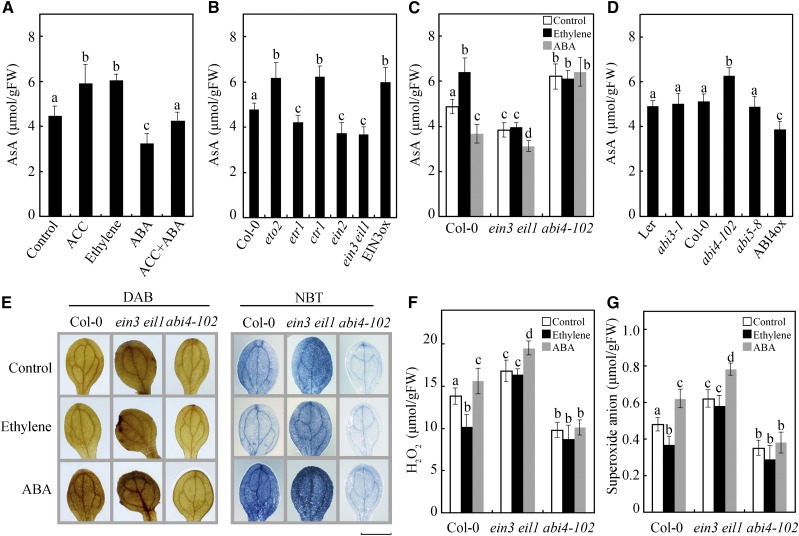
Ethylene and ABA antagonistically regulate AsA biosynthesis via EIN3 and ABI4. A, AsA contents in Col-0 treated with ACC, ethylene, ABA, or both ACC and ABA. B, AsA contents in Col-0, eto2, etr1-1, ctr1-1, ein2, ein3 eil1, and EIN3ox. C, AsA contents in Col-0, ein3 eil1, and abi4-102 in response to treatment with ethylene or ABA. D, AsA contents in Landsberg erecta (Ler), abi3-1, Col-0, abi4-102, and abi5-8. E, DAB and NBT staining of ROS in Col-0, ein3 eil1, and abi4-102 seedlings treated with ethylene or ABA. Bar = 0.2 cm. F and G, Measurements of H2O2 (F) and O2− (G) in Col-0, ein3 eil1, and abi4-102 in response to treatment with ethylene or ABA. Seven-day-old seedlings were treated with the indicated conditions for 12 h. Values in A to D, F, and G are means ± sd (n = 3). Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test). FW, Fresh weight.
To further study the relationship between ethylene and ABA in the regulation of AsA biosynthesis, the AsA content was evaluated in genetic backgrounds where ethylene or ABA signaling are perturbed. Our results indicated that the ethylene overproduction mutant eto2, the ethylene constitutive response mutant ctr1, and the EIN3 overexpression line EIN3ox produced much greater amounts of AsA than did wild-type Col-0, while ethylene-sensitive mutants ethylene response1-1 (etr1-1), ein2, and ein3 eil1 produced less (Fig. 2B), indicating that endogenous ethylene or enhanced ethylene signaling promote AsA biosynthesis. More importantly, the promotional effect of ethylene on AsA biosynthesis was disabled in the ein3 eil1 mutant (Fig. 2C), demonstrating the positive role of ethylene, especially the downstream ethylene-related transcription factor EIN3, in AsA biosynthesis. In addition, the ROS levels in these mutants were also evaluated. The high-AsA-content mutants eto2, ctr1, and EIN3ox consistently presented low ROS levels, while the low-AsA-content mutants etr1, ein2, and ein3 eil1 presented high ROS levels (Supplemental Fig. S1, A and B), indicating that ethylene-regulated AsA production reduces the accumulation of ROS.
ABI3, ABI4, and ABI5 are key downstream transcription factors involved in ABA signaling (Finkelstein et al., 2002; Hauser et al., 2011). To demonstrate how ABA modulates AsA production, we measured the contents of AsA and ROS in ABA-related mutants. Interestingly, compared with Col-0, only abi4-102 exhibited greater AsA production and lower ROS accumulation, whereas abi3-1 and abi5-8 did not impact their accumulation (Fig. 2D; Supplemental Fig. S1, C and D). In addition, ABA could not suppress AsA biosynthesis or promote ROS accumulation in abi4-102 (Fig. 2, C and E–G). This result was further demonstrated by the ABI4 overexpression line ABI4ox, which displayed lower AsA levels and higher ROS accumulation than did Col-0 (Fig. 2D; Supplemental Fig. S1, C and D). Thus, our data demonstrate that ABI4 is required for the regulation of ABA in AsA biosynthesis and ROS accumulation.
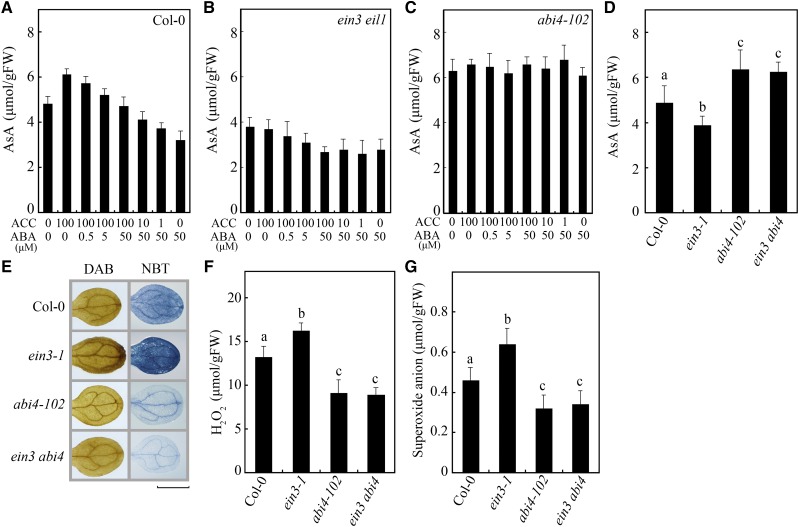
Because EIN3 and ABI4 play important roles in the regulation of AsA biosynthesis and ROS accumulation, EIN3 and ABI4 probably mediate the cross talk between ethylene and ABA. To verify this hypothesis, ein3 eil1 plants were treated with ABA, and abi4-102 plants were treated with ethylene. As shown in Figure 2, C and E–G, the regulatory functions of ethylene in AsA biosynthesis and ROS accumulation were inhibited in the abi4-102 plants, indicating that ABI4 mediates the cross talk between ethylene and ABA in the regulation of AsA biosynthesis and ROS accumulation. In the ein3 eil1 mutant, although ethylene signaling is disrupted, ABA still affected the levels of AsA and ROS (Fig. 2, C and E–G). These results were further demonstrated through the measurement of AsA contents in response to treatment with different concentrations of ACC and/or ABA in Col-0, ein3 eil1, and abi4-102 mutants. As shown in Figure 3A, in wild-type Col-0, the positive effect of high concentrations of ACC on AsA production was inhibited by ABA, while the inhibition of ABA on AsA production was relieved by increased ACC applications. In the ein3 eil1 mutant, although the function of ACC was inhibited, increased ABA still suppressed AsA production, with or without ACC treatment (Fig. 3B). The effects of both ACC and ABA on the regulation of AsA production were disabled in the abi4-102 mutant (Fig. 3C). Thus, EIN3 probably acts upstream of ABI4 in ethylene- and ABA-regulated AsA biosynthesis.
Figure 3.
ABI4 acts downstream of EIN3 in the regulation of AsA biosynthesis and ROS accumulation. A to C, AsA contents in Col-0 (A), ein3 eil1 (B), and abi4-102 (C) in response to treatment with different concentrations of ACC and ABA. Seven-day-old seedlings were treated with the indicated conditions for 12 h. D, AsA contents in Col-0, ein3-1, abi4-102, and ein3 abi4. E, DAB and NBT staining of ROS in Col-0, ein3-1, abi4-102, and ein3 abi4 seedlings. Bar = 0.2 cm. F and G, Measurements of H2O2 (F) and O2− (G) in Col-0, ein3-1, abi4-102, and ein3 abi4. Values in A to D, F, and G are means ± sd (n = 3). Different letters indicate statistically significant differences among the indicated data (P < 0.05, ANOVA with Tukey’s test). FW, Fresh weight.
