ABSTRACT
Aposematic theory has historically predicted that predators should select for warning signals to converge on a single form, as a result of frequency‐dependent learning. However, widespread variation in warning signals is observed across closely related species, populations and, most problematically for evolutionary biologists, among individuals in the same population. Recent research has yielded an increased awareness of this diversity, challenging the paradigm of signal monomorphy in aposematic animals. Here we provide a comprehensive synthesis of these disparate lines of investigation, identifying within them three broad classes of explanation for variation in aposematic warning signals: genetic mechanisms, differences among predators and predator behaviour, and alternative selection pressures upon the signal. The mechanisms producing warning coloration are also important. Detailed studies of the genetic basis of warning signals in some species, most notably Heliconius butterflies, are beginning to shed light on the genetic architecture facilitating or limiting key processes such as the evolution and maintenance of polymorphisms, hybridisation, and speciation. Work on predator behaviour is changing our perception of the predator community as a single homogenous selective agent, emphasising the dynamic nature of predator–prey interactions. Predator variability in a range of factors (e.g. perceptual abilities, tolerance to chemical defences, and individual motivation), suggests that the role of predators is more complicated than previously appreciated. With complex selection regimes at work, polytypisms and polymorphisms may even occur in Müllerian mimicry systems. Meanwhile, phenotypes are often multifunctional, and thus subject to additional biotic and abiotic selection pressures. Some of these selective pressures, primarily sexual selection and thermoregulation, have received considerable attention, while others, such as disease risk and parental effects, offer promising avenues to explore. As well as reviewing the existing evidence from both empirical studies and theoretical modelling, we highlight hypotheses that could benefit from further investigation in aposematic species. Finally by collating known instances of variation in warning signals, we provide a valuable resource for understanding the taxonomic spread of diversity in aposematic signalling and with which to direct future research. A greater appreciation of the extent of variation in aposematic species, and of the selective pressures and constraints which contribute to this once‐paradoxical phenomenon, yields a new perspective for the field of aposematic signalling.
Keywords: aposematism, continuous variation, polymorphism, polytypism
I. INTRODUCTION
Aposematic prey use warning signals to advertise their defences or unprofitability to potential predators (Poulton, 1890; Cott, 1940). Since Fritz Müller's (1879) first insights into the dynamics of aposematic species, selection from predators has generally been assumed to favour convergence in warning signals, as this decreases prey mortality during predator avoidance learning (Endler & Greenwood, 1988; Ruxton, Sherratt & Speed, 2004; Sherratt, 2008). Traditional theory holds that aposematic prey benefit from ‘strength in numbers’, as predators should learn an association between a signal and an aversive stimulus more rapidly and more effectively if they encounter it with greater frequency. Conversely, any aberrant forms of the warning signal, deviating from the ‘normative’ pattern (the average pattern or most common morph in the population) should increase mistaken attacks by predators, decreasing the effectiveness and speed of predator learning. Individuals with the ‘normative’ pattern thus benefit from the frequency of that phenotype and incur a reduced predation rate, whereas aberrant individuals do not have this benefit. Therefore, natural selection is thought to disfavour variation in aposematic patterns and favour monomorphism in warning signals (Poulton, 1890) – a hypothesis supported by many examples from the field (e.g. Mallet & Barton, 1989; Borer et al., 2010; Chouteau, Arias & Joron, 2016). As a result, variation in aposematic signals has historically been considered paradoxical.
Nevertheless, variation in warning signals is found at several levels, from individual to population and species‐level differences, and recent research has led to a renewed interest in this diversity (Arenas & Stevens, 2017). The degree to which any one aposematic pattern enhances fitness is a product of many different selective pressures, ranging from predator–prey interactions and environmental conditions to trade‐offs with other signal functions (Ojala, Lindström & Mappes, 2007). In this review, we bring together some of the latest findings of experimental and theoretical work to address the role of these selection pressures, and help resolve the apparent paradox of variation in aposematic phenotypes. While aposematic signallers can utilise multiple modalities (e.g. visual displays, odours, sounds, behaviours), simultaneously or sequentially (Rowe & Halpin, 2013), visual signals have received the most attention, so we have focused our discussion on variation in colour and pattern in aposematic animals (see examples of aposematic variation in Fig. 1).
Figure 1.

(A) White, yellow, and yellow/red morphs of the wood tiger moth (Arctia plantaginis) each vary in the extent of their melanisation. (B) The two‐spot ladybird (Adalia bipunctata) has numerous morphs including the typical melanic and non‐melanic forms shown here. (C) Morphs of the polytypic poison frog Ranitomeya imitator. (D) Continuous variation in stripe length and width in the North American striped skunk (Mephitis mephitis).
Before addressing the processes underpinning variation in warning signals, it is necessary to understand the levels at which it occurs (Fig. 2). Warning coloration can vary allopatrically between different populations of the same species [e.g. polytypism (Mayr, 1963)] or sympatrically within populations [e.g. polymorphism (Ruxton et al., 2004)]. The conspicuousness of signals, influenced by traits such as luminance and saturation, may also vary continuously among individuals of the same morph, temporally within a single individual across seasons or its life cycle, and among populations across a species' distribution range, forming a cline. Adding further complexity, more than one form of variation can occur simultaneously, and different components of the warning signal, such as size, pattern and hue, can vary independently, according to separate proximate mechanisms. Variation can be genetically determined (i.e. fixed), plastic, or shaped by the interaction of genes and the environment. The harlequin ladybird (Harmonia axyridis) for example, has multiple genetically determined morphs (Komai, 1956), but the extent of melanism within morphs has been shown to vary with developmental temperature (Knapp & Nedvěd, 2013). Considerable variation in aposematic signals is most difficult to explain at the intra‐population level, when alternative warning signal phenotypes co‐occur in single location (polymorphism, polyphenism, and continuous variation; Fig. 2). We have therefore focused our review on making sense of this poorly understood yet remarkably common phenomenon.
Figure 2.
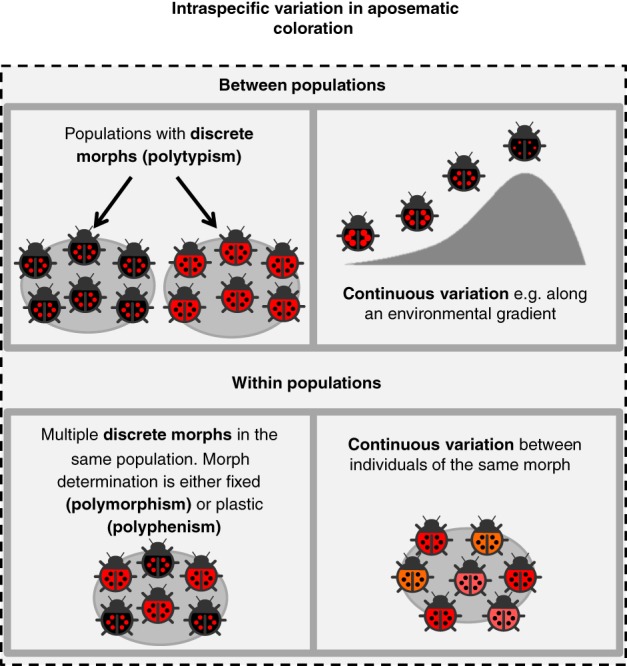
The levels of diversity in warning coloration discussed herein and associated terminology, with a hypothetical example using a single species of ladybird beetle.
