Abstract
Phytoparasitic nematodes use multiple tactics to influence phytohormone physiology and alter plant developmental programs to establish feeding sites.
Plant-parasitic nematodes are microscopically small animals that cause global annual crop losses of at least 80 billion dollars (Nicol et al., 2011). The evolution of nematodes into plant parasites occurred several times, resulting in diverse interaction modes with the plant (Smant et al., 2018). We will focus this review on the sedentary cyst nematodes (CN) and root-knot nematodes (RKN), as they are the foremost studied due to their economic importance (Jones et al., 2013) and fascinating liaison with plants in the form of nematode feeding sites (Box 1).
Figure 1.
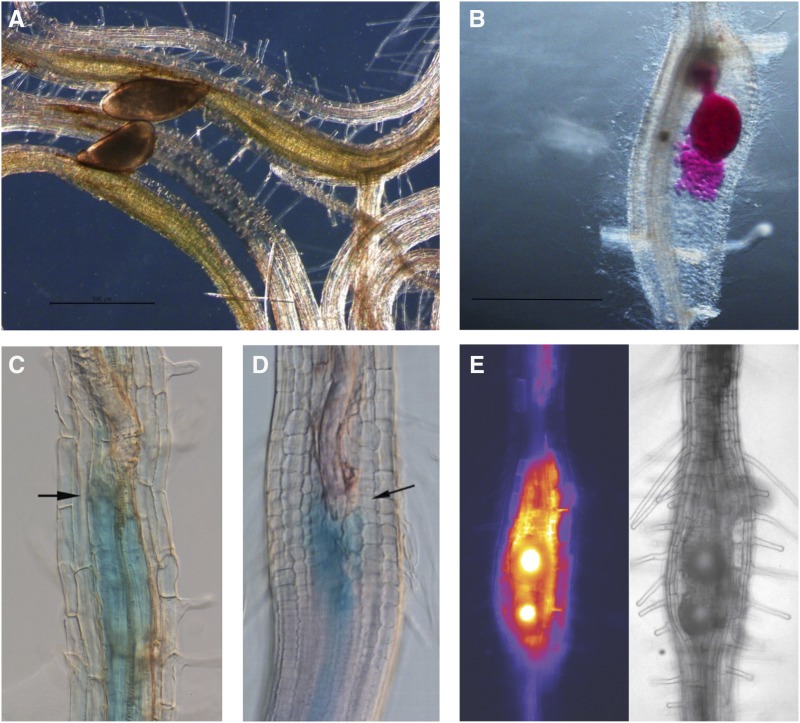
Plant-parasitic nematodes and their impact on plant gene regulation. A, Two females of the beet CN H. schachtii feeding on Arabidopsis roots. Picture courtesy of Anju Verma. B, Female of the rice RKN M. graminicola on rice roots, with egg mass, stained pink with acid fuchsin staining. Picture courtesy of Zobaida Lahari. C and D, GUS assays on Arabidopsis roots infected with H. schachtii showing up-regulation of the DR5-promoter (blue color; C) at 24 h after inoculation and the IAA14-promoter (D) at 2 d post inoculation. Black arrows point to nematode heads. Reprinted with permission from Grunewald et al., 2008. E, Up-regulation of the LAX3-promoter after H. schachtii infection in Arabidopsis roots with LAX3-YFP construct: (left) fluorescence image; (right) brightfield image. Pictures courtesy of Chris Lee.
Nematodes establish feeding sites by recruiting specific plant developmental pathways, involving hormonal cross talk. At the same time, nematodes need to suppress plant defense and its interacting hormone pathways. This interface between development and defense results in a complex pattern in which it is difficult to unravel the specific roles of different plant hormones. Therefore, we present a simplified model for describing the roles of hormones in plant-nematode interactions in this review. Auxin, as the key regulator, and cytokinin, as its modulator, are the primary plant hormones involved in cell division and differentiation (Benková et al., 2003; Pernisová et al., 2009). Jasmonate (JA) and salicylate (SA) are the principal plant defense hormones (Mur et al., 2006). Other hormones modulate and cross talk with these principal hormones and with each other, and can have different effects depending on the specific host-nematode interaction.
Besides the classical phytohormones, small, secreted peptide hormones shape plant development; remarkably, nematodes have evolved plant peptide hormone (PPH) effector mimics to facilitate parasitism. Recent studies have revealed a diversity of these peptides, which we will discuss in the latter half of this review.
NEMATODES MANIPULATE PHYTOHORMONE PATHWAYS FOR FEEDING SITE FORMATION
Auxin is Key to NFS Formation
Auxin, or indole-3 acetic acid (IAA), is a key regulator of plant organogenesis. Hence, it is not surprising that the initiation and maturation of NFS is associated with local accumulation of auxin (Karczmarek et al., 2004). Congruently, auxin mutants are significantly less susceptible to both CN and RKN (for review, see Grunewald et al., 2009b; Gleason et al., 2016). Auxin could be underpinning many of the changes occurring during feeding site development, such as hypertrophy, cell wall ingrowths, and cell cycle activation (de Almeida Engler et al., 1999). Auxin is known for its role in cell expansion via the up-regulation of cell wall-modifying proteins and plasma membrane proton pumps that regulate acid growth (Majda and Robert, 2018). During transfer cell formation, auxin and ethylene (ET) cooperatively bring about the development of cell wall ingrowths (Yuan et al., 2016). In addition, auxin is not only an important trigger for cell-cycle entry but also acts in various other cell cycle phases (Perrot-Rechenmann, 2010).
Transcriptome and promoter-reporter analyses of NFS reveal a complex temporal and spatial pattern of up- and down-regulation of auxin biosynthesis and signaling-related genes and miRNAs influencing mRNA stability (e.g., Ithal et al., 2007; Barcala et al., 2010; Ji et al., 2013; Hewezi et al., 2014; Cabrera et al., 2015, 2016). Soon after nematode infection, auxin biosynthesis and auxin-response genes are mainly up-regulated, whereas genes encoding repressors are turned off, supporting a role for auxin at this early stage of infection. The accumulation of auxin at the initiating NFS could be due to secretion by the nematodes, locally induced plant biosynthesis, and changes in auxin transport. Auxin, mainly in its conjugated form, has been detected in RKN (Meloidogyne incognita) and beet CN (Heterodera schachtii) secretions (De Meutter et al., 2005), but its impact on NFS formation is unknown.
The role of auxin transport during nematode infection of Arabidopsis (Arabidopsis thaliana) roots has been established by analyses of the involved genes at the levels of expression, protein localization, and mutant phenotypes (Grunewald et al., 2009a; Lee et al., 2011; Kyndt et al., 2016). A substantial amount of auxin is produced in plant shoots, with polar auxin transport generating morphogenic auxin gradients. An interacting network of influx (AUXIN/LIKE AUXIN [AUX/LAX]) and efflux (PIN-formed [PIN]) transmembrane proteins with temporally and spatially adjusted subcellular locations mediate this auxin flow (Vieten et al., 2007). The AUX1 and LAX3 influx proteins appear to be essential to the formation of galls and syncytia, as the expression of corresponding genes is strongly up-regulated in the early infection stages, and mutants are less susceptible to infection. PIN4 is needed for proper expansion of both syncytia and galls, with pin4 mutants resulting in the development of smaller cysts (Grunewald et al., 2009a; Kyndt et al., 2016). However, the contributions of some other PIN proteins appear to be quite different between the two types of feeding sites. PIN1 is necessary for delivering auxin from the shoots to the initiating syncytium, where its expression is strongly down-regulated to prevent the pumping out of auxin. Inside syncytia, PIN3 is relocated from the basal to the lateral plasma membranes to redirect auxin to the neighboring cells, stimulating the radial expansion of the syncytium. While a pin1 mutant has fewer and smaller cysts than wild-type plants, the pin3 mutation only affects syncytium and female cyst size (Grunewald et al., 2009a). In contrast to syncytia, giant cells express PIN1. The pin1 mutants only show a slightly reduced number of galls and no difference in nematode development. In contrast to CN, PIN2 and PIN3 appear to be more important for the delivery of auxin into the initiating giant cells induced by RKN, as illustrated by a nearly halved number of galls in the pin2 and pin3 mutants but no difference in female development (Kyndt et al., 2016).
How do nematodes manipulate auxin transport and signaling to provoke the necessary changes for NFS development? For the CN H. schachtii, two effector proteins have been pinpointed as facilitators of auxin effects in syncytia. The effector 19C07 targets the Arabidopsis LAX3 auxin import protein, possibly increasing its activity and thus enhancing auxin influx into the syncytium and adjacent cells (Lee et al., 2011). The effector 10A07 interacts with the auxin regulator protein INDOLEACETIC ACID-INDUCED16 (IAA16) from Arabidopsis (Hewezi et al., 2015). AUX/IAA constitute a gene family of 29 members in Arabidopsis that negatively regulate the auxin response factors (ARFs). Upon removal of specific AUX/IAA by the proteasome, the corresponding ARFs can activate auxin-responsive genes (Chapman and Estelle, 2009). Therefore, binding of 10A07 to IAA16 could prevent it from repressing auxin response genes. Congruently, plants overexpressing 10A07 show enhanced expression of ARF6-8 and 19 and are more susceptible to H. schachtii than control plants. Unexpectedly, however, overexpression of IAA16 has similar effects, indicating a more complex regulation than anticipated from an IAA repressor.
Cytokinins Modulate NFS Formation
Cytokinins are N6-substituted adenine derivatives that, in concert with auxin, control cell division and differentiation in plants (Schaller et al., 2014; Di Mambro et al., 2017). Cytokinins are critical for cell cycle control, and the timing and amplitude of their oscillating levels may be important for the decision of cells to go into mitosis or endoreduplication. Cytokinins delay senescence and convert tissues into sinks by modulating nutrient translocation.
Due to their involvement in cell cycle control and nutrient mobilization, cytokinins have long been assumed to play a role in NFS development. De Meutter et al. (2003) detected cytokinins in secretions from the CN H. schachtii and the RKN M. incognita. For H. schachtii, this finding was corroborated with the identification of a cytokinin-synthesizing nematode gene being expressed in the early infection stages (Siddique et al., 2015); silencing of this gene results in reduced infectivity by the nematode. On the other hand, cytokinin biosynthesis Arabidopsis mutants show significantly smaller syncytia compared to wild-type plants (Siddique et al., 2015). This observation implies that both plant- and nematode-produced cytokinins are needed for the optimal formation of syncytia. Such detailed analyses have not been performed for RKN, but a similar scenario is very likely. Cytokinin signaling mutants and plants with reduced cytokinin levels are less susceptible to both types of nematodes (Lohar et al., 2004; Siddique et al., 2015; Shanks et al., 2016; Dowd et al., 2017). Nevertheless, expression of cytokinin biosynthesis, signaling, and catabolism genes is different in syncytia and galls (Dowd et al., 2017), which could be underlying divergent types of cell cycle progression. This hypothesis was confirmed by the analyses of cytokinin perception mutants, demonstrating that Ahk4 is the main Ahk gene (coding for Arabidopsis His kinases) involved in syncytium development, while Ahk2 and Ahk3 are important for gall formation (Siddique et al., 2015; Dowd et al., 2017). Comparing gene expression of young syncytia and galls with callus, Cabrera et al. (2015) found that, due to a higher cytokinin/auxin ratio, syncytia resemble shoot-forming calli and galls are similar to solid callus. However, it is still unknown how cytokinin signaling relates to the different abnormal cell cycles in syncytia and giant cells.
