Loliolide, an end-product of carotenoid pathways, induces plant resistance to herbivores independently of jasmonic acid.
Abstract
Jasmonic acid (JA) plays an important role in the induction of herbivore resistance in many plants. However, JA-independent herbivore resistance has been suggested. An herbivore-resistance-inducing substance was isolated from Tobacco mosaic virus-infected tobacco (Nicotiana tabacum) leaves in which a hypersensitive response (HR) was induced and identified as loliolide, which has been identified as a β-carotene metabolite. When applied to tomato (Solanum lycopersicum) leaves, loliolide decreased the survival rate of the two-spotted spider mite, Tetranychus urticae, egg deposition by the same pest, and the survival rate of larvae of the common cutworm Spodoptera litura without exhibiting toxicity against these herbivores. Endogenous loliolide levels increased not only with an infestation by S. litura larvae, but also with the exogenous application of their oral secretions in tomato. A microarray analysis identified cell-wall–associated defense genes as loliolide-responsive tomato genes, and exogenous JA application did not induce the expression of these genes. Suppressor of zeaxanthin-less (szl), an Arabidopsis (Arabidopsis thaliana) mutant with a point mutation in a key gene of the β-carotene metabolic pathway, exhibited the decreased accumulation of endogenous loliolide and increased susceptibility to infestation by the western flower thrip (Frankliniella occidentalis). A pretreatment with loliolide decreased susceptibility to thrips in the JA-insensitive Arabidopsis mutant coronatine-insensitive1. Exogenous loliolide did not restore reduced electrolyte leakage in szl in response to a HR-inducing bacterial strain. These results suggest that loliolide functions as an endogenous signal that mediates defense responses to herbivores, possibly independently of JA, at least in tomato and Arabidopsis plants.
Plants have developed resistance response systems to defend against attacks by herbivores and pathogens. These systems have mainly been classified into two types: constitutive resistance and inducible resistance (Kiraly et al., 2007; War et al., 2012). In the latter case, primary and secondary metabolites, such as sugars, organic acids, phenylpropanoids, flavonoids, terpenes, alkaloids, and cyanogenic glycosides, are directly or indirectly involved in the induction of resistance (Fürstenberg-Hägg et al., 2013; Berens et al., 2017). These defense-related substances have been attracting attention as practical materials for the chemical control of plant diseases or herbivorous pests because of their potential to reduce the environmental burden in crop cultivation. A practical example is plant activators that are characterized by the capability to protect plants against pathogens through the activation of defense mechanisms without exhibiting direct antimicrobial or insecticidal activity. Several natural or chemically synthesized compounds have been identified as plant-activator or plant-activator–like compounds for plant diseases (Friedrich et al., 1996; Noutoshi et al., 2012; Seo et al., 2012, 2016; Sun et al., 2015).
Although information concerning plant activators for herbivorous pests is limited, one well-characterized example is the phytohormone jasmonic acid (JA). Several studies have shown that JA functions as an endogenous signal that activates defense responses to herbivore pests and wounding in plants (Wasternack and Strnad, 2016). However, JA-independent defense systems to herbivore pests or wounding have been detected in plants. Many wounding-responsive genes were found to still be enhanced in an Arabidopsis (Arabidopsis thaliana) jasmonic acid resistant1 mutant with reduced levels of JA-Ile, a biologically active derivative of JA (Suza and Staswick, 2008). Transgenic Nicotiana attenuata, in which the stress-responsive MAPK, MPK4, was silenced, exhibited increased resistance to the insect herbivore Manduca sexta independently of CORONATINE-INSENSITIVE1 (COI1)-mediated JA signaling (Hettenhausen et al., 2013). These findings also suggest the existence of defense-related substances other than JA or JA-related compounds in the induction of plant resistance to herbivores.
Some events in herbivore defense responses are also noted when plants are attacked by pathogens. For example, the accumulation of JA occurred at an early stage of the hypersensitive response (HR), a type of plant disease resistance response (Kenton et al., 1999; Andersson et al., 2006). HR is characterized by rapid cell death at the site of pathogen invasion in plants, typically resulting in the formation of necrotic lesions in which the pathogen is considered to localize (Dickman and Fluhr, 2013). The expression of herbivore-attack- or wounding-responsive genes, including PROTEINASE INHIBITORs, was induced during HR (Walling, 2000). These findings led to the hypothesis that unknown defense-related substances involved in herbivore resistance are produced during HR.
Here, we report the isolation and identification of a natural substance that induces resistance to three herbivore species with different feeding habits. We also provide evidence for this substance functioning as an endogenous signal that activates defense responses to herbivores.
RESULTS
Isolation and Identification of an Herbivore-Resistance-Inducing Substance from Tobacco
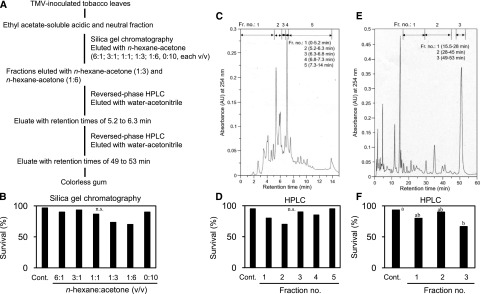
In the search for defense-related substances involved in the induction of resistance to herbivores, we used a combination of Tobacco mosaic virus (TMV) and tobacco (N. tabacum) carrying the TMV resistance gene N. To guide the fractionation of TMV-inoculated tobacco plants, we used a previously developed herbivore resistance assay (Kawazu et al., 2012). This assay system consists of the combination of Micro-Tom, a model tomato (Solanum lycopersicum) plant, and the two-spotted spider mite (Tetranychus urticae), a serious agricultural pest of many crops, such as Solanaceae and Fabaceae. The TMV-inoculated leaves of NN tobacco, a tobacco cultivar carrying the N gene, were extracted with methanol. The ethyl-acetate–soluble acidic and neutral fractions prepared from the methanol extract were loaded onto a column of silica gel and eluted in a stepwise manner by increasing concentrations of acetone in N-hexane (Fig. 1A). Each fraction was applied to 30 detached tomato leaves by floating them on the solution (a final concentration of 100 μL L−1) in petri dishes for 24 h (Supplemental Fig. S1). Each treated leaf was confined within a Munger cell, and one female mite was released onto the leaf surface. Resistance was assessed by counting the survival rate of the mites 5 d after the inoculation. Although there was no significant difference in activity for decreasing the survival rate of T. urticae mites between fractions (Fig. 1B), we focused on two fractions eluted with N-hexane/acetone ratios of 1:3 (v/v) and 1:6 (v/v), which tended to show activity. These two fractions were combined and subjected to reversed-phase high-performance liquid chromatography (HPLC; Fig. 1C). Fractions separated by an isocratic elution using water and acetonitrile were assayed in the same manner. Although there was no significant difference in activity between fractions (Fig. 1D), we collected an eluate with retention times of 5.2–6.3 min, which tended to exhibit activity and was subjected to further purification. In the second step of HPLC (Fig. 1E), a major peak eluate with retention times of 49–53 min showed activity (Fig. 1F) and was collected to yield a colorless gum.
Figure 1.
Flow diagram for purification and isolation of an herbivore-resistance-inducing substance from tobacco leaves. A, Extraction and purification procedure. B, Inhibitory activity against the survival rate of T. urticae mites in fractions obtained by silica gel column chromatography. A χ2 test detected a significant difference between control fraction and 1:3 and 1:6 fractions (χ2 = 15.09, P < 0.05), but the subsequent Ryan’s multiple range test for proportions did not detect any statistical difference. C, HPLC chromatogram of the active fractions obtained by silica gel column chromatography. D, Activity in fractions obtained by HPLC (χ2 = 7.74, P > 0.05). E, Chromatogram of the second HPLC step. F, Activity in fractions obtained by the second HPLC step (P < 0.01). Thirty mites were used for each fraction in (B), (D), and (F). Different letters indicate significant differences among treatments (Ryan’s multiple range test for proportions after a χ2 test).