To further analyze the relationship between EIN3 and ABI4 in the regulation of AsA and ROS levels, ein3-1 and abi4-102 were crossed to generate an ein3 abi4 double mutant. Similar to abi4-102, compared with wild-type Col-0, the double mutant ein3 abi4 presented increased AsA content and decreased ROS levels (Fig. 3, D–G), demonstrating that ABI4 genetically acts downstream of EIN3 in the regulation of AsA biosynthesis and ROS accumulation.
ABI4 Is the Direct Downstream Target of EIN3 in the Regulation of AsA Biosynthesis and ROS Accumulation
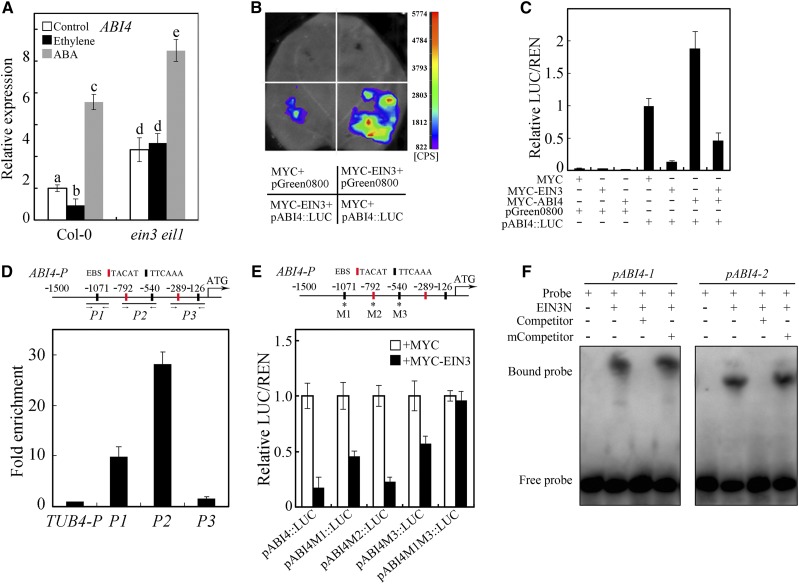
Studies have proven that genes encoding ERF proteins act as EIN3 targets in ethylene-regulated plant development and stress responses (Solano et al., 1998; Zhang et al., 2011; Chang et al., 2013). Therefore, the ERF gene ABI4 was considered to be regulated by ethylene via EIN3. This hypothesis was verified by reverse transcription quantitative PCR (RT-qPCR) analysis, in that ABI4 expression was suppressed by ethylene (Fig. 4A). This conclusion was further demonstrated by the high level of ABI4 transcripts in ein3 eil1 plants (Fig. 4A). And as previously reported (Bossi et al., 2009), ABI4 was induced by ABA treatment (Fig. 4A). Interestingly, the expression of ABI4 in ein3 eil1 plants was not regulated by ethylene but was promoted by ABA (Fig. 4A), consistent with the function of EIN3 and ABI4 in the regulation of AsA biosynthesis and ROS accumulation.
Figure 4.
ABI4 is a direct target gene of EIN3 in the regulation of AsA biosynthesis. A, Expression of ABI4 in Col-0 and ein3 eil1 in response to treatment with ethylene or ABA. Seven-day-old seedlings were treated with ethylene or ABA for 2 h. B and C, Transient expression assays testing the capability of EIN3 to regulate the expression of ABI4 in tobacco leaves (B) and Arabidopsis protoplasts (C). D, Top, sequence analysis of the ABI4 promoter. EBSs (TACAT and TTCAAA) are shown. The numbers indicate the distance away from the start codon. DNA fragments (P1, P2, and P3) were used for the ChIP assay. Bottom, ChIP-qPCR assays of EIN3 binding to the ABI4 promoter. The AtTUB4 promoter (TUB4-P) was used as an internal control. E, Transient expression assays testing the capability of EIN3 to regulate the expression of ABI4 in tobacco leaves when the ABI4 promoter contained mutations in the binding motif (M1, M2, and M3 indicate the ABI4 promoter motifs that contain a mutation). F, EMSA testing the binding of EIN3N to the promoter of ABI4 in vitro. As indicated, unlabeled probe was used as Competitor and those with a mutated binding motif as mCompetitor. Values in A and C to E are means ± sd (n = 3). Statistically significant differences are indicated by different letters in A (P < 0.05, ANOVA with Tukey’s test).
To further demonstrate the negative role of EIN3 in ABI4 expression, we used a transient expression system involving tobacco (Nicotiana tabacum) leaves and Arabidopsis protoplasts with a reporter vector pABI4::LUC (Luciferase) and/or effector vector MYC-EIN3. As shown in Figure 4, B and C, a certain amount of LUC activity was observed when the pABI4::LUC construct and the control 35S::MYC were combined together. However, the LUC activity clearly decreased when the control was replaced by the effector MYC-EIN3 (Fig. 4, B and C). Moreover, the inhibitory effect of EIN3 on ABI4 expression was partly relieved by addition of the MYC-ABI4 vector (Fig. 4C), which is consistent with the inductive function of ABI4 on its own gene expression (Bossi et al., 2009). Thus, our results indicate that ABI4 expression is antagonistically regulated by EIN3 and ABI4.
Analysis of the ABI4 promoter indicated that it contains at least five putative EIN3-binding sites (EBSs; Fig. 4D), implying a possible direct interaction between EIN3 and ABI4. We then performed a chromatin immunoprecipitation (ChIP) assay to investigate the interaction between EIN3 and the promoter of ABI4. Specific primers were designed for the three core regions of the ABI4 promoter (P1, P2, and P3; Fig. 4D). The results showed that the P1 and P2 regions, rather than the P3 region, were enriched in (ChIP)DNA (Fig. 4D), suggesting that EIN3 directly binds to the promoter of ABI4. In the P1 and P2 regions, there were two TTCAAA motifs (−1,071 and −-540) and one TACAT motif (−792); we subsequently introduced mutations in these three motifs. As shown in Figure 4E, only mutation of the two TTCAAA motifs (M1 and M3) weakened the inhibitory effect of MYC-EIN3 on LUC expression, especially when both motifs were mutated, indicating that EIN3 binds to the TTCAAA motif (−1,071 and −540) to repress ABI4 transcription. This result was further demonstrated in vitro by electrophoretic mobility shift assay (EMSA) with the purified N terminus of EIN3 (EIN3N), which contains a DNA-binding domain. As shown in Figure 4F, two fragments of the ABI4 promoter (pABI4-1 and pABI4-2), corresponding to the P1 and P2 regions, were bound by EIN3N. Unlabeled probe (Competitor) competed for EIN3N binding, unless the EBS motifs were mutated in the competitor (mCompetitor; Fig. 4F), further demonstrating that EIN3 interacts with the promoter of ABI4. Therefore, our evidence reveals that ABI4 is the direct downstream target of EIN3 in the regulation of AsA biosynthesis and ROS accumulation.
