Plant leaves optimize the quantity of PSI to maintain P700 oxidation to protect PSI against photo-oxidative damage.
Abstract
PSI has the potential to generate reactive oxygen species and be oxidatively inactivated by the reactive oxygen species. The photo-oxidative damage of PSI (also called PSI photoinhibition) causes the inhibition of the plant growth and is a lethal event for plants. It has been reported that PSI photoinhibition does not occur as long as the reaction-center chlorophyll (P700) remains oxidized, even in excess light conditions. This process is termed P700 oxidation and is supported by various regulatory mechanisms and likely also by the stoichiometric quantities of photosynthetic apparatus. In this study, we assessed how decreased photochemically active PSI in Arabidopsis (Arabidopsis thaliana) affected a variety of photosynthetic parameters, including P700 oxidation. Inactivation of PSI was rapidly and selectively induced by repetitive short-pulse illumination. PSI photoinhibition correlated linearly with decreases in effective quantum yield of PSII and nonphotochemical quenching; however, the photosynthetic CO2 assimilation rate was less affected, as exemplified by ∼50% of the normal CO2 assimilation rate maintained with an 80% loss in PSI photochemical activity. In contrast, effective quantum yield of PSI was enhanced following PSI photoinhibition, mainly owing to a decrease in the electron donor-side limitation of PSI. Based on these results, we propose that the stoichiometric quantity of PSI is optimized to induce P700 oxidation for dissipating excess light energy in PSI, thus avoiding inhibition of photosynthetic CO2 assimilation caused by PSI photoinhibition.
Plants carry out photosynthesis, at the risk of photo-oxidative damage, by converting the energy from sunlight to reducing power in the photosynthetic electron transport system, which begins with photo-excitation of the reaction-center chlorophylls in PSII and PSI, i.e. P680 and P700, respectively, in the thylakoid membrane in chloroplasts. Electrons originating from the oxidation of water on the luminal side of PSII are transported into PSI via the plastoquinone (PQ) pool, the cytochrome b6/f complex (Cyt b6/f), and plastocyanin, accompanied by the formation of a proton gradient across the thylakoid membrane, to produce ATP. Photo-excited P700 gives electrons to the acceptor side of PSI and finally to NADP+. Both ATP and NADPH are consumed in the Calvin-Benson cycle for photosynthetic CO2 assimilation. However, the production and consumption of reducing power is easily unbalanced because of various environmental fluctuations. If photo-excitation energy originating from P700 is more than the demand for NADPH production, it can cause reactive oxygen species–derived photo-oxidative damage to PSI (Terashima et al., 1994; Sejima et al., 2014). Once reactive oxygen species are generated, PSI is rapidly inactivated and thereafter takes days or even weeks to recover completely, thus inhibiting photosynthesis and growth of oxygenic photoautotrophs (Zivcak et al., 2015a; Shimakawa et al., 2016). This photo-oxidative damage to PSI is termed PSI photoinhibition and is recognized as a lethal event for oxygenic photoautotrophs.
Generally, PSI photoinhibition rarely occurs because plants suppress excessive photo-excitation of P700 by keeping it in its oxidized form that functions as the quencher of light energy (Foyer et al., 1990; Klughammer and Schreiber, 1994; Trissl, 1997; Bukhov and Carpentier, 2003; Golding and Johnson, 2003; Miyake et al., 2005). This is the mechanism by which oxygenic photoautotrophs safely carry out photosynthesis under the sun. Oxidation of P700 is controlled by various molecular mechanisms, known collectively as the P700 oxidation system (Shimakawa et al., 2017; Takagi et al., 2017; Shimakawa and Miyake, 2018). Conversely, it is supposed that P700 can be easily kept oxidized, even without any regulatory mechanisms, due to the stoichiometric quantities of photosynthetic apparatus, including PSII, Cyt b6/f, and PSI. The stoichiometric quantity (mol per leaf area) of Cyt b6/f is modulated and kept lower than those of PSII and PSI in plant leaves (Yamori et al., 2011; Schöttler and Tóth, 2014), which is one of the reasons why the electron transport in Cyt b6/f is usually recognized as the limiting step in the photosynthetic electron transport system (Anderson, 1992; Schöttler et al., 2015). Oxidized P700 is reduced with electrons predominantly from PSII, which clearly suggests that PSII quantity larger than that of PSI potentially impairs the maintenance of P700 in an oxidized state. Indeed, Bukhov et al. (2004) observed a faster reduction rate of oxidized P700 in those plant leaves in which PSI photoinhibition occurred under chilling stress (Bukhov et al., 2004). Additionally, a barley mutant having a decreased amount of PSII showed higher P700 oxidation than that of the wild type (Pfündel et al., 2008), whereas a chlorophyll b–deficient mutant showing a low PSI/PSII ratio was impaired in P700 oxidation (Brestic et al., 2015). Furthermore, PSI photoinhibition was reported in a mutant that contained a lower quantity of PSI than that of PSII (Ivanov et al., 2015). These facts imply that the stoichiometric quantity of PSI is optimized to induce P700 oxidation to protect PSI from photoinhibition and ensure robust photosynthesis. In this study, we sought to characterize the effects of quantitative decrease of the photochemically active PSI on a variety of photosynthetic parameters using the intact leaves of the Arabidopsis wild type.