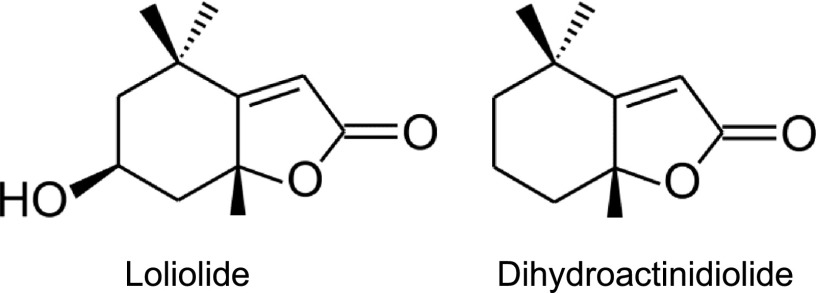
Mass and 1H and 13C nuclear magnetic resonance (NMR) spectra (see “Materials and Methods” and Supplemental Fig. S2 for details) for the peak were assigned to loliolide (Fig. 2; Kimura and Maki, 2002; He et al., 2010). Loliolide, a C11-terpene lactone, has been found in many algae and plants, including tobacco (Behr et al., 1973; Tanaka and Matsunaga, 1989; Pan et al., 2009), and is regarded as a photo-oxidative or thermal degradation product of carotenoids (Repeta, 1989; Mori and Khlebnikov, 1993; Rios et al., 2008). An earlier study identified loliolide as a natural repellent compound against herbivorous pest leaf cutter ants (Okunade and Weimer, 1985). Loliolide has also been shown to exhibit various physiological activities, such as growth inhibition, germination inhibition, and phytotoxic activities, for plants and antitumor activities and antimicrobial activities for animals and microorganisms (Grabarczyk et al., 2015; Islam et al., 2017). However, limited information is currently available on the physiological role of loliolide in plants.
Figure 2.
Chemical structures of loliolide and dihydroactinidiolide.
Exogenously Applied Loliolide Decreases the Survival of and Egg Deposition by T. urticae and the Survival of Spodoptera litura in Tomato Plants
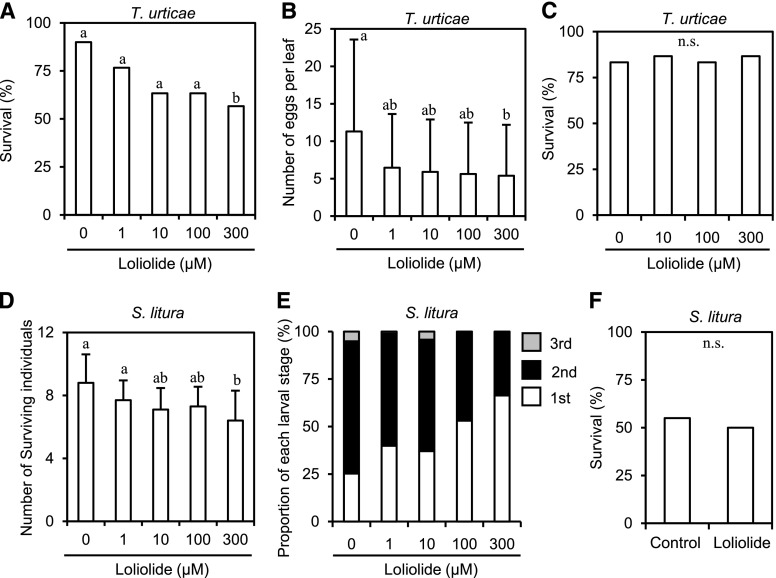
Although foliar sprays or absorption through roots using intact plants is an ideal method for applying a potential compound to plants without any influence of wound stresses, such as the detachment of leaves, it requires a large quantity of the compound. Due to the difficulties associated with preparing sufficient amounts of loliolide available for various assays by purification from natural resources or organic synthesis, we applied loliolide to detached leaves or leaf discs from intact leaves in this study. To examine the effects of the exogenous application of loliolide on the survival of female T. urticae, detached tomato leaves were treated by floating them on a solution containing various concentrations of loliolide or 0.1% methanol as a control for 24 h, and one female mite per leaf was released onto the leaf surface in Munger cells. Loliolide decreased the survival of female T. urticae when applied at 300 μM to tomato leaves (Fig. 3A). Dihydroactinidiolide (Fig. 2), a C11 terpene that is structurally similar to loliolide (Havaux, 2014), did not exhibit the same inhibitory activity against T. urticae survival (Supplemental Fig. S3). Loliolide was synthesized from lutein, a carotenoid, and exerted similar effects to the natural form (Supplemental Fig. S4); therefore, synthetic loliolide was used in subsequent analyses. Loliolide at 300 μM also decreased the number of eggs laid by female mites on detached tomato leaves (Fig. 3B). To examine whether loliolide exhibits direct insecticidal activity against T. urticae, we employed an assay system used for evaluating the toxicity of insecticides. Female mites were dipped into a solution containing 300 μM loliolide, and the number of surviving mites was counted 48 h after dipping. No significant differences were observed in survival rates between treatments (Fig. 3C).
Figure 3.
Effects of loliolide on the infestation of tomato leaves by T. urticae and S. litura and pesticidal activities for herbivores. A and B, Tomato leaves treated with different concentrations of loliolide for 24 h were used for the infestation assay using T. urticae or S. litura. Female T. urticae mites were placed on the leaf surface for 5 d, and the numbers of surviving mites (A) and laid eggs (B) were counted (for A, χ2 = 10.16, P > 0.005; for B, P < 0.05). C, Insecticidal activity assay. The numbers of surviving T. urticae mites 48 h after dipping into a solution containing 300 μM loliolide were counted (χ2 = 0.26, P > 0.05). D and E, The first-stage larvae of S. litura were placed on the leaf surface for 5 d, and the numbers of surviving larvae (D) and their growth stages (E) were counted (for D, P < 0.01; for E, P < 0.005 for first-instar larvae). F, Insecticidal activity assay. The numbers of surviving S. litura first-stage larvae 9 d after the application of an artificial diet containing 300 μM loliolide or 0.1% methanol (Control) were counted (P > 0.05, n = 100 larvae). Values for (A) and (B) are the mean ± sd (n = 30 mites), values for (C) are the mean of 30 mites, and values for (D) are the mean ± sd (n = 10 replicates). Different letters indicate significant differences among treatments (Ryan’s multiple range test for proportions after a χ2 test for A and C; Tukey-Kramer HSD test for B, D, and E; Fisher’s exact probably test for F).
We also assayed resistance using another pest. The leafworm moth S. litura is an important agricultural pest of many crops. We used the first-instar larvae of S. litura as an inoculum. Detached tomato leaves were floated on a solution containing various concentrations of loliolide or 0.1% methanol as a control for 24 h, and 10 hatchlings per two leaves were released onto the leaf surface and incubated in a sealed cup. Five days after the inoculation, we counted the number of surviving larvae, and the developmental stages of dead larvae were recorded. The survival rate of larvae was decreased by loliolide at 300 μM (Fig. 3D). The proportion of the first-instar larvae increased proportionally to the concentration of loliolide (Fig. 3E), suggesting that the treatment with loliolide delayed the development of larval growth. To assess whether loliolide exhibits insecticidal activity against S. litura, first-stage larvae were reared on an artificial diet including 300 μM loliolide. No significant differences were noted in larval survival between the control diets and diets with loliolide (Fig. 3F).
