Inactivation of a transcription factor lowers gluten in wheat and may provide a wheat alternative for some individuals with gluten sensitivities.
Abstract
Celiac disease is the most common food-induced enteropathy in humans, with a prevalence of approximately 1% worldwide. It is induced by digestion-resistant, proline- and glutamine-rich seed storage proteins, collectively referred to as gluten, found in wheat (Triticum aestivum). Related prolamins are present in barley (Hordeum vulgare) and rye (Secale cereale). The incidence of both celiac disease and a related condition called nonceliac gluten sensitivity is increasing. This has prompted efforts to identify methods of lowering gluten in wheat, one of the most important cereal crops. Here, we used bulked segregant RNA sequencing and map-based cloning to identify the genetic lesion underlying a recessive, low-prolamin mutation (lys3a) in diploid barley. We confirmed the mutant identity by complementing the lys3a mutant with a transgenic copy of the wild-type barley gene and then used targeting-induced local lesions in genomes to identify induced single-nucleotide polymorphisms in the three homeologs of the corresponding wheat gene. Combining inactivating mutations in the three subgenomes of hexaploid bread wheat in a single wheat line lowered gliadin and low-molecular-weight glutenin accumulation by 50% to 60% and increased free and protein-bound lysine by 33%.
Gluten is the common name for a complex mixture of approximately 100 Pro- and Gln-rich seed storage proteins found in wheat (Triticum aestivum) endosperm. In hexaploid bread wheat, in particular, gluten is responsible for the unique viscoelastic properties of dough used for making leavened bread. Related prolamins known as hordeins in barley (Hordeum vulgare) and secalins in rye (Secale cereale), but lacking the viscoelastic properties of wheat gluten, are found in the endosperms of these related cereals. Among gluten proteins, the most important for the elasticity and strength of bread dough are the polymeric high-molecular-weight (HMW) glutenins (Delcour et al., 2012). Monomeric gluten proteins contribute to the viscosity of gluten (Shewry, 2009; Kucek et al., 2015). Among the most prominent monomeric gluten proteins are the gliadins. These are classified into α, β, γ, and ω types based on their size, charge, and repetitive amino acid sequences (Shewry, 2009).
Gluten is the causative factor of celiac disease, the most common food-induced autoimmune enteropathy in humans (Lohi et al., 2007; Sapone et al., 2012; Green et al., 2015). Celiac disease is found primarily in individuals expressing human leukocyte antigen alleles DQ2 and/or DQ8. Immunodominant peptide epitopes have been found in α-gliadins (Shan et al., 2002) and ω-gliadins of wheat and in C-hordeins of barley (Tye-Din et al., 2010). Additional epitopes are also found in low-molecular-weight (LMW) and HMW glutenins, γ-gliadins from wheat, and B- and γ-hordeins from barley (Tye-Din et al., 2010; Sollid et al., 2012). Celiac disease patients exhibit a range of intestinal and extraintestinal symptoms, of which the most common is flattening of the villi of the small intestine, which can lead to poor absorption of nutrients and other pathological consequences. The only current treatment is a life-long avoidance of all gluten in the diet, which can be challenging given the prevalent use of gluten in diverse processed food products.
Given gluten’s negative consequences for celiac and nonceliac gluten sensitivity patients, efforts have been made to lower gluten by combining gliadin-deletion mutants (Camerlengo et al., 2017) or by using RNA interference transgenic methods in wheat (Gil-Humanes et al., 2010, 2014; Sánchez-León et al., 2017) as well as by conventional breeding of known low-hordein mutants in barley (Tanner et al., 2016). One such mutant is lys3a, a low-hordein barley that is also known as Risø 1508 or shrunken endosperm xenia (Ullrich and Eslick, 1977). lys3a was induced by ethylenimine mutagenesis of the two-rowed spring malting variety cv Bomi (Doll et al., 1974) close to 50 years ago in a program of barley mutagenesis and screening for increased Lys at the Risø agricultural experiment station in Roskilde, Denmark (Køie and Doll, 1979; Miflin and Shewry, 1979). The lys3a mutant is almost completely lacking in class C hordeins and accumulates considerably reduced amounts of several B class hordeins, while having a 45% increase in the accumulation of free and protein-bound Lys in the seed compared with the parental control (Shewry et al., 1977, 1978a; Munck et al., 2001). These phenotypes are the result of a single recessive allele (Doll, 1973) that has been variously mapped to barley chromosome 5H (Karlsson, 1977; Ullrich and Eslick, 1977; Jensen, 1979) or, more recently, to chromosome 1H (Druka et al., 2011; Rustgi and von Wettstein, 2015), but the underlying mutant gene is unknown.
We undertook to identify the lys3a mutant gene in barley using bulked segregant RNA sequencing (BSR-seq; Liu et al., 2012) and genetic fine mapping with the objective to determine if an analogous low-gluten wheat could be developed by inactivation of the homeologs in wheat. Here, we show that the lys3a mutation is due to a missense allele in a domain of one finger (DOF) zinc-finger transcription factor known as Barley Prolamin-box Binding Factor (BPBF; Mena et al., 1998). Transformation of the lys3a barley mutant with the wild-type BPBF gene restored hordein levels, which confirms the fact that a mutation in BPBF is responsible for the lys3a phenotype. Finally, we show that mutating the wheat homeologs of BPBF, the Wheat Prolamin-box Binding Factors (WPBFs), using the nontransgenic targeting-induced local lesions in genomes (TILLING) method (McCallum et al., 2000), results in a decreased-gluten, high-Lys wheat. To our knowledge, this is the first demonstration of a decreased-gluten, high-Lys wheat developed without the use of transgenic methods.
RESULTS
Identification of lys3a as a Missense Mutation in BPBF
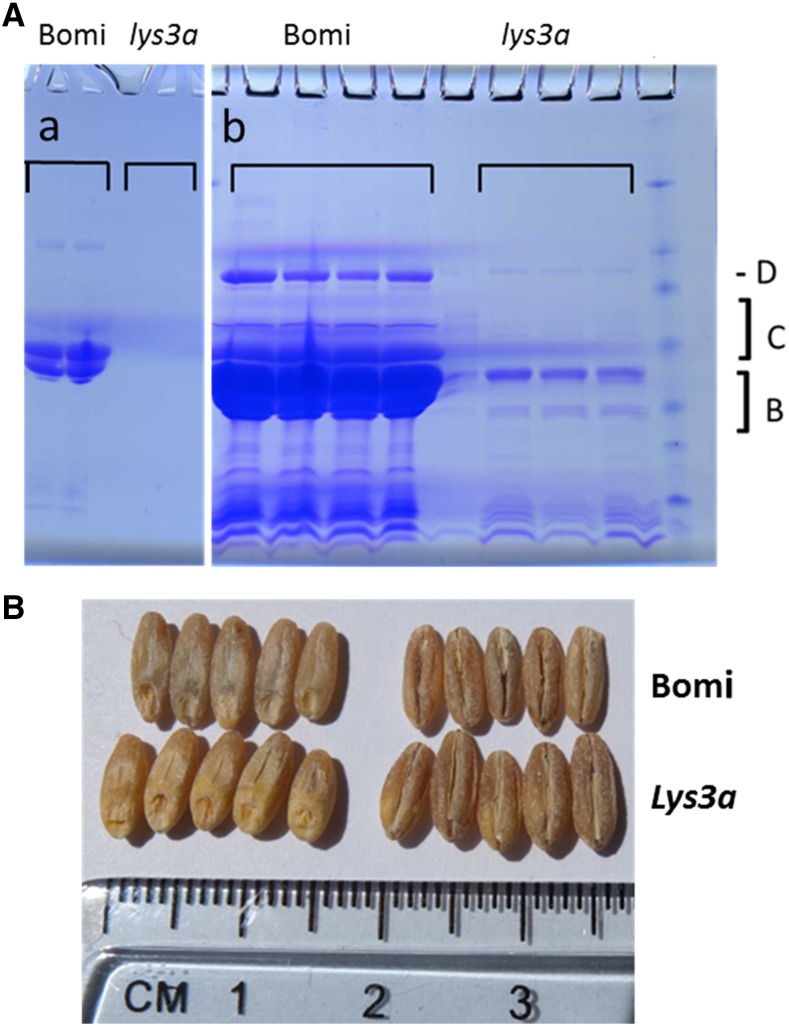
To gain insight into the molecular mechanisms responsible for the lys3a mutant’s effects, we conducted a BSR-seq experiment (Liu et al., 2012) to identify the underlying lesion. Our BSR-seq experiment was conducted on the F3 progeny of a cross between lys3a and cv Bowman. Separation of the F2 seeds derived from this cross into normal and mutant phenotypes was facilitated by the ease of distinguishing the homozygous recessive mutant from the normal phenotype by analysis of hordeins extracted from endosperm tissue, as shown in Figure 1. When loading equal fractions of extracted hordeins from normal and mutant endosperms, hordeins from the homozygous lys3a mutant parent, including B-, C-, and D-hordeins, are not visible on a Coomassie blue-stained SDS-PAGE gel compared with the conventional nonmutant cv Bowman barley cultivar. Only when the gel is overloaded 4-fold (Fig. 1A, gel b) do hordeins become visible in the mutant. This facile SDS-PAGE screen of F2 half-seeds was used to identify wild-type and homozygous mutant progeny of the cross. The tissue bulks used for RNA isolation were derived from 25 to 35 individual F3 seedlings, each derived from 25 to 35 independent F2s determined to be either homozygous wild type or homozygous mutant. To ensure that the wild-type F3 bulks were derived from homozygous wild-type F2 seeds, we ran SDS-PAGE gels of hordeins extracted from F3 progeny of the wild-type F2s and discarded those that segregated mutants. Illumina sequencing of cDNA derived from RNA isolated from three biological replicates of both mutant and wild-type whole-seedling tissue bulks identified single-nucleotide polymorphisms (SNPs) and gene expression variation tightly linked with the mutation.