VTC2 Is Regulated by EIN3-ABI4 in Ethylene- and ABA-Modulated AsA Biosynthesis
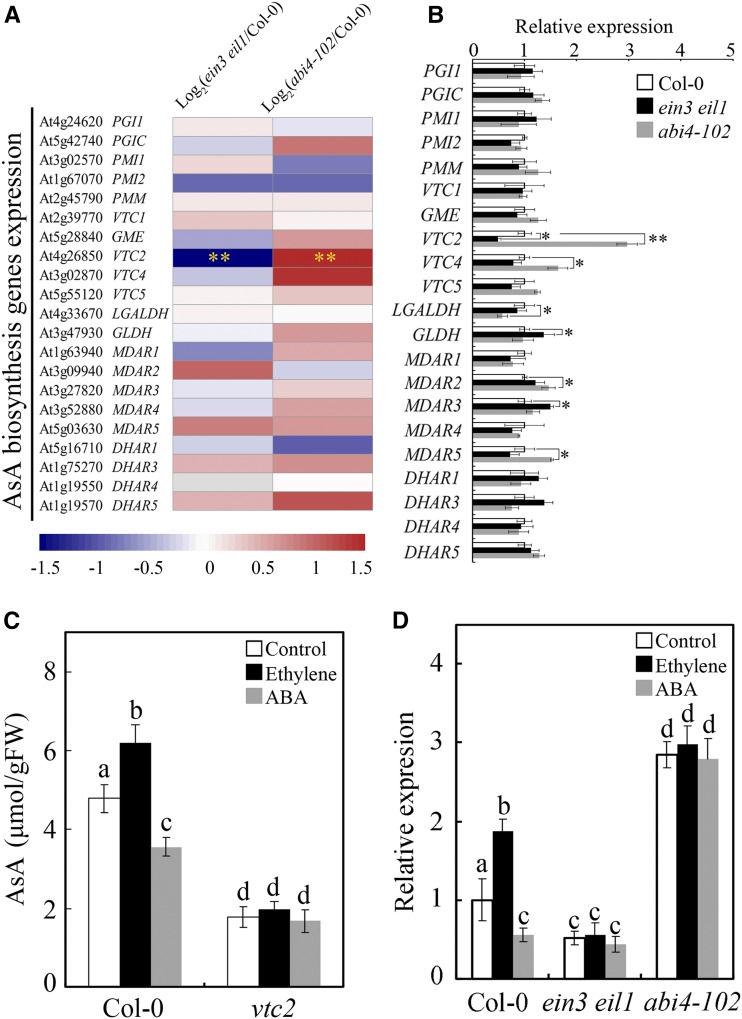
To identify the downstream genes of EIN3 and ABI4 in the regulation of AsA biosynthesis, RNA sequencing was conducted to investigate the transcriptome dynamics in ein3 eil1 and abi4-102. As shown in Supplemental Figure S2, 140 genes were identified as coregulated by EIN3 and ABI4. Interestingly, more than 60% of the genes that were coregulated by ethylene and ABA exhibited opposite regulatory trends in the ein3 eil1 and abi4-102 mutants (Supplemental Fig. S2, B and C), probably because of the inhibitory effect of EIN3 on ABI4 expression. Only one of the oppositely expressed genes, VTC2, is involved in AsA biosynthesis. The expression of the AsA biosynthesis genes was also tested by RT-qPCR, demonstrating that the expression of VTC2 was highly significantly different and opposite between the ein3 eil1 and abi4-102 mutants (Fig. 5, A and B). Thus, VTC2 is probably the key target gene of EIN3 and ABI4 in the regulation of AsA biosynthesis.
Figure 5.
VTC2 is required in EIN3-ABI4-regulated AsA biosynthesis. A, Transcriptome data of AsA biosynthesis genes in ein3 eil1 and abi4-102 mutants. The bar represents the log2 fold change. Double asterisks indicate statistically significant differences between mutants and Col-0 with a fold change > 2 and P < 0.01. B, Expression of AsA biosynthesis genes in Col-0, ein3 eil1, and abi4-102. ABI4 transcript levels were detected by RT-qPCR and normalized to reference gene ACTIN2. Asterisks indicate statistically significant differences between mutants and Col-0 using two-tailed Student’s t test (*, P < 0.05 and **, P < 0.01). C, AsA contents in Col-0 and vtc2 in response to treatment with ethylene or ABA. D, Expression of VTC2 in Col-0, ein3 eil1, and abi4-102 cells treated with ethylene or ABA. Seven-day-old seedlings were treated with ethylene or ABA for 12 h (C) or 2 h (D). Values are means ± sd (n = 3). Different letters in C and D indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test). FW, Fresh weight.
To identify the role of VTC2 in the regulation of AsA biosynthesis in response to ethylene and ABA, the vtc2 mutants were treated with ethylene or ABA. As previously reported (Dowdle et al., 2007), the AsA level in the vtc2 mutant was much lower than that in Col-0 and was not affected by ethylene or ABA treatment (Fig. 5C), demonstrating that VTC2 is required for the regulation of AsA biosynthesis by ethylene and ABA. Consistent with the function of ethylene and ABA in AsA biosynthesis, the expression of VTC2 was promoted by ethylene but suppressed by ABA (Fig. 5D). Importantly, the changes in VTC2 expression in response to ethylene and ABA were strongly suppressed by ein3 eil1 and abi4-102 (Fig. 5D), implying that the regulation of VTC2 expression by ethylene and ABA is mediated by EIN3 and ABI4.
ABI4 Transcriptionally Targets the AsA Biosynthesis Gene VTC2
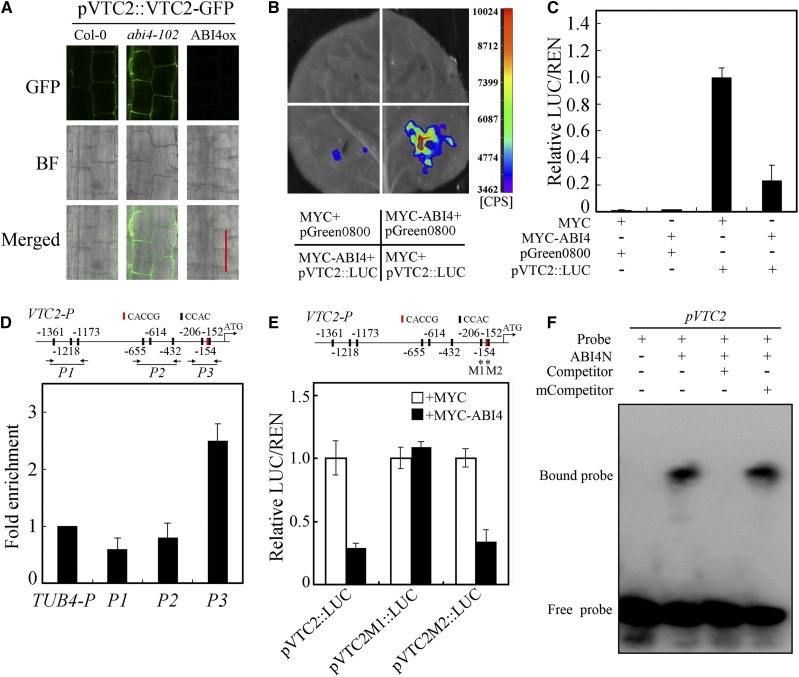
Since the effects of ethylene and ABA on AsA biosynthesis and ROS accumulation were entirely disabled in the abi4-102 mutant and ABI4 is downstream of EIN3 (Figs. 2, C and E–G, and 3), the ABI4-mediated transcriptional regulation of the VTC2 gene was initially investigated. The genomic DNA of VTC2 was fused with that of GFP under the control of the VTC2 native promoter, and the construct was then expressed in Col-0, abi4-102, and ABI4ox. The GFP fluorescence indicated that the expression of VTC2-GFP was higher in abi4-102 than in Col-0 and ABI4ox (Fig. 6A), demonstrating that ABI4 transcriptionally represses the expression of VTC2. In addition, transient expression systems involving tobacco leaves and Arabidopsis protoplasts were used to verify the regulatory patterns of ABI4 and VTC2. The LUC activity (on the basis of its fluorescence) of pVTC2::LUC was markedly suppressed in response to MYC-ABI4 addition (Fig. 6, B and C), demonstrating that ABI4 transcriptionally suppresses the expression of VTC2 in vivo.
Figure 6.
ABI4 binds to the VTC2 promoter to suppress VTC2 transcription. A, GFP fluorescence in the roots of pVTC2::VTC2-GFP transgenic lines in Col-0, abi4-102, and ABI4ox backgrounds. BF, Bright field. Bar = 10 μm. B and C, Transient expression assays testing the capability of ABI4 to regulate the expression of VTC2 in tobacco leaves (B) and Arabidopsis protoplasts (C). D, Top, sequence analysis of the VTC2 promoter. CACCG and CCAC elements are shown. The numbers indicate the distance away from the start codon. DNA fragments (P1, P2, and P3) were used for the ChIP assay. Bottom, ChIP-qPCR assays of ABI4 binding to the VTC2 promoter. The AtTUB4 promoter (TUB4-P) was used as an internal control. E, Transient expression assays testing the capability of ABI4 to regulate the expression of VTC2 in tobacco leaves when the VTC2 promoter contained mutations in the binding motif. M1 and M2 indicate the VTC2 promoter motifs that contained a mutation. F, EMSA testing the binding of ABI4N to the promoter of VTC2 in vitro. As indicated, unlabeled probe was used as Competitor and those with a mutated binding motif as mCompetitor. Values in C to E are means ± sd (n = 3).