Here we show how the complex biotic and abiotic environments in which species live give rise to a myriad of different selection pressures, which in turn lead to diversity in warning signals. This provides a general conceptual framework to explain when and why variation in aposematic patterns might exist. We begin by discussing the theory behind warning signal variation, then the demographic and genetic architecture that underpins it, before moving on to consider how variability in predation pressures can favour variation in warning signals, as opposed to monomorphy, even in mimicry systems (see Fig. 3 for mimicry). We then review how the multifunctionality of colour patterns can shape and favour diversity in aposematic signals. Finally, we summarise known cases of signal variation in aposematic species and discuss the taxonomic limitations of our current understanding of the diversity of warning signals. To showcase where and when warning signal variation occurs, and highlight possible systems in need of further study, we compiled a table of aposematic species in which variation has been described in the existing literature (see online Appendix S1 and Table S1). We find examples of warning signal variation in nearly every taxon in which we find aposematism (see online Table S1), suggesting that variation in warning signals is far more widespread than previously appreciated. Altogether, this review aims to demonstrate that variation in aposematic signalling should no longer be considered paradoxical, a new perspective that stands to advance our understanding of aposematic signalling.
Figure 3.

Definitions of the forms of mimicry discussed in this review.
II. THEORY
Explaining the existence of phenotypic variation in the face of selection has long challenged evolutionary biologists and theoreticians (Bull, 1987; Roulin, 2004). The outstanding colour variation in aposematic species has been viewed as particularly problematic due to the pervasive view of predators as a ‘purifying’ selective pressure moving warning coloration towards monomorphism (Mallet & Joron, 1999). The majority of theoretical work investigating the factors that determine such colour variation focuses on Müllerian mimicry (Joron & Mallet, 1998; Sherratt, 2008), involving the evolution and maintenance of a shared warning signal in sympatric, aposematic species (Müller, 1879). While it may seem counterintuitive to discuss the theory behind the evolution of similarity to understand how variation might arise and be maintained, the factors responsible for creating or reducing variation in signal form are likely to be closely linked. That is, selection pressures for or against mimicry and within‐species ‘purifying’ selection may have many features in common.
Early models predicted that when there are multiple morphs present (whether they belong to one species or multiple species), an adaptive landscape characterized by multiple fitness peaks is generated, and predators should act to push the population as a whole to the highest adaptive peak by removing morphs defining lower adaptive peaks (generally the less common morphs), particularly when there are numerous prey types (e.g. Sherratt, 2002; Beatty, Beirinckx & Sherratt, 2004; Ruxton et al., 2004). In a similar fashion, if variation within a population is not discrete, and the peaks are short with wide tails, then predators should push the population's adaptive peak up by removing outliers, i.e. those individuals most different from the ‘norm’ (Sherratt, 2006). Furthermore, where discrete variation occurs, the different phenotypes should evolve towards similarity as long as there is protective overlap between these distinct phenotypes in peak space, except when the phenotype is determined by a single locus (Turner, 1983). This occurs because overlapping space in the fitness landscape increases survival, and individuals that become increasingly more similar have overall higher survival (Mallet & Joron, 1999). This situation should only arise where there is a sufficient amount of overlap in fitness peaks in the adaptive landscape – if there is barely any overlap then the selection acting against phenotypes in the overlap area should be similar to that of a novel, unprotected form. In general, this scenario is more likely when there is one adaptive peak that is higher than others due to either population size or higher toxin load, in which case it should ‘capture’ the alternative species/morph (Turner, 1983).
These models predict that intraspecific warning signal variation would only persist under certain conditions. Firstly, variation can be maintained where population sizes are large (Plowright & Owen, 1980) and there is spatial or temporal variation in local predator communities combined with simple drift, resulting in a mosaic of different phenotypes (Franks & Noble, 2004; Ruxton et al., 2004; Sherratt, 2006). Secondly, and slightly more contentiously, new morphs could arise and reach high local frequency through mechanisms such as bottlenecks, drift, mutation, via fluctuations in local ecological factors, or through relaxed selection due to a decline in predator abundance, causing peak shifts and the creation of new adaptive peaks (Turner & Mallet, 1996). Notably, the exact mechanisms by which this occurs are rarely covered in any greater detail than the above list, and are often treated as a ‘black box’. Herein, we attempt to flesh out both the mechanisms and circumstances that may lead to the creation and maintenance of these new phenotypes and corresponding adaptive peaks.
Once new peaks are created, theory posits that local predators should exert uniform, frequency‐dependent selection for all conspicuous species/morphs towards this new peak (Sheppard et al., 1985). This stabilising selection can then work on surrounding populations via movement of hybrid clines or individuals migrating into new populations. This idea is known as shifting balance, and has been implicated in the evolution of geographical mosaic patterns in aposematic species and mimicry rings (Brown, Sheppard & Turner, 1974; Turner, 1983; Mallet, 2010; Chouteau & Angers, 2012). A key prediction of the shifting balance idea is that any form of polymorphism should be strongly selected against, and therefore temporary. Similarly, continuous variation in the aposematic signal should be generally selected against as stabilising selection should remove the most‐different individuals (i.e. those furthest from the ‘average’ appearance). This, of course, depends on predators being able to discriminate against and remember subtle differences in aposematic signal over time (see Section IV and Sherratt & Peet‐Paré, 2017).
Unfortunately, very little of the warning colour variation observed in wild populations meets the conditions outlined above. For example, multiple morphs of the same species are frequently found existing in the same locality (e.g. Brown & Benson, 1974; Borer et al., 2010), often at low densities and/or low frequencies within a population (Chouteau et al., 2017). Furthermore, the idea that such polymorphisms are likely to be transient and unstable has also been empirically challenged; for example, polymorphism in the poison frog Oophaga pumilio has been persistent on Bastimentos Island in Panama (Richards‐Zawacki, Yeager & Bart, 2013) and relaxed selection resulting from a decrease in predators produces a vastly reduced predation rate even on novel or intermediate forms (Chouteau & Angers, 2012). The mismatch between theory and empirical examples is in part due to the overly simplistic assumptions made about predator behaviour in earlier models. It is increasingly apparent that predator behaviour is more complex than early evolutionary models of warning coloration and mimicry allowed (Sherratt, 2008; Skelhorn, Halpin & Rowe, 2016), such as the early (and incorrect) assumption that predators sample a fixed number of prey to learn the association between signals and unprofitability (Rowland et al., 2010a). The incorporation of some of this complexity in predator behaviour into models, e.g. optimal predator sampling strategies based on exploration–exploitation trade‐offs (Sherratt, 2011), has started to close the gap between theory and empirical examples resulting in scenarios where warning colour variation is predicted to arise within and among species (Aubier & Sherratt, 2015; Kikuchi & Sherratt, 2015).