Endogenous Loliolide Increases with an Infestation by an Herbivore Insect in Tomato Plants
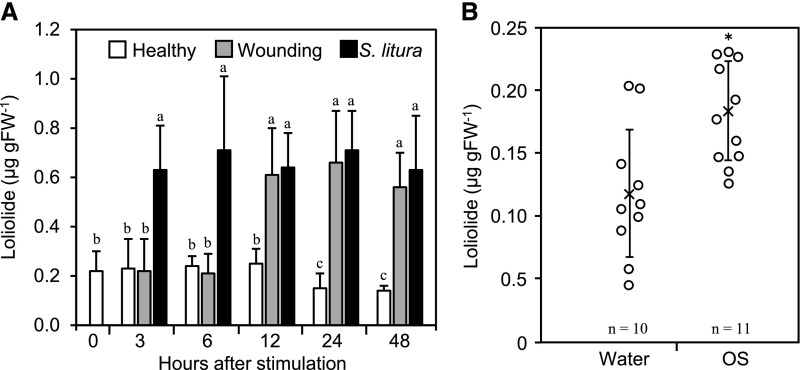
The herbivore resistance assay and toxicity assay suggested that reductions in the survival of female T. urticae and S. litura larvae on tomato leaves were due to host defense responses induced by loliolide. If loliolide functions as an endogenous signal mediating these defense responses, its endogenous amount may be increased by herbivore attacks. To test this possibility, we established a method for quantifying endogenous loliolide. Tomato leaves were inoculated with the first-instar larvae of S. litura, and the endogenous amounts of loliolide in inoculated leaves were assessed. Loliolide levels began to increase 3 h after the inoculation and remained at almost constant levels between 6 and 48 h (Fig. 4A). Mechanical wounding also enhanced the accumulation of loliolide; however, the accumulation of loliolide after wounding occurred later than that after the inoculation with S. litura larvae. Herbivores produce oral secretions that often elicit defense responses in plants (Schmelz, 2015). To examine whether loliolide accumulates in response to such an elicitor, we applied an oral secretion collected from S. litura larvae to tomato leaves by dropping onto an area punctured with a needle and measured endogenous amounts of loliolide 6 h later. Loliolide levels were significantly higher in oral-secretion-treated leaves than in leaves treated with water (Fig. 4B).
Figure 4.
Changes in the amount of endogenous loliolide by herbivore infestation, wounding, and treatments with insect oral secretions. A, Tomato leaves were inoculated with the first-stage larvae of S. litura or mechanically wounded, harvested at the times indicated, and used for the quantification of loliolide. Healthy leaves that had not been wounded were used as a control (Healthy). Values for (A) are the mean ± sd (n = 10 replicates). Different letters indicate significant differences among treatments (P < 0.05, Tukey-Kramer HSD test). B, Oral secretions (OS) from S. litura larvae or water were applied to tomato leaves that had previously been wounded by punctuation with a needle. Leaves were harvested 6 h after the application and used for the quantification of loliolide. Individual data points and the mean ± sd (from n = 10 replicates for water and n = 11 replicates for OS) are shown as open circles and crosses, respectively. Asterisks denote significant differences from the water sample (P < 0.01, t test).
Identification of Loliolide-Responsive Tomato Genes
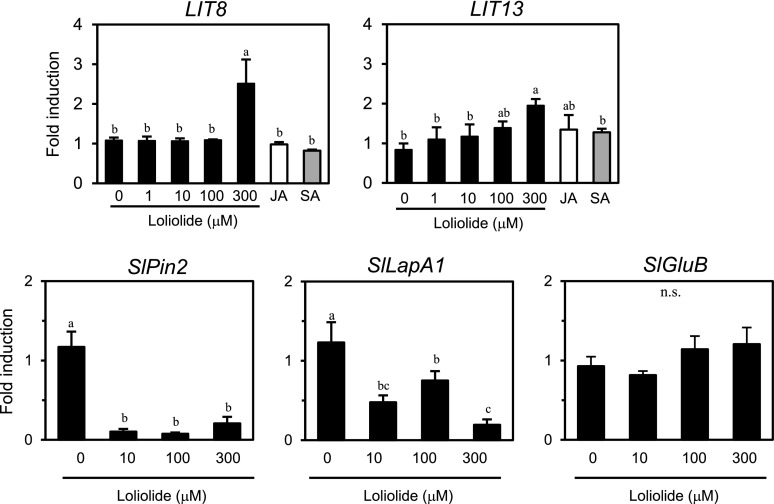
To identify host factors involved in defense responses induced by loliolide, we performed a microarray analysis of loliolide-responsive tomato genes using an Agilent Tomato Oligo DNA Microarray. Tomato leaves were treated with 300 μM loliolide or 0.1% methanol as a control for 12 h. We selected up-regulated genes with a fold change >2 over the methanol treatment and identified 27 clones as candidates for loliolide-induced tomato genes (LIT genes; Supplemental Table S1). We confirmed our microarray results using a real-time PCR analysis. Two out of the 27 clones (LIT8 and LIT13) showed highly reproducible expression profiles. LIT8, which is a gene-encoding cell wall invertase, showed up-regulated expression in response to the exogenous application of 300 μM loliolide (Fig. 5). The expression of LIT13, which is predicted to encode wall-associated receptor kinase 2, was increased by 300 μM. None of these two LIT genes showed up-regulated expression in response to JA or salicylic acid (SA). We also examined the induction kinetics of JA-responsive PROTEINASE INHIBITOR II (SlPin2) and Leu aminopeptidase (SlLapA1) genes and a herbivore- and SA-responsive basic β-1,3-glucanase gene (SlGluB; Van Kan et al., 1995; Chao et al., 1999). The exogenous application of loliolide at 10–300 μM reduced the expression of SlPin2 and SlLapA1 (Fig. 5). The expression of SlGluB was not induced by loliolide at 10–300 μΜ. A quantitative analysis of endogenous phytohormones in tomato indicated that JA and SA levels were not changed by exogenous loliolide (Supplemental Fig. S5).
Figure 5.
Gene expression analysis of tomato plants after a treatment with loliolide. Reverse transcription quantitative-PCR analysis of the indicated genes in tomato leaves 12 h after the treatment with different concentrations of loliolide, 10 μM methyl JA, 100 μM SA, or 0.1% methanol (0 μM) as a control. Values are the mean ± sd (n = three replicates). Different letters indicate significant differences among treatments (for LIT8, LIT13, SlPin2, and SlLapA1, P < 0.05, Tukey-Kramer HSD test; for SlGluB, P > 0.05, Tukey-Kramer HSD test).
Role of Loliolide in Thrips Resistance in Arabidopsis
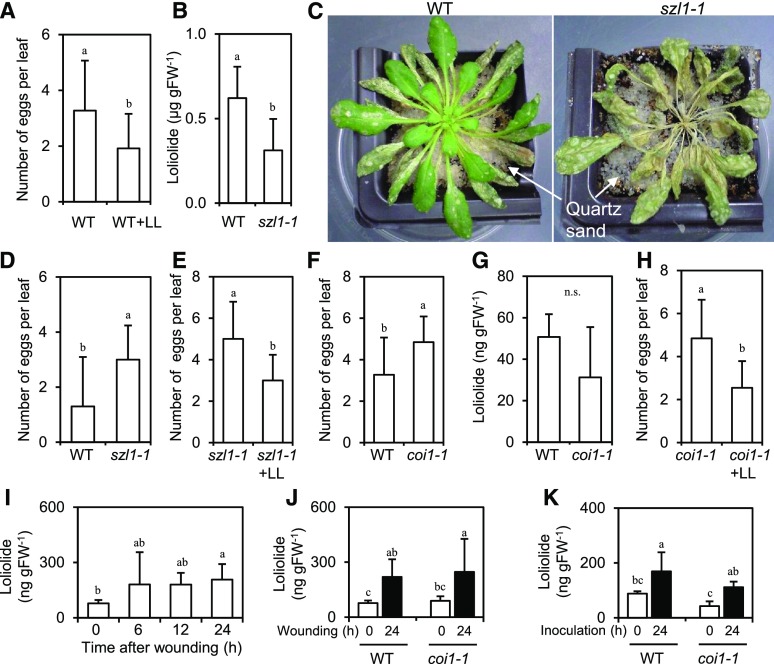
To further examine the importance of loliolide in host defenses to herbivores in plants, we used Arabidopsis mutants for carotenoid production. We initially examined whether exogenously applied loliolide induces herbivore protection in Arabidopsis using wild-type Columbia (Col-0) and the western flower thrips (Frankliniella occidentalis, one of the most serious insect pests of many crops), by assessing the asexual oviposition performance of thrips. Leaf discs punched out from intact Col-0 plants were floated on a solution containing 300 μM loliolide, and one adult female thrip per leaf disc was released onto the leaf surface. Resistance was assessed by counting the numbers of eggs on the leaves 3 d after the inoculation. Exogenously applied loliolide decreased the number of eggs laid by female thrips on Arabidopsis leaves (Fig. 6A). Because loliolide has been implicated as a degradation product of β-carotene, we focused on szl—an Arabidopsis mutant that contains a point mutation in the lycopene β-cyclase gene, a key gene of the β-carotene biosynthetic and metabolic pathways (Li et al., 2009). The quantification of endogenous loliolide indicated that szl1-1 had ∼50% of the wild-type loliolide level (Fig. 6B). szl1-1 plants were more severely damaged by the western flower thrips than wild-type plants (Fig. 6C). The western flower thrips laid larger numbers of eggs on szl1-1 plants than on wild-type plants (Fig. 6D). The loliolide treatment of szl1-1 plants resulted in a decrease in the number of eggs laid by the pest (Fig. 6E). To examine whether the inhibition of egg deposition by loliolide is mediated by JA, we assayed coi1, an Arabidopsis mutant defective in JA perception. We confirmed that the western flower thrips laid larger numbers of eggs on coi1-1 plants than on wild-type plants (Fig. 6F), which is consistent with previous findings (Abe et al., 2009). No marked differences were observed in endogenous loliolide levels in healthy leaves between wild-type and coi1-1 plants (Fig. 6G). The loliolide treatment of coi1-1 plants resulted in a smaller number of eggs laid by the pest than on coi1-1 plants treated with methanol only as the control (Fig. 6H). Whether loliolide increases in response to mechanical wounding or herbivore attacks was examined. Increases in endogenous loliolide were detected 24 h after wounding of leaves of wild-type (Fig. 6I) and coi1-1 (Fig. 6J) plants. Inoculation of wild-type plants with the western flower thrips resulted in an increase in endogenous loliolide (Fig. 6K). Thrips-induced accumulation of loliolide was also observed in coi1-1 plants.