ET has Diverse Roles in Plant Susceptibility to CN and RKN
ET (H2C = CH2) is a gaseous hormone involved in many plant processes, but is famous for its role in senescence and fruit ripening (including the activation of cell wall degradation). In other plant processes, ET can result in different outcomes through its positive cross talk with either the auxin pathway (Strader et al., 2010) or the JA pathway (Nahar et al., 2011).
The available information on the role of ET in nematode infection seems contradictory, but some major features can be distinguished. ET consistently inhibits RKN infection but has a positive effect on CN infection. Early reports by Glazer et al. (1983, 1985) showed that ET has a positive effect on gall weight and giant cell hypertrophy, but this effect does not necessarily equate to increased nematode infection. Indeed, all later studies across multiple plant species convincingly show that ET inhibits RKN infection, possibly through a decrease in nematode attraction to the roots (Nahar et al., 2011; Fudali et al., 2013; Mantelin et al., 2013). Consistent with ET playing a role in plant defense to RKN infection, resistant plants show more up-regulation of ET biosynthesis and response genes than susceptible plants (Kumari et al., 2016; Shukla et al., 2018).
In contrast, ET enhances the attraction of H. schachtii to Arabidopsis roots as shown by higher levels of infection in plants with more ET (response), while mutants in ET response (or treated with ET inhibitors) have fewer nematodes (Goverse et al., 2000; Wubben et al., 2001; Kammerhofer et al., 2015). Higher ET levels also have been linked to increased syncytium expansion (Goverse et al., 2000) and Bent et al. (2006) found fewer soybean CN (SCN) H. glycines females developing on ET-insensitive soybean roots. On the other hand, detailed attraction studies using SCNs yielded dissimilar results (Hu et al., 2017b). H. glycines juveniles are attracted more to soybean root tips pretreated with an ET biosynthesis inhibitor than to control roots. The attraction of H. glycines to roots of Arabidopsis (nonhost for SCN) is enhanced in ET-insensitive mutants and diminished in ET-overproducing mutants (Hu et al., 2017b). Recent work by Piya et al. (2018) elucidates how ET perception in Arabidopsis can result in higher or lower susceptibility to H. schachtii (measured as the number of developing females), depending on the receptor and its downstream pathway. The canonical ET signaling pathway causes suppression of SA-based defense, resulting in higher susceptibility to the CN, fitting the idea that ET enhances CN infection. The second pathway acts via the ETHYLENE RECEPTOR1, with ET inhibiting cytokinin signaling and thus reducing susceptibility to H. schachtii infection (Piya et al., 2018). Depending on the specific host-nematode interaction and timing or location of ET effects, cross talk with other hormone pathways could, therefore, have different effects on the host response to CN infection.
Habash et al. (2017) identified a tyrosinase-like protein secreted by H. schachtii (Hs-Tyr) that, upon ectopic expression in Arabidopsis, increases susceptibility to the CN but not to the RKN M. incognita. Hs-Tyr expression in the plant is correlated with higher auxin (IAA-conjugates) and 1-aminocyclopropane-1-carboxylic acid (ET-precursor) levels, two hormones involved in susceptibility to CN.
DEFENSE HORMONE PATHWAYS ACTIVATED BY THE PLANT AND DAMPENED BY THE PARASITE
An investigation of the plant response to organisms invading aerial plant parts identified SA and JA as important defense hormones interacting either antagonistically or synergistically (Mur et al., 2006). Findings from Arabidopsis led to the paradigm that SA generally protects against biotrophic pathogens, whereas JA inhibits necrotrophic micro-organisms and munching insects (Glazebrook, 2005; Beckers and Spoel, 2006). Gutjahr and Paszkowski (2009) concluded that SA also appears to activate defense against biotrophic pathogens in roots, but JA presented a complex picture, and more research was needed to dissect the role of both hormones in root defense signaling. Ten years and many publications later, this conclusion still stands true for plant-nematode interactions.
SA Activates Basal Defenses Against Nematodes
The application of SA, or chemicals with similar action, reduces nematode infection (e.g., Wubben et al., 2008; Nahar et al., 2011; Molinari et al., 2014; Kammerhofer et al., 2015; Molinari, 2016 ). In many cases, the effect is modest and has been explained by the capability of the nematodes to suppress the SA pathway (Sanz-Alférez et al., 2008; Barcala et al., 2010; Uehara et al., 2010; Ji et al., 2013; Shukla et al., 2018). Although the effect is not always significant, mutants and transgenics with lower SA levels or signaling generally are more susceptible to nematodes (Wubben et al., 2008; Nahar et al., 2011), whereas enhanced SA levels or signaling results in lower nematode infections (Priya et al., 2011; Lin et al., 2013; Youssef et al., 2013). Nguyen et al. (2016), however, did not find enhanced susceptibility to H. schachtii in Arabidopsis SA signaling mutants.
Many nematode effectors suppress plant defenses (Haegeman et al., 2012), but in only a few cases has this effect been specifically linked to suppression of the SA pathway. Effectors of fungal and oomycete pathogens have been implicated in the manipulation of SA biosynthesis. Some of these microbes secrete chorismate mutase and isochorismatase that convert chorismate and isochorismate, respectively, away from the main SA biosynthesis pathway, in this way lowering SA levels and defenses (Djamei et al., 2011; Liu et al., 2014). Similar genes have been identified in plant-parasitic nematodes (see Table 1). Wang et al. (2018) demonstrated that transient expression of an M. incognita chorismate mutase effector in Nicotiana benthamiana causes a decline in SA levels and larger lesions upon infection with Phytophthora capsici. Transgenic N. benthamiana plants expressing M. incognita chorismate mutase effector are more susceptible to M. incognita. Overexpression of a Hirschmanniella oryzae chorismate mutase or an isochorismatase in rice also enhances susceptibility to this nematode (L. Bauters, unpublished data).
Table 1. Nematode effectors mimicking PPHs and influencing phytohormone physiology and signaling at feeding sites.
| Effector Mimics of PPHs | ||
|---|---|---|
| CLE-like Peptides | ||
| HgCLE | Heterodera glycines | Wang et al., 2001, 2005, 2010a; Gao et al., 2003 |
| HsCLE | H. schachtii | Wang et al., 2011 |
| GrCLE | Globodera rostochiensis | Lu et al., 2009; Guo et al., 2011; Chen et al., 2015 |
| RrCLE | Rotylenchulus reniformis | Wubben et al., 2015 |
| MhCLE | Meloidogyne hapla | Bird et al., 2015 |
| CEP-like Peptides | ||
| MhCEP | M. hapla | Bobay et al., 2013; Bird et al., 2015 |
| RrCEP | R. reniformis | Eves-Van Den Akker et al., 2016 |
| IDA-like Peptides | ||
| MiIDL | M. incognita | Tucker and Yang, 2013; Kim et al., 2018 |
| MhIDL | M. hapla | Kim et al., 2018 |
| MfIDL | M. floridensis | Kim et al., 2018 |
| Effectors Influencing Phytohormone Physiology and Signaling | ||
| Auxins | ||
| Conjugated forms | H. schachtii | De Meutter et al., 2005 |
| Conjugated forms | M. incognita | De Meutter et al., 2005 |
| Cytokinins | ||
| iP, Z, BA-types | H. schachtii | De Meutter et al., 2003; Siddique et al., 2015 |
| iP, Z, BA-types | M. incognita | De Meutter et al., 2003 |
| Chorismate Mutase | ||
| HgCM | H. glycines | Bekal et al., 2003 |
| HsCM | H. schachtii | Vanholme et al., 2009 |
| GrCM | G. rostochiensis | Lu et al., 2008 |
| GpCM | G. pallida | Jones et al., 2003; Yu et al., 2011 |
| GtCM | G. tabacum | Yu et al., 2011 |
| GeCM | G. ellingtonae | Chronis et al., 2014 |
| MjCM | M. javanica | Lambert et al., 1999; Doyle and Lambert, 2003 |
| MiCM | M. incognita | Huang et al., 2005; Wang et al., 2018 |
| MaCM | M. arenaria | Long et al., 2006a, 2006b |
| HoCM | Hirschmanniella oryzae | Bauters et al., 2014 |
| PcCM | Pratylenchus coffeae | Haegeman et al., 2011 |
| Tyrosinase | ||
| HsTYR | H. schachtii | Habash et al., 2017 |
| Isochorismatase | ||
| GrICM | G. rostochiensis | Eves-Van Den Akker et al., 2016 |
| MhICM | M. hapla | Opperman et al., 2008 |
| RrICM | R. reniformis | Wubben et al., 2010 |
| HoICM | H. oryzae | Bauters et al., 2014 |
| Novel Proteins | ||
| Hg19C07 | H. glycines | Gao et al., 2003 |
| Hs19C07 | H. schachtii | Lee et al., 2011 |
| Hg10A07 | H. glycines | Hewezi et al., 2015 |
| Hs10A07 | H. schachtii | Hewezi et al., 2015 |
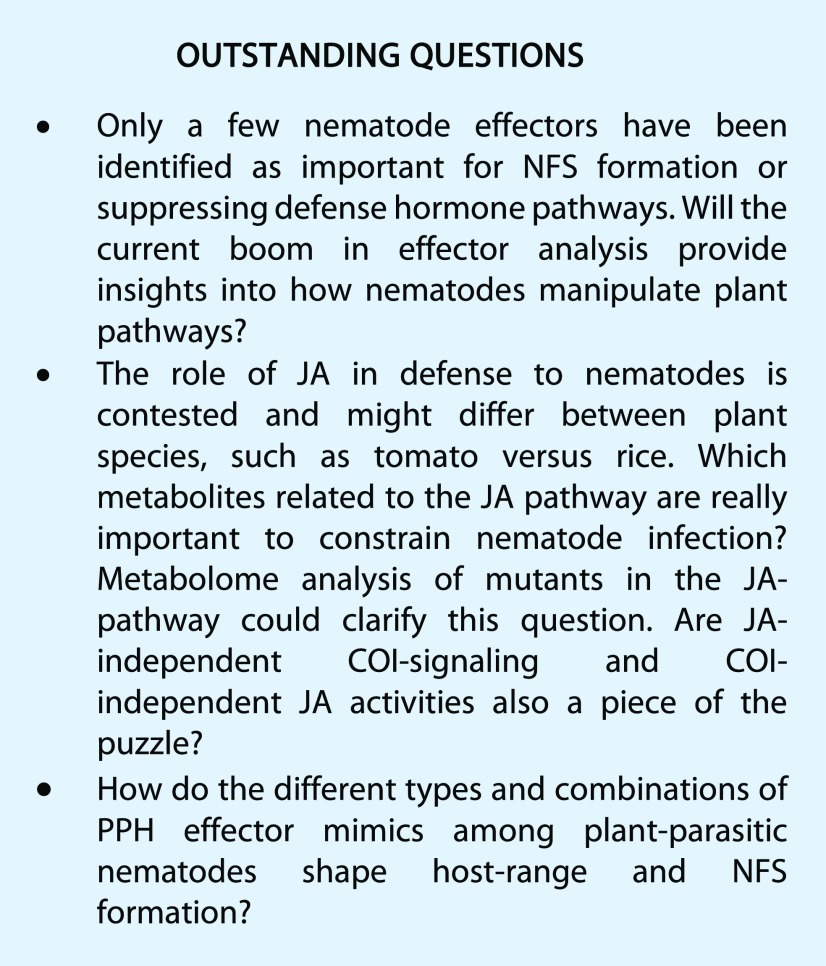
The JA Pathway has a Polemical Role in Nematode Infection
The release of JA during plant defense was first discovered as a response to insect attack. JA enhances the expression of protease inhibitors and pathways producing secondary metabolites with antiherbivore activity. Protease inhibitors constrain the proteolytic activity of the insects’ digestive enzymes to debilitate their growth and reproduction. Nematodes, being animals, also rely on proteases for obtaining sufficient nutrients from their food source. Therefore, it is not surprising that JA would play a role in defense to plant-parasitic nematodes. However, data on the role of the JA pathway (see Fig. 2 for an overview) in nematode infection are not unequivocal, at least not for RKN.