As these more recent theoretical models demonstrate, less‐paradoxical predictions about the emergence and maintenance of multiple fitness peaks in warningly coloured species can be generated by incorporating predictions derived from empirical work on the complexity of predator behaviour. However, there is still a great deal of nuance in predator behaviour that has yet to be captured in theoretical models (see Section IV). It is also important to note that genetic mechanisms may facilitate or constrain variation (McLean & Stuart‐Fox, 2014; see Section III) and that independent fitness peaks can easily be reinforced by alternative biotic and abiotic selection pressures (other than predation) that may also act upon warning coloration (Calsbeek, Hasselquist & Clobert, 2010; see Section V). Below we outline these and other factors that future models could take into consideration, hopefully facilitating convergence of model predictions with the variation observable in the warning coloration of aposematic species.
III. EVOLUTIONARY AND GENETIC CONSTRAINTS ON WARNING‐COLOUR DIVERSITY
Studies of the proximate mechanisms underlying aposematic variation have a limited taxonomic scope (but see Section VI and see online Table S1 for more possibilities), focusing primarily on Heliconius butterflies. Thus our review of the genetic and developmental pathways engendering diversity in warning colours is similarly largely based on insights gained from Heliconius.
(1). Geographic isolation and range shifts
Many of the well‐studied polymorphic/polytypic aposematic species occur in the Neotropics, and consequently early hypotheses explaining polymorphisms and polytypisms relied on the Pleistocene refugium theory (Turner, 1965; Brown, 1979). This theory states that high rates of allopatric speciation/subspeciation resulted from fragmentation of tropical forests during climate warming, and then when climate cooled, and forests became continuous, species became sympatric (for discussion see Merrill et al., 2015). The Pleistocene refugium theory has been invoked to explain the diversity of warning colours observed in poison frogs, neotropical Lepidoptera, and other tropical species, with refugia in Europe potentially playing a similar role for temperate species. However, this theory has recently been criticised and, in the case of Heliconius, time‐calibrated phylogenies indicate that diversity was present before the Pleistocene (Nelson et al., 1990; Whinnett et al., 2005; Dasmahapatra et al., 2010; Kozak et al., 2015; Merrill et al., 2015). The current working hypothesis for how geographic or microhabitat variation has led to polymorphisms includes several stages. First, polytypisms arise through parapatric populations (populations with a narrow contact zone and low levels of gene flow) via a variety of non‐climatic mechanisms, such as genetic drift or adaptation to the local abiotic environment (Mallet, Jiggins & McMillan, 1998). Then, once populations are established, either gene flow continues or they eventually become sympatric, producing polymorphisms that may be transient (Mallet et al., 1998; Joron & Iwasa, 2005). Polymorphisms/polytypisms can similarly arise due to earlier divergence of one clade, followed by subsequent mimicry by another clade (e.g. Symula, Schulte & Summers, 2001, 2003; Sanders, Malhotra & Thorpe, 2006).
(2). Genetic basis of warning coloration
Investigations into both Heliconius and Papilio (swallowtail butterflies) species have shown that a handful of specific genetic loci and associated regulatory elements are responsible for the varied phenotypes these genera present (Kunte et al., 2014; Kronforst & Papa, 2015; Nishikawa et al., 2015). While a limited number of loci controlling colour and pattern would seem to be a fairly large constraint on the evolution of phenotypes, in both groups it is in fact the basis for extensive phenotypic diversity, resulting from repeated selection (Nadeau, 2016). For example, a number of key loci are known to control switches in pattern elements within the mimetic radiation of Heliconius butterflies [e.g. WntA (Martin et al., 2012), optix (Reed et al., 2011; Supple et al., 2013) and cortex (Nadeau et al., 2016)]. Kronforst & Papa (2015, p. 12) suggest that in Heliconius the phenotypic lability resulting from the influence of a small number of loci under strong selection creates a ‘virtually unlimited number of possible wing‐pattern phenotypes’. Intuitively, this makes sense as a smaller number of loci will increase each locus' contribution to the phenotype and thus each locus will be under stronger selection (Gavrilets & Vose, 2005). Ultimately a simplified genomic architecture facilitates the diversification of warning coloration.
Hybridisation and adaptive introgression among species have also contributed to the diversity of warning coloration in Heliconius (Mallet et al., 1990; Gilbert, 2003; Heliconius Genome Consortium, 2012; Pardo‐Diaz et al., 2012; Wallbank et al., 2016). Although adaptive introgression and hybrid speciation both involve crossing individuals of different species, there is a difference that is worth noting as they are evolutionarily different mechanisms (Grant, Grant & Petren, 2005). Adaptive introgression results from gene flow from one species into the gene pool of another species through backcrossing of a hybrid with one of its parent species and can result in adaptive genes becoming incorporated back into the parental species (Grant et al., 2005; Kronforst & Papa, 2015). Examples of adaptive introgression in natural systems are rare although reported cases do exist. Among Heliconius butterflies, H. cydno can hybridise with H. melpomene, and Pardo‐Diaz et al. (2012) found repeated introgression of adaptive alleles from H. melpomene in H. timareta. Hybrid speciation differs from adaptive introgression in that novel genomes are created from two parental species, which can lead to novel adaptive peaks in the landscape (Kronforst & Papa, 2015). Known hybrids include H. heurrippa, a hybrid of H. melpomene and H. cydno in the wild (Salazar et al., 2005, 2008, 2010). Furthermore, H. elevatus was formed during a hybrid speciation event but is thought to have the colour patterns of H. melpomene introgressed into its genetic pool (Heliconius Genome Consortium, 2012), thus revealing a fine line between the dichotomy of introgression and hybridisation. There is also strong evidence that such hybrid‐trait speciation in Heliconius is promoted by tight genetic linkage between mate‐choice and colour‐pattern loci resulting in assortative mating based on wing colour patterns (Mavárez et al., 2006; Kronforst, Kapan & Gilbert, 2006a; Melo et al., 2009; Merrill et al., 2011). For example, H. cydno and H. pachinus mate preference segregates with forewing colour in hybrids, indicating that colour preference and wing colour are controlled by loci that are pleiotropic effects of a single locus (Kronforst et al., 2006b). Although our knowledge of hybrid speciation and adaptive introgression has come from Müllerian mimics, it is possible that non‐mimetic polymorphic aposematic coloration has resulted from both mechanisms.