RESULTS
Relationship between the Quantity of the Photochemically Active PSI and Photosynthetic Parameters of PSI in Arabidopsis
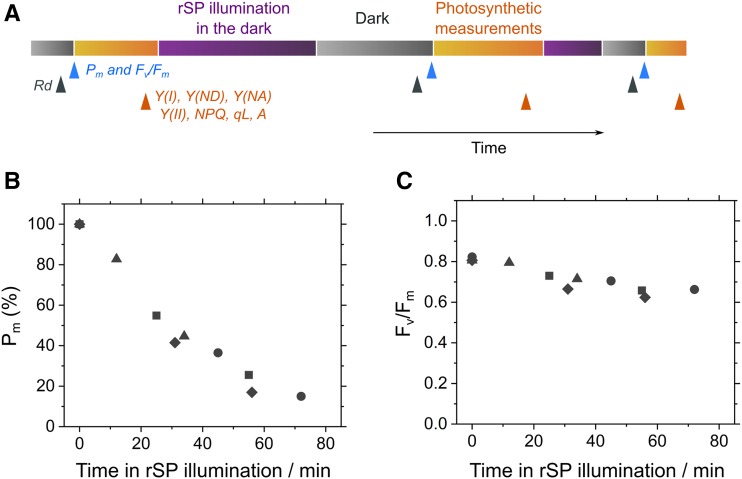
For experimentally modifying the quantity of photochemically active PSI in the plant leaves, we used the method “repetitive short-pulse (rSP) illumination,” which facilitates rapid and selective induction of PSI photoinhibition in the plant leaves in vivo. For this method, rSP (300 ms, 20,000 µmol photon m−2 s−1, every 10 s) is applied to the plant leaves of angiosperms in darkness, which instantaneously fills the photosynthetic electron transport system with electrons in the absence of stimulus for any regulatory mechanism of P700 oxidation (Sejima et al., 2014; Zivcak et al., 2015a, 2015b; Kono et al., 2017; Takagi et al., 2017; Shimakawa et al., 2019; Tikkanen and Grebe, 2018). Using rSP illumination, we created plant leaves partially impaired in PSI and evaluated the relationship between the quantity of photochemically active PSI with various photosynthetic parameters in Arabidopsis, as follows.
In Arabidopsis, rSP illumination caused PSI photoinhibition (Fig. 1). The quantity of photochemically active PSI was spectroscopically evaluated in vivo as the total quantity of photo-oxidizable P700 (Pm) before and after rSP illumination (Fig. 1A; Klughammer and Schreiber, 1994). In the leaves of Arabidopsis wild-type plants, Pm decreased to ∼20% of the intact value after rSP illumination in the dark (300 ms, 20,000 μmol photons m−2 s−1, every 10 s) for 1 h in ambient air (Fig. 1B). In contrast, maximum quantum efficiency of PSII photochemistry (Fv/Fm) was sustained, i.e. ∼80% of the intact value, even after rSP illumination (Fig. 1C), indicating that the method selectively induces inactivation of PSI in Arabidopsis like in other angiosperms (Sejima et al., 2014). After rSP illumination, we incubated the plants in darkness for 30 min so as to relax qE quenching and state transition (Baker, 2008) before the determination of Pm and Fv/Fm (Fig. 1A).
Figure 1.
Effects of PSI photoinhibition on the photochemical activities of PSI and PSII. A, Schematic representation of the experiment to induce PSI photoinhibition. Repetitive short-pulse (rSP) illumination (300 ms, 20,000 µmol photons m−2 s−1, every 10 s) was applied to Arabidopsis leaves in the dark as indicated by purple bars. Total oxidizable P700 (Pm), maximum quantum efficiency of PSII photochemistry (Fv/Fm), and dark respiration rate (Rd) were periodically determined after a 30-min dark incubation (gray bars), with the timing shown by blue (Pm and Fv/Fm) and black (Rd) arrows, and thereafter a variety of photosynthetic parameters were measured in the light (orange bars), with the timing indicated by orange arrows. The abbreviations of photosynthetic parameters were defined as follows: Y(I), effective quantum yield of PSI; Y(ND) and Y(NA), quantum yield of nonphotochemical energy dissipation of PSI due to the donor- and acceptor-side limitation; Y(II), effective quantum yield of PSII; NPQ, nonphotochemical quenching; qL, inferred oxidation level of PQ pool; and A, net photosynthetic CO2 assimilation rate. B, Time course of Pm during rSP illumination. C, Time course of Fv/Fm during rSP illumination. Data are independent measurements, with quadruple replicates indicated by circles, triangles, squares, and diamonds.