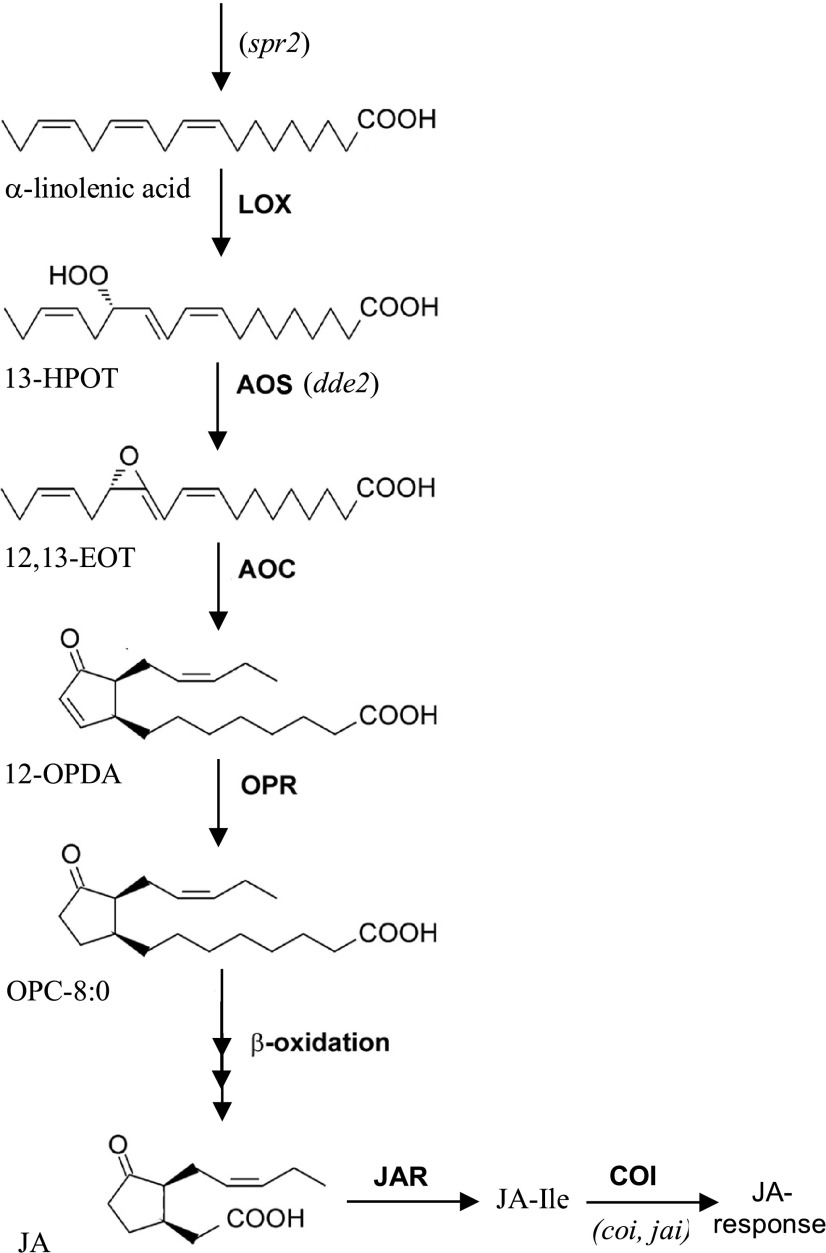
Figure 2.
Overview of JA biosynthesis pathway and related mutants. Only the main pathway of oxylipin synthesis to jasmonate is shown. Several branches occur that give rise to many other metabolites. In addition, several enzymes are encoded by multiple genes from a gene family, although only one is shown. Intermediates and derivates: 13-HPOT, 13-hydroperoxy-octadecatrienoic acid; 12,13-EOT, 12,13-epoxy octadecatrienoic acid; OPC-8:0, 3-oxo-2-(2-pentenyl)-cyclopentane-1-octanoic acid; JA-Ile, jasmonoyl-Ile. Enzymes: LOX, 13-lipoxygenase; OPR, 12-oxophytodienoate reductase; JAR, jasmonate response locus encoding a jasmonic acid-amido synthetase that converts JA into the bio-active JA-Ile. Mutants: spr2, suppressor of prosystemin response 2 mutant; coi1, the mutant in COI F-box protein involved in jasmonate signaling; jai, jasmonate insensitive, also mutant in COI.
For CN, the available data are consistent with JA enhancing defense. Application of Methyl-JA to Arabidopsis leaves reduces H. schachtii infection on the roots, and the JA biosynthesis mutants delayed-dehiscence2 (dde2) and lipoxygenase 6 (lox6) show enhanced female development compared to control plants (Kammerhofer et al., 2015). Arabidopsis mutants with higher JA levels/signaling are less susceptible to H. schachtii (Ali et al., 2013; Nguyen et al., 2016; Sidonskaya et al., 2016), and soybean roots overexpressing (E,E)-a-farnesene synthase (a gene up-regulated upon JA treatment) support lower levels of H. glycines infection, indicating an additional possible mechanism of JA action (Lin et al., 2017). At first sight, the results of Ozalvo et al. (2014) fit the “JA = defense” picture with the JA biosynthesis mutant lox4 being more susceptible to H. schachtii, but a closer look contradicts this conclusion (see below).
Over the past 10 years, more than 20 papers have been published on the role of JA in RKN infections, and the data overwhelmingly support JA as a defense molecule. Application of MeJA on tomato (Solanum lycopersicum), rice (Oryza sativa), and soybean (Glycine max) invariably reduces RKN infection (Cooper et al., 2005; Shimizu and Mazzafera, 2007; Fujimoto et al., 2011; Nahar et al., 2011; Zhang et al., 2011; Zinovieva et al., 2013; Vieira Dos Santos et al., 2014; Zhou et al., 2015; Hu et al., 2017a; Kyndt et al., 2017), while inhibitors of JA biosynthesis enhance infection (Nahar et al., 2011; Zhou et al., 2015). In contrast, analyses of mutants and transgenics modified in JA signaling or biosynthesis yield brain-twisting results.
The first indication of the complexity of the JA pathway was the report that a JA-insensitive mutant in tomato is less susceptible to M. incognita than the wild type (Bhattarai et al., 2008). This observation led to the conclusion that, whereas the hormone JA results in defense, JA signaling is needed for successful infection. However, other experiments with JA-signaling mutants do not support this conclusion: specifically, M. incognita infection of the Arabidopsis mutant coronatine insensitive (coi) does not differ from the wild type (Gleason et al., 2016), and the rice mutant jar1 is slightly more susceptible to the rice RKN Meloidogyne graminicola (T Kyndt and R singh, unpublished data). To augment the complexity, Gleason et al. (2016) demonstrated that coi is not needed for JA-induced defense against M. incognita infection in Arabidopsis.
What about mutant/transgenic plants with changes in JA biosynthesis? Tomato suppressor of prosystemin-mediated responses2 mutants, affected in the production of linolenic acid needed for JA biosynthesis, are more susceptible to M. incognita (Sun et al., 2011; Fan et al., 2015). Tomato plants overexpressing miR319 show lower JA levels and are highly susceptible to M. incognita (Zhao et al., 2015). Rice plants overexpressing allene oxide synthase (AOS) are less susceptible to M. graminicola (Kyndt et al., 2017), and the Arabidopsis AOS mutant dde2 shows more galling by M. hapla than wild type (Gleason et al., 2016).
However, not all mutants in JA biosynthesis corroborate the role of JA in defense. Depending on the Lox or allene oxide cyclase (Aoc) gene, mutants are more (lox4-1, Ozalvo et al., 2014; aoc-3, Naor et al., 2018) or less (lox3-1, Ozalvo et al., 2014) susceptible to RKN infection. As Naor et al. (2018) explain, the oxylipin biosynthesis pathway branches into many metabolites with differing levels of toxicity to RKN; therefore, mutants likely affect more than just the JA level. Gleason et al. (2016), for instance, showed that the intermediate 12-oxo-phytodienoic acid (OPDA) is much more important than JA for defense against RKN, which is consistent with JA and OPDA having different signaling roles (Dave and Graham, 2012). In contrast to Gleason et al. (2016), Naor et al. (2018) found the Arabidopsis dde2 mutant to be less susceptible to M. javanica.Ozalvo et al. (2014) add further to the confusion by demonstrating that the highly susceptible biosynthesis mutant lox4 has not lower but higher JA levels upon nematode infection and also higher JA, ET, and SA- regulated transcription, all thought to be involved in defense to RKN.
It is difficult to compare the different results, as some papers report gall numbers (the initial infection stage) and others describe female numbers or measure percent female development. In addition, numbers per root system can give a different conclusion compared to numbers per gram of root, especially if using mutants that are affected in their root morphology. Unfortunately, most authors do not describe the root phenotype of the mutants. An example of these complications are the results of Gao et al. (2008) on the lox3-4 mutant in maize (Zea mays). This mutant has elevated JA, SA, and ET levels in its roots and is highly susceptible to M. incognita infection, based on increased nematode attraction to roots and a higher number of eggs per gram of root compared to wild type. The lox3-4 mutant has much shorter roots, but the number of root tips needed for nematode invasion is most likely unaltered or even higher (nematode attraction and invasion per plant are higher). As a consequence, calculation of the number of eggs per invaded nematode is much lower in the lox3-4 than in wild type, which could be interpreted as less susceptible if susceptibility is measured as the ability of the host to allow nematode multiplication.
In conclusion, while spraying JA enhances plant defense to nematodes, it is not JA itself that is responsible, but its effects on the production of proteins (such as proteinase inhibitors) and metabolites (such as terpenes and oxylipins). Depending on how mutations in JA-related genes affect these antiherbivore compounds, the plant is rendered more or less susceptible to nematode infection.