Figure 6.
Analysis of the importance of loliolide in herbivore resistance using Arabidopsis. A, D, E, F, and H, F. occidentalis females were inoculated on the leaves of wild-type (Col-0) or the indicated mutant plants floated on a solution containing 300 μM loliolide or 0.1% methanol as a control, and the numbers of laid eggs were counted 3 d after the inoculation. Values for (A), (D), (E), (F), and (H) are the mean ± sd (n = 10–19 replicates). B and G, Endogenous loliolide contents in the leaves of wild-type, szl1-1 (B), and coi1-1 (G) plants. Values for (B) and (G) are the mean ± sd (n = five to six plants). C, Photographs taken 14 d after the inoculation of wild-type and szl1-1 plants with F. occidentalis. I, Time course of loliolide accumulation after wounding of wild-type Col-0 plants. J and K, Endogenous loliolide contents in the leaves of wild-type and coi1-1 plants 0 and 24 h after wounding (J) or inoculation with F. occidentalis (K). For (I) n = 10 replicates for each time point, (J) n = 8 replicates, and (K) n = 5 replicates, values are the mean ± sd. Different letters indicate significant differences among treatments (for A, B, D, E, F, and H, P < 0.05, t test; for G, P > 0.05; t test; for I, J, and K, P < 0.05, Tukey-Kramer HSD test). LL, loliolide; WT, wild type.
Role of Loliolide in HR Cell Death in Arabidopsis
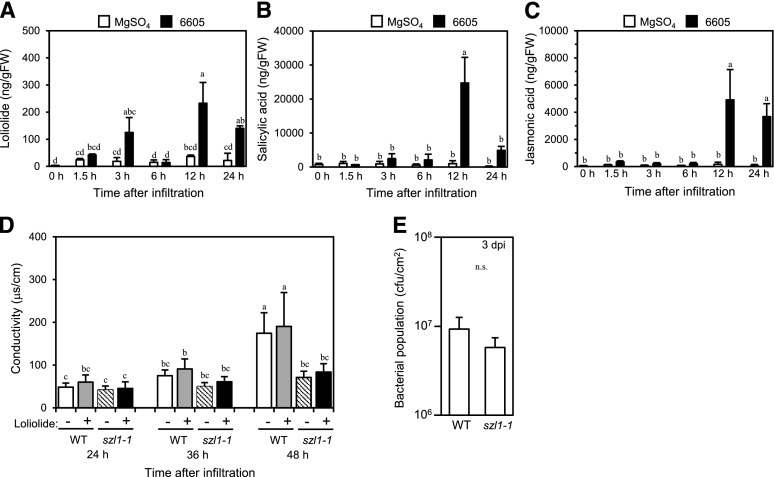
To examine whether loliolide is involved in HR cell death, we used the combination of Arabidopsis and Pseudomonas syringae pv. tabaci 6605, a bacterial strain that causes HR in Arabidopsis (Taguchi and Ichinose, 2011). We initially examined whether loliolide increases in response to P. syringae pv. tabaci 6605 by assessing endogenous loliolide levels after infiltration with the bacterial suspension. Significantly higher loliolide levels were detected 12 and 24 h after infiltration with the strain than after infiltration with MgSO4 (Fig. 7A). We also detected increases in SA (Fig. 7B) and JA (Fig. 7C) in leaves 12 or 24 h after infiltration with P. syringae pv. tabaci 6605. We compared electrolyte leakage, a hallmark of HR cell death, in the P. syringae pv. tabaci 6605-infiltrated leaves of wild-type and szl1-1 plants in the presence or absence of 300 μM loliolide. In the absence of exogenous loliolide, szl1-1 plants exhibited less electrolyte leakage 48 h after infiltration than the wild type (Fig. 7D). The exogenous application of loliolide did not increase electrolyte leakage in wild-type and szl1-1 plants. No significant differences were observed in the bacterial population in P. syringae pv. tabaci 6605-inoculated leaves 3 d after the inoculation between wild-type and szl1-1 plants (Fig. 7E).
Figure 7.
A–C, Analysis of the role of loliolide in HR cell death using Arabidopsis. Concentrations of loliolide (A), SA (B), and JA (C) in Col-0 leaves before (0 h) or after the infiltration with P. syringae pv. tabaci 6605 or 10 mM MgSO4 as a control. D, Conductivity in wild-type and szl1-1 leaves after the infiltration with P. syringae pv. tabaci 6605 in the presence or absence of 300 μM loliolide. E, Bacterial population in wild-type and szl1-1 leaves 3 d after the inoculation with P. syringae pv. tabaci 6605. Values for (A) to (C) are the mean ± sd (n = three replicates), values for (D) are the mean ± sd (n = 12 leaf discs), and values for (E) are the mean ± sd (n = six replicates, t test). Different letters indicate significant differences among treatments (for A–D, P < 0.05, Tukey-Kramer HSD test; for E, P > 0.05, t test). WT, wild type.
DISCUSSION
T. urticae, S. litura, and F. occidentalis exhibit different feeding behaviors. T. urticae feed on leaves, resulting in the appearance of chlorotic spots on the leaf surface (Park and Lee, 2002). S. litura chew leaves, resulting in severe leaf damage resembling the wounds caused by mechanical wounding. F. occidentalis feed on many plant organs, such as leaves, stems, and fruits, resulting in fruit distortion or leaf damage (Mouden et al., 2017). JA has been shown to play an important role in regulating the defense responses of tomato and Arabidopsis to these three different herbivore pests (Kant et al., 2004; Abe et al., 2008, 2009). Our results suggest that loliolide functions as an endogenous signal that mediates host defense responses to these three herbivores and that JA does not appear to be involved in loliolide-induced resistance, at least to F. occidentalis. However, exogenously applied loliolide inhibited the expression of JA- and herbivore-responsive genes, such as SlPin2 and SlLapA1, in tomato. Because endogenous JA levels were not decreased by exogenous loliolide in tomato, loliolide may act to prevent JA signaling pathways, leading to the induction of defense-related genes, at least in this plant species. Further studies are needed to clarify the role of loliolide in JA signaling pathways.