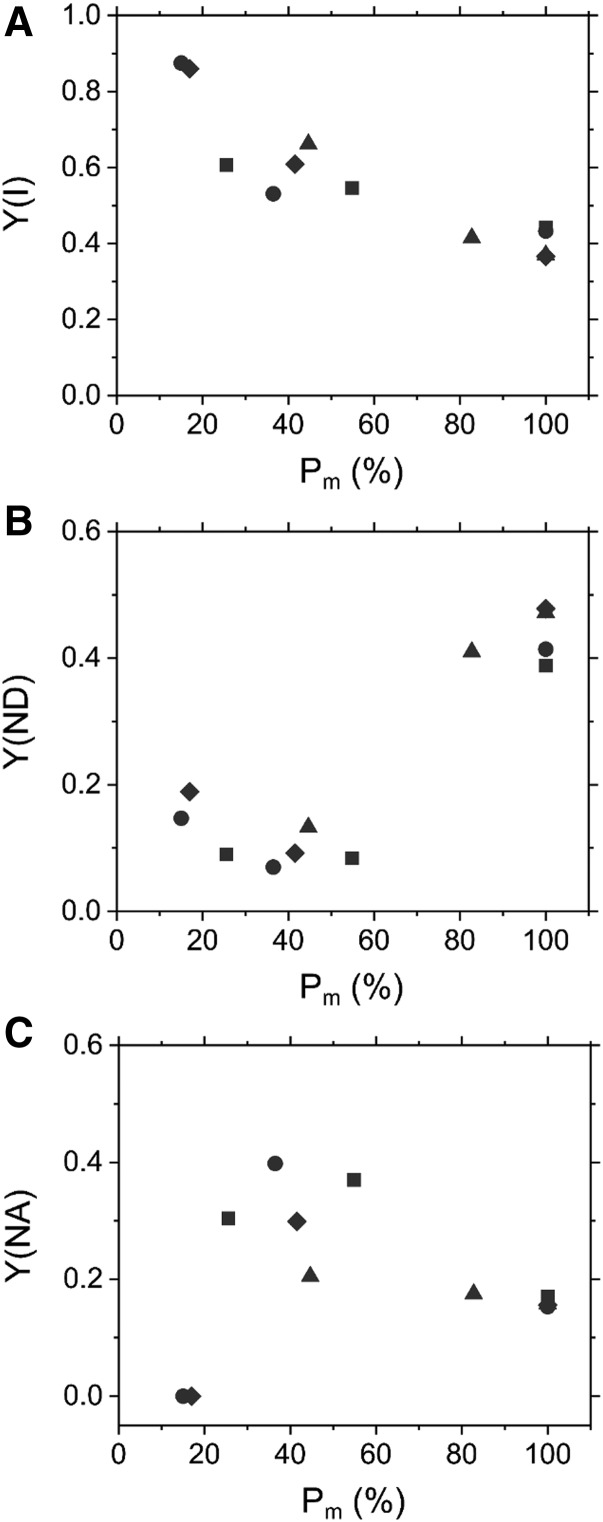
Photosynthetic parameters of PSI were measured in Arabidopsis at a steady state of photosynthesis under red actinic light (820 μmol photons m−2 s−1) after the determination of Pm and Fv/Fm (Fig. 1A). Commonly in C3 plants, photorespiration functions as a large alternative electron sink (Badger et al., 2000; Driever and Baker, 2011; Hanawa et al., 2017), likely affecting a variety of photosynthetic parameters. In this study, the measurements of photosynthetic parameters were performed under high CO2 conditions (21 kPa O2, 100 Pa CO2) to exclude the effects of photorespiration on these parameters. With a 80% loss of PSI photochemical activity, effective quantum yield of PSI [Y(I)] increased from 0.4 to 0.9 (Fig. 2A). The photochemical reaction in PSI is limited at both electron donor and acceptor-sides. Quantum yield of nonphotochemical energy dissipation due to donor-side limitation [Y(ND)] indicates the ratio of oxidized P700 to total photo-oxidizable P700; that is, Y(ND) is the indicator of P700 oxidation. At the steady state of photosynthesis, in intact leaves of Arabidopsis under actinic light, Y(ND) was calculated as 0.5, which reflects that 50% of P700 remained oxidized (Fig. 2B). However, Y(ND) dramatically decreased from 0.5 to 0.1 with a decrease in Pm from 100% to 60% (Fig. 2B). These data suggest that PSI photoinhibition in this range prevented oxidation of P700. Additionally, we plotted quantum yield of nonphotochemical energy dissipation due to acceptor-side limitation [Y(NA)] against Pm. When Pm decreased from 100% to 60%, Y(NA) increased with the inactivation of PSI (Fig. 2C). However, when the amount of photochemically active PSI was less than 20% of the intact value, we could not detect Y(NA) at all (Fig. 2C).
Figure 2.
Effects of PSI photoinhibition on photosynthetic parameters of PSI in Arabidopsis. A, Relationship between effective quantum yield of PSI [Y(I)] and residual total oxidizable P700 (Pm). B and C, Relationship between quantum yield of nonphotochemical energy dissipation of PSI due to the donor- and acceptor-side limitation [Y(ND) and Y(NA), respectively] and Pm. Evaluations were made before and after rSP illumination (300 ms, 20,000 µmol photons m−2 s−1, every 10 s). Photosynthetic parameters of PSI were measured at the steady state of photosynthesis (820 µmol photons m−2 s−1) under 21 kPa O2 and 100 Pa CO2. Data are independent measurements, with quadruple replicates indicated by circles, triangles, squares, and diamonds.