ABI4 promotes the expression of genes by binding to the CACCG motif and inhibits the expression of other genes by binding the CCAC element (Shu et al., 2013). As expected, our results indicated that the VTC2 promoter contains one CACCG motif (−154) and eight CCAC elements, as shown in Figure 6D. We then performed a ChIP assay to investigate the interaction between ABI4 and the promoter of VTC2 in vivo using a transgenic line expressing an ABI4-MYC fusion protein. Specific primers were designed for the three core regions of the VTC2 promoter (P1, P2, and P3; Fig. 6D). We discovered that the P3 region, rather than the P1 and P2 regions, was enriched in (ChIP) DNA (Fig. 6D). In the P3 region, there are two CCAC motifs (−206 and −152), and one of them overlaps with a CACCG motif (−154). We then mutated these motifs, as shown in Figure 6E, and analyzed the transient expression in Arabidopsis protoplasts. The results showed that the addition of MYC-ABI4 still suppressed the expression of pVTC2M2::LUC but had no effect on the expression of pVTC2M1::LUC (Fig. 6E), indicating that the −206 CCAC motif is required for the regulation of VTC2 expression by ABI4. This result was further demonstrated by EMSA in vitro. With the addition of the DNA-binding N-terminal portion of ABI4 (ABI4N), shifted bands were clearly detected when the probe was incubated in a solution that contained CCAC oligonucleotides (Fig. 6F). This binding could be competed by the unlabeled oligonucleotides unless mutated in the CCAC motif (Fig. 6F). These results indicated that ABI4 directly binds to the promoter of VTC2, especially the −206 CCAC motif, to repress the transcription of VTC2.
To investigate whether EIN3 directly regulates the expression of VTC2, the reporter vector pVTC2::LUC and/or the effector vector MYC-EIN3 were transiently expressed in Arabidopsis protoplasts. Unexpectedly, the addition of MYC-EIN3 did not increase the signaling generated by the reporter pVTC2::LUC (Supplemental Fig. S3A). Although several EBSs exist in the promoter of the VTC2 gene, the results of the ChIP assay involving transgenic lines expressing the EIN3-Flag fusion protein indicated that EIN3 does not directly bind to the VTC2 promoter (Supplemental Fig. S3B); these findings suggest that EIN3 does not directly regulate VTC2 expression and that additional cofactors are required for EIN3 to promote VTC2 expression in AsA biosynthesis and ROS accumulation.
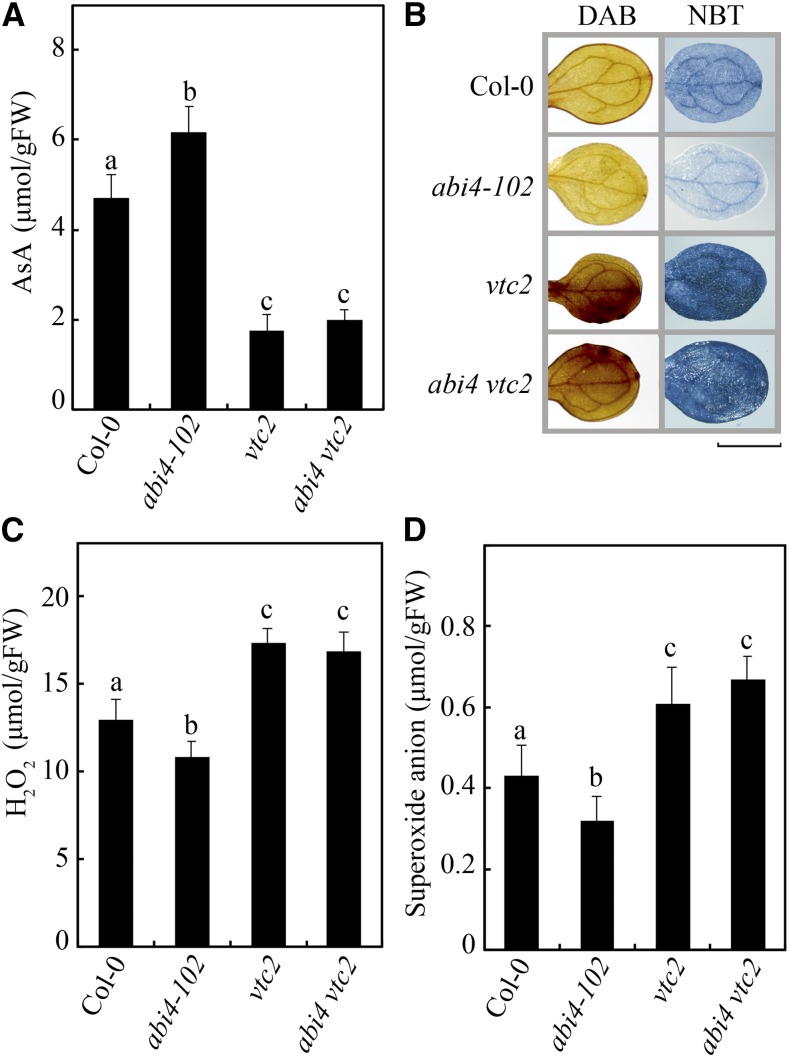
To substantiate the premise that ethylene- and ABA-regulated AsA production and ROS accumulation are modulated via an ABI4-VTC2 regulatory cascade, vtc2 was crossed with abi4-102 to generate the abi4 vtc2 double mutant. Intriguingly, compared with wild-type Col-0 and the abi4-102 mutant, the double mutant presented reduced AsA content and increased ROS levels (Fig. 7), demonstrating that ABI4 genetically acts upstream of VTC2 in the regulatory cascade leading to AsA biosynthesis and ROS accumulation.
Figure 7.
VTC2 acts downstream of ABI4 in the regulation of AsA biosynthesis and ROS accumulation. A, AsA contents in Col-0, vtc2, abi4-102, and abi4 vtc2. B, DAB and NBT staining of ROS in Col-0, abi4-102, vtc2, and abi4 vtc2 seedlings. Bar = 0.2 cm. C and D, Measurements of H2O2 (C) and O2− (D) in Col-0, abi4-102, vtc2, and abi4 vtc2 seedlings. Values in A, C, and D are means ± sd (n = 3). Different letters indicate statistically significant differences (P < 0.05, ANOVA with Tukey’s test). FW, Fresh weight.
EIN3-ABI4-VTC2 Coordinates the Function of Ethylene and ABA in AsA Production
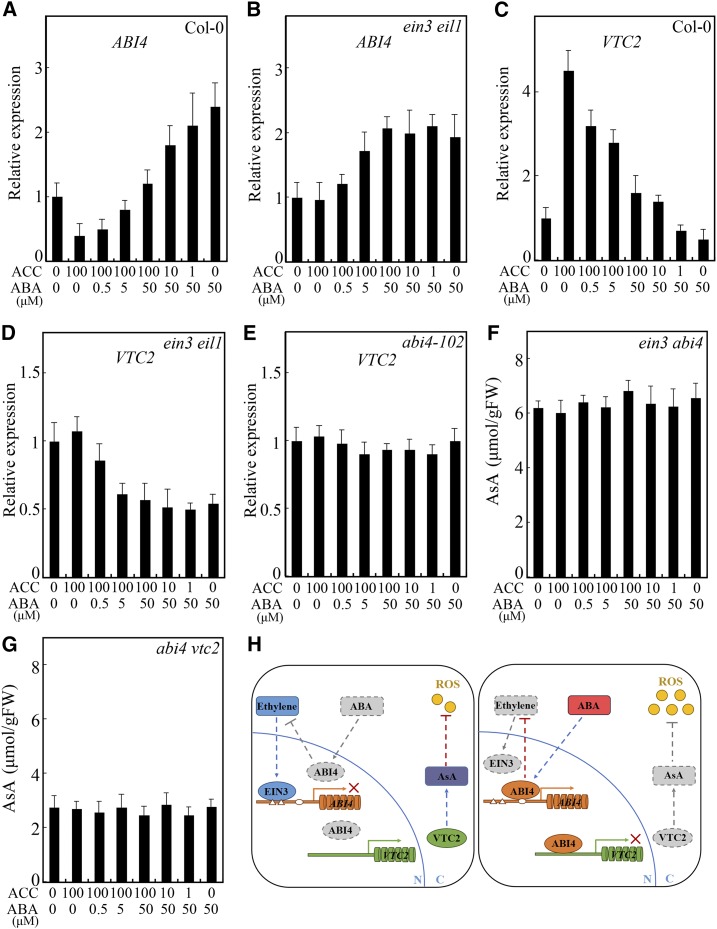
As both ethylene and ABA function via ABI4 to regulate AsA biosynthesis, we assessed ABI4 expression levels in response to treatment with different concentrations of ACC and/or ABA in Col-0 and ein3 eil1 mutants. As shown in Figure 8A, in the wild-type Col-0, the inhibitory effect of high concentrations of ACC on ABI4 expression was relieved by increasing the concentration of ABA. The down-regulation of ABI4 expression under ACC treatment was relieved by treatment with gradually increased ABA concentrations (Fig. 8A), indicating that ethylene and ABA antagonistically regulate ABI4 at the transcriptional level. Moreover, in the ein3 eil1 mutant, increasing the concentration of ABA up-regulated ABI4 expression in both the presence and absence of ACC (Fig. 8B), indicating that ABA inhibits the effects of ethylene on ABI4 expression downstream of EIN3.