In view of the importance of JA in defense, we could expect nematode effectors that interfere with this pathway. Indeed, transcriptome analyses have found suppression of JA-related genes in syncytia and giant cells (Ithal et al., 2007; Ji et al., 2013). Nematode-secreted fatty acid and retinol (FAR)-binding proteins have been proposed to interfere with lipid signaling in host defense, for animal (e.g., Garofalo et al., 2003) as well as plant parasites. The FAR protein of the potato (Solanum tuberosum) CN Globodera pallida is located on the cuticle surface and interferes with plant LOX-mediated defense (Prior et al., 2001). Tomato roots expressing the M. javanica MjFAR are more susceptible to RKN infection, and this observation is correlated with lower expression of the JA-responsive proteinase inhibitor2 (Iberkleid et al., 2013); however, some genes in the JA pathway are expressed at higher levels in these roots (Iberkleid et al., 2015).
OTHER PLANT HORMONES PLUG INTO THE DEFENSE CORE
In contrast to the ample studies on the importance of auxin and jasmonate in susceptibility and defense, respectively, very little research has been done on the role of gibberellic acid (GA), abscisic acid (ABA), brassinosteroids, and strigolactones in nematode infection. The available knowledge is limited mainly to the rice-M. graminicola system.
GA is well known for its role in stimulating plant growth by the degradation of DELLAs, a class of growth-repressing nuclear proteins. Studies in Arabidopsis revealed that GA antagonizes JA action and promotes SA signaling and/or perception (Navarro et al., 2008). In rice, GA interacts antagonistically with both JA and SA signaling pathways (De Vleesschauwer et al., 2016). Congruently, GA is important for susceptibility of rice to M. graminicola, as shown in a detailed study using the application of GA or a GA-biosynthesis inhibitor and a series of mutants (Yimer et al., 2018). In contrast, foliar application of GA to tomato increases resistance against M. javanica (Moosavi, 2017). However, these latter results were not confirmed by analysis of GA-mutants, and the GA concentration applied might have influenced the outcome (Bauters et al., 2018; Yimer et al., 2018).
The application of ABA increases the susceptibility of rice and tomato to RKN infection (Kyndt et al., 2017; Moosavi, 2017), and brassinosteroids suppress rice defense to M. graminicola (Nahar et al., 2013). In rice, ABA (Kyndt et al., 2017), brassinosteroids (Nahar et al., 2013), and strigolactones (Lahari et al., 2018) all appear to enhance susceptibility to M. graminicola through antagonism with the JA pathway.
NEMATODES SECRETE PPH EFFECTOR MIMICS FOR FEEDING SITE FORMATION
Besides the classical phytohormones discussed so far, small, secreted peptide hormones are also potent modulators of plant growth and development. It has become increasingly evident that secreted peptides play critical roles in mediating a range of plant-microbe interactions, either by induction of PPH gene expression, for instance during legume-rhizobium symbioses, or by secreting PPH effector mimics (Yamaguchi et al., 2016; Ronald and Joe, 2018; Taleski et al., 2018). Here, we focus on PPH effector mimics secreted by nematodes, the first animal-pathogen model identified to secrete such molecules for parasitism. The different classes of PPH effector mimics identified from nematodes have expanded to include CLAVATA3/EMBRYO SURROUNDING REGION (CLE)-like, C-TERMINALLY ENCODED PEPTIDE (CEP)-like, and INFLORESCENCE DEFICIENT IN ABSCISSION (IDA)-like peptides.
CLE-like Peptides
Plant CLEs play important roles in shoot, root, and vascular meristem maintenance and are classified as either A-type or B-type peptides (for review, see Yamaguchi et al., 2016). The A-type peptides promote cell differentiation, whereas the B-type peptides suppress differentiation of tracheary elements and promote procambial cell division. Comprehensive clustering analysis has categorized plant CLEs into groups with potentially shared function (Goad et al., 2017). Aside from plants, CLE-like peptide effector mimics have been identified from multiple genera of CN, RKN, and more recently, from the reniform nematode (a semiendoparasite that induces syncytia; Fig. 3). In the case of CN and reniform nematodes, the domain architecture of CLE-like peptide effector mimics resembles that of plant CLE proteins (Lu et al., 2009; Wang et al., 2010a, 2011; Wubben et al., 2015). Plant CLEs are produced as prepropeptides harboring an N-terminal secretion signal peptide that directs them through the plant secretory pathway for delivery to the apoplast. A central “pro” domain, referred to as the “variable” domain because of its lack of sequence homology among family members, separates the secretion signal peptide and C-terminal CLE domain. Similarly, CN and reniform produce CLEs as prepropeptides, but this occurs in the dorsal esophageal gland cell of the nematode (Wang et al., 2010a; Wubben et al., 2015). The N-terminal secretion signal peptide directs these effector proteins through the gland cell secretory pathway for packaging into secretory granules. They are then delivered as propeptides (comprised of a central variable domain and a C-terminal CLE domain with homology to plant CLE peptides) to the cytoplasm of host root cells through the stylet (Lu et al., 2009; Wang et al., 2010a; Mitchum et al., 2012, 2013). Once in the cytoplasm of host root cells, they are redirected through the plant secretory pathway to the apoplast by an unknown posttranslational trafficking mechanism mediated by a conserved “cryptic signal peptide” sequence in the N-terminal portion of the variable domain (Wang et al., 2010b). The proteins subsequently undergo posttranslational modification by hydroxyproline (Hyp) arabinosylation and proteolytic cleavage down to the 12-amino acid CLE peptide to release one or more bioactive ligands (Chen et al., 2015). These ligands interact with plant Leu-rich repeat (LRR) receptor kinases, including CLV1, CLV2, and BARELY ANY MERISTEMs, to positively regulate NFS development (Guo et al., 2010, 2015; Chen et al., 2015). Silencing of nematode CLE genes or their cognate plant receptors delays nematode development by impairing NFS formation (Replogle et al., 2011, 2013; Chen et al., 2015; Guo et al., 2015, 2017). In contrast to CN, the absence of a “pro” domain from RKN CLEs suggests this nematode may deliver bioactive CLE peptide mimics directly into the apoplast (Mitchum et al., 2012; Bird et al., 2015).
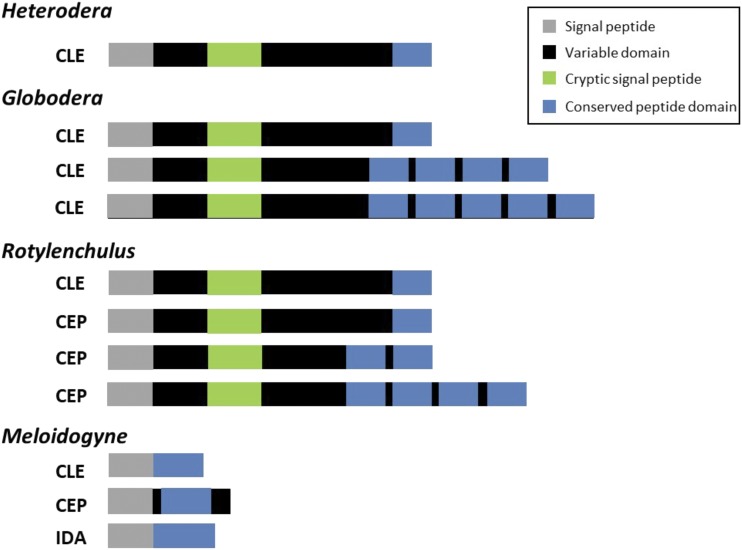
Figure 3.
Representative structures of nematode CLE-like, CEP-like, and IDA-like proteins. Cyst and reniform nematode CLE and CEP-like proteins contain an N-terminal signal peptide, a variable domain, and either single or multiple conserved C-terminal peptide motifs similar to plant CLE or CEPs, respectively. The green box in the variable domain of cyst and reniform nematode CLE and CEP-like proteins denotes a cryptic signal peptide sequence. Root-knot nematode CLE, CEP, and IDA-like proteins lack a variable domain sequence.
Based on the findings that nematode CLE peptide effector mimics belong to multigene families, are coordinately expressed, and can encode proteins with multiple CLE domains (Mitchum et al., 2012), it appears that nematodes may require the simultaneous secretion of a mixture of CLE peptide mimics for NFS formation. Multiple single-domain CLEs have been identified from Heterodera, RKN, and reniform, whereas Globodera species harbor multidomain CLEs. Another fascinating observation is that no two identical CLE sequences have been identified within a single genus or across genera. Whether these differences play a significant role in plant host adaptation is still unknown. Until recently, only A-type CLE-like peptide effector mimics had been identified from nematodes (Mitchum et al., 2012). However, mining of the H. glycines early parasitic stage transcriptome (Gardner et al., 2018) revealed B-type CLE peptide effector mimics nearly identical to tracheary element differentiation inhibitory factor (TDIF), encoded by CLE41 and CLE44, in Arabidopsis (Guo et al., 2017). In plants, the TDIF peptide regulates vascular stem cell maintenance through an interaction with the TDIF RECEPTOR (TDR)/PHLOEM INTERCALATED WITH XYLEM receptor kinase to activate two independent downstream pathways. The TDIF-TDR-WOX4 pathway promotes procambial cell proliferation, whereas the TDIF-TDR-Glycogen Synthase Kinase3-BRI1-EMS SUPPRESSOR1 pathway inhibits xylem differentiation from procambial cells. Procambial-associated genes are activated in both CN and RKN feeding sites (Guo et al., 2017; Yamaguchi et al., 2017). The TDIF-TDR-WOX4 procambial cell proliferation pathway is required for CN feeding site formation (Guo et al., 2017); however, further research is needed to assess whether the TDIF-TDR-Glycogen Synthase Kinase3-BRI1-EMS SUPPRESSOR1 signaling is equally important. As of yet, B-type CLE peptide effector mimics have not been identified from RKN, and a potential role of these vascular stem cell signaling pathways in RKN feeding site formation remains to be confirmed. Of note is the low abundance of nematode B-type CLEs relative to A-type CLEs in early CN parasitic stages (Guo et al., 2017). A detailed analysis assessing if nematodes tightly control the expression and release of specific peptide effectors during the phases of NFS formation will help gauge whether there is any potential biological significance of peptide synergism. Other than a role for WOX4, little is known about the downstream intracellular nematode peptide signaling cascades. Additional research is needed to dissect what appears to be a complex network of nematode CLE-receptor interactions to understand fully their specific contribution to NFS formation.