Relationship between the Quantity of the Photochemically Active PSI and Photosynthetic Parameters of PSII in Arabidopsis
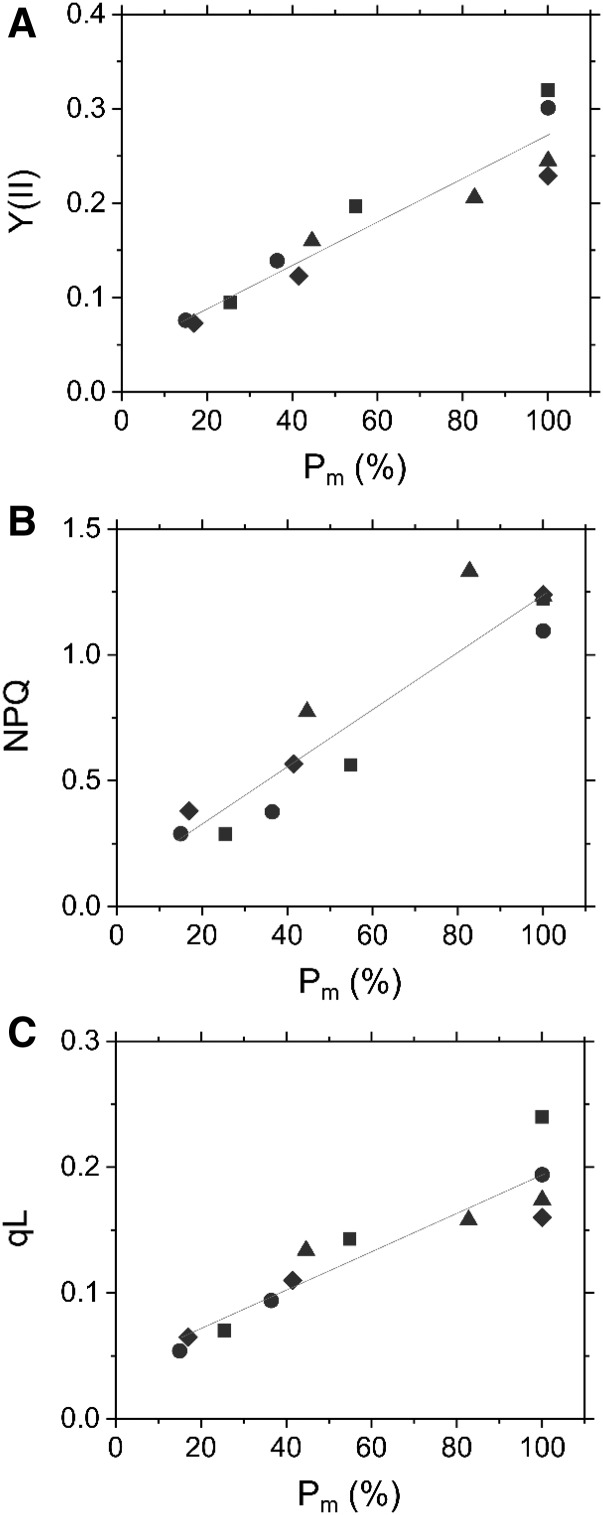
Next, we assessed the impact of the quantity of photochemically active PSI on the photosynthetic parameters of PSII in Arabidopsis. Effective quantum yield of PSII [Y(II)] showed an approximately linear relationship with Pm (Fig. 3A), which suggests that the operating efficiency of the photochemical reaction in PSII is correlated to the quantity of the photochemically active PSI in plant leaves. Similarly, nonphotochemical quenching of chlorophyll fluorescence in PSII (non-photochemical quenching), the indicator of a thermal dissipation of excess light energy in PSII, was proportional to Pm (Fig. 3B). Furthermore, photochemical quenching of chlorophyll fluorescence based on the lake model of the antenna system in PSII (represented as qL), reflecting the oxidation level of QA and possibly the PQ pool, showed a trend similar to that of Y(II) (Fig. 3C).
Figure 3.
Effects of PSI photoinhibition on photosynthetic parameters of PSII in Arabidopsis. A, Relationship between effective quantum yield of PSII [Y(II)] and residual total oxidizable P700 (Pm). B, Relationship between nonphotochemical quenching (NPQ) and Pm. C, Relationship between inferred oxidation level of PQ pool (qL) and Pm. Evaluations were made before and after rSP illumination (300 ms, 20,000 µmol photons m−2 s−1, every 10 s). Photosynthetic parameters of PSII were measured at the steady state of photosynthesis (820 µmol photons m−2 s−1) under 21 kPa O2 and 100 Pa CO2. Data are independent measurements, with quadruple replicates indicated by circles, triangles, squares, and diamonds. Red lines show linear regressions (A, R2 = 0.896; B, R2 = 0.897; C, R2 = 0.860, respectively).