Figure 8.
Ethylene and ABA promote VTC2 transcription and AsA biosynthesis via EIN3 and ABI4. A to E, Expression of ABI4 in Col-0 (A) and ein3 eil1 (B) and expression of VTC2 in Col-0 (C), ein3 eil1 (D), and abi4-102 (E) in response to treatment with different concentrations of ACC and ABA. F and G, AsA contents in ein3 abi4 (F) and abi4 vtc2 (G) in response to treatment with different concentrations of ACC and ABA. Seven-day-old seedlings were treated with the indicated conditions for 2 h (A–E) or 12 h (F and G). Values in A to G are means ± sd (n = 3). FW, Fresh weight. H, Model of ethylene- and ABA-coregulated AsA biosynthesis and ROS accumulation via EIN3-ABI4-VTC2. Left, when the effect of ethylene is dominant, ethylene-stabilized EIN3 binds to the promoter of ABI4 and inhibits its expression. Under this situation, a high level of VTC2 expression leads to more AsA production, thus low ROS level. Right, when the effect of ABA is dominant, the expression of ABI4 is probably promoted by ABA through ABI4 itself. Then, ABI4 binds to the promoter of VTC2 to suppress its expression. Also, ABI4 has a negative role in ethylene production. Under this situation, a low level of VTC2 expression leads to less AsA production, thus high ROS level.
VTC2 was identified as the key target gene of EIN3 and ABI4 in the regulation of AsA biosynthesis; thus, we measured VTC2 expression in Col-0, ein3 eil1, and abi4-102 in response to different concentrations of ACC and/or ABA. The induction of VTC2 expression in Col-0 in response to ACC was inhibited by treatment with ABA; however, increased ACC relieved the inhibitory effect of ABA (Fig. 8C), further suggesting counteracting effects of ethylene and ABA. In the ein3 eil1 mutant, the effects of ACC on the regulation of VTC2 expression and AsA production diminished, while ABA still suppressed both processes (Figs. 3B and 8D). Neither ACC nor ABA could affect VTC2 expression in the abi4-102 mutant (Fig. 8E). Furthermore, in abi4-102, ein3 abi4, and abi4 vtc2, the regulation of both ACC and ABA in AsA biosynthesis was disabled (Figs. 3C and 8, F and G). These results indicate that EIN3-ABI4-VTC2 coordinates the function of ethylene and ABA in AsA production.
DISCUSSION
The regulation of ROS accumulation by ethylene and ABA impacts many different physiological processes (Pei et al., 2000; Overmyer et al., 2003; Wang and Song, 2008; Zhong et al., 2009; Mersmann et al., 2010; Jiang et al., 2013; Tintor et al., 2013; Peng et al., 2014), but the underlying mechanisms governing the relationship between ethylene and ABA remain poorly understood. Here, at the Arabidopsis early seedling stage, we reveal that AsA plays an essential role in the regulation of ROS accumulation in response to ethylene and ABA. During this process, ethylene and ABA antagonistically regulate AsA biosynthesis and ROS accumulation via EIN3 and ABI4; and this regulation is mediated by the direct targeting of EIN3 to ABI4, which further transcriptionally represses the expression of the AsA biosynthesis gene VTC2. As a crucial node, ABI4 coordinates the cross talk between ethylene and ABA in the regulation of AsA biosynthesis and ROS accumulation.
ROS contents in plants are dynamically regulated by ethylene and ABA, although this regulation most likely differs among physiological processes. For example, ABA increases ROS production in guard cells to promote stomatal closure but reduces ROS levels in imbibed seeds to inhibit germination (Pei et al., 2000; Wang and Song, 2008; Ye et al., 2012). Interestingly, it is reported that ethylene not only promotes ROS production but also accelerates ROS scavenging, which enhance seedling salt tolerance through different mechanisms (Jiang et al., 2013; Peng et al., 2014). Moreover, an increasing number of studies also have proved that ethylene could promote ROS accumulation under stress conditions, especially in senescent leaves where direct regulation of RBOH expression by ethylene might be more important in ROS production (Pei et al., 2000; Wang and Song, 2008; Jiang et al., 2013). This is probably because of the dual roles and different levels of ROS under stress conditions and in older plants. Therefore, more investigations are required to explore the function of ethylene and ABA in the regulation of ROS accumulation in different tissues and developmental stages. In our research, we found that ABA promotes while ethylene suppresses ROS accumulation in Arabidopsis at the early seedling stage. This regulation is achieved by the nonenzymatic antioxidant AsA, which is induced by ethylene but suppressed by ABA, and plays an important role in ethylene- and ABA-coregulated ROS accumulation.
It is well known that AsA is a primary antioxidant and a key enzyme cofactor in both animals and plants (Smirnoff and Wheeler, 2000; De Tullio and Arrigoni, 2004; Smirnoff, 2018). Despite controversy, AsA has been considered as a promising anticancer agent (Shenoy et al., 2018). Due to mutation of the last enzyme in the AsA biosynthesis pathway, some animals, including humans, have to obtain AsA from plants to ensure essential metabolic levels and oxidative protection (Chatterjee, 1973). Moreover, increasing numbers of investigations have focused on the regulation of AsA production (Gallie, 2013; Wang et al., 2013b; Bulley and Laing, 2016). For instance, Arabidopsis ERF98 binds to the promoter of VTC1 and increases its expression and, thereby, AsA production (Zhang et al., 2012). CSN5B interacts with the VTC1 protein to modulate AsA biosynthesis (Wang et al., 2013a). As the enzyme of the committed step, VTC2 is considered a key regulator of AsA biosynthesis in many plant species (Dowdle et al., 2007; Bulley et al., 2009; Li et al., 2013). In this research, we showed that EIN3 and ABI4 consequently regulate the expression of VTC2 rather than that of VTC1. Thus, our results in this research demonstrate that VTC2, as a key regulatory point, is transcriptionally and antagonistically modulated by ethylene and ABA. Although another study found that the vtc2 mutant contains two independent mutations, one that confers AsA biosynthesis and another that affects seedling growth (Dowdle et al., 2007; Lim et al., 2016), the mutant we used in this research clearly effectively controls AsA production. Further research will be required to clarify whether the VTC2-mediated regulation of AsA biosynthesis by phytohormones participates in plant growth or other developmental processes.
The cross talk between ethylene and ABA has been studied for a long time. For example, Ghassemian et al. (2000) and Beaudoin et al. (2000) proved that ethylene and ABA act synergistically or antagonistically in the regulation of root growth and seed germination, respectively. In this report, we reveal that ABI4 is a novel integrating node in the interaction between ethylene and ABA in AsA biosynthesis and ROS accumulation; ABI4 is transcriptionally repressed by the upstream factor EIN3, releasing the transcriptional inhibition of VTC2 to coordinate the cross talk between ethylene and ABA in AsA biosynthesis. Moreover, EIN3 is considered a key regulator of ethylene networks in other signaling pathways such as the light and salt responses (Zhong et al., 2009, 2012; Chang et al., 2013); consistent with this fact, our data showed that EIN3 regulates ABI4 transcription. In addition, ABI4 and AsA have been reported to contribute to the regulation of the biosynthesis of ethylene (Smirnoff and Wheeler, 2000; Dong et al., 2016), gibberellins (Shu et al., 2013), and ABA and cytokinins (Smirnoff and Wheeler, 2000; Huang et al., 2017); thus, a complex regulatory loop might exist in the ABI4-integrated regulation of ethylene and ABA in AsA biosynthesis.