Conversely, whilst some level of recombination can facilitate diversity in warning signals, too high a level has the potential to have a homogenising effect (Mayr, 1963), and hybridisation is not always adaptive (Arias et al., 2016). In polymorphic populations, there should be tight linkage between loci to facilitate the coexistence of several combinations of congruous alleles, thus producing several different phenotypes (Merrill et al., 2015). Genes that are closely linked (i.e. supergenes) facilitate multiple functional elements to segregate as a single Mendelian locus despite recombination elsewhere in the genome, and have been found to be associated with polymorphic mimicry (Brown & Benson, 1974; Charlesworth & Charlesworth, 1975; Turner, 1977b; Joron et al., 2006; Thompson & Jiggins, 2014). Heliconius numata has several coexisting discrete mimetic phenotypes in the same population that are coded for by a single supergene (Joron et al., 2011; Merrill et al., 2015). Unsurprisingly, similar supergene architecture is not present in the sister species of H. numata, which do not have local polymorphisms (Huber et al., 2015).
Many of the genes identified in Heliconius as controlling coloration are conserved across Lepidoptera (Nadeau, 2016; Nadeau et al., 2016), which comprise a significant proportion of aposematic species and their mimics (see online Table S1). Whether similar genetic architecture underlies warning coloration polymorphisms in aposematic species outside this taxon is not yet clear. Work in ladybirds (Tan & Li, 1934; Komai, 1956; Majerus, 1994), colubrid snakes that are Batesian mimics (Davis Rabosky, Cox & Rabosky, 2016a), and a poison frog (Vestergaard et al., 2015) indicate that morph variation in these species is also determined by a small number of gene loci. However, in contrast to the more complex supergene organisation seen in H. numata, mimetic warning coloration in colubrid snakes is the result of a much simpler multilocus system (Davis Rabosky et al., 2016a). These differences can have important implications for evolutionary dynamics in mimicry, for example via their influence on evolutionary rate or even a subsequent evolutionary shift from warning coloration to crypsis, a phenomenon common in snakes but not in Heliconius (Davis Rabosky et al., 2016b).
Given this evidence, it is clear that in order to understand how the genetic architecture of warning coloration enables or constrains morphological variation we need more information about the genes and gene networks at play, as well as a broader taxonomic coverage of the genetic architecture. Alongside the work already carried out on snakes, promising taxa include wasps (Perrard et al., 2014) and ladybird beetles (Lee et al., 2011). The latter are particularly intriguing as, unlike Heliconius spp., there is scant evidence of hybridisation, and for two highly polymorphic species (H. axyridis and A. bipunctata) multiple morphs have been produced in the laboratory that are scarce in the field (Majerus, 1994; Hodek, van Emden & Honek, 2012). Furthermore, recent work on the wood tiger moth Arctia plantaginis has revealed a negative genetic correlation between the efficacy of larval and adult warning coloration that likely contributes to the maintenance of observed variation in aposematic coloration at both life stages (Lindstedt et al., 2016). Investigations into other such genetic correlations outside of Heliconius, for example between different components of the warning signals themselves (e.g. in Pieris butterflies; Kingsolver & Wiernasz, 1991), may therefore also prove fruitful to further our understanding of warning‐signal variation.
IV. PREDATION AND SIGNAL VARIATION
Interactions between predators and defended prey lie at the heart of the paradox surrounding diversity in aposematism. While predation has traditionally been considered to favour monomorphy in warning signals, a growing appreciation of the differences in physiology, psychology and habitat use between predator species, populations, and individuals suggests that predator communities are in fact heterogeneous and dynamic selective agents. This generates diversity in predation risk and creates a significant opportunity for the maintenance of variation in aposematic prey.
(1). Predators vary spatially, temporally, taxonomically, and individually
A predator's response to warningly coloured prey depends on both the prey's relative unprofitability and the conspicuousness of their visual signals (Mappes, Marples & Endler, 2005), so aposematic prey must carefully balance their investment in these two strategic components (Speed & Ruxton, 2007). Yet predators are also highly variable in their response to both chemical defences and visual cues. Therefore, the most adaptive tactic for defended prey will largely depend on the specific predator community in their immediate environment. Variation among predators and predator guilds can occur at several levels: among species, spatially among populations, temporally across seasons or an individual's lifetime, and at a finer scale among individuals (whether based on a stable behavioural type/syndrome or variable factors such as motivation), creating a mosaic of different selective pressures. In the following section, we suggest how variation in multiple predator traits, at different spatial and temporal scales, can facilitate the maintenance of different patterns of variation in prey signals (summarised in Fig. 4).
Figure 4.

Types of variation in predators and the forms of warning‐signal variation they may promote: 1, temporal variation (for example seasonal polyphenism); 2, polytypism; 3, polymorphism within a metapopulation; 4, polymorphism; 5, continuous variation.
(a). Types of variation in predators, and potential consequences
For a given predator (species or individual), defended prey vary in their degree of unprofitability (Brower et al., 1968), from mere distastefulness to deadly toxin loads. The impact of this difference is in part dependent on the specific predator and thus will differ among predators according to their susceptibility to specific toxins (Endler & Mappes, 2004; Mappes et al., 2005), while the willingness of any individual to attack and consume defended prey will further be modulated by other factors, such as motivation and experience. Specialist predators, such as grosbeaks and orioles feeding on defended monarch butterflies, Danaus plexippus (Fink & Brower, 1981; Brower, 1988) or raptors preying on vipers (Vipera spp.; Valkonen et al., 2012), can overcome the defences of aposematic animals, whether through resistance to their defences or careful handling. As such, attracting their attention with bright aposematic signals would be detrimental to prey survival. Tolerance of prey defences can vary across species but also among populations of predators; for example, some populations of garter snakes, Thamnophis sirtalis, have evolved resistance to newt tetrodotoxin (Geffeney, 2002). This may lead to polytypic or polymorphic variation in the conspicuousness of defended prey, following the distribution of more‐ or less‐tolerant predators across populations and microhabitats.