Relationship between the Quantity of the Photochemically Active PSI and Photosynthetic CO2 Assimilation in Arabidopsis
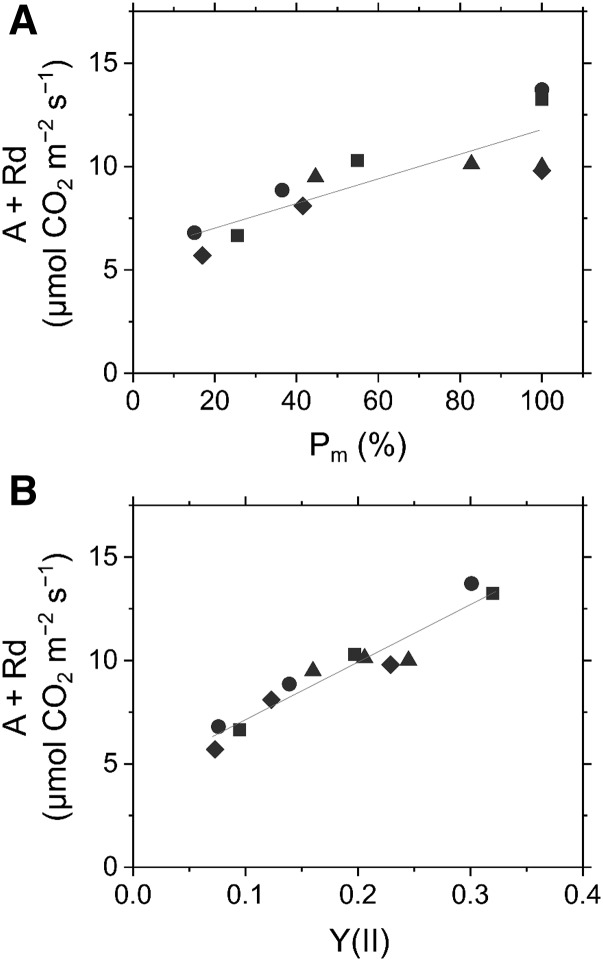
We measured photosynthetic CO2 assimilation rate, calculated as the sum of net CO2 assimilation rate (A) and dark respiration rate, in intact and damaged leaves of Arabidopsis. When compared with how Y(II), NPQ, and qL responded to PSI photoinhibition, a less linear correlation was observed in the inactivation of photosynthetic CO2 assimilation when Pm decreased (Fig. 4A). The inhibition of CO2 assimilation rate might be suppressed until the residual Pm was less than 60%, and thereafter, it started to decrease with the inactivation of PSI (Fig. 4A). However, ∼50% of the normal CO2 assimilation rate was maintained even with an 80% reduction in PSI photochemical activity in the leaves of Arabidopsis wild-type plants (Fig. 4A). Additionally, when Y(II) was plotted against the CO2 assimilation rate (Fig. 4B), the resultant relationship indicated that the effective quantum yield of PSII can relatively reflect the photosynthetic CO2 assimilation rate when PSI photoinhibition occurs.
Figure 4.
Effects of PSI photoinhibition on photosynthetic CO2 assimilation of PSI in Arabidopsis. A, Relationship between photosynthetic CO2 assimilation rate (A + dark respiration rate [Rd]) with residual total oxidizable P700 (Pm). B, Relationship between A + Rd and effective quantum yield of PSII [Y(II)]. Evaluations were made before and after rSP illumination (300 ms, 20,000 µmol photons m−2 s−1, every 10 s). Photosynthetic parameters of PSI were measured at the steady state of photosynthesis (820 µmol photons m−2 s−1) under 21 kPa O2 and 100 Pa CO2. Data are independent measurements, with quadruple replicates indicated by circles, triangles, squares, and diamonds. Red lines show linear regressions (A, R2 = 0.718; B, R2 = 0.920, respectively).
DISCUSSION
Sufficient Quantity of PSI Supports P700 Oxidation in Plant Leaves
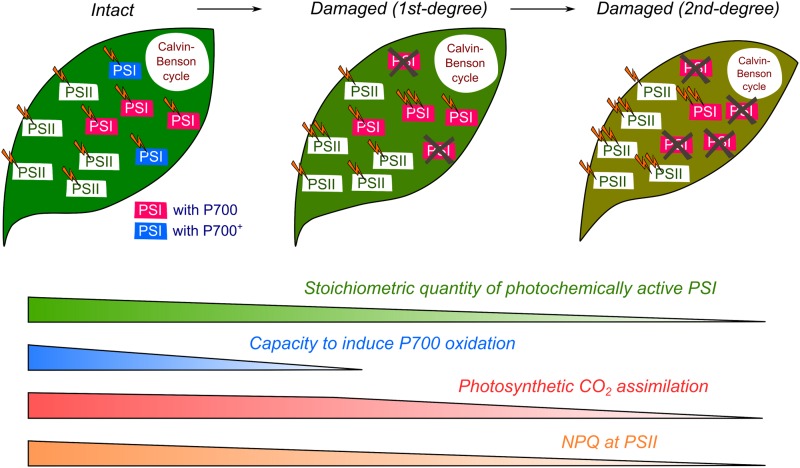
In the current study, we investigated the relationship between the stoichiometric quantity of the photochemically active PSI and P700 oxidation in vivo during steady-state photosynthesis in Arabidopsis. Although diverse regulatory mechanisms for the oxidation of P700 have been reported in oxygenic photoautotrophs (Shimakawa and Miyake, 2018), the effect of the quantity of PSI on P700 oxidation was poorly understood. rSP illumination is a useful tool to rapidly and selectively induce PSI photoinhibition in plant leaves (Sejima et al., 2014; Zivcak et al., 2015a, 2015b; Kono et al., 2017; Takagi et al., 2017; Shimakawa et al., 2019; Tikkanen and Grebe, 2018). This method enabled us to decrease the quantity of active PSI in the leaves of Arabidopsis wild-type plants. With PSI inactivated, Y(I) in principle increased (Fig. 2A); that is, the residual photochemically active PSI efficiently drives the photooxidation/reduction cycle, which is reasonable considering that the electron donor (i.e. PSII) and acceptor (i.e. the Calvin-Benson cycle) of PSI are relatively larger in these situations. In contrast, when Pm was decreased from 100% to 60%, Y(ND) decreased from 0.5 to 0.1 (Fig. 2B), indicating that plant leaves cannot keep P700 oxidized when 40% of PSI is inactivated even in the presence of a variety of regulatory mechanisms (i.e. P700 oxidation system). In other words, the leaves of Arabidopsis contain a quantity of PSI that is likely sufficient to enable P700 oxidation in excess light conditions, which may be regulated at the genetic level and modulated to suit any variation in environmental conditions. In the illustrated model of the photosynthetic electron transport system in the processes of PSI photoinhibition in Arabidopsis leaves (Fig. 5), we summarized the effects of a decrease in the stoichiometric quantity of PSI on P700 oxidation as well as on the other physiological states as follows.