ABI4 encodes an AP2/ERF transcription factor that can be transcriptionally activated by itself and was initially characterized as a crucial element in the ABA signaling pathway; ABI4 is involved in many plant developmental processes, including seed development and germination (Finkelstein et al., 1998; Söderman et al., 2000; Bossi et al., 2009), the Glc response (Arenas-Huertero et al., 2000; Laby et al., 2000), lipid mobilization (Penfield et al., 2006), and salt stress and chloroplast-nucleus retrograde signaling pathways (Koussevitzky et al., 2007; Jagadeeswaran et al., 2009; Xu et al., 2016). ABI4 is required for AsA-regulated Arabidopsis growth (Kerchev et al., 2011); however, the effects of ABI4 on AsA biosynthesis have not been studied in depth. Here, we found that ABI4 suppresses AsA production via transcriptional inhibition of the VTC2 gene. Moreover, this process is affected by ethylene and ABA and probably mediates phytohormone-regulated plant growth and stress responses. EIN3 and ABI4 can regulate the expression of target genes by acting as repressors or activators (Wind et al., 2013; Ju and Chang, 2015). In our study, the inhibitory effect of the two transcription factors was demonstrated via transient expression assays. In tobacco leaves and Arabidopsis protoplasts, EIN3 and ABI4 significantly inhibited the expression of the target genes ABI4 and VTC2, respectively. In the presence of ethylene, this successive transcriptional inhibition was activated by the accumulation of EIN3 and could be suppressed by ABA via activation of the core element ABI4. This phenomenon might be a strategy by which plants subtly and delicately control AsA production in natural environments.
In conclusion, our findings offer novel insights into how plant hormones regulate AsA biosynthesis and propose a model of the cross talk between ethylene and ABA in Arabidopsis at the early seedling stage (Fig. 8H): ethylene and ABA antagonistically regulate the expression of ABI4, which affects the transcription of the VTC2 gene, AsA biosynthesis, and ROS accumulation, in Arabidopsis seedlings. This regulatory mechanism might provide a delicate way for plants to control AsA production and ROS accumulation. Under environmental stimuli, such as salt stress, both ethylene and ABA are induced, which will antagonistically control ROS levels through AsA, and this regulation is integrated by the promotion of ethylene and repression of ABA. Our previous work proved that ABA suppresses ethylene biosynthesis via ABI4 (Dong et al., 2016), leading to changes in the endogenous concentrations of ethylene and ABA in plants subjected to environmental stimuli. The interactions of ethylene and ABA will be balanced via the control of the key integrator ABI4, subsequently affecting AsA biosynthesis. To verify this hypothesis, we observed the growth of plants, in which the functions of EIN3 and ABI4 were genetically perturbed, under salt stress. The abi4-102 mutant, which displays high AsA levels and low ROS accumulation, was less sensitive to salt stress than Col-0; whereas ABI4ox and ein3 eil1, which present low levels of AsA and high ROS accumulation, were more sensitive (Supplemental Fig. S4). Importantly, the suppression of growth in ABI4ox and ein3 eil1 under salt stress was largely reversed by supplementation with AsA (Supplemental Fig. S4), strongly suggesting a key role for AsA in ROS accumulation under salt stress. Thus, the EIN3-ABI4-VTC2 transcriptional cascade coordinates the function of ethylene and ABA in AsA production and ROS accumulation in response to complex environmental stimuli.
MATERIALS AND METHODS
Plant Materials and Chemicals
All Arabidopsis (Arabidopsis thaliana) transgenic lines and mutants were generated in the Col-0 background, with the exception that abi3-1 was generated in the Landsberg erecta background. Homozygous lines of abi3-1 (CS24), abi5-8 (SALK_013163), ctr1-1 (CS8057), etr1-1 (CS237), ein2 (CS8844), ein3-1 (CS8052), eto2 (CS8059), and abi4-102 (CS3837) were obtained from the Arabidopsis Biological Resource Center. EIN3ox (Zhong et al., 2009), ABI4ox (Dong et al., 2016), and vtc2 and abi4 vtc2 (Kerchev et al., 2011) have been previously described.
The plants were grown on MS medium (Murashige and Skoog, 1962) containing 0.5% (w/v) phytagel under a 16-h-white light (150 µmol m−2 s−1)/8-h-dark cycle at 22°C. For the hormone treatments, 7-d-old seedlings were transferred into MS liquid medium supplemented with or without 10 μm ACC, 10 μL L−1 ethylene gas, or 1 μm ABA for 12 h (for AsA and ROS measurements) or 2 h (for RT-qPCR) under light condition. For the ethylene gas treatment, the plates were sealed and injected with 10 μL L−1 ethylene gas. All chemicals used were obtained from Sigma-Aldrich.
Generation of Transgenic Plants
The DNA sequence of VTC2, including 1,903 bp upstream of the start codon, was amplified via PCR and then cloned into the modified expression vector pCAMBIA1300-GFP using a pEASY-Uni Seamless Cloning and Assembly Kit (Transgen Biotech). The vector carrying pVTC2::VTC2-GFP was introduced into different genotypes of Col-0 and the abi4-102 and ABI4ox backgrounds using an Agrobacterium tumefaciens-mediated transformation assay. The transgenic lines were selected with hygromycin and confirmed by PCR amplification. The roots of T3 homozygous lines that contained a single insertion were used to observe GFP fluorescence. The sequences of the primers used in this study are listed in Supplemental Table S1.
Genetic Manipulation
The double mutant ein3 abi4 was generated by crossing the recessive ethylene mutant ein3-1 with abi4-102. The F2 progeny from the crosses were grown in the dark for 4 d in medium supplemented with 10 μm ACC to isolate individual ethylene-insensitive plants that were homozygous for ein3-1. The individual isolated plants were subsequently screened for resistance to ABA, and their homozygous nature was confirmed by sequencing.
The double mutant abi4 vtc2 was generated by crossing abi4-102 with vtc2 (Kerchev et al., 2011). The F2 progeny from the crosses were grown on medium supplemented with ABA to isolate homozygous abi4-102 lines by phenotype. The individual plants were subsequently screened by sequencing. Information about the primers used is summarized in Supplemental Table S1.
ROS Staining
Whole plants were infiltrated with 0.1% (w/v) DAB (Sigma-Aldrich) in 10 mm MES (pH 6.5) for H2O2 staining or with 0.1% (w/v) NBT (Sigma-Aldrich) in 50 mm potassium phosphate buffer (pH 6.4) for superoxide staining. After about 10 min to several hours (based on the staining degree) of incubation in the dark at 37°C, the plants were destained in 95% (v/v) ethanol.
ROS Measurement
The titanium-peroxide complex method was used to measure the contents of H2O2 as described by Patterson et al. (1984) with minor modifications. First, 0.1 g (fresh weight, about 40 seedlings) of treated seedlings was weighed after removing excess MS liquid medium by paper towel. Then the seedlings were ground in liquid nitrogen and extracted with 1 mL of cooled acetone, after which the samples were centrifuged for 10 min at 8,000g. One milliliter of the supernatant was then added to a solution consisting of 0.1 mL of 5% (w/v) titanium sulfate and 0.2 mL of ammonia. The mixture was centrifuged for 10 min at 4,000g. After removal of the supernatant, the precipitate was dissolved in 1 mL of 2 m H2SO4. The A415 was then immediately measured. The H2O2 content was determined using a standard curve generated from known concentrations of H2O2.
The O2− content in the seedlings was measured as described by Li and Gong (2005) with minor modifications. First, 0.1 g (fresh weight) of treated seedlings was ground in liquid nitrogen and added to 1 mL of potassium phosphate buffer (65 mm, pH 7.8). Then the mixture was centrifuged for 20 min at 12,000g at 4°C. One milliliter of 0.1 m hydroxylamine was added to the supernatant and incubated at 25°C for 20 min. Then 1 mL of 7 mm α-naphthalenamine and 1 mL of 58 mm 4-aminobenzenesulfonic acid were added and incubated at 25°C for 20 min. An equal volume of trichloromethane (3 mL) was added to eliminate the pigments, and the mixture was centrifuged at 10,000g for 5 min. The absorbance of the supernatant at 530 nm was then immediately measured. The O2− content was determined using a standard curve generated from known concentrations of sodium nitrite.