Figure 1.
SDS-PAGE of cv Bomi and lys3a barley endosperm hordeins and appearance of seeds. A, Four percent to 20% gradient SDS-PAGE experiment in which endosperm hordeins of parental cv Bomi, and the lys3a mutant derived from it, are shown. Equivalent fractions of extracted hordeins from both are shown. Gel b represents a 4-fold overloading of the hordeins compared with gel a. D refers to the position of the D-hordein, C shows the location of the C-hordeins, and B indicates the location of the B-hordeins on the gel. B, Image of seeds (husks removed) of cv Bomi and lys3a mutant derived from cv Bomi (top and bottom rows, respectively).
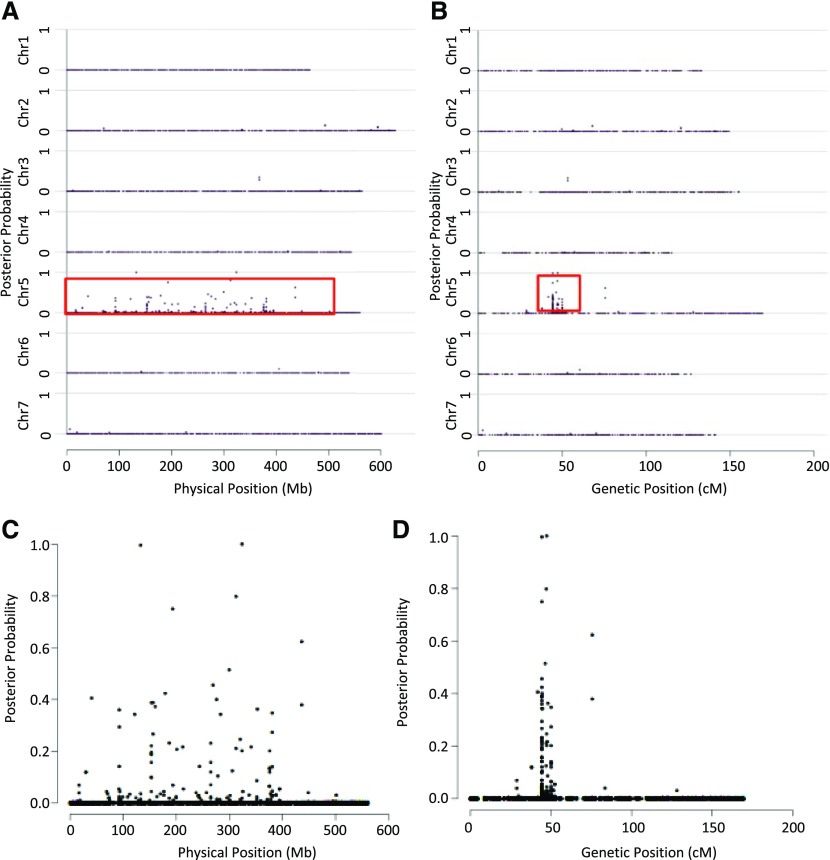
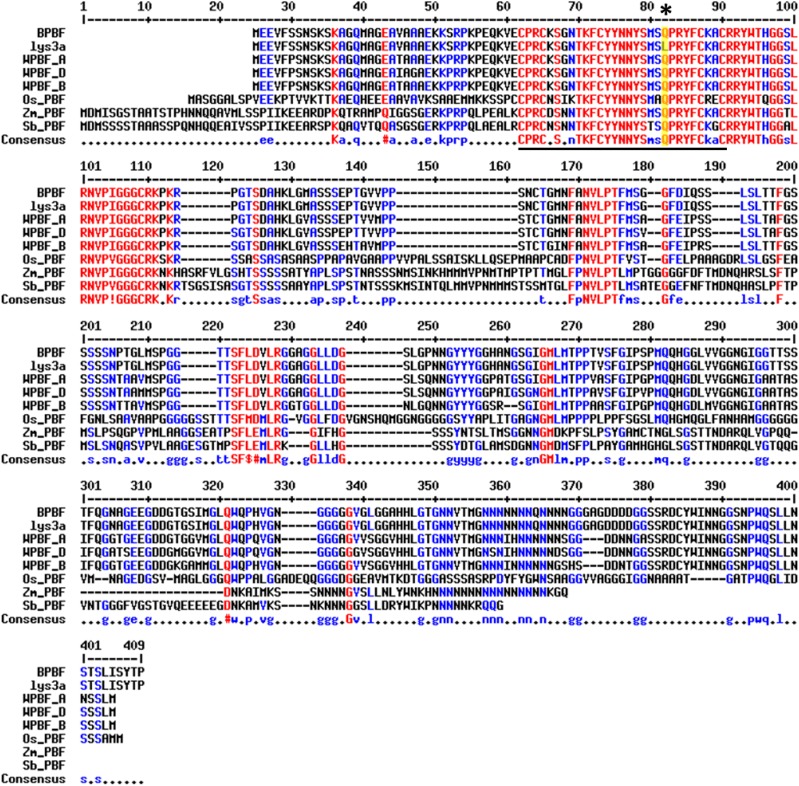
This experiment allowed us to definitively link the lys3a mutant to an approximately 7-centimorgan interval in the pericentromeric region of chromosome 5H (Fig. 2). Utilizing the first (preliminary) version of the barley genome assembly (Mayer et al., 2012), this area appears to encompass a physical distance of approximately 200 Mb of DNA in a region of suppressed recombination. We generated 25 kompetitive allele specific PCR (KASP) genotyping probes (LGC; Supplemental Table S1) from some of the 123 SNPs linked to the mutant on chromosome 5H and mapped them in a larger mapping population of 1,814 individuals. Half-seed hordein analysis of all seeds identified homozygous mutant individuals, while KASP genotyping identified cosegregating markers. Two markers, approximately 1 Mb apart, were in absolute linkage with the mutant phenotype with no recombinants (Supplemental Fig. S1). Examination of this region of barley chromosome 5H using Ensembl Barley (http://plants.ensembl.org/Hordeum_vulgare/Info/Index; Kersey et al., 2016) and BarleyMap (http://floresta.eead.csic.es/barleymap/; Mascher et al., 2013; Cantalapiedra et al., 2015) identified approximately 30 high-confidence and 30 low-confidence genes. We examined this region for putative regulatory genes such as transcription factors and determined that a low-confidence gene was identical to the known BPBF gene. Sequencing of this gene in the lys3a and cv Bomi backgrounds identified a novel SNP in lys3a compared with the cv Bomi parental line. This SNP resulted in a missense mutation in the DOF-DNA-binding zinc finger, and we considered it to be a likely candidate for the barley lys3a mutant. In lys3a, an A-to-T transversion at nucleotide 173 (numbering beginning with the ATG start codon) leads to a missense mutation in which Gln at amino acid 58 is replaced by Leu (Fig. 3). Barley BPBF was cloned in 1998 (Mena et al., 1998) on the basis of the homology of its DOF-DNA-binding domain to the previously isolated maize DOF transcription factor that was named Prolamin-box Binding Factor, since it binds to a highly conserved seven-nucleotide motif called the P-box (prolamin box) that is found in the promoters of multiple seed storage protein genes (Vicente-Carbajosa et al., 1997). Mena et al. (1998) also cloned a cDNA of a wheat homolog of the barley DOF transcription factor. Subsequent research showed that the wheat homolog indeed plays a role in the transcription of gliadin genes (Dong et al., 2007), and other researchers cloned and sequenced the three genomic homeologs of hexaploid bread wheat WPBF (Ravel et al., 2006).
Figure 2.
BSR-seq localizes lys3a to chromosome 5H. Posterior probabilities of SNPs putatively cosegregating with lys3a are depicted on the y axis, while physical position (A and C) and genetic position (B and D) are shown on the x axis. A and B show all seven barley chromosomes, while C and D depict chromosome 5H only.
Figure 3.
Alignment of cereal PBF proteins. A sequence alignment of the barley, the lys3a barley mutant, the wheat A, B, and D subgenome homeologs, as well as the rice (Oryza sativa), maize (Zea mays), and sorghum (Sorghum bicolor) prolamin box-binding factor proteins is shown. The zinc finger is underlined, and the asterisk and yellow highlight denote the absolutely conserved Gln, which is mutated to a Leu in the lys3a barley mutant. The alignment was made using default parameters of the program multalin (Corpet, 1988). Accession numbers are as follows: rice PBF gene, AK107294 (Yamamoto et al., 2006); maize PBF, AAB70119.1 (Vicente-Carbajosa et al., 1997); and sorghum PBF, XP_002448852.1.