CEP-like Peptides
As the genomes and transcriptomes of more plant-parasitic nematodes have been released, computational scans have identified additional classes of PPH effector mimics, lending further support for peptide hormone mimicry as a signature adaptation to plant parasitism (Bird et al., 2015). CEP-like peptide effector mimics have been identified from Meloidogyne genomes and, more recently, from reniform nematode but not from CN genomes. In plants, CEPs are small, secreted peptide hormones implicated in nitrogen-demand signaling, nodulation, and lateral root development (reviewed by Taleski et al., 2018). CEP propeptides are cleaved by amino- and carboxypeptidases to release 15-amino acid bioactive peptides that signal through LRR-RK CEPR1. Like CLEs, CEP activity is regulated by posttranslational modifications in the form of Hyp arabinosylation. Despite the widespread identification of CEPs in plants, downstream signaling mediated by CEP-CEPR remains unknown. Twelve reniform CEP gene family members have been identified to date. They are unique in that they harbor one intron per domain sequence, whereas all other CEPs identified from animals and plants are encoded by a single exon, suggesting an independent evolutionary origin (Eves-Van Den Akker et al., 2016). Similar to plant CEPs, the reniform CEPs are produced as prepropeptides. Remarkably, the “pro” domain harbors a cryptic signal peptide with similarity to CN and reniform CLE-like effectors, not only suggesting that these effector proteins may be indirectly routed to the apoplast upon delivery as propeptides to host root cells but that the trafficking mechanism by which this occurs may be conserved across genera and span to different classes of effectors. CEPs are produced within the dorsal gland cell of sedentary reniform females, suggesting a prominent role in NFS formation. Interestingly, the RKN CEPs, like their CLE counterparts, lack the “pro” domain, lending further support for a mechanism of direct delivery to the apoplast to exert their function in giant cell formation (Bobay et al., 2013; Bird et al., 2015). Although the role of CEP-like effector mimics in nematode parasitism remains unknown, Arabidopsis primary root length and lateral root number are inhibited in a dose-dependent manner upon exogenous application of RrCEP1, similar to the application of plant CEP peptides. In addition, feeding sites induced by the CN H. schachtii are smaller in size in the RrCEP1-treated roots, suggesting that one potential function of nematode CEPs may be to regulate NFS size (Eves-Van Den Akker et al., 2016). Further studies are needed to clarify the unique role of CEP-like PPH effector mimics in plant-nematode interactions and any potential role in host nitrate uptake like their plant counterparts.
IDA-like Peptides
The broad spectrum of PPH effector mimics identified from Meloidogyne species may aid RKN to parasitize a broad range of host plant species. In addition to CLE-like and CEP-like PPH effector mimics, several IDA-like (IDL) family members have been identified from multiple Meloidogyne species (Tucker and Yang, 2013; Kim et al., 2018). An exhaustive search of CN and reniform sequence data for IDL peptides remains to be conducted; however, no IDL peptides were identified from existing sequence data for Heterodera and Globodera spp. (Kim et al., 2018). In plants, IDA signaling through the LRR-RKs HAESA (HAE) and HAESA-like2 activates a MAP kinase signaling cascade that leads to the expression of KNOX transcription factors, which regulate a suite of cell wall-modifying proteins important for cell separation during floral organ abscission and lateral root emergence. More recently, IDL peptides were shown to modulate plant stress and defense responses to pathogens (Vie et al., 2017; Wang et al., 2017). Like CLEs and CEPs, IDAs harbor an N-terminal secretion signal peptide and undergo proteolytic cleavage to 14-amino acid bioactive peptides in the apoplast. RKN IDL effector mimics have a similar domain architecture. A synthetic M. incognita IDL1 (MiIDL1) peptide applied exogenously to the Arabidopsis mutant ida is able to rescue floral abscission and lateral root phenotypes in an HAE/HAESA-like2-dependent manner (Kim et al., 2018). However, direct binding of MiIDL1 to these receptors has not been demonstrated. Similarly, transgenic Arabidopsis ida mutant plants expressing MiIDL1 exhibit wild-type floral abscission. Host-derived RNAi targeting of MiIDL1 leads to fewer and smaller galls compared to control plants, demonstrating a critical role in parasitism. Together, these data provide evidence of a specific role of IDL PPH mimics in giant cell formation and point to a potentially unique adaptation for RKN parasitism.
INTEGRATION OF PEPTIDE AND HORMONE SIGNALING FOR NFS FORMATION
Cross talk between peptide and hormone signaling regulates developmental processes and responses to external stimuli (for review, see Wang et al., 2016). Evidence for such cross talk governing NFS formation is amassing in the literature. Alterations to phytohormone physiology and signaling, induced in response to nematode feeding, may be coordinately regulated by PPH effector mimics and hormones to fine-tune root developmental programs in favor of NFS formation. Studies showing that a low Mr peptide (s) from G. rostochiensis secretions costimulates the proliferation of protoplasts together with auxin and cytokinin, provided some of the first evidence for potential cross talk between nematode-secreted peptides and hormonal signaling (Goverse et al., 1999). Recent studies suggest CN may be co-opting early signaling events in vascular cell patterning, a process controlled by CLE peptides and hormonal signaling, for the successful formation of NFS. For instance, the beet (Beta vulgaris) CN H. schachtii secretes HsCLE2, an A-type CLE peptide mimic that is identical to AtCLE5/6 while simultaneously secreting HsCLEB, a B-type CLE peptide mimic nearly identical to Arabidopsis CLE41/TDIF (Guo et al., 2017). These peptides act synergistically in an auxin-dependent manner to suppress differentiation and promote vascular stem cell proliferation. They also activate the expression of numerous auxin-responsive genes known to be up-regulated in NFS (Whitford et al., 2008). Though plant CLE peptides exhibit cell-type specific expression patterns, overlapping expression domains may be critical for developmental programs requiring the synergistic action of multiple CLE peptides. Nematodes appear to have adapted to exploit this by controlling both the timing and quantity of A- and B-type peptides secreted into a chosen cell to potentially bypass the plant’s own cell type-specific and negative feedback regulation mechanisms. Aside from auxin, there are also reports of intersections among CLE signaling and BR, CK, and GA signaling both locally and systemically. TDIF signaling suppresses xylem differentiation from procambial cells through integration with BR signaling (Kondo et al., 2014); GA positively regulates the expression of CLE6 and overexpression of this peptide partially rescues GA-deficiency (Bidadi et al., 2014); and CLEs are regulators of type-A ARRs to promote CK signaling (Kondo et al., 2011). A similarly complex cross talk is likely at play for other classes of plant peptides and hormones. For instance, the developmental programs underlying lateral root emergence requires the integration of auxin and IDA signaling to regulate cell-wall–modifying proteins involved in cell separation (Kumpf et al., 2013). These studies illuminate the incredibly complex network of peptide and hormone signaling pathways likely active in NFS formation.
CONCLUSION
Substantial progress has been made in our understanding of how plant hormones shape the interface between plants and nematodes and how nematode effector proteins contribute to this interaction. However, the few effectors that have been identified as participating in NFS formation cannot explain the myriad of complex changes that lead to a mature feeding cell (see "Outstanding Questions"). Undoubtedly, we still have much to learn about the interplay among peptide, phytohormone, and defense signaling pathways in NFS formation. Moreover, the field has expanded, as nematodes are no longer unique among plant pathogens in their ability to secrete mimics of PPHs (Ronald and Joe, 2018). It was recently discovered that the fungal pathogen Fusarium oxysporum and the bacterial pathogen Xanthomonas oryzae pv. oryzae secrete functional peptide mimics of plant rapid alkalinization factor and plant peptide containing sulfated tyrosine peptides, respectively (Masachis et al., 2016; Pruitt et al., 2017). Thus, as we continue to uncover the complex interplay between peptide and hormone signaling in plant-nematode interactions, the findings are likely to have much broader applicability in molecular plant-microbe interactions than previously thought.
Acknowledgments
The authors wish to thank the various funding agencies that have supported their research programs on hormone and peptide signaling through the years, as well as the postdoctoral fellows, students, staff scientists, and many colleagues who have contributed to the research findings described in this review. The authors also thank Nagabhushana Ithal for the nematode picture of the vTOC icon.
Footnotes
This work was supported by the Research Foundation-Flanders (project no. 3G008718), the Special Research Fund of Ghent University (grant nos. BOF13/GOA/030 and 01G01318), the National Science Foundation (grant no. IOS-1456047), the U.S. Department of Agriculture National Institute of Food and Agriculture, and the United Soybean Board.
Articles can be viewed without a subscription.