The minimum concentration (300 μM) of exogenously applied loliolide that induced resistance to T. urticae, S. litura, and F. occidentalis was 30-fold greater than the effective concentration (10 μM) of methyl jasmonate (MeJA) that induced resistance to the same herbivores (Kawazu et al., 2012). Thus, the minimum effective concentration of loliolide appears to be relatively low. This may be partially explained by the inhibitory effects of loliolide on the expression of JA-responsive genes, as described above. Alternatively, the stress caused by the detachment of leaves or scission of leaf discs used for our assays may have influenced the effects of loliolide on the induction of host defenses. Further experiments with foliar sprays or absorption through roots using intact plants are needed to clarify the actual minimum effective concentration of exogenous loliolide.
Carotenoids function as antioxidants, photosynthetic components, and precursors of phytohormones, including abscisic acid and strigolactones, in plants (Nisar et al., 2015). They are a family of isoprenoid molecules that are synthesized from isopentenyl phosphate. Isopentenyl phosphate is converted into C20 geranylgeranyl diphosphate through successive condensation reaction steps. The condensation of two geranylgeranyl diphosphates, which is catalyzed by phytoene synthase, produces the first carotenoid C40 phytoene. Phytoene is enzymatically converted into lycopene, a precursor of α-carotene and β-carotene. Our quantitative analysis of endogenous loliolide revealed that szl, an Arabidopsis mutant that carries a point mutation in the lycopene β-cyclase gene, had lower levels of loliolide. This result suggests that loliolide is produced via the β-carotene metabolic pathway and is supported by previous findings showing that loliolide is a degradation product of β-carotene (Repeta, 1989; Rios et al., 2008). However, we synthesized loliolide from lutein, a carotenoid derived from α-carotene, suggesting the ability to produce it via the α-carotene metabolic pathway. Loliolide may be produced via both pathways. β-carotene undergoes oxidation by environmental stresses, such as light and reactive oxygen species, to produce small volatile substances, including β-cyclocitral and β-ionone (Havaux, 2014). β-Cyclocitral has been implicated in reactive oxygen species signaling in plants (Ramel et al., 2012). β-Cyclocitral and β-ionone have been shown to inhibit infestation by herbivore pests (Wei et al., 2011; Nyalala et al., 2013; Cáceres et al., 2016). β-Cyclocitral is known to induce SA signaling in Arabidopsis, and MeJA enhances the emission of β-cyclocitral in rice (Oryza sativa; Tanaka et al., 2014; Lv et al., 2015). Loliolide may have a different mode of action from β-cyclocitral. Dihydroactinidiolide did not exhibit the same activity as loliolide, which suggests a relationship between the structures of loliolide-related compounds and their biological activities.
Our microarray analysis of loliolide-responsive tomato genes suggests the existence of a signal transduction pathway that connects loliolide to the plant cell wall. Cell wall invertases are sucrose-degrading enzymes that bind to the plant cell wall and have been implicated in plant defenses against biotic stresses (Tauzin and Giardina, 2014). Wall-associated receptor kinases are found in the plant cell wall and have been shown to play an important role in regulating defense responses to abiotic and biotic stresses (Kohorn, 2016). Loliolide may be involved in defenses against herbivores through, at least, the activation of cell wall-associated responses.
Despite the accumulation of endogenous loliolide in response to infection with P. syringae pv. tabaci 6605 in wild-type Arabidopsis plants, electrolyte leakage in szl1-1 plants was not restored to wild-type levels by exogenously applied loliolide. This suggests that loliolide only makes a minor contribution to HR cell death caused by this pathogen. The reduction in electrolyte leakage observed in szl1-1 plants may have been due to carotenoid metabolites other than loliolide. Because we originally isolated loliolide from TMV-inoculated tobacco leaves, further studies are needed to clarify whether loliolide is involved in TMV-induced HR cell death.
MATERIALS AND METHODS
Plant Materials, Herbivores, and a Bacterial Strain
Two-month-old tobacco (Nicotiana tabacum cv. NN) plants grown in pots containing disinfected soil in a temperature-controlled greenhouse at 25°C under natural sunlight were used. Four-week-old tomato plants (Solanum lycopersicum cv. Micro-Tom) grown in pots containing disinfected soil under 16 h light/8 h dark at ∼100 μmol photons m−2 s−1 at 25°C were used. Eight-week-old Arabidopsis (Arabidopsis thaliana) plants grown in pots containing disinfected soil under 10-h light/14-h dark at 100–120 μmol photons m−2 s−1 at 22°C were used. In the assay using intact Arabidopsis plants and Frankliniella occidentalis, Arabidopsis plants were grown in pots containing disinfected soil covered with a thin layer of quartz sand to identify thrips that dropped from plants. Arabidopsis szl1-1 was obtained from the Arabidopsis Biological Resource Center. coi1-1 has been described in Seo et al. (2012).
The two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), and the western flower thrip, F. occidentalis (Pergande; Thysanoptera: Thripidae), have been described in Kawazu et al. (2012). The eggs of Spodotera litura (Fabricius; Lepidoptera: Noctuidae) were purchased from Sumika Technoservice.
Pseudomonas syringae pv. tabaci 6605 has been described in Taguchi and Ichinose (2011).
Extraction, Fractionation, and Purification
TMV-inoculated leaves that had been incubated at 20°C for 120 h were cut into small pieces with a razor blade, homogenized in three volumes of cold methanol with a Polytron (Kinematica), and extracted at 4°C for 2 h. After the filtration and concentration of the extract, the remaining aqueous phase was adjusted to pH 3.0 with HCl and partitioned three times with equal volumes of ethyl acetate. The ethyl acetate phase was partitioned twice with equal volumes of 5% (w/v) sodium bicarbonate, and the upper organic phase containing neutral substances was recovered. The lower sodium bicarbonate phase was acidified to pH 2.0 and partitioned with ethyl acetate, while the upper organic phase containing acidic substances was recovered. The two organic phases recovered were combined, dried over anhydrous sodium sulfate, evaporated to dryness, and loaded onto the column (3 × 20 cm) of a silica gel (Wakogel C-200, particle size of 75–150 μm; Wako Pure Chemical). The column was successively eluted with a mixture of N-hexane-acetone (6:1; 3:1; 1:1; 1:3; 1:6, 0:10, each v/v). Fractions eluted with N-hexane-acetone (1:3) and N-hexane-acetone (1:6) were combined and separated on a reversed-phase HPLC column (Atlantis Prep T3 OBD, particle size of 5 μm, 19 mm, 15 cm; Waters). The column was eluted with a mixture of H2O-CH3CN (6:4, v/v) at a flow rate of 10 mL/min, with monitoring at 254 nm. A fraction with retention times of 5.2–6.8 min was separated further on an HPLC column (LiChrospher 100 RP-18, particle size of 5 μm, 4 mm, 25 cm; Agilent) eluted with mobile phase B (H2O:CH3CN, 4:1, v/v) at a flow rate of 0.8 mL/min. A peak fraction with a retention time of 49–53 min was collected to yield 7.5 mg of a colorless gum.
All fractions obtained were evaporated, dissolved in methanol, diluted to appropriate concentrations with water, and used in the infestation assay employing T. urticae.
Spectrometry Analyses of Loliolide
The electron ionization-mass spectrum (MS) of the purified compound showed a molecular ion (M+) at m/z (relative intensity %) 196 (11%) and fragment ions at mass-to-charge ratio (m/z) 178 (67%), 163 (33%), 153 (19%), 140 (45%), 111 (100%), 95 (28%), 85 (22%), 67 (25%), and 57 (25%). The high resolution-electron ionization-MS of the purified compound showed a [M+Na]+ ion at m/z 219.1003, corresponding to a molecular formula of C11H16O3Na with a calculated molecular mass of 219.0992. 1H and 13C NMR (NMR) of the purified compound were recorded at 500 and 125 MHz, respectively, with CDCl3 as solvent. 1H NMR spectra data were as follows: δ1.30 (3H, s, H10), 1.49 (3H, s, H11), 1.56 (1H, dd, H3b), 1.81 (3H, s, H12), 1.81 (1H, dd, H3a), 2.00 (1H, ddd, H1b), 2.48 (1H, ddd, H1a), 4.36 (1H, sept, H2), and 5.78 (1H, s, H6). 13C NMR spectra data were as follows: δ26.5, 27.0, 30.7, 36.0, 45.6, 47.3, 66.6, 86.8, 113.0, 171.9, and 183.0.