The Gln that is altered to Leu in lys3a barley is an absolutely conserved residue in the zinc-finger DNA-binding region of the DOF domain (Fig. 3; Gupta et al., 2015), and this alteration from Gln, an amino acid with a polar side chain, to Leu, an amino acid with a hydrophobic side chain, is predicted to severely negatively affect the DNA-binding properties of the DOF domain using the PROVEAN (Protein Variation Effect Analyzer) bioinformatics server (Choi and Chan, 2015). This Gln-to-Leu alteration of the DOF domain in lys3a barley was also found upon sequencing the same gene in cv Lysiba, a barley cultivar developed by outcrossing the original mutant at least three times to unrelated cultivars and selecting at each cross for high-Lys segregants. Based on the known properties of BPBF, we hypothesized that BPBF represented a strong candidate for the underlying lys3a mutation, and we performed additional experiments to verify this hypothesis.
Transgenic Complementation of the lys3a Mutant with the Wild-Type BPBF Gene
We transformed the mutant cv Lysiba with a construct in which the BPBF gene from the cv Bomi parent was driven by the strong seed-specific D-hordein gene promoter (Cho et al., 2002). This promoter was chosen because it had been previously shown to result in strong endosperm-specific transgene expression and because the D-hordein gene is not itself regulated by the BPBF gene (Sørensen et al., 1996). Of six independent transformation events recovered, two lines exhibited T1 seeds with increased hordein levels (Supplemental Figs. S2–S4). In these two transgenic lines, some seeds exhibited a more than 2-fold overall increase in hordein levels, particularly C-hordein. Transgenic complementation did not restore all missing hordein proteins in these two transgenic lines. This result may be due to differences in the expression pattern of the native BPBF gene compared with the expression pattern of the D-hordein promoter driving expression of the BPBF gene in the transgenic lines.
Genetic Variation in the Barley BPBF Gene in the U.S. Department of Agriculture Barley Core Germplasm Collection
To assess the degree of genetic variation in the barley PBF gene and to determine whether any similar alleles that alter the DOF domain in this gene exist in barley germplasm collections, we obtained a set of 186 barley accessions from the U.S. Department of Agriculture’s (USDA) National Small Grains Germplasm Collection that represent a mini-core of the larger 1,860-member iCore (informative Core) collection of diverse barley germplasm (Muñoz-Amatriaín et al., 2014). This 10% subset of the iCore was chosen based on its contribution to the polymorphism information content of the larger core set (Supplemental Table S2). Genomic DNA was prepared from seedlings of each of the members of the mini-core, and the BPBF gene was PCR amplified using Phusion high-fidelity polymerase and sequenced from all 186 members (for primers, see Supplemental Table S3). High-quality sequence was obtained from 180 of the 186 members of this mini iCore. The sequence extended from 434 bases 5′ of the ATG protein start codon to 580 bases 3′ of the stop codon (2,017 bp total). Twelve distinct haplotypes were identified; seven haplotypes were represented by a single accession, and two haplotypes were found in two accessions each (Supplemental Table S2; Supplemental Fig. S5). The remaining accessions (169) were represented by three haplotypes: haplotype_1 with a sequence identical to cv Bomi was the most abundant. It was found in 91 accessions. Haplotype_11, which differed from the sequence of cv Bowman at a single position, was the second most abundant haplotype. It was present in 63 accessions. The haplotype represented by cv Bowman (haplotype_9) was found in 15 accessions. The two parents, cv Bomi and Bowman (haplotype_1 and haplotype_9, respectively), in our mapping population differ at 10 SNPs in the 999-bp BPBF protein-coding sequence, five of which affect the protein sequence (A21V, A154G, S196G, S238N, and V266G; Supplemental Fig. S6). When analyzed with the PROVEAN program (Choi and Chan, 2015), none of these changes are anticipated to alter transcription factor function. Six accessions contained additional SNPs that affect the protein sequence, but none of the SNPs (P32L, P91T, G118R, and G306D) were found in the zinc-finger DNA-binding domain (Supplemental Fig. S6). Analysis of these variants with the PROVEAN server suggested that the P91T, G118R, and G306D alleles were neutral and unlikely to affect BPBF function. However, the P32L allele was scored as moderately deleterious. Further analysis of the two accessions containing this allele will be required to assess whether it influences hordein accumulation.
A previous study, using a different collection of barley accessions, sequenced two shorter fragments of the BPBF gene in a candidate gene association study of barley endosperm agronomic traits and identified some of the same haplotypes (Haseneyer et al., 2010). Consistent with our results, these authors identified SNPs in BPBF associated with crude protein content.
TILLING of WPBF, the Wheat Homeolog of the Barley PBF Gene
To determine whether inactivating mutations of the homeologs of WPBF, the wheat PBF gene, affect wheat seed storage protein accumulation in a manner similar to the lys3a allele in barley, we used TILLING in a hexaploid wheat TILLING library containing more than 10,000 individual M2 lines (Slade et al., 2005) to identify novel alleles in all three wheat subgenomes. TILLING primers (Supplemental Table S3) were devised that were specific for each of the three genomes of wheat. In total, 789 TILLING alleles were found; however, due to redundancy in the library, of these 789 alleles, only 488 were unique (numbers of unique mutants are shown in parentheses in Table 1). In the A genome, 170 unique alleles were identified, 144 unique alleles were identified in the B genome, and 174 unique alleles were identified in the D genome (Fig. 4; Table 1; Supplemental Table S4). In several instances, lines were identified that contained more than one induced SNP in the B or D homeologs (Supplemental Table S4). The wheat PBF gene consists of two exons separated by an intron of approximately 1 kb. The first exon consists of an untranslated sequence, while the entire protein-coding sequence of the gene is present in the second exon (Ravel et al., 2006). We identified lines containing novel alleles primarily in the N-terminal zinc-finger DNA-binding region, although in the case of the A and D genome homeologs, we also identified novel alleles in the C-terminal region, which is also thought to play a role in DNA binding and in protein-protein interactions (Noguero et al., 2013). In addition to identifying premature stop mutations, we also identified missense mutations that were expected to inactivate the WPBF transcription factor by preventing the formation of the zinc finger due to alterations of the Cys residues that coordinate the zinc atom necessary for DNA binding. Similar mutations of zinc-coordinating Cys residues in DOF genes isolated from pumpkin (Cucurbita maxima) and maize have been created by site-directed mutagenesis, and the corresponding proteins have been shown to lose their DNA-binding ability using gel-shift and southwestern-blotting methods (Yanagisawa, 1995; Shimofurutani et al., 1998; Umemura et al., 2004). All of the novel alleles identified in these WPBF homeologs provide a rich resource for further analysis that will help to determine critical residues for their function.
Table 1. Numbers of novel TILLING alleles in A, B, and D genome WPBF homeologs.
Numbers of TILLING alleles of the different classes in wheat are listed. Numbers are total alleles, with numbers of unique, nonredundant alleles in parentheses.
| Gene | Noncoding | Silent | Missense | Severe Missense | Nonsense | Total |
|---|---|---|---|---|---|---|
| WPBF _A | 37 (24) | 65 (42) | 126 (72) | 37 (27) | 8 (5) | 273 (170) |
| WPBF_B | 6 (3) | 104 (55) | 82 (43) | 68 (40) | 4 (3) | 264 (144) |
| WPBF_D | 18 (13) | 74 (48) | 100 (69) | 60 (44) | 0 | 252 (174) |
| Total | 61 (40) | 243 (145) | 308 (184) | 165 (111) | 12 (8) | 789 (488) |
Figure 4.
Overview of novel TILLING alleles found in WPBF homeologs. The red-outlined rectangles represent the A, B, and D genome-coding regions of the WPBF homeologs, and the arrowheads show the locations of the TILLING alleles. Red arrowheads represent premature stop alleles, black arrowheads refer to missense alleles, and purple arrowheads represent silent alleles. Depiction of mutants was made using the program PARSESNP (Taylor and Greene, 2003).