References
- Ali MA, Abbas A, Kreil DP, Bohlmann H (2013) Overexpression of the transcription factor RAP2.6 leads to enhanced callose deposition in syncytia and enhanced resistance against the beet cyst nematode Heterodera schachtii in Arabidopsis roots. BMC Plant Biol 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcala M, García A, Cabrera J, Casson S, Lindsey K, Favery B, García-Casado G, Solano R, Fenoll C, Escobar C (2010) Early transcriptomic events in microdissected Arabidopsis nematode-induced giant cells. Plant J 61: 698–712 [DOI] [PubMed] [Google Scholar]
- Bauters L, Haegeman A, Kyndt T, Gheysen G (2014) Analysis of the transcriptome of Hirschmanniella oryzae to explore potential survival strategies and host-nematode interactions. Mol Plant Pathol 15: 352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauters L, Nahar K, Hossain M, Gheysen G (2018) Gibberellin reduces susceptibility of rice (Oryza sativa) against the migratory nematode Hirschmanniella oryzae. Nematology 20: 703–709 [Google Scholar]
- Beckers GJM, Spoel SH (2006) Fine-tuning plant defence signalling: Salicylate versus jasmonate. Plant Biol (Stuttg) 8: 1–10 [DOI] [PubMed] [Google Scholar]
- Bekal S, Niblack TL, Lambert KN (2003) A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol Plant Microbe Interact 16: 439–446 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bent AF, Hoffman TK, Schmidt JS, Hartman GL, Hoffman DD, Xue P, Tucker ML (2006) Disease- and performance-related traits of ethylene-insensitive soybean. Crop Sci 46: 893–901 [Google Scholar]
- Bhattarai KK, Xie Q-G, Mantelin S, Bishnoi U, Girke T, Navarre DA, Kaloshian I (2008) Tomato susceptibility to root-knot nematodes requires an intact jasmonic acid signaling pathway. Mol Plant Microbe Interact 21: 1205–1214 [DOI] [PubMed] [Google Scholar]
- Bidadi H, Matsuoka K, Sage-Ono K, Fukushima J, Pitaksaringkarn W, Asahina M, Yamaguchi S, Sawa S, Fukuda H, Matsubayashi Y, et al. (2014) CLE6 expression recovers gibberellin deficiency to promote shoot growth in Arabidopsis. Plant J 78: 241–252 [DOI] [PubMed] [Google Scholar]
- Bird DM, Jones JT, Opperman CH, Kikuchi T, Danchin EG (2015) Signatures of adaptation to plant parasitism in nematode genomes. Parasitology 142(Suppl 1): S71–S84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobay BG, DiGennaro P, Scholl E, Imin N, Djordjevic MA, Mck Bird D (2013) Solution NMR studies of the plant peptide hormone CEP inform function. FEBS Lett 587: 3979–3985 [DOI] [PubMed] [Google Scholar]
- Cabrera J, Fenoll C, Escobar C (2015) Genes co-regulated with LBD16 in nematode feeding sites inferred from in silico analysis show similarities to regulatory circuits mediated by the auxin/cytokinin balance in Arabidopsis. Plant Signal Behav 10: e990825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera J, Barcala M, García A, Rio-Machín A, Medina C, Jaubert-Possamai S, Favery B, Maizel A, Ruiz-Ferrer V, Fenoll C, et al. (2016) Differentially expressed small RNAs in Arabidopsis galls formed by Meloidogyne javanica: A functional role for miR390 and its TAS3-derived tasiRNAs. New Phytol 209: 1625–1640 [DOI] [PubMed] [Google Scholar]
- Chapman EJ, Estelle M (2009) Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 43: 265–285 [DOI] [PubMed] [Google Scholar]
- Chen S, Lang P, Chronis D, Zhang S, De Jong WS, Mitchum MG, Wang X (2015) In planta processing and glycosylation of a nematode CLAVATA3/ENDOSPERM SURROUNDING REGION-like effector and its interaction with a host CLAVATA2-like receptor to promote parasitism. Plant Physiol 167: 262–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis D, Chen SY, Skantar AM, Zasada IA, Wang XH (2014) A new chorismate mutase gene identified from Globodera ellingtonae and its utility as a molecular diagnostic marker. Eur J Plant Pathol 139: 239–246 [Google Scholar]
- Cooper WR, Jia L, Goggin L (2005) Effects of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. J Chem Ecol 31: 1953–1967 [DOI] [PubMed] [Google Scholar]
- Dave A, Graham IA (2012) Oxylipin signaling: A distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front Plant Sci 3: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Engler J, De Vleesschauwer V, Burssens S, Celenza JL Jr., Inzé D, Van Montagu M, Engler G, Gheysen G (1999) Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11: 793–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meutter J, Tytgat T, Witters E, Gheysen G, Van Onckelen H, Gheysen G (2003) Identification of cytokinins produced by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita. Mol Plant Pathol 4: 271–277 [DOI] [PubMed] [Google Scholar]
- De Meutter J, Tytgat T, Prinsen E, Gheysen G, Van Onckelen H, Gheysen G (2005) Production of auxin and related compounds by the plant parasitic nematodes Heterodera schachtii and Meloidogyne incognita. Commun Agric Appl Biol Sci 70: 51–60 [PubMed] [Google Scholar]
- De Vleesschauwer D, Seifi HS, Filipe O, Haeck A, Huu SN, Demeestere K, Höfte M (2016) The DELLA protein SLR1 integrates and amplifies salicylic acid- and jasmonic acid-dependent innate immunity in rice. Plant Physiol 170: 1831–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mambro R, De Ruvo M, Pacifici E, Salvi E, Sozzani R, Benfey PN, Busch W, Novak O, Ljung K, Di Paola L, et al. (2017) Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proc Natl Acad Sci USA 114: E7641–E7649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djamei A, Schipper K, Rabe F, Ghosh A, Vincon V, Kahnt J, Osorio S, Tohge T, Fernie AR, Feussner I, et al. (2011) Metabolic priming by a secreted fungal effector. Nature 478: 395–398 [DOI] [PubMed] [Google Scholar]
- Dowd CD, Chronis D, Radakovic ZS, Siddique S, Schmülling T, Werner T, Kakimoto T, Grundler FMW, Mitchum MG (2017) Divergent expression of cytokinin biosynthesis, signaling and catabolism genes underlying differences in feeding sites induced by cyst and root-knot nematodes. Plant J 92: 211–228 [DOI] [PubMed] [Google Scholar]
- Doyle EA, Lambert KN (2003) Meloidogyne javanica chorismate mutase 1 alters plant cell development. Mol Plant Microbe Interact 16: 123–131 [DOI] [PubMed] [Google Scholar]
- Eves-Van Den Akker S, Lilley CJ, Yusup HB, Jones JT, Urwin PE (2016) Functional C-TERMINALLY ENCODED PEPTIDE (CEP) plant hormone domains evolved de novo in the plant parasite Rotylenchulus reniformis. Mol Plant Pathol 17: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JW, Hu CL, Zhang LN, Li ZL, Zhao FK, Wang SH (2015) Jasmonic acid mediates tomato’s response to root knot nematodes. J Plant Growth Regul 34: 196–205 [Google Scholar]
- Fudali SL, Wang C, Williamson VM (2013) Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol Plant Microbe Interact 26: 75–86 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Tomitaka Y, Abe H, Tsuda S, Futai K, Mizukubo T (2011) Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. J Plant Physiol 168: 1084–1097 [DOI] [PubMed] [Google Scholar]
- Gao B, Allen R, Maier T, Davis EL, Baum TJ, Hussey RS (2003) The parasitome of the phytonematode Heterodera glycines. Mol Plant Microbe Interact 16: 720–726 [DOI] [PubMed] [Google Scholar]
- Gao X, Starr J, Göbel C, Engelberth J, Feussner I, Tumlinson J, Kolomiets M (2008) Maize 9-lipoxygenase ZmLOX3 controls development, root-specific expression of defense genes, and resistance to root-knot nematodes. Mol Plant Microbe Interact 21: 98–109 [DOI] [PubMed] [Google Scholar]
- Gardner M, Dhroso A, Johnson N, Davis EL, Baum TJ, Korkin D, Mitchum MG (2018) Novel global effector mining from the transcriptome of early life stages of the soybean cyst nematode Heterodera glycines. Sci Rep 8: 2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo A, Kennedy MW, Bradley JE (2003) The FAR proteins of parasitic nematodes: Their possible involvement in the pathogenesis of infection and the use of Caenorhabditis elegans as a model system to evaluate their function. Med Microbiol Immunol (Berl) 192: 47–52 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu Rev Phytopathol 43: 205–227 [DOI] [PubMed] [Google Scholar]
- Glazer I, Orion D, Apelbaum A (1983) Interrelationships between ethylene production, gall formation, and root-knot nematode development in tomato plants infected with Meloidogyne javanica. J Nematol 15: 539–544 [PMC free article] [PubMed] [Google Scholar]
- Glazer I, Apelbaum A, Orion D (1985) Effect of inhibitors and stimulators of ethylene production on gall development in Meloidogyne javanica-infected tomato roots. J Nematol 17: 145–149 [PMC free article] [PubMed] [Google Scholar]
- Gleason C, Leelarasamee N, Meldau D, Feussner I (2016) OPDA has key role in regulating plant susceptibility to the root-knot nematode Meloidogyne hapla in Arabidopsis. Front Plant Sci 7: 1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad DM, Zhu C, Kellogg EA (2017) Comprehensive identification and clustering of CLV3/ESR-related (CLE) genes in plants finds groups with potentially shared function. New Phytol 216: 605–616 [DOI] [PubMed] [Google Scholar]
- Goverse A, Rouppe van der Voort J, Roppe van der Voort C, Kavelaars A, Smant G, Schots A, Bakker J, Helder J (1999) Naturally induced secretions of the potato cyst nematode co-stimulate the proliferation of both tobacco leaf protoplasts and human peripheral blood mononuclear cells. Mol Plant Microbe Interact 12: 872–881 [DOI] [PubMed] [Google Scholar]
- Goverse A, Overmars H, Engelbertink J, Schots A, Bakker J, Helder J (2000) Both induction and morphogenesis of cyst nematode feeding cells are mediated by auxin. Mol Plant Microbe Interact 13: 1121–1129 [DOI] [PubMed] [Google Scholar]
- Grunewald W, Karimi M, Wieczorek K, Van de Cappelle E, Wischnitzki E, Grundler F, Inzé D, Beeckman T, Gheysen G (2008) A role for AtWRKY23 in feeding site establishment of plant-parasitic nematodes. Plant Physiol 148: 358–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, Cannoot B, Friml J, Gheysen G (2009a) Parasitic nematodes modulate PIN-mediated auxin transport to facilitate infection. PLoS Pathog 5: e1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald W, van Noorden G, Van Isterdael G, Beeckman T, Gheysen G, Mathesius U (2009b) Manipulation of auxin transport in plant roots during Rhizobium symbiosis and nematode parasitism. Plant Cell 21: 2553–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Chronis D, De La Torre CM, Smeda J, Wang X, Mitchum MG (2015) Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol J 13: 801–810 [DOI] [PubMed] [Google Scholar]
- Guo X, Wang J, Gardner M, Fukuda H, Kondo Y, Etchells JP, Wang X, Mitchum MG (2017) Identification of cyst nematode B-type CLE peptides and modulation of the vascular stem cell pathway for feeding cell formation. PLoS Pathog 13: e1006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Han L, Hymes M, Denver R, Clark SE (2010) CLAVATA2 forms a distinct CLE-binding receptor complex regulating Arabidopsis stem cell specification. Plant J 63: 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Ni J, Denver R, Wang X, Clark SE (2011) Mechanisms of molecular mimicry of plant CLE peptide ligands by the parasitic nematode Globodera rostochiensis. Plant Physiol 157: 476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutjahr C, Paszkowski U (2009) Weights in the balance: Jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol Plant Microbe Interact 22: 763–772 [DOI] [PubMed] [Google Scholar]
- Habash SS, Radakovic ZS, Vankova R, Siddique S, Dobrev P, Gleason C, Grundler FMW, Elashry A (2017) Heterodera schachtii Tyrosinase-like protein—A novel nematode effector modulating plant hormone homeostasis. Sci Rep 7: 6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman A, Joseph S, Gheysen G (2011) Analysis of the transcriptome of the root lesion nematode Pratylenchus coffeae generated by 454 sequencing technology. Mol Biochem Parasitol 178: 7–14 [DOI] [PubMed] [Google Scholar]