Figure 5.
Hypothetical overview of the progression of PSI photoinhibition in plant leaves. Stoichiometric quantity of photochemically active PSI decreases with the degree of PSI photoinhibition. As PSI photoinhibition progresses, P700 oxidation is not maintained, which is the turning point between first and second degree PSI photoinhibition.
Capacity of Remaining PSI Can Suppress the Inhibition of Photosynthesis
When Pm decreased to 60% of that present in untreated leaves, the photosynthetic CO2 assimilation rate was likely to show a smaller decrease than that in the phase whereby Pm further decreased from 60% to 20% of normal levels, as supported by the low linear correlation for the relationship between Pm and CO2 assimilation rate (Fig. 4A). The former phase of PSI photoinhibition, termed as “first-degree” in Figure 5, corresponds to the phase in which oxidized P700 can be still observed as reflected in Y(ND). In the latter phase (“second-degree” in Fig. 5), P700 oxidation is no longer maintained in the steady-state photosynthesis (Fig. 2B). In principle, oxidized P700 should not cause charge separation in PSI, and rather it should engage in thermal dissipation of light energy (Trissl, 1997; Bukhov and Carpentier, 2003). This may be the reason why the decrease in photosynthetic CO2 assimilation rate was alleviated when Pm ranged from 100% to 60% (Fig. 4A); that is, the fractions of PSI with oxidized P700 reflect the capacity of remaining PSI complexes following PSI photoinhibition and can be recognized as surplus PSI for photosynthesis in intact leaves (Fig. 5).
Oxidized P700 Functions as a Quencher of Light Energy to Balance the Photo-Excitation between PSII and PSI
Theoretically, oxidized P700 thermally dissipates the light energy in PSI, which can mitigate the pressure of photo-excitation in PSII. As PSI photoinhibition advanced and Pm ranged from 100% to 40%, Y(NA) slightly increased; and thereafter, it disappeared (Fig. 2C). As mentioned above, with a decrease in photochemically active PSI, the limitation on the electron acceptor side should in principle be relieved. Therefore, the slight increase in Y(NA; Fig. 2C) suggests that the electron influx into PSI on the donor side was enhanced. Light energy absorbed by chloroplasts in the plant leaves is distributed into PSII and PSI (Fig. 5), which suggests that a decrease in the quantity of the photochemically active PSI can cause an increase in the light energy flow into PSII. Indeed, Bukhov et al. (2004) reported accelerated rereduction of oxidized P700 in PSI-damaged cucumber leaves. In this study, the observed increase in Y(NA) when Pm ranged from 100% to 40% (Fig. 2C) supports the theory that oxidized P700 functions as the quencher of light energy in PSI. In PSII, Y(II) decreased with PSI photoinhibition (Fig. 3A), but did not show a linear relationship with photosynthetic CO2 assimilation rate passing through the graph origin, different from that in intact C3 plant leaves (Fig. 4A; Genty et al., 1989). This can be explained by an increase in the absorbance of PSII fraction (Genty et al., 1989; Baker et al., 2007). These data also demonstrate the effects of the change in the distribution of light energy between PSII and PSI on the chlorophyll fluorescence parameters. Simultaneously, the decrease in qL is assumed to reflect two effects: a decrease in the electron acceptor (i.e. PSI) and an increase in the photo-excitation pressure (Fig. 3C). Evidence for the hypothesis that oxidized P700 suppresses the charge separation of PSII by quenching the light energy in PSII through light harvesting complex and/or spillover-dependent mechanisms (Ueno et al., 2018; Yokono and Akimoto, 2018) might be also present within the results of the current study.
Decrease in PSI Is Correlated with a Decrease in NPQ
NPQ showed a linear inverse correlation with the extent of PSI photoinhibition in Arabidopsis (Fig. 3B). In the chlorophyll fluorescence measurement, NPQ reflects a thermal dissipation of excess light energy in PSII to protect PSII against photoinhibition (Li et al., 2002). Thus, a reduction of the electron acceptor side of PSII at steady-state photosynthesis, following PSI photoinhibition, as reflected in a decrease in qL (Fig. 3C), might be contributed also by the decrease in NPQ. These results suggest that PSI photoinhibition possibly causes PSII photoinhibition as a secondary effect under illuminated conditions. To our knowledge, the linear relationship between PSI/PSII ratio and NPQ was first characterized by Brestic and coworkers in early growth stages of chlorophyll b–deficient wheat mutant lines (Brestic et al., 2015). In C3 plants, NPQ is mainly composed of qE quenching, which is believed to correlate with the lumen acidification produced by photosynthetic linear and possibly cyclic electron flow, both of which depend on PSI (Kanazawa and Kramer, 2002; Li et al., 2002). Therefore, a loss of PSI can decrease the thylakoid membrane potential to lower qE quenching. In fact, PSI photoinhibition causes a decrease in the proton gradient across the thylakoid membrane in wheat leaves (Brestic et al., 2015; Zivcak et al., 2015a). On the other hand, the thylakoid membrane potential is modulated also by the narrowing proton conductance of chloroplast ATP synthase and thylakoid ion transporters (Rott et al., 2011; Armbruster et al., 2014). However, proton conductance is not affected by PSI photoinhibition (Zivcak et al., 2015a). In this study, we present a correlation between the amount of photochemically active PSI and NPQ in the wild-type plant leaves (Fig. 3B). The contribution of PSI to NPQ should be further quantitatively characterized in future works.