Effects on Protein and Amino Acid Accumulation Due to Inactivating Mutations in WPBF Homeologs
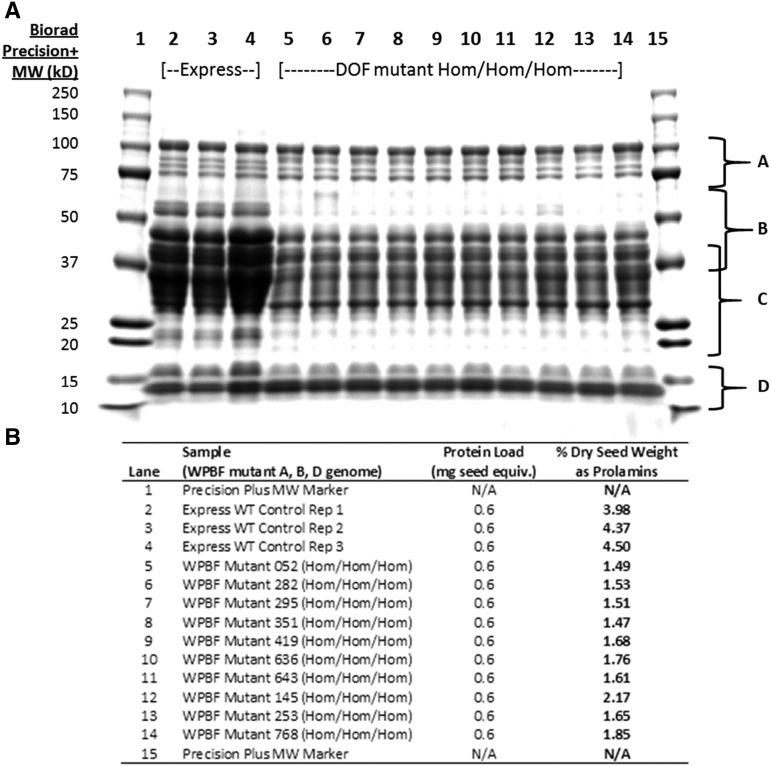
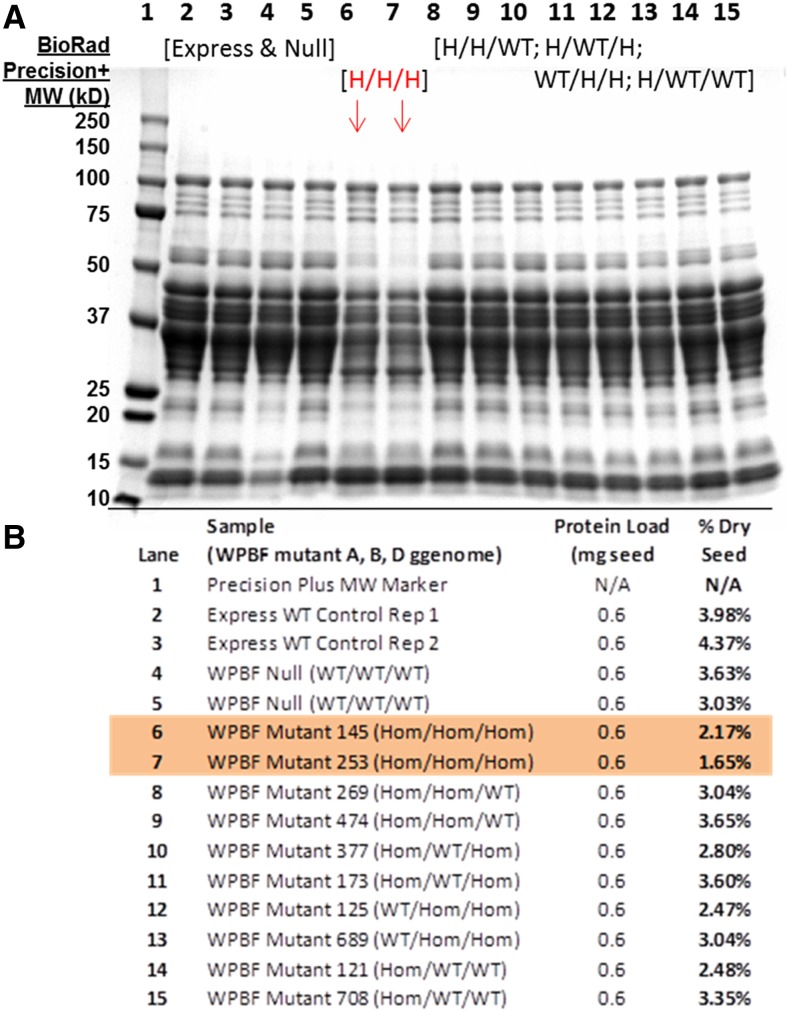
Because of the hexaploid nature of bread wheat and the expectation that WPBF-inactivating mutations, like the lys3a BPBF mutant, would be genetically recessive, we crossed wheat plants containing mutations in the A, B, and D genomes to each other to establish lines containing putative inactive WPBF homeologs in all three genomes. The first lines we chose to cross to each other included one containing a premature stop mutation in the WPBF gene in the B genome (W70*) and two lines containing Tyr residues in place of zinc-coordinating Cys residues in the A (C66Y) and D (C63Y) genomes. These were chosen because they were the first TILLING alleles identified in each of the three homeologs that putatively inactivated the WPBF. Additional severe and, in the case of the A genome, premature stop mutations were identified later. Figure 5A depicts the results of SDS-PAGE analysis of storage proteins extracted from individual seeds of the parental control cv Express and from individual F3 seeds, each derived from an independent individual F2 triple null seed. Strikingly, each triple null homozygous seed exhibited an overall decrease of about 50% to 60% in the accumulation of a variety of gliadins and low-molecular-weight glutenins, as assessed by quantitative analysis of proteins extracted from individual seeds (Fig. 5B). No decrease in seed storage protein accumulation was observed in F3 seeds derived from triple wild-type F2 segregants lacking the mutations (Fig. 6A). Highlighting the recessive nature of the mutations and the redundancy of the WPBF homeologs, Figure 6A also shows that there is no storage protein reduction in individual F3 seeds from segregants in which one unmutated WPBF homeolog is paired with two mutated homeologs. These data demonstrate that a single wild-type copy on any of the subgenomes can compensate for missing function on any of the other two subgenomes.
Figure 5.
SDS-PAGE of wheat prolamins from parental cv Express and from WPBF mutants. A, A 12% SDS-PAGE gel of seed extracts from parental wheat cv Express (lanes 2–4); lanes 5 to 14 represent seed extracts from lines in which all three homeologs of WPBF are inactivated. Each lane shows proteins derived from a single F3 seed, and each F3 seed was harvested from a separate F2 plant. Regions of the gel labeled A through D refer to different seed storage protein fractions: A are HMW glutenins; B are LMW glutenins; C are α, α-β, and γ-gliadins; and D are gliadin/avenin-like proteins as well as trypsin and α-amylase inhibitor proteins. B, Amount of prolamins in each F3 seed as a percentage of dry seed weight, determined as described in the text. WT, Wild type.
Figure 6.
SDS-PAGE of wheat prolamins from multiple genotypic classes of WPBF mutants. A, A 12% SDS-PAGE gel of seed extracts from parental wheat cv Express (lanes 2 and 3), sibling wild-type (WT) segregants (lanes 4 and 5), triple WPBF-inactivating alleles (lanes 5 and 6), and genotypic classes in which one or two homeologs are not mutant (lanes 8–15). B, Genotypes in each lane and the amount of prolamins in each seed as a percentage of dry seed weight.
The lys3a barley mutant notably fails to accumulate C-hordeins and has drastically reduced accumulation of B-hordeins. To assess which proteins are absent or reduced in the WPBF triple null mutants, we conducted liquid chromatography-mass spectrometry (LC-MS) of excised SDS-gel fragments (Supplemental Table S5). Bands that fall within the region of 30 to 55 kD, which make up approximately 70% to 80% of the total wheat seed storage proteins, are expected to contain α- and γ-gliadins as well as LMW glutenins, and this was confirmed by the putative identities of the proteins we identified by LC-MS in gel bands excised from this region of the gel (Supplemental Table S5). Two prominent bands at about 55 kD, which were drastically reduced in the triple null line, were identified as β-amylase and an LMW glutenin protein (Bromilow et al., 2017). In general, the gliadins and LMW glutenins that are reduced in accumulation in wheat by the triple null WPBF mutations are homologous to the B- and C-hordeins, whose accumulation is reduced by the lys3a mutation in barley. These results provide evidence that the A and D genome Cys-to-Tyr mutants are inactivating mutations, as expected, and provide additional evidence that the barley mutation in the BPBF gene underlies the lys3a phenotype.
In addition to affecting seed storage protein accumulation, the WPBF triple null line also alters amino acid accumulation in seeds. We extracted total amino acids from seeds of three independent triple null F3 lines, each derived from an independent F2 seed, and compared the content of free amino acids in these seeds with three independent triple wild-type segregants. As shown in Supplemental Figure S7 and Supplemental Table S6, the triple null wheat lines contain considerably reduced amounts of Pro, Glu (deamidated Gln), and Phe, as expected based on their lower levels of gliadins and LMW glutenins, which contain repetitive sequences rich in these amino acids. Decreases in these amino acids were also observed in lys3a barley (Tallberg, 1977). Notably, among the essential amino acids, the triple null lines exhibit 33% and 14% significantly increased amounts of Lys and Thr, respectively, when compared with their wild-type siblings. These two amino acids are generally limiting for human nutrition in cereals; efforts have been made to increase Lys in other cereals, and this has led to the development of quality protein maize, for example. Researchers have concluded that quality protein maize provides health benefits in undernourished populations (Gunaratna et al., 2010; Nuss and Tanumihardjo, 2011).