Predator sensory systems, including their perception of visual cues and other cognitive functions (e.g. ability to learn, remember and generalise between signals), may also facilitate the maintenance of polytypic and polymorphic variation among aposematic prey. The key sensory systems used for hunting differ among predator taxa, so, for the same defensive effect, prey may need to employ a diversity of signal forms to maximise their ‘avoid me’ signal efficacy (Guilford & Dawkins, 1991). Predation experiments with artificial prey demonstrate that only some predators respond to visual cues; for example, while avian predators avoid warningly coloured dendrobatid frog models, crabs and lizards do not (Willink et al., 2014). Variation in the effectiveness of warning coloration when confronted with different predator communities may lead to conflicting selective pressures on prey signals. In Japan, the relative abundance of avian predators, which rely on vision when hunting, compared to mammalian predators, for whom visual properties are less relevant, may be responsible for the variation in the extent of red coloration in Cynops pyrrhogaster newts between island and mainland populations (Mochida, 2011). Among visually oriented predators themselves, there is considerable variation in perceptual abilities (Osorio & Vorobyev, 2008), suggesting that some predators could perceive or distinguish visual signals that others may not. In addition, sensory processing in the brain plays a role in determining key features influencing the effectiveness of warning signals, such as detectability, discriminability and memorability (Guilford & Dawkins, 1991). Finally, environmental conditions also affect the visibility and effectiveness of warning colours, dependent on ambient light and the characteristics of natural backgrounds (Endler, 1990, 1993; Bond & Kamil, 2006; Rojas, Rautiala & Mappes, 2014b); so aposematism overall, or some specific colour morphs, may be more effective in particular habitats.
Beyond perception of the signals, higher‐level cognitive processes may also influence predator responses to prey signals, and thus ultimately impact the adaptive value of conspicuousness and warning coloration. Generalisation between visual signals, whether they cannot be perceptually distinguished or are grouped together by higher‐order cognitive processes, is especially interesting, as it would effectively allow different colour morphs to co‐occur with equal fitness (Amézquita et al., 2013; Richards‐Zawacki et al., 2013; Stuckert, Venegas & Summers, 2014b; Rönkä et al., 2018). For example, tests with multiple passerine species suggest that they differ in their ability to generalise prior experience of red firebugs (Pyrrhocoris apterus) to yellow morphs of this species (Exnerová et al., 2006). Although it would not itself select for variation, generalisation between morphs could facilitate the maintenance of different forms (which could provide other selective benefits; see alternative selection pressures in Section V) in populations where predators tend not to distinguish between morphs.
Even if predators classify signals as distinct, further differences in their response will arise due to variation in general neophobia, cautiousness when handling novel prey, and dietary conservatism. These effects can potentially facilitate the evolution of novel conspicuous morphs (Marples, Roper & Harper, 1998; Thomas et al., 2003, 2004; Exnerová et al., 2006); although experimental evidence suggests dietary conservatism may not be sufficient to counteract positive frequency‐dependent selection against novel morphs when these are rare and conspicuous (Marples & Mappes, 2010). In some cases, innate avoidance of specific patterns plays an important role, as demonstrated by the aversion of naive turquoise‐browed motmots (Eumomota superciliosa) and great kiskadees (Pitangus sulphuratus) to coral snake (Micrurus spp.) patterns (Smith, 1975, 1977). Strong innate responses may allow polymorphisms in warning signals to evolve if the predators avoid a broad class of visual signals, such as all ringed patterns in the case of coral snakes. Finally, variability in the learning abilities of predators will affect the benefit of aposematic signalling for defended prey (Endler & Mappes, 2004; Mappes et al., 2005). Recent work on domestic chicks (Gallus gallus domesticus) showed variation in avoidance learning among different breeds of this species. Chickens bred for high productivity were initially less wary of aposematic prey, but also formed weaker associations between signals and defences over time than the other breeds of chicken, leading to differential prey survival in laboratory experiments (Rowland, Fulford & Ruxton, 2017). Predators in the wild may also differ in their learning abilities, leading to variation in predation risk for aposematic prey with different signals, and are also likely to differ from domestic chickens. Further research on learning in more relevant predators could alter our expectations of predator capabilities and responses to aposematic prey; for example, evidence that predators can rapidly memorise many different signal forms would challenge the assumption of strong selection for aposematic signal monomorphy.
Classic experiments on neophobia and dietary conservatism in passerine birds also reveal further intraspecific variation, which cannot be attributed to factors such as differences in territory, experience or sex (Marples et al., 1998). These could be linked to personality, known to affect both initial reactions to aposematic prey and the learning process (Exnerová et al., 2010), or individual condition. A predator's level of hunger and current condition will determine its motivation and willingness to attack and consume risky prey, including warningly coloured individuals, which will impact the relative benefit of aposematic displays. Rather than rejecting aposematic prey outright, predators consider all available prey types to make adaptive foraging decisions, based on the relative costs of ingesting toxins versus the nutritional gain from consuming the prey (Barnett et al., 2012). Experiments with European starlings (Sturnus vulgaris) suggest they can distinguish not only undefended from toxic prey, but also different levels of chemical defences, via taste‐rejection (Skelhorn & Rowe, 2006, 2009), as well as gaining nutritional information about the prey (Skelhorn et al., 2016). This allows them to make educated decisions while foraging, depending on their motivation to feed; accordingly, starlings are more willing to consume defended prey when their own reserves are experimentally reduced (Barnett, Bateson & Rowe, 2007), early‐life or current conditions are harsher (Chatelain, Halpin & Rowe, 2013; Bloxham et al., 2014), or the prey have greater nutritional value relative to their toxicity (Halpin, Skelhorn & Rowe, 2014; Smith, Halpin & Rowe, 2016). While there is a growing body of evidence, primarily from laboratory experiments, suggesting that varying levels of motivation affect prey choice by predators, how this may impact the survival of aposematic prey and selection pressures on signal form in the wild is not yet clear. The physiological mechanisms and cognitive processes responsible for these adaptive decisions are still relatively poorly known, but there is scope for mediation of this toxicity–nutrition trade‐off to vary among species, populations and personalities (Skelhorn et al., 2016). Exploring how different predators deal with the trade‐offs associated with foraging in a natural setting, such as balancing the time required to assess the profitability of warningly coloured prey accurately, while managing their own exposure to predators and efficient foraging, would be extremely valuable for obtaining a more well‐rounded picture of predation risk for aposematic prey.
Motivation is not the only highly variable trait affecting predator responses to aposematic prey. Prior experience is critical in determining whether a predator will choose to attack and consume a prey item. This can vary widely across species and populations of predators, as traits such as dietary specialisations (Exnerová et al., 2003; Ihalainen et al., 2012) and territoriality (Endler & Rojas, 2009) affect which prey assemblages a predator may experience. For example, omnivorous and more specialised passerine birds respond differently when presented with aposematic invertebrates (Exnerová et al., 2003). Similarly, great tits (Parus major) from Finland are more reluctant to attack aposematic prey than great tits from Bohemia, possibly due to a reduced exposure to warningly coloured invertebrates, and a higher proportion of neophobic and migratory birds in the population (Exnerová et al., 2015). On a finer scale, a predator's level of experience will depend on the number of encounters with defended prey, so may differ between age classes (Lindström, Alatalo & Mappes, 1999). Seasonal fluctuations in overall predator naivety may occur as young predators learn to forage for themselves and sample aposematic prey for the first time, thereby impacting the relative benefits of conspicuousness and crypsis for defended prey at different times of the year (Mappes et al., 2014) and potentially favouring seasonal polyphenism, as seen in striated shieldbugs, Graphosoma lineatum (Tullberg et al., 2008; Johansen et al., 2010).