MATERIALS AND METHODS
Arabidopsis Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild type plant (Columbia ecotype) was grown under a long-day condition (16-h light, 23°C, 100 µmol photons m−2 s−1, white fluorescent lamp; 8-h dark, 21°C) with a relative humidity of 60% ± 10%. Seeds were planted in pots that contained a 1:1 mixture of vermiculite and Metro-Mix 350 (Sun Gro Horticulture); 1000-fold diluted Hyponex solution (Hyponex) was used as a watering solution.
Measurements of Gas Exchange, Chlorophyll Fluorescence, and P700
Gas exchange (CO2 and water) was measured using a LI-7000 gas analyzer (Li-COR) equipped with a 3010 DUAL gas exchange leaf chamber (Walz, Effeltrich, Germany), in which the flowing gas was saturated with water vapor at 18.0 ± 0.1°C and the leaf temperature was maintained at 25°C. Chlorophyll fluorescence and oxidized P700 were simultaneously measured using a Dual-PAM-100 fluorometer (Walz).
The photosynthetic parameters of PSII were calculated using chlorophyll fluorescence parameters as follows (Baker, 2008): PSII operating efficiency (quantum yield of photochemical energy conversion in PSII), Y(II) = (Fmʹ – Fʹ)/Fmʹ; nonphotochemical quenching, NPQ = (Fm – Fmʹ)/Fmʹ; fraction of “open” PSII centers (with QA oxidized), on the basis of a lake model, for the PSII photosynthetic apparatus, qL = [(Fmʹ – Fʹ)/(Fmʹ – Foʹ)] × (Foʹ/Fʹ), where, Fo represents minimum fluorescence from a dark-adapted leaf; Foʹ, minimum fluorescence from a light-adapted leaf; Fm, maximum fluorescence from a dark-adapted leaf; Fmʹ, maximum fluorescence from a light-adapted leaf; and Fʹ, fluorescence emission from a light-adapted leaf. Pulse-amplitude modulated red measuring light (620 nm, 0.1 µmol photons m−2 s−1) was used to determine Fo. Red actinic light was supplied using a chip-on-board light emitting diode (LED) array (635 nm). Short-saturation pulse light (8,000 µmol photons m−2 s−1, 300 ms) was also provided by the LED array for the determinations of Fm and Fmʹ.
The photosynthetic parameters of PSI were calculated from the redox state of P700 as follows (Klughammer and Schreiber, 1994; Schreiber and Klughammer, 2008): quantum yield of photochemical energy conversion in PSI, Y(I) = (Pmʹ − P)/Pm; quantum yield of nonphotochemical energy dissipation due to donor-side limitation, Y(ND) = P/Pm; quantum yield of nonphotochemical energy dissipation due to acceptor-side limitation, Y(NA) = (Pm − Pmʹ)/Pm, where, Pm represents total quantity of photo-oxidizable P700; Pmʹ, maximum quantity of photo-oxidized P700 by a saturation pulse; and P, quantity of photo-oxidized P700 at steady state. Pulse-amplitude modulated near-infrared measuring lights (830 and 870 nm) were applied to measure the transmittance of oxidized P700.
Statistical Analysis
All statistical analyses were performed using Origin 2017 (LightStone).
Acknowledgments
The authors thank Editage (www.editage.jp) for providing English corrections.
Footnotes
This work was supported by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (CREST, JST) (grant no. AL65D21010 to C.M.) and by Japan Society for the Promotion of Science (JSPS) (grant no. 16J03443 to G.S., who is also a JSPS research fellow).