DISCUSSION
We describe a lowered-gluten, high-Lys wheat developed with the nontransgenic mutation breeding technique known as TILLING. These lines contain inactivating mutations in the three homeologs of WPBF, which is a transcription factor that plays a regulatory role in the accumulation of seed storage reserves in cereals. This scientific advance was enabled by our identification of a missense mutation in BPBF as the genetic basis of the low-hordein lys3a barley mutant. Prior to our wheat TILLING results, no null mutations were known in PBF homologs in any cereal, a fact that led to the speculation that null mutations might be inviable (Wu and Messing, 2012).
Using BSR-seq and genetic fine mapping, we determined that the BPBF Q58L missense mutation is responsible for the lys3a mutant’s pleiotropic defects. In addition to a drastic reduction in the accumulation of B- and C-hordeins (Doll, 1983), this mutant exhibits multiple other effects, including an increase in the accumulation of free and protein-bound Lys (Køie and Doll, 1979), an increase in the embryo size (Cook et al., 2018), a reduction in starch accumulation, shrunken seeds, and a decrease in yield (Munck and Jespersen, 2009). However, concerted breeding efforts aimed at increasing yield while maintaining the high-Lys phenotype, without regard to the hordein phenotype, minimized the negative effects of the mutation. Due to the linked nature of the high-Lys and low-hordein phenotypes, these efforts also maintained the low-hordein phenotype (Munck and Jespersen, 2009). Given the major effects of the lys3a mutation, we hypothesize that the missense mutation we identified in the DNA-binding domain of the BPBF transcription factor likely inactivates it. Experiments to investigate the DNA-binding properties of this BPBF mutant using electrophoretic mobility shift assays were unsuccessful (data not shown); however, transgenic expression of wild-type BPBF partially complemented the lys3a mutation, which supports our identification of this gene as the basis of the lys3a mutation.
The results of the BSR-seq analysis conclusively showed that the lys3a mutation maps to the pericentromeric region of chromosome 5H (previously known as chromosome 7). This is consistent with earlier mapping experiments (Ullrich and Eslick, 1977; Jensen, 1979). Nevertheless, in a recent article describing the systematic backcrossing and mapping of a large collection of barley mutants (Druka et al., 2011), the lys3a mutant was mapped to chromosome 1H (line BW496; Rustgi and von Wettstein, 2015). We obtained the described putatively lys3a-containing line BW496 (Druka et al., 2011) from the USDA Small Grains Germplasm Collection and extracted hordeins from endosperms of multiple seeds. SDS-PAGE of hordeins appeared to be normal, with none of the obvious, characteristic hordein alterations found in the lys3a mutant (data not shown). We conclude that this mutation was not present in the seeds of line BW496 that we obtained.
Due to studies by Sørensen (1992) showing that B-hordein promoters remained methylated in the endosperm of lys3a mutants while B-hordein promoters were unmethylated in the wild-type endosperm, it was proposed that the lys3a mutation might be in a 5-methylcytosine glycosylase (Demeter) enzyme that removes methyl groups from methylated DNA (Wen et al., 2012). However, the main barley Demeter homolog is found at approximately 400 Mb on the long arm of chromosome 5H, a considerable physical distance from the lys3a mutation. Nevertheless, it is apparent that DNA methylation also plays a role in affecting seed storage protein accumulation in cereals (Lund et al., 1995; Radchuk et al., 2005; Wen et al., 2012). The possible connection between PBF genes and DNA methylation in cereal endosperms merits further investigation.
Although the degree of prolamin reduction in our current wheat PBF TILLING lines (50%–60%) is not sufficient to make them appropriate for celiac patients, additional TILLING approaches are underway that we anticipate will reduce gluten even further. In addition, the wheat PBF TILLING lines may be combined with other approaches, such as gluten-degrading enzyme supplements (Wolf et al., 2015; Syage et al., 2017), to benefit individuals with gluten sensitivities. In our previously published work, we provided evidence for this synergistic approach using a nonhuman primate model of celiac disease (Bethune et al., 2008). We showed that the lys3a barley mutant reduces prolamin-related symptoms when it replaces conventional barley in the feed given to gluten-sensitive rhesus macaques (Sestak et al., 2015). Furthermore, symptoms due to prolamin consumption in these animals were not observed when this low-hordein barley feed was supplemented with a prolamin-degrading enzyme (Sestak et al., 2016). Future research using decreased-gluten wheat will be necessary to expand on these findings.
Because the accumulation of the HMW glutenins did not appear to be substantially affected in our WPBF mutant lines, this decreased-gluten wheat may retain some of the important viscoelastic functional properties of normal wheat. We are currently increasing seed of these lines in order to address this question. Because wheat can tolerate a high mutation load (Uauy et al., 2009), each TILLING line contains a large number of background mutations that must be removed by genetic crosses. Like the barley lys3a mutant, the triple homozygous mutant wheat lines exhibit some negative pleiotropic effects, such as smaller seed size (Supplemental Fig. S8) and somewhat reduced total protein and starch levels (Supplemental Table S7). Future breeding will be required to determine if these effects can be overcome. Due to incompletely understood mechanisms of seed proteome rebalancing (Wu and Messing, 2014), the decreased-gluten wheat lines we developed also contain higher levels of Lys and Thr, making them more nutritious for all consumers of wheat and products made with wheat. The potential agronomic and functional utility of these wheat lines can be determined using the molecular markers developed in this study for marker-assisted breeding.
MATERIALS AND METHODS
Genetic Crosses and Hordein Extraction
The original lys3a mutant and its parent, cv Bomi, were obtained from the USDA’s National Small Grains Germplasm Collection in Aberdeen, Idaho (https://www.ars.usda.gov/pacific-west-area/aberdeen-id/small-grains-and-potato-germplasm-research/docs/national-small-grains-collection/). The lys3a mutant was crossed to cv Bowman as well as to cv Lysiba, a cultivar developed with the original lys3a allele. To evaluate the presence of the recessive lys3a allele in seeds, F2 and subsequent generation seeds were cut in half, and hordeins from endosperms were extracted according to Shewry et al. (1978b) and evaluated by SDS-PAGE. The embryo halves of the seeds were saved for planting.
Plant Material
Hexaploid wheat (Triticum aestivum ‘Express’) with mutations in the WPBF gene in each of the A, B, and D subgenomes was crossed to each other and to the parental cultivar. Different genetic classes were identified by KASP genotyping (LGC) using probes developed to specific SNPs and genomic DNA isolated from seedling leaf tissue. To study restoration of hordein accumulation in the lys3a mutant through complementation by BPBF expression, the lys3a-containing cv Lysiba was used for transformation. Barley (Hordeum vulgare) and wheat plants were sown in Sunshine Mix #3 and grown in pots in Conviron chambers and in the greenhouse under conditions described by Slade et al. (2012).
Protein Extraction
Prolamin proteins were extracted from mature wheat and barley seeds as described (Marchylo, 1987). Wheat seeds (1 g) were ground to a fine powder using a Retsch model 301 mixer mill. Due to segregation of alleles in the T1 barley transgenic for the BPBF gene, 12 individual seeds were separately ground, extracted, and analyzed.
For protein extraction, 40 mg of ground seed was extracted with 1 mL of 60% (v/v) isopropanol and 2% (v/v) β-mercaptoethanol in 50 mm Tris-Borate buffer, pH 8, by heating at 60°C for 1 h with mixing. Extracted samples were centrifuged (18,000g for 10 min), and a 0.525-mL aliquot was added to 1.5 mL of 20% (v/v) sodium chloride and stored in a −20°C freezer overnight to precipitate prolamin proteins. Precipitated proteins were pelleted at 18,000g for 2 min, the supernatant was decanted, and residual solution was removed by vacuum evaporation using a SpeedVac concentrator. Protein pellets were then dissolved in Sigma Total Plant Protein Reagent Type 4 containing 1% Sigma Plant Protease Inhibitor Cocktail. The protein concentrations were determined using either the Pierce 660-nm protein assay or the Qubit protein assay (Thermo Fisher Scientific).
Protein Gel Electrophoresis
The extracted prolamins were denatured and reduced with Novex Tris-Gly SDS Sample Buffer and Reducing Agent (Thermo Fisher Scientific) and heating to 70°C for 10 min. SDS-PAGE was performed using Invitrogen WedgeWell Tris-Gly gels and Bio-Rad Kaleidoscope Precision Plus MW Standards with Novex Tris-Gly Running Buffer. Developed gels were fixed in 50% methanol/10% acetic acid solution for 15 min and stained for 3 h using the Colloidal Blue Staining Kit (Thermo Fisher Scientific). Gel images were obtained using a Bio-Rad ChemiDoc XRS+ system with Image Lab software.