Synthesis of Loliolide
A mixture of lutein (500 mg; Ark Pharm) and methylene blue (10 mg; Merck) in 500 mL of chloroform was photooxidized under irradiation with a 37-W fluorescent lamp with vigorous stirring for 24 h. Reaction products were extracted with ethyl acetate. After evaporation, the ethyl acetate extract was subjected to column chromatography with silica gel and eluted with N-hexane-acetone, as described in “Extraction, Fractionation, and Purification.” Fractions eluted with N-hexane-acetone (1:3, v/v) and N-hexane-acetone (1:6, v/v) were combined and subjected to HPLC purification with a reversed-phase column (Sunfire, particle size of 10 μm, 10 mm, 15 cm; Waters) and a solvent mixture (H2O:CH3CN, 7:3, v/v) as a mobile phase to yield 26 mg of loliolide.
Chemicals and Chemical Treatments
Loliolide, dihydroactinidiolide (Santa Cruz Biotechnology), MeJA (Wako Pure Chemical), and SA (Nacalai) were dissolved in methanol and diluted to appropriate concentrations with water. Methanol concentrations did not exceed 0.1% (v/v) in any experiment.
Herbivore Infestation
Tomato leaves were excised with scissors from the 3- to 5-leaf positions of plants, and 15–20 leaves were floated on 80 mL of a solution containing the fraction, loliolide, or dihydroactinidiolide in a glass dish (16 cm in diameter, 4 cm in depth) at 25°C for 24 h under 16-h light/8-h dark. After briefly washing with distilled water to remove the chemical solution, each leaf was used for the assays with T. urticae or S. litura.
In the assay using T. urticae, newly emerged adult females were allowed to couple with males for 3 d, and 3-d-old females were then used in assays. We defined the day of adult emergence as 1 d old in this study. One female mite per leaf was released onto the surface of the leaf confined within modified Munger cells (Supplemental Fig. S1; Munger, 1942) and incubated at 25°C under 16-h light/8-h dark. The numbers of surviving individuals and eggs laid were counted 5 d after the inoculation. We used 30 mites and 30 detached leaves for each chemical concentration.
In the assay using S. litura, 10 hatchlings were released onto the surface of two tomato leaves with petioles that were inserted into a 1.5-mL microtube filled with distilled water to prevent water loss from leaves during the incubation and then incubated in a sealed plastic cup (9 cm in diameter; 14 cm in height) at 25°C under 16-h light/8-h dark. The numbers of surviving individuals and larval instars were counted 5 d after the inoculation. We regarded the combination of 10 hatchlings and two detached leaves as one biological replicate and used 10 replicates for each chemical concentration in Figure 3, D and E. In the measurement of loliolide in Figure 4A, 10 hatchlings were released onto the surface of two detached tomato leaves that had not been treated with loliolide and incubated as described above, and 10 replicates (100 hatchlings and 20 leaves) were used for the measurement.
The assay using F. occidentalis was conducted using the method described by Abe et al. (2009) with slight modifications. Leaf discs (1 cm in diameter) were punched out from intact Arabidopsis plants (three to five discs from one plant), and one 7- to 14-d-old adult female was allowed to lay eggs on the surface of one leaf disc that was floated on 0.8 mL of distilled water, 300 μM loliolide solution, or 0.1% (v/v) methanol in one well of a 48-well polystyrene plate (1.0 cm2/well; CELLSTAR 48W, Greiner Bio-One). The plate was covered with a plastic film (ABI Prism Optical Adhesive Cover, Applied Biosystems), and seven small holes per well were punctuated with a 27G injection needle for ventilation. The plate was incubated at 25°C. Three days after the inoculation, leaf discs were stained with trypan blue, and the numbers of stained eggs were counted. We regarded the combination of one female and one leaf disc as one biological replicate.
In the experiments shown in Figure 6K using F. occidentalis, 30 adult females were allowed to feed on each whole plant in an acryl cylinder chamber with air ventilation windows covered with a fine mesh, and the leaves were harvested 24 h after inoculation and used to measure loliolide. We regarded the combination of 30 females and one plant as one biological replicate.
Insecticidal Activity Assays
Adult female T. urticae mites were dropped into a solution containing 300 μM loliolide for 5 s, and the number of surviving mites 48 h after the treatment was counted.
In the assay using S. litura, loliolide (final concentration of 300 μM) or methanol (final concentration of 0.1% [v/v]) was added to an artificial diet (Insecta LFM, Nosan; 10 larvae per diet), and the diet was divided into 10 pieces. The first-instar larvae of S. litura were reared on the pieces (10 larvae per piece). The number of surviving larvae from 100 larvae was counted 9 d after the application of the diet.
Oral Secretion Application and Wound Stress Treatments
Oral secretions from S. litura larvae were collected and stored at −80°C until use. In the application of oral secretions, two leaves were detached from one tomato plant, and 20 holes (0.5 mm in diameter) per leaf were made using a needle. Punctuated leaves were floated on a 100-fold-diluted aqueous solution (5 mL per leaf) of oral secretions or water as a control in a petri dish at 25°C for 6 h. Six to eight leaf discs (8 mm in diameter) were punched out from the leaf area with holes from two treated leaves and used to measure loliolide. Six to eight leaf discs were regarded as one biological replicate.
The wounding of tomato leaves was performed by puncturing with a needle as described above, and the wounded leaves were floated on 0.1% (v/v) methanol solution.
In the experiments shown in Figure 6, I and J, three to four leaves per one Arabidopsis plant were wounded with forceps (∼30% of the leaf area). The wounded plants were incubated in a plastic box with 100% humidity, and the wounded leaves were harvested at the indicated time intervals and used to measure loliolide. We regarded one plant as one biological replicate.
Measurements of Loliolide and Phytohormones
In the measurement of loliolide, plant materials were ground in liquid nitrogen with a mortar and pestle and suspended in five volumes of cold 80% (v/v) acetone in 50 mM anhydrous citric acid. In this step, 100 ng of coumarin-5,6,7,8-d4 (CDN Isotopes) was added to the extract to estimate the recovery rate of loliolide during the purification procedure. The extract was concentrated under a stream of nitrogen gas, and the remaining aqueous phase was extracted three times with diethyl ether. After passing through anhydrous sodium sulfate and concentrating, as described above, the remaining residue was dissolved in ethyl acetate and subjected to gas chromatography–mass spectrometry analysis. Gas chromatography–mass spectrometry was performed on an Agilent 7890 gas chromatography system equipped with a 5975C mass selective detector (Agilent). Separation was performed on a capillary column (HP-1MS, length of 30 m, i.d. of 0.25 mm, thickness of 0.25 mm, Agilent) with He as the carrier gas at a flow rate of 1 mL/min. The oven temperature was held at 40°C for 1 min, increased to 200°C at 10°C/min, then increased to 280°C at 8°C/min, and held for 5 min. The injection temperature was 250°C. Selected ion monitoring was used for data acquisition at m/z 196, 178, and 111 for loliolide and m/z 150, 122, and 94 for coumarin-5,6,7,8-d4. The retention times of loliolide and coumarin-5,6,7,8-d4 were 16.8 and 13.1 min, respectively.
In the measurement of JA and SA, six leaf discs (8 mm in diameter) were punched out from three intact tomato plants, floated on 3 mL of a solution containing 300 μM loliolide or 0.1% (v/v) methanol in one well of a 6-well polystyrene plate (34.8 mm in diameter, 17.4 mm depth), incubated at 25°C for 12 h, and used for the extraction of JA and SA. We regarded six leaf discs as one biological replicate. The extraction and quantification of JA and SA were performed in accordance with the procedure described by Seo et al. (2012).
Microarray Analysis
The leaves of tomato plants were treated with 300 μM loliolide or 0.1% (v/v) methanol and incubated for 12 h. Total RNA was extracted using TRIzol reagent (Invitrogen) followed by RNA purification columns (RNeasy, Qiagen) and labeled with cyanine dye 3 (Cy3) using Quick Amp Labeling (Agilent) for a one-color experiment. The hybridization of individually labeled cRNAs to microarrays (Tomato Oligo DNA microarray, Agilent), scanning of the hybridized arrays, and the extraction of data from the scanned images were performed in accordance with the procedure described by Seo et al. (2012). Fold changes were calculated as a ratio between the signal averages of three biological replicates of the loliolide treatment and methanol. Microarray data were deposited in the public Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) and have been assigned the accession number GSE115942.