Finally, variation in predator traits interacts with other forms of variation in the whole community of organisms in a given habitat, such that the characteristics of this community, and the interactions between all its members, will ultimately shape the selective pressures acting on warning signal form. From the predators' perspective, the presence, abundance and nutritional value of alternative prey, as well as the effort required to locate them and the toxin load already ingested, all impact the net benefits of attack (Turner & Speed, 1999; Sherratt, 2003; Rowland et al., 2010c; Carle & Rowe, 2014; Skelhorn et al., 2016), and the strength of selection for convergence in prey signals (Fig. 4; Kokko, Mappes & Lindström, 2003; Lindström et al., 2004). The diversity of prey coloration within populations is equally important, not only in shaping predator experience, but also because of the demands it places on predators' cognitive skills. Selective pressures for signal uniformity may be relaxed in more complex communities, as predator learning is limited by their ability to memorise multiple signals and their associated risks and benefits (Ihalainen et al., 2012). In an even broader ecological context, the predation risk experienced by the predators of aposematic prey themselves may also contribute to their response to warning signals (Lima & Dill, 1990), due to variable costs of exposure to predators incurred by longer prey‐handling times, or increased searching behaviour to find alternative prey. As such, differences in both prey and predator communities among populations, as well as spatio‐temporal heterogeneity within populations, combine to produce variable selection pressures affecting warning signal form.
(b). Predator response to variation in prey toxicity, and its implications for aposematic variation
Just as variation in predator communities was originally underappreciated, the variability of secondary defences, particularly chemical defences, in natural populations has long been neglected (Speed et al., 2012). At the extreme end of this spectrum is automimicry, a phenomenon whereby some individuals within a population of aposematic animals have either extremely low levels of toxins or none at all (Brower, Brower & Corvino, 1967; Ruxton et al., 2004). This seems to occur primarily in species that acquire either toxins or toxin precursors from their diet. Automimicry poses a problem for defended individuals because, similar to Batesian mimicry, it degrades the efficiency of the aposematic signal and thus any given individual in the population is more likely to be attacked (Fig. 3). Further, automimicry poses a problem for predators that may also experience negative side effects, for example by unintentionally consuming toxic prey after previous experience with a palatable individual of the same species (Ruxton et al., 2004). Nevertheless, models indicate that automimicry may persist when there are two discrete levels of defence within a population and low predation pressures (Broom, Speed & Ruxton, 2005), or when defence is a continuous trait (and especially when defence levels trade off with fecundity; Svennungsen & Holen, 2007). Additionally, evidence indicates that automimicry may in fact not affect overall predation rates in a population when automimics are below 25% of the population (Skelhorn & Rowe, 2007). With respect to this review, automimicry is of interest as a potential intermediate step towards polymorphism, if the population of automimics begins to diverge into two different aposematic strategies. For example, in insects, females could evolve a preference for different host plants to oviposit on, which produces differential toxicity in the population and potentially different peaks in the adaptive landscape. Broom et al. (2005) have shown this to be a stable strategy and it could function as an intermediate step towards polymorphism via ecological mechanisms. Although theory would predict that the phenotype in the lower adaptive peak should evolve towards similarity with the higher peaked phenotype (e.g. Turner, 1983), there are alternative mechanisms that may maintain this (see Section V). Over time, this behaviour could become canalised and correlate with the aposematic signal as well. How common this is, or whether it occurs at all, is unknown. Automimicry may also be capable of creating polymorphisms in situations in which toxicity and colour are linked via some environmental trait. A plausible mechanism would be something akin to the resource‐allocation theory that has been supported by work on ladybird beetles (Blount et al., 2009, 2012; see Section V), wherein some individuals acquire a chemical defence and others do not.
Similar to automimicry within a species, mimetic species are often unequally protected. This brings about a scenario known as quasi‐Batesian mimicry, occasionally referred to as Speedian mimicry (Speed, 1990; Fig. 3). Although mimicry has often been described as a binary scenario, i.e. either Batesian or Müllerian, there is evidence that it may be better represented as a spectrum, much as visual strategies are now perceived as a continuum ranging from crypsis to aposematism. Mimicry appearing to be Müllerian in nature may in fact be detrimental to one species and lead to quasi‐Batesian mimicry if there is a difference in the level of defence between the two mimetic species (Speed, 1990). Crucially, it is as yet unclear whether differences in toxicity and associated unpalatability actually produce quasi‐Batesian systems, or if variation between mimetic species with differing levels of toxins is ecologically irrelevant and these species have functionally mutualistic relationships (e.g. Rowland et al., 2007; Stuckert et al., 2014a). Similar to Batesian mimicry, local polymorphism may be beneficial to individuals of species with a low level of defence; if they can mimic different established aposematic species, they would gain a greater survival advantage, as the costs of mimicry would be spread across several model species (Speed, 1993; Ruxton et al., 2004). Quasi‐Batesian mimicry may also put selective pressure on the less‐defended species to be more similar to the phenotype of the better‐defended species. This in turn may be sufficiently detrimental to the better‐defended species that they may experience selection away from the shared form (similar to Batesian mimicry). This could, theoretically, lead to an evolutionary chase between the model and the quasi‐Batesian mimics in a red queen chase scenario (Van Valen, 1973), particularly if selective pressures promote similar rates of adaptation in the two species. Furthermore, as discussed above, predators can make decisions based on both their nutritional level and toxin load, and therefore the availability of alternative, palatable prey may strongly influence the relationship between mimetic species, particularly if they differ in toxicity (Rowland et al., 2010b). Including information on predator state in models of mimicry can lead to surprising outcomes; for example, two species that are visually distinct may both still benefit from the other species' presence even when toxins are not costly for predators to detoxify (Halpin, Skelhorn & Rowe, 2012; Halpin et al., 2017). Additionally, differences in chemical defences (i.e. Batesian or quasi‐Batesian mimicry) could cause populations of a defended species to experience different coevolutionary trajectories (Laine, 2009), particularly when they are in geographic isolation. This could lead to polytypism, or polymorphism if the populations eventually become sympatric once more.
In reality, the role that variation in chemical defence has on populations and the evolution and maintenance of variation in colour phenotypes is largely speculative. This, in part, derives from a general uncertainty as to whether or not these differences in toxicity actually make ecological differences to predators. In general, we lack the empirical data to determine what this variation means to predators, or even why this variation occurs. This is a fairly substantial gap in our knowledge, one which could lead to a burgeoning subdiscipline.