LC-MS
Gluten proteins in SDS-PAGE gel bands were identified by data-dependent LC-MS (Steen and Mann, 2004). Excised gel bands were diced, destained, reduced, and alkylated using the Pierce in-gel protein digestion kit prior to mass spectrometric analysis (Lahm and Langen, 2000). In-gel digestion of proteins was done with chymotrypsin (Promega) for 16 h at 37°C followed by trypsin (Promega) for 6 h. Peptides were separated on an ACE C18 column (0.3 × 150 mm; Advanced Chromatography Technologies) in a 5% to 55% gradient of acetonitrile in 0.1% formic acid. Spectra were obtained on an LCQ Deca XP plus mass spectrometer (Thermo Scientific) using one survey MS scan (350–2,000 D) followed by four data-dependent MS/MS scans of the most abundant ions in the MS survey scan. Parameters for data-dependent MS/MS included a default charge state of 2, an isolation width of 2 D, and collision energy of 35% for collision-induced decay of ions with abundance greater than 1 × 105.
Mass spectral data were processed using the GPM manager application (GPM extreme edition, v. 2.2.1.0; Beavis Informatics) and Scaffold software (v. 3.00.03; Proteome Software) with comparison with proteins in the UniProtKB Triticum aestivum database (downloaded on May 25, 2016) to which the sequence of common contaminants was appended (e.g. human keratins, trypsin, BSA, and others in the cRAP database from GPM) prior to reverse concatenation using a Perl script (provided by Dr. Brett Phinney, University of California Davis Genome Center Proteomics Core Facility). To assert whether a protein is present in a given sample, a minimum of two peptides with greater than 80% probability of being correctly identified was required as well as a minimum 99% probability of protein presence. Probabilities were assigned by the Peptide Prophet and Protein Prophet algorithms within the Scaffold bioinformatics software (Keller et al., 2002; Nesvizhskii et al., 2003; Searle et al., 2008; Searle, 2010).
Amino Acid Analysis
Total amino acid content of 1 g of powdered whole wheat seed was determined by Covance Labs using automated precolumn derivatization of hydrolysates followed by HPLC analysis according to the methods of Schuster (1988) and Henderson and Brooks (2010) and included the analysis of Cys (Barkholt and Jensen, 1989). The analyses were conducted on three biological replicates.
BSR-seq
BSR-seq was conducted according to Liu et al. (2012). RNA was isolated from three independent biological replicate pools of the wild type and three independent biological replicate pools of homozygous mutant tissue. Each pool consisted of 7-d-old etiolated shoot and root tissue from between 20 and 30 germinated F3 seeds, each derived from a separate F2 seed from a lys3a × cv Bowman cross. In the mutant pool, F2 recessive mutant seeds were identified by half-seed hordein analysis as described above. To ensure that individuals in the wild-type pool were not segregating for the recessive lys3a allele, F2 embryos whose endosperm half-seed hordein analysis indicated a wild-type phenotype were planted, and half-seed hordein analysis was conducted on approximately 20 F3 seeds from each wild-type F2. In the wild-type pool, only tissue from germinated F3 seeds was used, none of whose siblings had any mutant segregants.
Marker Development, Fine Mapping, and Identification of BPBF
Using the sequence context information from SNPs that were exclusively linked to the mutant pool, KASP probes (LGC) were designed and used to genotype individuals from a larger mapping population. Hordein extracts from endosperm half-seeds of 1,814 seeds were evaluated to identify recessive lys3a mutants. DNA was isolated from seedling leaf tissue grown from the germinated embryos. Following fine mapping, PCR primers were designed to amplify the BPBF gene. PCR products were amplified from genomic DNA isolated from seedling leaf tissue of cv Bomi and lys3a using a proofreading polymerase (Phusion polymerase; New England Biolabs) and sequenced.
Transformation of Barley
Construct pARC1057 (Supplemental Fig. S9) was transformed into immature embryos of barley cv Lysiba, essentially as described (Matthews et al., 2001), with some modifications. The Agrobacterium tumefaciens strain used was EHA105 (Hood et al., 1993), timentin was added to the medium at 200 mg L−1 to suppress bacterial growth after cocultivation, and transgenic plant tissue was selected using geneticin at 100 mg L−1. After 3 weeks of rooting of shoots in medium containing 25 mg L−1 kanamycin, T0 plantlets were transferred into a mixture of Sunshine #2 and Sunshine #3, in a 1:1 ratio, and grown at day/night periods of 14 h at 25°C/10 h at 18°C. Expression of the NPTII gene was confirmed by analyzing ground T0 leaf material by NPTII immunostrip test (Agdia). Harvested T1 seed was planted and grown similarly as described above.
TILLING of WPBF Homeologs
Approximately 10,000 individual M2 mutant genomic DNAs from spring wheat cv Express were screened. TILLING of wheat was conducted according to Slade et al. (2005) as follows. The M2 wheat DNA was pooled into groups of two individual plants. The DNA concentration for each individual within the pool was approximately 2 ng mL−1, with a final concentration of 4 ng mL−1 for the entire pool. Then, 5 μL of the pooled DNA samples (or 20 ng of wheat DNA) was arrayed on microtiter plates and subjected to gene-specific PCR.
PCR amplification was performed in 15-μL volumes containing 20 ng of pooled DNA, 0.75× ExTaq buffer (Clontech), 1.1 mm additional MgCl2, 0.3 mm deoxyribonucleotide triphosphates, 0.3 mm primers, 0.009 units of Ex-Taq DNA polymerase (Clontech), 0.02 units of DyNAzyme II DNA Polymerase (Thermo Scientific), and, if necessary, 0.33 m Polymer-Aide PCR Enhancer (Sigma-Aldrich). PCR amplification was performed using an MJ Research thermal cycler as follows: 95°C for 2 min; 8 cycles of touchdown PCR (94°C for 20 s, followed by an annealing step starting at 70°C–68°C for 30 s and decreasing 1°C per cycle, and then a temperature ramp of 0.5°C s−1 to 72°C followed by 72°C for 1 min); 25 to 45 cycles of 94°C for 20 s, 63°C or 65°C for 30 s, ramp 0.5°C s−1 to 72°C, and 72°C for 1 to 2 min; 72°C for 8 min; 98°C for 8 min; 80°C for 20 s; and 60 cycles of 80°C for 7 s, –0.3°C per cycle.
PCR products (2–4 μL) were digested on 96-well plates. Three microliters of a solution containing 6 mm HEPES (pH 7), 6 mm MgCl2, 6 mm sodium chloride, 0.012× Triton X-100, 0.03 mg mL−1 BSA, 0.5× T-Digest buffer [Advanced Analytical Technologies (AATI)], 0.912 units each of Surveyor Endonuclease and Enhancer (Transgenomic), and 0.5× dsDNA Cleavage Enzyme (AATI) was added to the PCR product. Digestion reactions were incubated at 45°C for 45 min. The specific activity of the Surveyor enzyme was 800 units mL−1, where 1 unit was defined by the manufacturer as the amount of enzyme required to produce 1 ng of acid-soluble material from sheared, heat-denatured calf thymus DNA at pH 8.5 for 1 min at 37°C. Reactions were stopped by addition of 20 μL of Dilution Buffer E (AATI) or 1× Tris-EDTA. The reactions were stored in the freezer until they were run on the Fragment Analyzer (AATI) Capillary Electrophoresis System. Samples were run on the Fragment Analyzer utilizing the DNF-920-K1000T Mutation Discovery Kit (AATI) according to the manufacturer’s protocol.
After electrophoresis, the assays were analyzed using PROSize 2.0 Software (AATI). The gel image showed a sequence-specific pattern of background bands common to all 96 lanes. Rare events, such as mutations, create new bands that stand out above the background pattern. Plants with bands indicative of mutations of interest were evaluated by TILLING individual members of a pool mixed with wild-type DNA and then sequencing individual PCR products.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers MK468595 to MK468607.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Genetic mapping of lys3a.
Supplemental Figure S2. Protein levels in barley transgenic events.
Supplemental Figure S3. SDS-PAGE of T1 seeds of transgenic event 5.
Supplemental Figure S4. SDS-PAGE of T1 seeds of transgenic event 6.
Supplemental Figure S5. Alignment of iCore haplotype DNA sequences.
Supplemental Figure S6. Alignment of selected haplotype protein sequences.
Supplemental Figure S7. Amino acid analyses of wheat seeds.
Supplemental Figure S8. Seed phenotypes of wheat mutant lines, wild-type siblings, and parental cultivar.
Supplemental Figure S9. Transgenic construct.
Supplemental Table S1. KASP probes.
Supplemental Table S2. iCore members and haplotypes.
Supplemental Table S3. List of primers (Ng and Henikoff, 2003).
Supplemental Table S4. List of WPBF alleles (Pietrokovski et al., 1996).
Supplemental Table S5. LC-MS of endosperm proteins.
Supplemental Table S6. Amino acid analyses of wheat seeds.
Supplemental Table S7. Protein and starch analyses of WPBF F3 and F4 generation mutants, nonmutant siblings, and parental cultivar (McCleary et al., 1997).
Acknowledgments
For a gift of seeds of barley cv Lysiba and for friendly collaboration during the early stages of this research, we thank Dr. Diter von Wettstein (deceased). We thank Dr. Harold Bockelman at the USDA National Small Grains Collection in Aberdeen, Idaho, for seeds of cv Bomi, lys3a barley (Risø 1508), BW496, and the barley iCore. For excellent technical assistance, we thank Ennis Sandle and Yingzhi Lu. For critical reading of the article, we thank Dr. Ann Slade and Dr. Margaret Miller.