References
- Anderson JM. (1992) Cytochrome b6 f complex: Dynamic molecular organization, function and acclimation. Photosynth Res 34: 341–357 [DOI] [PubMed] [Google Scholar]
- Armbruster U, Carrillo LR, Venema K, Pavlovic L, Schmidtmann E, Kornfeld A, Jahns P, Berry JA, Kramer DM, Jonikas MC (2014) Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun 5: 5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger MR, von Caemmerer S, Ruuska S, Nakano H (2000) Electron flow to oxygen in higher plants and algae: Rates and control of direct photoreduction (Mehler reaction) and rubisco oxygenase. Philos Trans R Soc Lond B Biol Sci 355: 1433–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30: 1107–1125 [DOI] [PubMed] [Google Scholar]
- Brestic M, Zivcak M, Kunderlikova K, Sytar O, Shao H, Kalaji HM, Allakhverdiev SI (2015) Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth Res 125: 151–166 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Carpentier R (2003) Measurement of photochemical quenching of absorbed quanta in photosystem I of intact leaves using simultaneous measurements of absorbance changes at 830 nm and thermal dissipation. Planta 216: 630–638 [DOI] [PubMed] [Google Scholar]
- Bukhov NG, Govindachary S, Rajagopal S, Joly D, Carpentier R (2004) Enhanced rates of P700+ dark-reduction in leaves of Cucumis sativus L photoinhibited at chilling temperature. Planta 218: 852–861 [DOI] [PubMed] [Google Scholar]
- Driever SM, Baker NR (2011) The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ 34: 837–846 [DOI] [PubMed] [Google Scholar]
- Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25: 83–100 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subj 990: 87–92 [Google Scholar]
- Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218: 107–114 [DOI] [PubMed] [Google Scholar]
- Hanawa H, Ishizaki K, Nohira K, Takagi D, Shimakawa G, Sejima T, Shaku K, Makino A, Miyake C (2017) Land plants drive photorespiration as higher electron-sink: Comparative study of post-illumination transient O2-uptake rates from liverworts to angiosperms through ferns and gymnosperms. Physiol Plant 161: 138–149 [DOI] [PubMed] [Google Scholar]
- Ivanov AG, Morgan-Kiss RM, Krol M, Allakhverdiev SI, Zanev Y, Sane PV, Huner NPA (2015) Photoinhibition of photosystem I in a pea mutant with altered LHCII organization. J Photochem Photobiol B 152(Pt B): 335–346 [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Kramer DM (2002) In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc Natl Acad Sci USA 99: 12789–12794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Kono M, Yamori W, Suzuki Y, Terashima I (2017) Photoprotection of PSI by far-red light against the fluctuating light-induced photoinhibition in Arabidopsis thaliana and field-grown plants. Plant Cell Physiol 58: 35–45 [DOI] [PubMed] [Google Scholar]
- Li X-P, Müller-Moulé P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves--relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46: 629–637 [DOI] [PubMed] [Google Scholar]
- Pfündel E, Klughammer C, Schreiber U (2008) Monitoring the effects of reduced PSII antenna size on quantum yields of photosystems I and II using the Dual-PAM-100 measuring system. PAM Appl Notes 1: 21–24 [Google Scholar]
- Rott M, Martins NF, Thiele W, Lein W, Bock R, Kramer DM, Schöttler MA (2011) ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23: 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttler MA, Tóth SZ (2014) Photosynthetic complex stoichiometry dynamics in higher plants: Environmental acclimation and photosynthetic flux control. Front Plant Sci 5: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöttler MA, Tóth SZ, Boulouis A, Kahlau S (2015) Photosynthetic complex stoichiometry dynamics in higher plants: Biogenesis, function, and turnover of ATP synthase and the cytochrome b6f complex. J Exp Bot 66: 2373–2400 [DOI] [PubMed] [Google Scholar]
- Schreiber U, Klughammer C (2008) Saturation pulse method for assessment of energy conversion in PSI. PAM Appl Notes 1: 11–14 [Google Scholar]
- Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55: 1184–1193 [DOI] [PubMed] [Google Scholar]
- Shimakawa G, Miyake C (2018) Oxidation of P700 ensures robust photosynthesis. Front Plant Sci 9: 1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G, Shaku K, Miyake C (2016) Oxidation of P700 in photosystem I is essential for the growth of cyanobacteria. Plant Physiol 172: 1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G, Ishizaki K, Tsukamoto S, Tanaka M, Sejima T, Miyake C (2017) The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiol 173: 1636–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakawa G, Murakami A, Niwa K, Matsuda Y, Wada A, Miyake C (2019) Comparative analysis of strategies to prepare electron sinks in aquatic photoautotrophs. Photosynth Res 139: 401–411 [DOI] [PubMed] [Google Scholar]
- Takagi D, Ishizaki K, Hanawa H, Mabuchi T, Shimakawa G, Yamamoto H, Miyake C (2017) Diversity of strategies for escaping reactive oxygen species production within photosystem I among land plants: P700 oxidation system is prerequisite for alleviating photoinhibition in photosystem I. Physiol Plant 161: 56–74 [DOI] [PubMed] [Google Scholar]
- Terashima I, Funayama S, Sonoike K (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperatures is photosystem I, not photosystem II. Planta 193: 300–306 [Google Scholar]
- Tikkanen M, Grebe S (2018) Switching off photoprotection of photosystem I - a novel tool for gradual PSI photoinhibition. Physiol Plant 162: 156–161 [DOI] [PubMed] [Google Scholar]
- Trissl H-W. (1997) Determination of the quenching efficiency of the oxidized primary donor of Photosystem I, P700+: Implications for the trapping mechanism. Photosynth Res 54: 237–240 [Google Scholar]
- Ueno Y, Shimakawa G, Miyake C, Akimoto S (2018) Light-harvesting strategy during CO2-dependent photosynthesis in the green alga Chlamydomonas reinhardtii. J Phys Chem Lett 9: 1028–1033 [DOI] [PubMed] [Google Scholar]
- Yamori W, Takahashi S, Makino A, Price GD, Badger MR, von Caemmerer S (2011) The roles of ATP synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol 155: 956–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokono M, Akimoto S (2018) Energy transfer and distribution in photosystem super/megacomplexes of plants. Curr Opin Biotechnol 54: 50–56 [DOI] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kunderlikova K, Sytar O, Allakhverdiev SI (2015a) Repetitive light pulse-induced photoinhibition of photosystem I severely affects CO2 assimilation and photoprotection in wheat leaves. Photosynth Res 126: 449–463 [DOI] [PubMed] [Google Scholar]
- Zivcak M, Brestic M, Kunderlikova K, Olsovska K, Allakhverdiev SI (2015b) Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: Does activity of photosystem I play any role in OJIP rise? J Photochem Photobiol B 152(Pt B): 318–324 [DOI] [PubMed] [Google Scholar]