AsA Measurements
Measurements of AsA contents were conducted as previously described (Wang et al., 2013a) using nicotinic acid as an internal standard. Briefly, 0.1 g (fresh weight) of treated seedlings was homogenized, after which 20 μL of the extractions was injected into an LC-10A HPLC instrument (Shimadzu). Chromatographic separation was achieved using an Agilent TC-C18 (250 mm × 4.6 mm, 5 μm) column, and then absorbance was measured at 254 nm using an SPD M10A detector. The reported AsA content included both the oxidized and reduced forms.
RNA Extraction and RT-qPCR Analysis
The total RNA of the seedlings was extracted using a Plant RNA Isolation Mini Kit (CWBIO). RNA reverse transcription was performed using HiScript II Q RT Supermix for qPCR (Vazyme Biotech), and qPCR was performed using SYBR Green Master Mix (TaKaRa). RT-qPCR was performed using the iQ5 system (Bio-Rad). Four serial dilutions of complementary DNA were used to evaluate the specificity and efficiency of the primers. Three biological samples were each analyzed with three technical replicates. ACTIN2, UBQ5, and PP2A were used as reference genes for normalization, and the relative transcript levels were calculated by the 2−△△Ct method according to Vandesompele et al. (2002). Primers used for RT-qPCR and their efficiency are listed in Supplemental Table S1.
Transcriptome Profiling Analysis
The total RNA of 7-d-old seedlings was extracted as described above. Sequencing was conducted with an Illumina HiSeq 2000 sequencer, and the resulting reads were assembled via Bowtie v2.0.6 by mapping them to the reference genome of Arabidopsis (TAIR10; Langmead and Salzberg, 2012). Transcript expression was evaluated by cuffdiff v2.2.1 (http://cufflinks.cbcb.umd.edu; Trapnell et al., 2013). A value of q < 0.05 and a fold change > 2 between the wild-type Col-0 and mutants were considered assessment criteria for significantly different expression.
Transient Expression Assays
For the transient expression assays, tobacco (Nicotiana tabacum) leaf and Arabidopsis protoplast systems were used for qualitative and quantitative analyses, respectively. A. tumefaciens-mediated tobacco transient transformation was performed as previously described (Liu et al., 2010). The transformed plants were grown in the dark for 24 h, followed by a 16-h-light/8-h-dark cycle for 48 h. The leaves were subsequently sprayed with 100 μm luciferin (Promega) and placed in the dark for 5 min. LUC activity was observed with an iXon low-light cooled CCD imaging apparatus (Andor Technology).
Arabidopsis protoplasts were prepared and transfected with the corresponding constructs using a polyethylene glycol/calcium-mediated method as previously described (Wang et al., 2013a). The firefly LUC and REN activities were measured with a dual-luciferase reporter assay kit (Promega). The LUC activity was normalized to the REN activity, and the relative LUC/REN ratios were calculated. For each plasmid combination, five independent transformations were performed.
ChIP Assays
ChIP assays were performed using an Epiquik Plant ChIP Kit (Gepigentek) with 7-d-old 35S::EIN3-Flag or 35S::ABI4-Myc transgenic seedlings. Affinity-purified anti-Myc or anti-Flag monoclonal antibodies were used to immunoprecipitate the DNA complex, after which the precipitated DNA was analyzed via qPCR. The sequences of the primers used for ChIP-qPCR are listed in Supplemental Table S1, and TUBULIN4 was used as an internal control.
EMSA
The sequences encoding the 146 N-terminal amino acids of ABI4 (ABI4N) and 332 N-terminal amino acids of EIN3 (EIN3N) were cloned into pET28a or pGEX-6p-1 and transformed into Escherichia coli strain BL21 (DE3). The recombinant proteins were purified with His-Select Nickel Affinity Gel (Sigma-Aldrich) and ProteinIso GST Resin (Transgen Biotech) according to each manufacturer’s instructions. Oligonucleotide probes containing binding motifs were synthesized and labeled with biotin at the 3′ end (Thermo). EMSA was performed using a Lightshift Chemiluminescent EMSA Kit (Thermo). The probe sequences are listed in Supplemental Table S1.
Statistical Analysis
Unless otherwise noted, the data were analyzed with one-way ANOVA (Tukey’s test, P < 0.05) by using SPSS. RT-qPCR data were log transformed before analysis. Different letters were used to indicate statistically significant differences in the figures. Error bars represent sd (n = 3).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: ETR1 (AT1G66340), CTR1 (AT5G03730), EIN2 (AT5G03280), EIN3 (AT3G20770), EIL1 (AT2G27050), ABI3 (AT3G24650), ABI4 (AT2G40220), ABI5 (AT2G36270), ACTIN2 (AT3G18780), TUBULIN4 (AT5G44340), PGI1 (AT4G24620), PGIC (AT5G42740), PMI1 (AT3G02570), PMI2 (AT1G67070), PMM (At2G45790), VTC1 (AT2G39770), GME (AT5G28840), VTC2 (AT4G26850), VTC4 (AT3G02870), VTC5 (AT5G55120), LGALDH (AT4G33670), GLDH (AT3G47930), MDAR1 (AT1G63940), MDAR2 (AT3G09940), MDAR3 (AT3G27820), MDAR4 (AT3G52880), MDAR5 (AT5G03630), DHAR1 (AT5G16710), DHAR3 (AT1G75270), DHAR4 (AT1G19550), and DHAR5 (AT1G19570).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. ROS levels in ethylene- and ABA-related mutants.
Supplemental Figure S2. Transcriptome analysis of EIN3- and ABI4-coregulated genes.
Supplemental Figure S3. EIN3 does not directly bind to the promoter of VTC2.
Supplemental Figure S4. The phenotypes of EIN3- and ABI4-related materials under salt stress.
Supplemental Table S1. The primers used in this article.
Acknowledgments
We thank Dr. Christine H. Foyer (University of Leeds), Dr. Keke Yi (Chinese Academy of Agricultural Sciences), and Dr. Ying Zhu (Zhejiang Academy of Agricultural Sciences) for providing AsA-related mutant Arabidopsis seeds.
Footnotes
This work was supported by the National Natural Science Foundation of China (31670280), the China Postdoctoral Science Foundation (2017M610134), and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences.
Articles can be viewed without a subscription.