Footnotes
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (award nos. R42DK097976 and R42DK072721).
Articles can be viewed without a subscription.
References
- Barkholt V, Jensen AL (1989) Amino acid analysis: Determination of cysteine plus half-cystine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal Biochem 177: 318–322 [DOI] [PubMed] [Google Scholar]
- Bethune MT, Borda JT, Ribka E, Liu MX, Phillippi-Falkenstein K, Jandacek RJ, Doxiadis GGM, Gray GM, Khosla C, Sestak K (2008) A non-human primate model for gluten sensitivity. PLoS ONE 3: e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromilow SN, Gethings LA, Langridge JI, Shewry PR, Buckley M, Bromley MJ, Mills EC (2017) Comprehensive proteomic profiling of wheat gluten using a combination of data-independent and data-dependent acquisition. Front Plant Sci 7: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerlengo F, Sestili F, Silvestri M, Colaprico G, Margiotta B, Ruggeri R, Lupi R, Masci S, Lafiandra D (2017) Production and molecular characterization of bread wheat lines with reduced amount of α-type gliadins. BMC Plant Biol 17: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalapiedra CP, Boudiar R, Casas AM, Igartua E, Contreras-Moreira B (2015) BARLEYMAP: Physical and genetic mapping of nucleotide sequences and annotation of surrounding loci in barley. Mol Breed 35: 13 [Google Scholar]
- Cho MJ, Choi HW, Jiang W, Ha CD, Lemaux PG (2002) Endosperm-specific expression of green fluorescent protein driven by the hordein promoter is stably inherited in transgenic barley (Hordeum vulgare) plants. Physiol Plant 115: 144–154 [DOI] [PubMed] [Google Scholar]
- Choi Y, Chan AP (2015) PROVEAN web server: A tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31: 2745–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook F, Hughes N, Nibau C, Orman-Ligeza B, Schatlowski N, Uauy C, Trafford K (2018) Barley lys3 mutants are unique amongst shrunken-endosperm mutants in having abnormally large embryos. J Cereal Sci 82: 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16: 10881–10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour JA, Joye IJ, Pareyt B, Wilderjans E, Brijs K, Lagrain B (2012) Wheat gluten functionality as a quality determinant in cereal-based food products. Annu Rev Food Sci Technol 3: 469–492 [DOI] [PubMed] [Google Scholar]
- Doll H. (1973) Inheritance of the high-lysine character of a barley mutant. Hereditas 74: 293–294 [DOI] [PubMed] [Google Scholar]
- Doll H. (1983) Barley seed proteins and possibilities for their improvement. In Gottschalk W, Müller HP, eds, Seed Proteins: Biochemistry, Genetics, Nutritive Value. Martinus Nijhoff, The Hague, The Netherlands, pp 207–223 [Google Scholar]
- Doll H, Køie B, Eggum B (1974) Induced high lysine mutants in barley. Radiat Bot 14: 73–80 [Google Scholar]
- Dong G, Ni Z, Yao Y, Nie X, Sun Q (2007) Wheat Dof transcription factor WPBF interacts with TaQM and activates transcription of an alpha-gliadin gene during wheat seed development. Plant Mol Biol 63: 73–84 [DOI] [PubMed] [Google Scholar]
- Druka A, Franckowiak J, Lundqvist U, Bonar N, Alexander J, Houston K, Radovic S, Shahinnia F, Vendramin V, Morgante M, et al. (2011) Genetic dissection of barley morphology and development. Plant Physiol 155: 617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Humanes J, Pistón F, Tollefsen S, Sollid LM, Barro F (2010) Effective shutdown in the expression of celiac disease-related wheat gliadin T-cell epitopes by RNA interference. Proc Natl Acad Sci USA 107: 17023–17028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Humanes J, Pistón F, Altamirano-Fortoul R, Real A, Comino I, Sousa C, Rosell CM, Barro F (2014) Reduced-gliadin wheat bread: An alternative to the gluten-free diet for consumers suffering gluten-related pathologies. PLoS ONE 9: e90898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green PH, Lebwohl B, Greywoode R (2015) Celiac disease. J Allergy Clin Immunol 135: 1099–1106, quiz 1107 [DOI] [PubMed] [Google Scholar]
- Gunaratna NS, Groote HD, Nestel P, Pixley KV, McCabe GP (2010) A meta-analysis of community-based studies on quality protein maize. Food Policy 35: 202–210 [Google Scholar]
- Gupta S, Malviya N, Kushwaha H, Nasim J, Bisht NC, Singh VK, Yadav D (2015) Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 241: 549–562 [DOI] [PubMed] [Google Scholar]
- Haseneyer G, Stracke S, Piepho HP, Sauer S, Geiger HH, Graner A (2010) DNA polymorphisms and haplotype patterns of transcription factors involved in barley endosperm development are associated with key agronomic traits. BMC Plant Biol 10: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JW, Brooks A (2010) Improved amino acid methods using Agilent ZORBAX Eclipse Plus C18 columns for a variety of Agilent LC instrumentation and separation goals. Agilent Technologies, Wilmington, DE. http://www.chem.agilent.com/Library/applications/5990-4547EN.pdf
- Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Jensen J. (1979) Location of a high-lysine gene and the DDT-resistance gene on barley chromosome 7. Euphytica 28: 47–56 [Google Scholar]
- Karlsson K. (1977) Linkage studies in a gene for high lysine content in Riso barley mutant 1508. Barley Genet Newsl 7: 40–43 [Google Scholar]
- Keller A, Nesvizhskii AI, Kolker E, Aebersold R (2002) Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74: 5383–5392 [DOI] [PubMed] [Google Scholar]
- Kersey PJ, Allen JE, Armean I, Boddu S, Bolt BJ, Carvalho-Silva D, Christensen M, Davis P, Falin LJ, Grabmueller C, et al. (2016) Ensembl Genomes 2016: More genomes, more complexity. Nucleic Acids Res 44: D574–D580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Køie B, Doll H (1979) International symposium on seed protein improvement in cereals and grain legumes, Vol 1 International Atomic Energy Agency, Vienna, Austria, pp 205–214 [Google Scholar]
- Kucek LK, Veenstra LD, Amnuaycheewa P, Sorrells ME (2015) A grounded guide to gluten: How modern genotypes and processing impact wheat sensitivity. Compr Rev Food Sci Food Saf 14: 285–302 [DOI] [PubMed] [Google Scholar]
- Lahm HW, Langen H (2000) Mass spectrometry: A tool for the identification of proteins separated by gels. Electrophoresis 21: 2105–2114 [DOI] [PubMed] [Google Scholar]
- Liu S, Yeh CT, Tang HM, Nettleton D, Schnable PS (2012) Gene mapping via bulked segregant RNA-Seq (BSR-Seq). PLoS ONE 7: e36406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi S, Mustalahti K, Kaukinen K, Laurila K, Collin P, Rissanen H, Lohi O, Bravi E, Gasparin M, Reunanen A, et al. (2007) Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther 26: 1217–1225 [DOI] [PubMed] [Google Scholar]
- Lund G, Ciceri P, Viotti A (1995) Maternal-specific demethylation and expression of specific alleles of zein genes in the endosperm of Zea mays L. Plant J 8: 571–581 [DOI] [PubMed] [Google Scholar]
- Marchylo B. (1987) Barley cultivar identification by SDS gradient PAGE analysis of hordein. Can J Plant Sci 67: 927–944 [Google Scholar]
- Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, Muñoz-Amatriaín M, Close TJ, Wise RP, Schulman AH, et al. (2013) Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ). Plant J 76: 718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PR, Wang MB, Waterhouse PM, Thornton S, Fieg SJ, Gubler F, Jacobsen JV (2001) Marker gene elimination from transgenic barley, using co-transformation with adjacent ‘twin T-DNAs’ on a standard Agrobacterium transformation vector. Mol Breed 7: 195–202 [Google Scholar]
- Mayer KF, Waugh R, Brown JW, Schulman A, Langridge P, Platzer M, Fincher GB, Muehlbauer GJ, Sato K, Close TJ, et al. (2012) A physical, genetic and functional sequence assembly of the barley genome. Nature 491: 711–716 [DOI] [PubMed] [Google Scholar]
- McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeted screening for induced mutations. Nat Biotechnol 18: 455–457 [DOI] [PubMed] [Google Scholar]
- McCleary BV, Gibson TS, Mugford DC (1997) Measurement of total starch in cereal products by amyloglucosidase-alpha-amylase method: Collaborative study. J AOAC Int 80: 571–579 [Google Scholar]
- Mena M, Vicente-Carbajosa J, Schmidt RJ, Carbonero P (1998) An endosperm-specific DOF protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. Plant J 16: 53–62 [DOI] [PubMed] [Google Scholar]
- Miflin B, Shewry P (1979) The synthesis of proteins in normal and high lysine barley seeds. In Laidman DL, Wyn Jones RG, eds, Recent Advances in the Biochemistry of Cereals. Academic Press, London, pp 239–273 [Google Scholar]
- Munck L, Jespersen B (2009) The multiple uses of barley endosperm mutants in plant breeding for quality and for revealing functionality in nutrition and food technology. In Shu QY, ed, Induced Plant Mutations in the Genomics Era. Proceedings of an International Joint FAO/IAEA Symposium, Vienna, Austria, 2008. International Atomic Energy Agency, Vienna, pp 182–186 [Google Scholar]