(2). Predator diversity contributes to the maintenance of variation in aposematic prey
(a). The distribution of predator diversity shapes patterns of variation in prey
Population‐level differences in predation regimes may facilitate the maintenance of continuous variation between populations of warningly coloured species, as seen in the red coloration of newts on Japanese islands (Mochida, 2011), or polytypisms. Within populations, many studies demonstrate greater predation risks for rare and novel conspicuous forms relative to locally abundant ones (Lindström et al., 2001; Borer et al., 2010), particularly in poison frogs (e.g. Noonan & Comeault, 2009) and Heliconius butterflies (e.g. Mallet & Barton, 1989; Chouteau et al., 2016). These local predation pressures can produce a purifying selective force, driving populations towards distinct local phenotypes (Joron & Iwasa, 2005; Sherratt, 2006). In poison frogs, artificial predation experiments with models resembling distinct colour morphs of Ranitomeya imitator demonstrate that predation risk for these morphs varies geographically, favouring polytypisms (Chouteau & Angers, 2011).
On a smaller scale, differences between predator communities across microhabitats within a single population may facilitate the maintenance of polymorphisms in aposematic species and even contribute to speciation, as has been suggested for ithomiine butterflies (Mallet & Gilbert, 1995; Beccaloni, 1997; Elias et al., 2008). In a recent study in Ecuador, butterflies with particular wing patterns were found at different frequencies among distinct microhabitats in the canopy (Willmott et al., 2017). The community of avian predators likely to be encountered by these butterflies also covaried with these microhabitats, and artificial predation experiments suggested that predation risk experienced by specific wing patterns differed among microhabitats. Moreover, behavioural choices, such as temporal variation in activity or microhabitat selection, will enable aposematic prey to alter their conspicuousness and improve their chances of survival (Rojas, Devillechabrolle & Endler, 2014a; Arenas & Stevens, 2017), thus enabling multiple signal forms to coexist successfully.
(b). Dealing with predator diversity within a population
The presence of a diverse community of predators in a single location may favour variability in warning signals, so as to mitigate overall predation risk. Variation in the extent of conspicuousness may be employed as a compromise strategy, whereby signals of intermediate visibility, but still distinct and recognisable, may deter predators that heed the signal without attracting too much attention from others. For example, the polytypic poison frogs Oophaga granulifera and O. pumilio include morphs that are green and cryptic, others that are bright and truly ‘aposematic’, and intermediate phenotypes. This phenomenon seems to be related to behavioural phenotypes and attack rates by predators, as frogs from brighter populations are bolder and experience lower attack rates (Maan & Cummings, 2012; Willink et al., 2013, 2014). Alternatively, a given signal may vary depending on the position of the observer. In distance‐dependent signalling, aposematic species possess pattern elements that make them appear cryptic from afar, yet conspicuous up close (Barnett & Cuthill, 2014; Barnett, Scott‐Samuel & Cuthill, 2016). Examples include Vipera snakes (Valkonen et al., 2012), some butterfly larvae (Tullberg, Merilaita & Wiklund, 2005; Bohlin, Tullberg & Merilaita, 2008) and spotted skunks (Spilogale spp.), which are difficult to detect unless viewed closely (Caro et al., 2013). Thus, prey coloration is not always exclusively cryptic or aposematic, but rather forms a continuum between camouflage and warning coloration, which can be manipulated to the prey's advantage.
Diversity within a population of predators can also maintain fixed variation within an aposematic prey population, under certain circumstances. Contrary to traditional theories of Müllerian mimicry, positive frequency‐dependent selection is not ubiquitous (Greenwood, Wood & Batchelor, 1981; Amézquita et al., 2013; Richards‐Zawacki et al., 2013). Müller's more simplistic assumptions about the relations between predators and prey, such as the fixed numbers of prey encounters required for learning, have since been replaced by a greater understanding of the complexity of predator communities. Considering the number of variables potentially affecting the overall outcome of foraging decisions by predators, a broad range of different selection regimes should be expected (Stevens & Ruxton, 2012; Aubier & Sherratt, 2015) including spatiotemporal variation in selection even within a single population of prey.
In particular, several processes may lead to negative frequency‐dependent selection, facilitating the maintenance of polymorphisms within populations (Svensson, Abbott & Härdling, 2005; Olendorf et al., 2006). Foraging predators must constantly balance the costs and benefits of concentrating on prey they know to be profitable, or sampling unfamiliar prey items, which could be more valuable or potentially harmful. Optimal‐sampling theory predicts that these adaptive decisions will depend on the likelihood that a prey item is defended, and the probability that the predator will encounter this type of prey again. In the context of warning coloration, it suggests that rarer aposematic morphs should be avoided, as predators learn about profitability from their past experiences of more regularly encountered prey (Sherratt, 2011; Aubier & Sherratt, 2015). Search‐image formation, more‐efficient handling of commonly encountered prey, and the potential costs of gathering information about the profitability of unknown items will all encourage predation of common forms (Skelhorn et al., 2016). Whether a predator will decide to attack common defended prey will also depend on prey toxicity within the community; for example, relatively weak defences or few palatable alternatives will favour predation on common aposematic forms, thus promoting polymorphism (Greenwood et al., 1981).
The effect of predator community in different populations may even override expectations based on positive frequency‐dependent selection. Yellow and white morphs of male wood tiger moths occur at different frequencies across Europe, but local morph frequency does not always predict survival in artificial predation experiments. In one study, predation of the two morphs varied according to the community of bird species present, with yellow morphs being more successful in communities dominated by Paridae (tits, in Northern Europe), rather than Prunellidae, represented by the dunnock Prunella modularis (Nokelainen et al., 2014). This suggests that understanding the characteristics of the relevant predator community may be the most important means of predicting signal evolution. In a general framework, modelling the evolution of a simple polymorphic prey population, with two morphs differing in conspicuousness and facing a mix of predators that differ in their tolerance of the prey defences, demonstrates several possible outcomes (Endler & Mappes, 2004). Depending on the proportion of predators choosing to avoid the prey, the population may become monomorphic for either the more or less visible morph, or, if both predator types occur in similar numbers, the polymorphism may be maintained. Experiments with firebugs and wild‐caught birds suggests that if a new colour morph of a defended species appears within a population, neophobia alone is unlikely to overcome purifying selection and enable the persistence of the new form (Exnerová et al., 2006). However, evolutionary modelling suggests that a combination of dietary wariness, interacting with overall predation risk and signal conspicuousness will favour diversity in warning signals within populations, with or without frequency‐dependent selection (Franks & Oxford, 2009). Moreover, the results of simulations based on selection regimes observed in polymorphic species such as Cepaea land snails, Oophaga poison frogs, Sonora snakes and Heliconius butterflies suggest that differences in the range of predators, operating in small local populations or across multiple populations at a regional scale, can promote a mosaic of polymorphisms in prey, without invoking any additional mechanisms favouring diversity (Holmes, Grundler & Davis Rabosky, 2017). Multiple ways in which predators and predator communities may differ can thus ultimately affect selective pressures leading to diversity in warning coloration.