- Haegeman A, Mantelin S, Jones JT, Gheysen G (2012) Functional roles of effectors of plant-parasitic nematodes. Gene 492: 19–31 [DOI] [PubMed] [Google Scholar]
- Hewezi T, Piya S, Richard G, Rice JH (2014) Spatial and temporal expression patterns of auxin response transcription factors in the syncytium induced by the beet cyst nematode Heterodera schachtii in Arabidopsis. Mol Plant Pathol 15: 730–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewezi T, Juvale PS, Piya S, Maier TR, Rambani A, Rice JH, Mitchum MG, Davis EL, Hussey RS, Baum TJ (2015) The cyst nematode effector protein 10A07 targets and recruits host posttranslational machinery to mediate its nuclear trafficking and to promote parasitism in Arabidopsis. Plant Cell 27: 891–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, You J, Li C, Hua C, Wang C (2017a) Exogenous application of methyl jasmonate induces defence against Meloidogyne hapla in soybean. Nematology 19: 293–304 [Google Scholar]
- Hu Y, You J, Li C, Williamson VM, Wang C (2017b) Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci Rep 7: 41282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Dong R, Allen R, Davis EL, Baum TJ, Hussey RS (2005) Two chorismate mutase genes from the root-knot nematode Meloidogyne incognita. Mol Plant Pathol 6: 23–30 [DOI] [PubMed] [Google Scholar]
- Iberkleid I, Vieira P, de Almeida Engler J, Firester K, Spiegel Y, Horowitz SB (2013) Fatty acid-and retinol-binding protein, Mj-FAR-1 induces tomato host susceptibility to root-knot nematodes. PLoS One 8: e64586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iberkleid I, Sela N, Brown Miyara S (2015) Meloidogyne javanica fatty acid- and retinol-binding protein (Mj-FAR-1) regulates expression of lipid-, cell wall-, stress- and phenylpropanoid-related genes during nematode infection of tomato. BMC Genomics 16: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ, Mitchum MG (2007) Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol Plant Microbe Interact 20: 510–525 [DOI] [PubMed] [Google Scholar]
- Ji H, Gheysen G, Denil S, Lindsey K, Topping JF, Nahar K, Haegeman A, De Vos WH, Trooskens G, Van Criekinge W, et al. (2013) Transcriptional analysis through RNA sequencing of giant cells induced by Meloidogyne graminicola in rice roots. J Exp Bot 64: 3885–3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JT, Furlanetto C, Bakker E, Banks B, Blok V, Chen Q, Phillips M, Prior A (2003) Characterization of a chorismate mutase from the potato cyst nematode Globodera pallida. Mol Plant Pathol 4: 43–50 [DOI] [PubMed] [Google Scholar]
- Jones JT, Haegeman A, Danchin EG, Gaur HS, Helder J, Jones MG, Kikuchi T, Manzanilla-López R, Palomares-Rius JE, Wesemael WM, et al. (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol 14: 946–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerhofer N, Radakovic Z, Regis JM, Dobrev P, Vankova R, Grundler FM, Siddique S, Hofmann J, Wieczorek K (2015) Role of stress-related hormones in plant defence during early infection of the cyst nematode Heterodera schachtii in Arabidopsis. New Phytol 207: 778–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczmarek A, Overmars H, Helder J, Goverse A (2004) Feeding cell development by cyst and root-knot nematodes involves a similar early, local and transient activation of a specific auxin-inducible promoter element. Mol Plant Pathol 5: 343–346 [DOI] [PubMed] [Google Scholar]
- Kim J, Yang R, Chang C, Park Y, Tucker ML (2018) The root-knot nematode Meloidogyne incognita produces a functional mimic of the Arabidopsis INFLORESCENCE DEFICIENT IN ABSCISSION signaling peptide. J Exp Bot 69: 3009–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Hirakawa Y, Kieber JJ, Fukuda H (2011) CLE peptides can negatively regulate protoxylem vessel formation via cytokinin signaling. Plant Cell Physiol 52: 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo Y, Ito T, Nakagami H, Hirakawa Y, Saito M, Tamaki T, Shirasu K, Fukuda H (2014) Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signalling. Nat Commun 5: 3504. [DOI] [PubMed] [Google Scholar]
- Kumari C, Dutta TK, Banakar P, Rao U (2016) Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci Rep 6: 22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpf RP, Shi CL, Larrieu A, Stø IM, Butenko MA, Péret B, Riiser ES, Bennett MJ, Aalen RB (2013) Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proc Natl Acad Sci USA 110: 5235–5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Goverse A, Haegeman A, Warmerdam S, Wanjau C, Jahani M, Engler G, de Almeida Engler J, Gheysen G (2016) Redirection of auxin flow in Arabidopsis thaliana roots after infection by root-knot nematodes. J Exp Bot 67: 4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyndt T, Nahar K, Haeck A, Verbeek R, Demeestere K, Gheysen G (2017) Interplay between carotenoids, abscisic acid and jasmonate guides the compatible rice-Meloidogyne graminicola interaction. Front Plant Sci 8: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahari Z, Ullah C, Kyndt T, Gershenzon J, Gheysen G (2018) Strigolactone deficiency reduces root-knot nematode Meloidogyne graminicola infection in rice by enhancing the jasmonate pathway. Abstract. The Plant Biology meeting 2018, July 14–18 in Montreal. [Google Scholar]
- Lambert KN, Allen KD, Sussex IM (1999) Cloning and characterization of an esophageal-gland-specific chorismate mutase from the phytoparasitic nematode Meloidogyne javanica. Mol Plant Microbe Interact 12: 328–336 [DOI] [PubMed] [Google Scholar]
- Lee C, Chronis D, Kenning C, Peret B, Hewezi T, Davis EL, Baum TJ, Hussey R, Bennett M, Mitchum MG (2011) The novel cyst nematode effector protein 19C07 interacts with the Arabidopsis auxin influx transporter LAX3 to control feeding site development. Plant Physiol 155: 866–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Mazarei M, Zhao N, Zhu JJ, Zhuang X, Liu W, Pantalone VR, Arelli PR, Stewart CN Jr., Chen F (2013) Overexpression of a soybean salicylic acid methyltransferase gene confers resistance to soybean cyst nematode. Plant Biotechnol J 11: 1135–1145 [DOI] [PubMed] [Google Scholar]
- Lin J, Wang D, Chen X, Köllner TG, Mazarei M, Guo H, Pantalone VR, Arelli P, Stewart CN Jr., Wang N, et al. (2017) An (E,E)-α-farnesene synthase gene of soybean has a role in defence against nematodes and is involved in synthesizing insect-induced volatiles. Plant Biotechnol J 15: 510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Song T, Zhang X, Yuan H, Su L, Li W, Xu J, Liu S, Chen L, Chen T, et al. (2014) Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat Commun 5: 4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J 38: 203–214 [DOI] [PubMed] [Google Scholar]
- Long H, Wang X, Xu J (2006a) Molecular cloning and life-stage expression pattern of a new chorismate mutase gene from the root-knot nematode Meloidogyne arenaria. Plant Pathol 55: 559–563 [Google Scholar]
- Long H, Wang X, Xu JH, Hu YJ (2006b) Isolation and characterization of another cDNA encoding a chorismate mutase from the phytoparasitic nematode Meloidogyne arenaria. Exp Parasitol 113: 106–111 [DOI] [PubMed] [Google Scholar]
- Lu SW, Tian D, Borchardt-Wier HB, Wang X (2008) Alternative splicing: A novel mechanism of regulation identified in the chorismate mutase gene of the potato cyst nematode Globodera rostochiensis. Mol Biochem Parasitol 162: 1–15 [DOI] [PubMed] [Google Scholar]
- Lu SW, Chen S, Wang J, Yu H, Chronis D, Mitchum MG, Wang X (2009) Structural and functional diversity of CLAVATA3/ESR (CLE)-like genes from the potato cyst nematode Globodera rostochiensis. Mol Plant Microbe Interact 22: 1128–1142 [DOI] [PubMed] [Google Scholar]
- Majda M, Robert S (2018) The role of auxin in cell wall expansion. Int J Mol Sci 19: 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantelin S, Bhattarai KK, Jhaveri TZ, Kaloshian I (2013) Mi-1-mediated resistance to Meloidogyne incognita in tomato may not rely on ethylene but hormone perception through ETR3 participates in limiting nematode infection in a susceptible host. PLoS One 8: e63281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masachis S, Segorbe D, Turrà D, Leon-Ruiz M, Fürst U, El Ghalid M, Leonard G, López-Berges MS, Richards TA, Felix G, et al. (2016) A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol 1: 16043. [DOI] [PubMed] [Google Scholar]
- Mitchum MG, Wang X, Wang J, Davis EL (2012) Role of nematode peptides and other small molecules in plant parasitism. Annu Rev Phytopathol 50: 175–195 [DOI] [PubMed] [Google Scholar]
- Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M, Davis EL (2013) Nematode effector proteins: An emerging paradigm of parasitism. New Phytol 199: 879–894 [DOI] [PubMed] [Google Scholar]
- Molinari S. (2016) Systemic acquired resistance activation in solanaceous crops as a management strategy against root-knot nematodes. Pest Manag Sci 72: 888–896 [DOI] [PubMed] [Google Scholar]
- Molinari S, Fanelli E, Leonetti P (2014) Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol Plant Pathol 15: 255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi MR. (2017) The effect of gibberellin and abscisic acid on plant defense responses and on disease severity caused by Meloidogyne javanica on tomato plants. J Gen Plant Pathol 83: 173–184 [Google Scholar]
- Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006) The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140: 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Kyndt T, De Vleesschauwer D, Höfte M, Gheysen G (2011) The jasmonate pathway is a key player in systemically induced defense against root knot nematodes in rice. Plant Physiol 157: 305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K, Kyndt T, Hause B, Höfte M, Gheysen G (2013) Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Mol Plant Microbe Interact 26: 106–115 [DOI] [PubMed] [Google Scholar]
- Naor N, Gurung FB, Ozalvo R, Bucki P, Sanadhya P, Brown Miyara S (2018) Tight regulation of allene oxide synthase (AOS) and allene oxide cyclase-3 (AOC3) promote Arabidopsis susceptibility to the root-knot nematode Meloidogyne javanica. Eur J Plant Pathol 150: 149–165 [Google Scholar]
- Navarro L, Bari R, Achard P, Lisón P, Nemri A, Harberd NP, Jones JDG (2008) DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol 18: 650–655 [DOI] [PubMed] [Google Scholar]
- Nguyen PDT, Pike S, Wang J, Nepal Poudel A, Heinz R, Schultz JC, Koo AJ, Mitchum MG, Appel HM, Gassmann W (2016) The Arabidopsis immune regulator SRFR1 dampens defences against herbivory by Spodoptera exigua and parasitism by Heterodera schachtii. Mol Plant Pathol 17: 588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockland S, Maafi ZT (2011) Current nematode threats to world agriculture. In Jones JT, Gheysen G, Fenoll C, eds, Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer, Heidelberg, pp 21–43 [Google Scholar]
- Opperman CH, Bird DM, Williamson VM, Rokhsar DS, Burke M, Cohn J, Cromer J, Diener S, Gajan J, Graham S, et al. (2008) Sequence and genetic map of Meloidogyne hapla: A compact nematode genome for plant parasitism. Proc Natl Acad Sci USA 105: 14802–14807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozalvo R, Cabrera J, Escobar C, Christensen SA, Borrego EJ, Kolomiets MV, Castresana C, Iberkleid I, Brown Horowitz S (2014) Two closely related members of Arabidopsis 13-lipoxygenases (13-LOXs), LOX3 and LOX4, reveal distinct functions in response to early developmental steps of nematode feeding cells. Mol Plant Pathol 15: 319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, Válková M, Malbeck J, Souček P, Reichman P, Hoyerová K, Dubová J, Friml J, et al. (2009) Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux. Proc Natl Acad Sci USA 106: 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Rechenmann C. (2010) Cellular responses to auxin: Division versus expansion. Cold Spring Harb Perspect Biol 2: a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piya S, Binder BM, Hewezi T (2018) Canonical and noncanonical ethylene signaling pathways that regulate Arabidopsis susceptibility to the cyst nematode Heterodera schachtii. New Phytol 10.1111/nph.15400 [DOI] [PubMed] [Google Scholar]