Reverse Transcription Quantitative-PCR Analysis
Six leaf discs (8 mm in diameter) were punched out from three intact tomato plants, floated on 3 mL of a solution containing various concentrations of loliolide, MeJA, or SA in one well of a 6-well polystyrene plate (34.8 mm in diameter, 17.4 mm depth), incubated at 25°C for 12 h, and used for the extraction of total RNA. We regarded six leaf discs as one biological replicate and used three replicates for each chemical concentration.
A reverse transcription quantitative-PCR analysis using total RNA was performed in a two-step reaction using a SYBR Green kit (Bio-Rad) in accordance with the procedure described by Kawazu et al. (2012). Information concerning the primers used is shown in Supplemental Table S2. The expression levels of Slactin were used to normalize those of the target genes.
Bacterial Infiltration, Measurement of Electrolyte Leakage, and Bacterial Growth Assay
The leaves of Arabidopsis plants were infiltrated with a suspension of P. syringae pv. tabaci 6605 in 10 mM MgSO4 at a density of 2 × 108 cfu/mL or 10 mM MgSO4 as a control and used for measurements of electrolyte leakage, loliolide, or phytohormones. We used two leaves per plant for infiltration.
Leaf discs (8 mm in diameter) were punched out from infiltrated leaves (two discs from one plant), and one disc per well was floated on 2 mL of sterilized distilled water in a 12-well polystyrene plate (22.1 mm in diameter, 17.4 mm depth) at 22°C for 60 min. After discarding water using a pipette, 1 mL of a solution containing 300 μM loliolide or 0.1% (v/v) methanol was added to each well, and the plate was incubated. An aliquot of the sample solution was subjected to the measurement of electrolyte leakage using a portable conductivity meter at the indicated time intervals. We used 12 discs from six plants for each treatment.
In the measurement of loliolide and phytohormones, the plants were allowed to dry after infiltration for ∼30 min and incubated in a plastic box covered with a polyethylene wrap at 22°C. We regarded six infiltrated leaves (from three plants) as one biological replicate and used three replicates for the measurement.
In the bacterial growth assay, leaf discs (8 mm in diameter) punched out from Arabidopsis plants (four discs from one plant) were immersed into a bacterial suspension in 10 mM MgSO4 at a density of 2 × 108 cfu/mL for 1 h. The discs were placed on filter paper moistened with sterilized distilled water and incubated at 22°C for 3 d. After sterilizing the leaf surface with H2O2, four leaf discs were ground with 1 mL of sterilized distilled water in a mortar and serially diluted in water. An aliquot of diluted bacterial solutions was spread on a LB agar plate and incubated at 25°C for 2 d, and the numbers of colonies on the plate were then counted. Each diluted sample was analyzed in duplicate. Four leaf discs from one plant were regarded as one biological replicate.
Statistical Analyses
We compared survival rates in the infestation assay using the χ2 test followed by Ryan’s multiple range test for proportions (Ryan, 1960). We used Fisher’s exact probability test to compare survival rates in the dipping assay. These analyses were conducted using R version 3.3.3 (R Development Core Team, 2017). Differences in the number of eggs were tested by a one-way analysis of variance and then compared using the Tukey-Kramer honestly significant difference (HSD) test. This analysis was conducted with JMP version 9.0.2 (SAS Institute). The Student’s t test was used to compare the significance of the difference in the mean of two samples.
Accession Numbers
Sequence data from this article can be found in the GenBank/European Molecular Biology Laboratory data libraries under accession numbers K03291 (SlPin2), NM_001246933 (SlLapA1), M80608 (SlGluB), NM_001247864 (LIT8), XM_004246272 (LIT13), and BT012695 (Slactin).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Flow diagram showing key steps in fractionation and purification of herbivore-resistance–inducing substances.
Supplemental Figure S2. 1H and 13C NMR spectra of loliolide.
Supplemental Figure S3. Effects of dihydroactinidiolide on the infestation of tomato leaves by T. urticae.
Supplemental Figure S4. Effects of synthetic loliolide on the infestation of tomato leaves by T. urticae.
Supplemental Figure S5. Effects of loliolide on the accumulation of JA and SA in tomato leaves.
Supplemental Table S1. List of candidates for loliolide-responsive tomato genes identified by the microarray analysis.
Supplemental Table S2. List of primers used in this study.
Acknowledgments
We thank S. W. Hong and N. Tajima for their assistance with the purification, spectrometric analysis, and synthesis of loliolide; N. Hinomoto for providing T. urticae; Genebank Project, National Agriculture and Food Research Organization for providing F. occidentalis; M. Teruse for assistance with the gene expression analysis; the Leaf Tobacco Research Laboratory of Japan Tobacco Inc. for providing P. syringae pv. tabaci 6605; and the Arabidopsis Biological Resource Center for providing szl1-1.
Footnotes
This work was supported by The Japanese Program for the Promotion of Basic and Applied Researches for Innovations in Bio-oriented Industry (BRAIN) and the Ministry of Education, Culture, Sports, Science and Technology (MEXT KAKENHI grant no. 25292037).
Articles can be viewed without a subscription.
References
- Abe H, Ohnishi J, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M (2008) Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol 49: 68–80 [DOI] [PubMed] [Google Scholar]
- Abe H, Shimoda T, Ohnishi J, Kugimiya S, Narusaka M, Seo S, Narusaka Y, Tsuda S, Kobayashi M (2009) Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol 9: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MX, Hamberg M, Kourtchenko O, Brunnström A, McPhail KL, Gerwick WH, Göbel C, Feussner I, Ellerström M (2006) Oxylipin profiling of the hypersensitive response in Arabidopsis thaliana. Formation of a novel oxo-phytodienoic acid-containing galactolipid, arabidopside E. J Biol Chem 281: 31528–31537 [DOI] [PubMed] [Google Scholar]
- Behr D, Wahlberg I, Nishida T, Enzell CR (1973) Tobacco chemistry. 50. (3S,5R,8S,9ξ)-5,8-Epoxy-6-megastigmene-3,9-diol and (3S*,5R*,6R*,7E,9ξ)-3,6-Epoxy-7-megastigmene-5,9-diol. Two new nor-carotenoids of Greek tobacco. Acta Chem Scand B 33: 701–704 [Google Scholar]
- Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K (2017) Evolution of hormone signaling networks in plant defense. Annu Rev Phytopathol 55: 401–425 [DOI] [PubMed] [Google Scholar]
- Cáceres LA, Lakshminarayan S, Yeung KKC, McGarvey BD, Hannoufa A, Sumarah MW, Benitez X, Scott IM (2016) Repellent and attractive effects of α-, β-, and dihydro-β-ionone to generalist and specialist herbivores. J Chem Ecol 42: 107–117 [DOI] [PubMed] [Google Scholar]
- Chao WS, Gu YQ, Pautot V, Bray EA, Walling LL (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol 120: 979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman MB, Fluhr R (2013) Centrality of host cell death in plant-microbe interactions. Annu Rev Phytopathol 51: 543–570 [DOI] [PubMed] [Google Scholar]
- Friedrich L, Lawton K, Ruess W, Masner P, Specker N, Gut Rella M, Meier B, Dincher S, Staub T, Ukness S, et al. (1996) A benzothiadiazole derivative induces systemic acquired resistance in tobacco. Plant J 10: 61–70 [Google Scholar]
- Fürstenberg-Hägg J, Zagrobelny M, Bak S (2013) Plant defense against insect herbivores. Int J Mol Sci 14: 10242–10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarczyk M, Wińska K, Mączka W, Potaniec B, Anioł M (2015) Loliolide—the most ubiquitous lactone. Acta Univ Lodz Folia Biol Oecol 11: 1–8 [Google Scholar]