References
- Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid: A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci 8: 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164: 447–459 [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J (2000) Interactions between abscisic acid and ethylene signaling cascades. Plant Cell 12: 1103–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: A gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Bossi F, Cordoba E, Dupré P, Mendoza MS, Román CS, León P (2009) The Arabidopsis ABA-INSENSITIVE (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59: 359–374 [DOI] [PubMed] [Google Scholar]
- Bulley S, Laing W (2016) The regulation of ascorbate biosynthesis. Curr Opin Plant Biol 33: 15–22 [DOI] [PubMed] [Google Scholar]
- Bulley SM, Rassam M, Hoser D, Otto W, Schünemann N, Wright M, MacRae E, Gleave A, Laing W (2009) Gene expression studies in kiwifruit and gene over-expression in Arabidopsis indicates that GDP-L-galactose guanyltransferase is a major control point of vitamin C biosynthesis. J Exp Bot 60: 765–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KN, Zhong S, Weirauch MT, Hon G, Pelizzola M, Li H, Huang SSC, Schmitz RJ, Urich MA, Kuo D, et al. (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. eLife 2: e00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee IB. (1973) Evolution and the biosynthesis of ascorbic acid. Science 182: 1271–1272 [DOI] [PubMed] [Google Scholar]
- Davey MW, Van Montagu M, Inze D, Sanmartin M, Kanellis A, Smirnoff N, Benzie IJ, Strain JJ, Favell D, Fletcher J (2000) Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric 80: 825–860 [Google Scholar]
- De Tullio MC, Arrigoni O (2004) Hopes, disillusions and more hopes from vitamin C. Cell Mol Life Sci 61: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K-J, Mittler R, Noctor G (2016) Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol 171: 1535–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Yu Y, Li S, Wang J, Tang S, Huang R (2016) Abscisic acid antagonizes ethylene production through the ABI4-mediated transcriptional repression of ACS4 and ACS8 in Arabidopsis. Mol Plant 9: 126–135 [DOI] [PubMed] [Google Scholar]
- Dowdle J, Ishikawa T, Gatzek S, Rolinski S, Smirnoff N (2007) Two genes in Arabidopsis thaliana encoding GDP-L-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J 52: 673–689 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Wang ML, Lynch TJ, Rao S, Goodman HM (1998) The Arabidopsis abscisic acid response locus ABI4 encodes an APETALA 2 domain protein. Plant Cell 10: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Suppl) 14: S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR. (2013) L-Ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica (Cairo) 2013: 795964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Zhang L (2008) Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol 165: 138–148 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Waadt R, Schroeder JI (2011) Evolution of abscisic acid synthesis and signaling mechanisms. Curr Biol 21: R346–R355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang X, Gong Z, Yang S, Shi Y (2017) ABI4 represses the expression of type-A ARRs to inhibit seed germination in Arabidopsis. Plant J 89: 354–365 [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran G, Saini A, Sunkar R (2009) Biotic and abiotic stress down-regulate miR398 expression in Arabidopsis. Planta 229: 1009–1014 [DOI] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Mithani A, Visscher A, Ragoussis J, Mott R, Smith JAC, Harberd NP (2012) ROS-mediated vascular homeostatic control of root-to-shoot soil Na delivery in Arabidopsis. EMBO J 31: 4359–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Belfield EJ, Cao Y, Smith JAC, Harberd NP (2013) An Arabidopsis soil-salinity-tolerance mutation confers ethylene-mediated enhancement of sodium/potassium homeostasis. Plant Cell 25: 3535–3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Chang C (2015) Mechanistic insights in ethylene perception and signal transduction. Plant Physiol 169: 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchev PI, Pellny TK, Vivancos PD, Kiddle G, Hedden P, Driscoll S, Vanacker H, Verrier P, Hancock RD, Foyer CH (2011) The transcription factor ABI4 is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 23: 3319–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J (2007) Signals from chloroplasts converge to regulate nuclear gene expression. Science 316: 715–719 [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Laing WA, Martínez-Sánchez M, Wright MA, Bulley SM, Brewster D, Dare AP, Rassam M, Wang D, Storey R, Macknight RC, et al. (2015) An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 27: 772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZG, Gong M (2005) Improvement of measurement method for superoxide anion radical in plant. Yunnan Zhi Wu Yan Jiu 27: 211–216 [Google Scholar]
- Li J, Liang D, Li M, Ma F (2013) Light and abiotic stresses regulate the expression of GDP-L-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 238: 535–547 [DOI] [PubMed] [Google Scholar]
- Lim B, Smirnoff N, Cobbett CS, Golz JF (2016) Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front Plant Sci 7: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster CL, Clarke SG (2008) L-Ascorbate biosynthesis in higher plants: The role of VTC2. Trends Plant Sci 13: 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang Y, Tang S, Zhao Q, Zhang Z, Zhang H, Dong L, Guo H, Xie Q (2010) An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J 61: 893–903 [DOI] [PubMed] [Google Scholar]
- Mersmann S, Bourdais G, Rietz S, Robatzek S (2010) Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol 154: 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410 [DOI] [PubMed] [Google Scholar]
- Mittler R. (2017) ROS are good. Trends Plant Sci 22: 11–19 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473–497 [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H Jr, Kangasjärvi J (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosché M, Kangasjärvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium(IV). Anal Biochem 139: 487–492 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Penfield S, Li Y, Gilday AD, Graham S, Graham IA (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell 18: 1887–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Li Z, Wen X, Li W, Shi H, Yang L, Zhu H, Guo H (2014) Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genet 10: e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pető A, Lehotai N, Feigl G, Tugyi N, Ördög A, Gémes K, Tari I, Erdei L, Kolbert Z (2013) Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep 32: 1913–1923 [DOI] [PubMed] [Google Scholar]
- Shenoy N, Creagan E, Witzig T, Levine M (2018) Ascorbic acid in cancer treatment: Let the phoenix fly. Cancer Cell 34: 700–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu K, Zhang H, Wang S, Chen M, Wu Y, Tang S, Liu C, Feng Y, Cao X, Xie Q (2013) ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet 9: e1003577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AR, Bergmann DC (2016) Transcriptional control of cell fate in the stomatal lineage. Curr Opin Plant Biol 29: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. (2018) Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic Biol Med 122: 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL (2000) Ascorbic acid in plants: Biosynthesis and function. Crit Rev Biochem Mol Biol 35: 291–314 [DOI] [PubMed] [Google Scholar]
- Söderman EM, Brocard IM, Lynch TJ, Finkelstein RR (2000) Regulation and function of the Arabidopsis ABA-insensitive4 gene in seed and abscisic acid response signaling networks. Plant Physiol 124: 1752–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: A transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintor N, Ross A, Kanehara K, Yamada K, Fan L, Kemmerling B, Nürnberger T, Tsuda K, Saijo Y (2013) Layered pattern receptor signaling via ethylene and endogenous elicitor peptides during Arabidopsis immunity to bacterial infection. Proc Natl Acad Sci USA 110: 6211–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L (2013) Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol 31: 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: 0034.1–0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, et al. (2017) Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front Plant Sci 8: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Song CP (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178: 703–718 [DOI] [PubMed] [Google Scholar]
- Wang J, Yu Y, Zhang Z, Quan R, Zhang H, Ma L, Deng XW, Huang R (2013a) Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell 25: 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhang Z, Huang R (2013b) Regulation of ascorbic acid synthesis in plants. Plant Signal Behav 8: e24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Cao H, Sun Y, Li X, Chen F, Carles A, Li Y, Ding M, Zhang C, Deng X, et al. (2013c) Arabidopsis paired amphipathic helix proteins SNL1 and SNL2 redundantly regulate primary seed dormancy via abscisic acid-ethylene antagonism mediated by histone deacetylation. Plant Cell 25: 149–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczak C, Carmody M, Kangasjärvi J (2018) Reactive oxygen species in plant signaling. Annu Rev Plant Biol 69: 209–236 [DOI] [PubMed] [Google Scholar]
- Wind JJ, Peviani A, Snel B, Hanson J, Smeekens SC (2013) ABI4: Versatile activator and repressor. Trends Plant Sci 18: 125–132 [DOI] [PubMed] [Google Scholar]
- Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ (2015) Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J Exp Bot 66: 2839–2856 [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao H, Ruan W, Deng M, Wang F, Peng J, Luo J, Chen Z, Yi K (2017) ABNORMAL INFLORESCENCE MERISTEM1 functions in salicylic acid biosynthesis to maintain proper reactive oxygen species levels for root meristem activity in rice. Plant Cell 29: 560–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chi W, Sun X, Feng P, Guo H, Li J, Lin R, Lu C, Wang H, Leister D, et al. (2016) Convergence of light and chloroplast signals for de-etiolation through ABI4-HY5 and COP1. Nat Plants 2: 16066. [DOI] [PubMed] [Google Scholar]
- Ye N, Zhu G, Liu Y, Zhang A, Li Y, Liu R, Shi L, Jia L, Zhang J (2012) Ascorbic acid and reactive oxygen species are involved in the inhibition of seed germination by abscisic acid in rice seeds. J Exp Bot 63: 1809–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CC, Ma B, Collinge DP, Pogson BJ, He SJ, Xiong Q, Duan KX, Chen H, Yang C, Lu X, et al. (2015) Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 27: 1061–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CC, Zhao H, Ma B, Chen SY, Zhang JS (2017) Diverse roles of ethylene in regulating agronomic traits in rice. Front Plant Sci 8: 1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li Z, Quan R, Li G, Wang R, Huang R (2011) An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol 157: 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang J, Zhang R, Huang R (2012) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71: 273–287 [DOI] [PubMed] [Google Scholar]
- Zhong S, Zhao M, Shi T, Shi H, An F, Zhao Q, Guo H (2009) EIN3/EIL1 cooperate with PIF1 to prevent photo-oxidation and to promote greening of Arabidopsis seedlings. Proc Natl Acad Sci USA 106: 21431–21436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Shi H, Xue C, Wang L, Xi Y, Li J, Quail PH, Deng XW, Guo H (2012) A molecular framework of light-controlled phytohormone action in Arabidopsis. Curr Biol 22: 1530–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]