- Munck L, Pram Nielsen J, Møller B, Jacobsen S, Søndergaard I, Engelsen S, Nørgaard L, Bro R (2001) Exploring the phenotypic expression of a regulatory proteome-altering gene by spectroscopy and chemometrics. Anal Chim Acta 446: 169–184 [Google Scholar]
- Muñoz-Amatriaín M, Cuesta-Marcos A, Endelman JB, Comadran J, Bonman JM, Bockelman HE, Chao S, Russell J, Waugh R, Hayes PM, et al. (2014) The USDA barley core collection: Genetic diversity, population structure, and potential for genome-wide association studies. PLoS ONE 9: e94688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Aebersold R (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658 [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31: 3812–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguero M, Atif RM, Ochatt S, Thompson RD (2013) The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci 209: 32–45 [DOI] [PubMed] [Google Scholar]
- Nuss ET, Tanumihardjo SA (2011) Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv Nutr 2: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrokovski S, Henikoff JG, Henikoff S (1996) The Blocks database: A system for protein classification. Nucleic Acids Res 24: 197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radchuk VV, Sreenivasulu N, Radchuk RI, Wobus U, Weschke W (2005) The methylation cycle and its possible functions in barley endosperm development. Plant Mol Biol 59: 289–307 [DOI] [PubMed] [Google Scholar]
- Ravel C, Nagy IJ, Martre P, Sourdille P, Dardevet M, Balfourier F, Pont C, Giancola S, Praud S, Charmet G (2006) Single nucleotide polymorphism, genetic mapping, and expression of genes coding for the DOF wheat prolamin-box binding factor. Funct Integr Genomics 6: 310–321 [DOI] [PubMed] [Google Scholar]
- Rustgi S, von Wettstein D (2015) Breeding barley ornamented with the novel agronomical attributes. Med Aromat Plants 4: e158 [Google Scholar]
- Sánchez-León S, Gil-Humanes J, Ozuna CV, Giménez MJ, Sousa C, Voytas DF, Barro F (2017) Low-gluten, non-transgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J 16: 902–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PHR, Hadjivassiliou M, Kaukinen K, Rostami K, Sanders DS, Schumann M, et al. (2012) Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster R. (1988) Determination of amino acids in biological, pharmaceutical, plant and food samples by automated precolumn derivatization and high-performance liquid chromatography. J Chromatogr A 431: 271–284 [DOI] [PubMed] [Google Scholar]
- Searle BC. (2010) Scaffold: A bioinformatic tool for validating MS/MS-based proteomic studies. Proteomics 10: 1265–1269 [DOI] [PubMed] [Google Scholar]
- Searle BC, Turner M, Nesvizhskii AI (2008) Improving sensitivity by probabilistically combining results from multiple MS/MS search methodologies. J Proteome Res 7: 245–253 [DOI] [PubMed] [Google Scholar]
- Sestak K, Thwin H, Dufour J, Aye PP, Liu DX, Moehs CP (2015) The effects of reduced gluten barley diet on humoral and cell-mediated systemic immune responses of gluten-sensitive rhesus macaques. Nutrients 7: 1657–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, Thwin H, Dufour J, Liu DX, Alvarez X, Laine D, Clarke A, Doyle A, Aye PP, Blanchard J, et al. (2016) Supplementation of reduced gluten barley diet with oral prolyl endopeptidase effectively abrogates enteropathy-associated changes in gluten-sensitive macaques. Nutrients 8: 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C (2002) Structural basis for gluten intolerance in celiac sprue. Science 297: 2275–2279 [DOI] [PubMed] [Google Scholar]
- Shewry PR. (2009) Wheat. J Exp Bot 60: 1537–1553 [DOI] [PubMed] [Google Scholar]
- Shewry P, Pratt HM, Charlton M, Miflin B (1977) Two-dimensional separation of the prolamins of normal and high lysine barley (Hordeum vulgare L.). J Exp Bot 28: 597–606 [Google Scholar]
- Shewry P, Hill J, Pratt H, Leggatt M, Miflin B (1978a) An evaluation of techniques for the extraction of hordein and glutelin from barley seed and a comparison of the protein composition of Bomi and Risø 1508. J Exp Bot 29: 677–692 [Google Scholar]
- Shewry PR, Pratt HM, Miflin BJ (1978b) Varietal identification of single seeds of barley by analysis of hordein polypeptides. J Sci Food Agric 29: 587–596 [Google Scholar]
- Shimofurutani N, Kisu Y, Suzuki M, Esaka M (1998) Functional analyses of the Dof domain, a zinc finger DNA-binding domain, in a pumpkin DNA-binding protein AOBP. FEBS Lett 430: 251–256 [DOI] [PubMed] [Google Scholar]
- Slade AJ, Fuerstenberg SI, Loeffler D, Steine MN, Facciotti D (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat Biotechnol 23: 75–81 [DOI] [PubMed] [Google Scholar]
- Slade AJ, McGuire C, Loeffler D, Mullenberg J, Skinner W, Fazio G, Holm A, Brandt KM, Steine MN, Goodstal JF, et al. (2012) Development of high amylose wheat through TILLING. BMC Plant Biol 12: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid LM, Qiao SW, Anderson RP, Gianfrani C, Koning F (2012) Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 64: 455–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen MB. (1992) Methylation of B-hordein genes in barley endosperm is inversely correlated with gene activity and affected by the regulatory gene Lys3. Proc Natl Acad Sci USA 89: 4119–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen MB, Müller M, Skerritt J, Simpson D (1996) Hordein promoter methylation and transcriptional activity in wild-type and mutant barley endosperm. Mol Gen Genet 250: 750–760 [DOI] [PubMed] [Google Scholar]
- Steen H, Mann M (2004) The ABC’s (and XYZ’s) of peptide sequencing. Nat Rev Mol Cell Biol 5: 699–711 [DOI] [PubMed] [Google Scholar]
- Syage JA, Murray JA, Green PHR, Khosla C (2017) Latiglutenase improves symptoms in seropositive celiac disease patients while on a gluten-free diet. Dig Dis Sci 62: 2428–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallberg A. (1977) The amino-acid composition in endosperm and embryo of a barley variety and its high lysine mutant. Hereditas 87: 43–46 [Google Scholar]
- Tanner GJ, Blundell MJ, Colgrave ML, Howitt CA (2016) Creation of the first ultra-low gluten barley (Hordeum vulgare L.) for coeliac and gluten-intolerant populations. Plant Biotechnol J 14: 1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor NE, Greene EA (2003) PARSESNP: A tool for the analysis of nucleotide polymorphisms. Nucleic Acids Res 31: 3808–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye-Din JA, Stewart JA, Dromey JA, Beissbarth T, van Heel DA, Tatham A, Henderson K, Mannering SI, Gianfrani C, Jewell DP, et al. (2010) Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Sci Transl Med 2: 41ra51. [DOI] [PubMed] [Google Scholar]
- Uauy C, Paraiso F, Colasuonno P, Tran RK, Tsai H, Berardi S, Comai L, Dubcovsky J (2009) A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biol 9: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich SE, Eslick RF (1977) Inheritance of the shrunken endosperm character, sex3c, of Bomi Riso mutant 1508 and its association with lysine content. Barley Genet Newsl 7: 66–73 [Google Scholar]
- Umemura Y, Ishiduka T, Yamamoto R, Esaka M (2004) The Dof domain, a zinc finger DNA-binding domain conserved only in higher plants, truly functions as a Cys2/Cys2 Zn finger domain. Plant J 37: 741–749 [DOI] [PubMed] [Google Scholar]
- Vicente-Carbajosa J, Moose SP, Parsons RL, Schmidt RJ (1997) A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc Natl Acad Sci USA 94: 7685–7690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Wen N, Pang J, Langen G, Brew-Appiah RA, Mejias JH, Osorio C, Yang M, Gemini R, Moehs CP, et al. (2012) Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health. Proc Natl Acad Sci USA 109: 20543–20548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C, Siegel JB, Tinberg C, Camarca A, Gianfrani C, Paski S, Guan R, Montelione G, Baker D, Pultz IS (2015) Engineering of Kuma030: A gliadin peptidase that rapidly degrades immunogenic gliadin peptides in gastric conditions. J Am Chem Soc 137: 13106–13113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Messing J (2012) Rapid divergence of prolamin gene promoters of maize after gene amplification and dispersal. Genetics 192: 507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Messing J (2014) Proteome balancing of the maize seed for higher nutritional value. Front Plant Sci 5: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto MP, Onodera Y, Touno SM, Takaiwa F (2006) Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes. Plant Physiol 141: 1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S. (1995) A novel DNA-binding domain that may form a single zinc finger motif. Nucleic Acids Res 23: 3403–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]