- Havaux M. (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79: 597–606 [DOI] [PubMed] [Google Scholar]
- He Z, Zhang A, Ding L, Lei X, Sun J, Zhang L (2010) Chemical composition of the green alga Codium Divaricatum Holmes. Fitoterapia 81: 1125–1128 [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Baldwin IT, Wu J (2013) Nicotiana attenuata MPK4 suppresses a novel jasmonic acid (JA) signaling-independent defense pathway against the specialist insect Manduca sexta, but is not required for the resistance to the generalist Spodoptera littoralis. New Phytol 199: 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Iwasaki A, Suenaga K, Kato-Noguchi H (2017) Isolation and identification of two potential phytotoxic substances from the aquatic fern Marsilea crenata. J Plant Biol 60: 75–81 [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC (2004) Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol 135: 483–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu K, Mochizuki A, Sato Y, Sugeno W, Murata M, Seo S, Mitsuhara I (2012) Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod-Plant Interact 6: 221–230 [Google Scholar]
- Kenton P, Mur LAJ, Atzon R, Wasternack C, Draper J (1999) Jasmonic acid accumulation in tobacco hypersensitive response lesions. Mol Plant Microbe Interact 12: 74–48 [Google Scholar]
- Kimura J, Maki N (2002) New loliolide derivatives from the brown alga Undaria pinnatifida. J Nat Prod 65: 57–58 [DOI] [PubMed] [Google Scholar]
- Kiraly L, Barnaz B, Kiralyz Z (2007) Plant resistance to pathogen infection: Forms and mechanisms of innate and acquired resistance. J Phytopath 155: 385–396 [Google Scholar]
- Kohorn BD. (2016) Cell wall-associated kinases and pectin perception. J Exp Bot 67: 489–494 [DOI] [PubMed] [Google Scholar]
- Li Z, Ahn TK, Avenson TJ, Ballottari M, Cruz JA, Kramer DM, Bassi R, Fleming GR, Keasling JD, Niyogi KK (2009) Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell 21: 1798–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv F, Zhou J, Zeng L, Xing D (2015) β-cyclocitral upregulates salicylic acid signalling to enhance excess light acclimation in Arabidopsis. J Exp Bot 66: 4719–4732 [DOI] [PubMed] [Google Scholar]
- Mori K, Khlebnikov V (1993) Carotenoids and degraded carotenoids, VIII-synthesis of (+)-dihydroactinidiolide, (+)- and (–)-actinidiolide, (+)- and (–)-loliolide as well as (+)-and (–)-epiloliolide. Liebigs Ann Chem 1993: 77–82 [Google Scholar]
- Mouden S, Sarmiento KF, Klinkhamer PGL, Leiss KA (2017) Integrated pest management in western flower thrips: Past, present and future. Pest Manag Sci 73: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger F. (1942) A method of rearing thrips in the laboratory. J Econ Entomol 35: 373–375 [Google Scholar]
- Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8: 68–82 [DOI] [PubMed] [Google Scholar]
- Noutoshi Y, Okazaki M, Kida T, Nishina Y, Morishita Y, Ogawa T, Suzuki H, Shibata D, Jikumaru Y, Hanada A, et al. (2012) Novel plant immune-priming compounds identified via high-throughput chemical screening target salicylic acid glucosyltransferases in Arabidopsis. Plant Cell 24: 3795–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyalala SO, Petersen MA, Grout BWW (2013) Volatile compounds from leaves of the African spider plant (Gynandropsis gynandra) with bioactivity against spider mite (Tetranychus urticae). Ann Appl Biol 162: 290–298 [Google Scholar]
- Okunade AL, Weimer DF (1985) Loliolide, an ant-repellent compound from Xanthoxyllum setulosum. J Nat Prod 48: 472–473 [Google Scholar]
- Pan L, Sinden MR, Kennedy AH, Chai H, Watson LE, Graham TL, Kinghorn AD (2009) Bioactive constitutes of Helianthus tuberosus (Jerusalem artichoke). Phytochem Lett 2: 15–18 [Google Scholar]
- Park YL, Lee JH (2002) Leaf cell and tissue damage of cucumber caused by two-spotted spider mite (Acari: Tetranychidae). J Econ Entomol 95: 952–957 [DOI] [PubMed] [Google Scholar]
- Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylidès C, Havaux M (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci USA 109: 5535–5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. (March 8, 2017)
- Repeta DJ. (1989) Carotenoid diagenesis in recent marine sediments: II. Degradation of fucoxanthin to loliolide. Geochim Cosmochim Acta 53: 699–707 [Google Scholar]
- Rios JJ, Fernández-García E, Mínguez-Mosquera MI, Pérez-Gálvez A (2008) Description of volatile compounds generated by the degradation of carotenoids in paprika, tomato and marigold oleoresins. Food Chem 106: 1145–1153 [Google Scholar]
- Ryan TA. (1960) Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull 57: 318–328 [DOI] [PubMed] [Google Scholar]
- Schmelz EA. (2015) Impacts of insect oral secretions on defoliation-induced plant defense. Curr Opin Insect Sci 9: 7–15 [DOI] [PubMed] [Google Scholar]
- Seo S, Gomi K, Kaku H, Abe H, Seto H, Nakatsu S, Neya M, Kobayashi M, Nakaho K, Ichinose Y, et al. (2012) Identification of natural diterpenes that inhibit bacterial wilt disease in tobacco, tomato and Arabidopsis. Plant Cell Physiol 53: 1432–1444 [DOI] [PubMed] [Google Scholar]
- Seo S, Nakaho K, Hong SW, Takahashi H, Shigemori H, Mitsuhara I (2016) L-Histidine induces resistance in plants to the bacterial pathogen Ralstonia solanacearum partially through the activation of ethylene signaling. Plant Cell Physiol 57: 1932–1942 [DOI] [PubMed] [Google Scholar]
- Sun TJ, Lu Y, Narusaka M, Shi C, Yang YB, Wu JX, Zeng HY, Narusaka Y, Yao N (2015) A novel pyrimidin-like plant activator stimulates plant disease resistance and promotes growth. PLoS One 10: e0123227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suza WP, Staswick PE (2008) The role of JAR1 in Jasmonoyl-l-isoleucine production during Arabidopsis wound response. Planta 227: 1221–1232 [DOI] [PubMed] [Google Scholar]
- Taguchi F, Ichinose Y (2011) Role of type IV pili in virulence of Pseudomonas syringae pv. tabaci 6605: correlation of motility, multidrug resistance, and HR-inducing activity on a nonhost plant. Mol Plant Microbe Interact 24: 1001–1011 [DOI] [PubMed] [Google Scholar]
- Tanaka R, Matsunaga S (1989) Loliolide and olean-12-en-3β,9α,11α-triol from Euphorbia supina. Phytochemistry 28: 1699–1702 [Google Scholar]
- Tanaka K, Taniguchi S, Tamaoki D, Yoshitomi K, Akimitsu K, Gomi K (2014) Multiple roles of plant volatiles in jasmonate-induced defense response in rice. Plant Signal Behav 9: e29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kan JAL, Cozijnsen T, Danhash N, De Wit PJGM (1995) Induction of tomato stress protein mRNAs by ethephon, 2,6-dichloroisonicotinic acid and salicylate. Plant Mol Biol 27: 1205–1213 [DOI] [PubMed] [Google Scholar]
- Walling LL. (2000) The myriad plant responses to herbivores. J Plant Growth Regul 19: 195–216 [DOI] [PubMed] [Google Scholar]
- War AR, Paulraj MG, Ahmad T, Buhroo AA, Hussain B, Ignacimuthu S, Sharma HC (2012) Mechanisms of plant defense against insect herbivores. Plant Signal Behav 7: 1306–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasternack C, Strnad M (2016) Jasmonate signaling in plant stress responses and development—active and inactive compounds. N Biotechnol 33(5 Pt B): 604–613 [DOI] [PubMed] [Google Scholar]
- Wei S, Hannoufa A, Soroka J, Xu N, Li X, Zebarjadi A, Gruber M (2011) Enhanced β-ionone emission in Arabidopsis over-expressing AtCCD1 reduces feeding damage in vivo by the crucifer flea beetle. Environ Entomol 40: 1622–1630 [DOI] [PubMed] [Google Scholar]