- Prior A, Jones JT, Blok VC, Beauchamp J, McDermott L, Cooper A, Kennedy MW (2001) A surface-associated retinol- and fatty acid-binding protein (Gp-FAR-1) from the potato cyst nematode Globodera pallida: Lipid binding activities, structural analysis and expression pattern. Biochem J 356: 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya DB, Somasekhar N, Prasad J, Kirti P (2011) Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root-knot nematode, Meloidogyne incognita. BMC Res Notes 4: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt RN, Joe A, Zhang W, Feng W, Stewart V, Schwessinger B, Dinneny JR, Ronald PC (2017) A microbially derived tyrosine-sulfated peptide mimics a plant peptide hormone. New Phytol 215: 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replogle A, Wang J, Bleckmann A, Hussey RS, Baum TJ, Sawa S, Davis EL, Wang X, Simon R, Mitchum MG (2011) Nematode CLE signaling in Arabidopsis requires CLAVATA2 and CORYNE. Plant J 65: 430–440 [DOI] [PubMed] [Google Scholar]
- Replogle A, Wang J, Paolillo V, Smeda J, Kinoshita A, Durbak A, Tax FE, Wang X, Sawa S, Mitchum MG (2013) Synergistic interaction of CLAVATA1, CLAVATA2, and RECEPTOR-LIKE PROTEIN KINASE 2 in cyst nematode parasitism of Arabidopsis. Mol Plant Microbe Interact 26: 87–96 [DOI] [PubMed] [Google Scholar]
- Ronald P, Joe A (2018) Molecular mimicry modulates plant host responses to pathogens. Ann Bot 121: 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Alférez S, Mateos B, Alvarado R, Sánchez-Muñoz M (2008) SAR induction in tomato plants is not effective against root-knot nematode infection. Eur J Plant Pathol 120: 417–425 [Google Scholar]
- Schaller GE, Street IH, Kieber JJ (2014) Cytokinin and the cell cycle. Curr Opin Plant Biol 21: 7–15 [DOI] [PubMed] [Google Scholar]
- Shanks CM, Rice JH, Zubo Y, Schaller GE, Hewezi T, Kieber JJ (2016) The role of cytokinin during infection of Arabidopsis thaliana by the cyst nematode Heterodera schachtii. Mol Plant Microbe Interact 29: 57–68 [DOI] [PubMed] [Google Scholar]
- Shimizu MM, Mazzafera P (2007) Polyphenoloxidase is induced by methyljasmonate and Meloidogyne javanica in soybean roots but is not involved in resistance. Nematology 9: 625–634 [Google Scholar]
- Shukla N, Yadav R, Kaur P, Rasmussen S, Goel S, Agarwal M, Jagannath A, Gupta R, Kumar A (2018) Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-infected tomato (Solanum lycopersicum) roots reveals complex gene expression profiles and metabolic networks of both host and nematode during susceptible and resistance responses. Mol Plant Pathol 19: 615–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique S, Radakovic ZS, De La Torre CM, Chronis D, Novák O, Ramireddy E, Holbein J, Matera C, Hütten M, Gutbrod P, et al. (2015) A parasitic nematode releases cytokinin that controls cell division and orchestrates feeding site formation in host plants. Proc Natl Acad Sci USA 112: 12669–12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidonskaya E, Schweighofer A, Shubchynskyy V, Kammerhofer N, Hofmann J, Wieczorek K, Meskiene I (2016) Plant resistance against the parasitic nematode Heterodera schachtii is mediated by MPK3 and MPK6 kinases, which are controlled by the MAPK phosphatase AP2C1 in Arabidopsis. J Exp Bot 67: 107–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smant G, Helder J, Goverse A (2018) Parallel adaptations and common host cell responses enabling feeding of obligate and facultative plant parasitic nematodes. Plant J 93: 686–702 [DOI] [PubMed] [Google Scholar]
- Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64: 874–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yin J, Cao H, Li C, Kang L, Ge F (2011) Elevated CO2 influences nematode-induced defense responses of tomato genotypes differing in the JA pathway. PLoS One 6: e19751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski M, Imin N, Djordjevic MA (2018) CEP peptide hormones: Key players in orchestrating nitrogen-demand signalling, root nodulation, and lateral root development. J Exp Bot 69: 1829–1836 [DOI] [PubMed] [Google Scholar]
- Tucker ML, Yang R (2013) A gene encoding a peptide with similarity to the plant IDA signaling peptide (AtIDA) is expressed most abundantly in the root-knot nematode (Meloidogyne incognita) soon after root infection. Exp Parasitol 134: 165–170 [DOI] [PubMed] [Google Scholar]
- Uehara T, Sugiyama S, Matsuura H, Arie T, Masuta C (2010) Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol 51: 1524–1536 [DOI] [PubMed] [Google Scholar]
- Vanholme B, Kast P, Haegeman A, Jacob J, Grunewald W, Gheysen G (2009) Structural and functional investigation of a secreted chorismate mutase from the plant-parasitic nematode Heterodera schachtii in the context of related enzymes from diverse origins. Mol Plant Pathol 10: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vie AK, Najafi J, Winge P, Cattan E, Wrzaczek M, Kangasjärvi J, Miller G, Brembu T, Bones AM (2017) The IDA-LIKE peptides IDL6 and IDL7 are negative modulators of stress responses in Arabidopsis thaliana. J Exp Bot 68: 3557–3571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Dos Santos MC, Curtis RHC, Abrantes I (2014) The combined use of Pochonia chlamydosporia and plant defence activators—A potential sustainable control strategy for Meloidogyne chitwoodi. Phytopathol Mediterr 53: 66–74 [Google Scholar]
- Vieten A, Sauer M, Brewer PB, Friml J (2007) Molecular and cellular aspects of auxin-transport-mediated development. Trends Plant Sci 12: 160–168 [DOI] [PubMed] [Google Scholar]
- Wang G, Zhang G, Wu M (2016) CLE peptide signaling and crosstalk with phytohormones and environmental stimuli. Front Plant Sci 6: 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Joshi S, Korkin D, Mitchum MG (2010b) Variable domain I of nematode CLEs directs post-translational targeting of CLE peptides to the extracellular space. Plant Signal Behav 5: 1633–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lee C, Replogle A, Joshi S, Korkin D, Hussey R, Baum TJ, Davis EL, Wang X, Mitchum MG (2010a) Dual roles for the variable domain in protein trafficking and host-specific recognition of Heterodera glycines CLE effector proteins. New Phytol 187: 1003–1017 [DOI] [PubMed] [Google Scholar]
- Wang J, Replogle A, Hussey R, Baum T, Wang X, Davis EL, Mitchum MG (2011) Identification of potential host plant mimics of CLV3/ESR (CLE)-like peptides from the plant-parasitic nematode Heterodera schachtii. Mol Plant Pathol 12: 177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Allen R, Ding X, Goellner M, Maier T, de Boer JM, Baum TJ, Hussey RS, Davis EL (2001) Signal peptide-selection of cDNA cloned directly from the esophageal gland cells of the soybean cyst nematode Heterodera glycines. Mol Plant Microbe Interact 14: 536–544 [DOI] [PubMed] [Google Scholar]
- Wang X, Mitchum MG, Gao B, Li C, Diab H, Baum TJ, Hussey RS, Davis EL (2005) A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol Plant Pathol 6: 187–191 [DOI] [PubMed] [Google Scholar]
- Wang X, Hou S, Wu Q, Lin M, Acharya BR, Wu D, Zhang W (2017) IDL6-HAE/HSL2 impacts pectin degradation and resistance to Pseudomonas syringae pv tomato DC3000 in Arabidopsis leaves. Plant J 89: 250–263 [DOI] [PubMed] [Google Scholar]
- Wang X, Xue B, Dai J, Qin X, Liu L, Chi Y, Jones JT, Li H (2018) A novel Meloidogyne incognita chorismate mutase effector suppresses plant immunity by manipulating the salicylic acid pathway and functions mainly during the early stages of nematode parasitism. Plant Pathol 67: 1436–1448 [Google Scholar]
- Whitford R, Fernandez A, De Groodt R, Ortega E, Hilson P (2008) Plant CLE peptides from two distinct functional classes synergistically induce division of vascular cells. Proc Natl Acad Sci USA 105: 18625–18630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubben MJE II, Su H, Rodermel SR, Baum TJ (2001) Susceptibility to the sugar beet cyst nematode is modulated by ethylene signal transduction in Arabidopsis thaliana. Mol Plant Microbe Interact 14: 1206–1212 [DOI] [PubMed] [Google Scholar]
- Wubben MJE, Jin J, Baum TJ (2008) Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA-independent pathogenesis-related gene expression in roots. Mol Plant Microbe Interact 21: 424–432 [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Callahan FE, Scheffler BS (2010) Transcript analysis of parasitic females of the sedentary semi-endoparasitic nematode Rotylenchulus reniformis. Mol Biochem Parasitol 172: 31–40 [DOI] [PubMed] [Google Scholar]
- Wubben MJ, Gavilano L, Baum TJ, Davis EL (2015) Sequence and spatiotemporal expression analysis of CLE-motif containing genes from the reniform nematode (Rotylenchulus reniformis Linford & Oliveira). J Nematol 47: 159–165 [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi YL, Ishida T, Sawa S (2016) CLE peptides and their signaling pathways in plant development. J Exp Bot 67: 4813–4826 [DOI] [PubMed] [Google Scholar]
- Yamaguchi YL, Suzuki R, Cabrera J, Nakagami S, Sagara T, Ejima C, Sano R, Aoki Y, Olmo R, Kurata T, et al. (2017) Root-knot and cyst nematodes activate procambium-associated genes in Arabidopsis roots. Front Plant Sci 8: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimer HZ, Nahar K, Kyndt T, Haeck A, Van Meulebroek L, Vanhaecke L, Demeestere K, Höfte M, Gheysen G (2018) Gibberellin antagonizes jasmonate-induced defense against Meloidogyne graminicola in rice. New Phytol 218: 646–660 [DOI] [PubMed] [Google Scholar]
- Youssef RM, MacDonald MH, Brewer EP, Bauchan GR, Kim KH, Matthews BF (2013) Ectopic expression of AtPAD4 broadens resistance of soybean to soybean cyst and root-knot nematodes. BMC Plant Biol 13: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Chronis D, Lu SW, Wang XH (2011) Chorismate mutase: An alternatively spliced parasitism gene and a diagnostic marker for three important Globodera nematode species. Eur J Plant Pathol 129: 89–102 [Google Scholar]
- Yuan J, Bateman P, Gutierrez-Marcos J (2016) Genetic and epigenetic control of transfer cell development in plants. J Genet Genomics 43: 533–539 [DOI] [PubMed] [Google Scholar]
- Zhang L-N, Cheng J-H, Yang R, Sun Z-H, Wu C-X, Wang S-H (2011) Effects of JA synthesis-related genes Spr2 and LePrs on the resistance to root-knot nematodes in tomato. Zhongguo Nong Ye Ke Xue 44: 4022–4028 [Google Scholar]
- Zhao W, Li Z, Fan J, Hu C, Yang R, Qi X, Chen H, Zhao F, Wang S (2015) Identification of jasmonic acid-associated microRNAs and characterization of the regulatory roles of the miR319/TCP4 module under root-knot nematode stress in tomato. J Exp Bot 66: 4653–4667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jia F, Shao S, Zhang H, Li G, Xia X, Zhou Y, Yu J, Shi K (2015) Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front Plant Sci 6: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinovieva SV, Vasyukova NI, Udalova ZhV, Gerasimova NG (2013) The participation of salicylic and jasmonic acids in genetic and induced resistance of tomato to Meloidogyne incognita (Kofoid and White, 1919). Biol Bull 40: 297–303 [PubMed] [Google Scholar]