V. THE MULTIFUNCTIONALITY OF APOSEMATIC SIGNALS
While predation is – by definition – the selective pressure driving aposematism, warning coloration is also subject to many other, potentially antagonistic, factors. These can be abiotic or biotic, the latter including both intraspecific and interspecific interactions. Several, such as thermoregulation and sexual selection, are already well studied in the context of warning‐signal polymorphism and polytypism, while others, including parental and early‐life effects, have only recently been recognised as potential factors generating and maintaining variation in coloration. Such selection pressures may be complementary to predation, augmenting its effect on aposematic phenotype, or alternatively may oppose the effect of the selective pressure of predation, producing more than one phenotypic optimum and enabling signal variation. These conflicting selection pressures can influence the abundance of different, genetically determined, morphs among populations and within a population (polymorphism), specific morph expression (polyphenism), and also more continuous colour variation within morphs (e.g. variation in conspicuousness; Figs 1 and 2).
(1). Abiotic selection pressures
(a). Temperature and melanism
One clear example of a trait that is important for multiple aspects of an organism's fitness is melanisation. Melanin creates the black patterns seen in many of the classic aposematic signals across multiple taxa, from insects to mammals, as well as underlying the structural, iridescent, colours recently shown to act as aposematic signals in many bugs and beetles (Fabricant et al., 2013; Fabricant et al., 2014). The pigment also increases an organism's ability to absorb radiation (Clusella‐Trullas, van Wyk & Spotila, 2007; Hetem et al., 2009) providing fitness benefits for individuals in cooler environments through improved thermoregulation (de Jong, Gussekloo & Brakefield, 1996). However, while increased melanisation provides fitness benefits for aposematic species in some instances (Clusella‐Trullas et al., 2007; Lindstedt, Lindström & Mappes, 2009b), it also has associated costs. Melanic pigmentation often forms a key part of aposematic coloration, yet recent evidence suggests the contrast between a signal and its background (dictated by the chromatic component of the signal) as opposed to internal contrast, is the more important determinant of aposematic signal detectability (Arenas, Troscianko & Stevens, 2014). This may help to explain the much higher level of predation risk associated with melanism in aposematic species (Hegna et al., 2013; Arenas, Walter & Stevens, 2015).
The trade‐off between the positive/thermoregulatory benefits and negative/predation‐risk costs of melanisation are well explored in aposematic species in relation to temperature (e.g. Arctia plantaginis; Hegna et al., 2013). Variation in temperature is known to contribute to within‐ morph plastic adjustment of the levels of melanism in warning signals, for example spot‐size in ladybirds (Michie et al., 2010, 2011), contributing to continuous variation in signal expression within and among populations. Seasonal fluctuations in temperature and changes in predation (see Section IV) likely promote melanism‐based polymorphism within populations of aposematic species. Even when the predation costs associated with the pigment are high, asymmetrical mate preferences, such that more‐melanic individuals have higher mating success, may contribute to the persistence of melanic morphs within populations (Saino et al., 2013; Culumber et al., 2014; Mishra & Omkar, 2014). The relative abundance of these melanic morphs within a population also increases with the benefits of improved thermoregulation (i.e. decreasing temperature), leading to both altitudinal and latitudinal clines in morph abundance (Clusella‐Trullas et al., 2007). For example, the proportion of melanic morphs in populations of the two‐spot ladybird (Adalia bipunctata) is greater in higher, and therefore colder, latitudes (Brakefield, 1984) and these clines in morph abundance have been shown to alter in response to climate change (de Jong & Brakefield, 1998).
Melanin also has benefits associated with ultraviolet (UV) protection (Ortonne, 2002), immunocompetence (Dubovskiy et al., 2013), and desiccation (King & Sinclair, 2015) and its abundance is plastically adjusted in response to increases in these challenges (Wilson et al., 2001; Abram et al., 2015; Välimäki et al., 2015). Variation in these factors may act, like temperature, to enable the persistence of variation in the abundance of melanic morphs across spatial (polytypism) and temporal (polymorphism) scales. The selection landscape determining both the abundance of melanic morphs within aposematic populations and the degree of melanism within morphs themselves will thus consist of multiple competing selection pressures, beyond simply temperature and predation risk.
(b). Resource availability
The production of warning signals requires adequate resources to deal with the associated cost of signal production, both the overall increase in energy expenditure and the associated oxidative stress burden (McGraw, 2005; Galván & Alonso‐Alvarez, 2008; Blount et al., 2009), and in many cases specific access to dietary pigments such as carotenoids (Blount et al., 2012). Experiments in red‐eyed tree frogs (Agalychnis callidryas), a non‐aposematic species, have shown that the amount of carotenoid pigments available at critical times during development influences the redness of their dorsum (Ogilvy, Preziosi & Fidgett, 2012). Signal expression in aposematic species is therefore likely to be strongly influenced by the availability and quality of food, particularly during early development (Monaghan, 2008; Blount et al., 2009). Research indicates that this most commonly occurs in terms of variation in morph conspicuousness (i.e. saturation and luminance; Blount et al., 2012) as opposed to the dietary determination of differently coloured morphs observed in the camouflaged caterpillars of numerous lepidoptera species (Greene, 1989; Fink, 1995). High‐quality diets during development can lead to the production of larger, brighter, and more colourful warning signals compared to low‐quality diets (Grill & Moore, 1998; Ojala et al., 2007; Lindstedt et al., 2009a). The way that individual foraging areas and populations map onto the landscape of differing resource availability is likely to determine the scale at which the consequent variation in conspicuousness occurs, such as among individuals (continuous within‐morph variation) or among populations, for example along a resource gradient (polytypism). Furthermore, early‐life diet does not always affect warning coloration (Grill & Moore, 1998; Flores et al., 2013), the relationship between the two is likely complicated by the fact that warning coloration advertises an associated defence (Poulton, 1890; Summers et al., 2015).
(c). The resource‐allocation hypothesis and quantitative honesty
The nature of the relationship between an aposematic signal and defence is complex and likely to play a role in the way resource availability shapes aposematic signal variation, especially within‐morph variation in conspicuousness. While aposematic species are inherently qualitatively honest, they may not necessarily be quantitatively honest (i.e. show a positive relationship between the level of signal and the level of defence). For example, positive relationships between conspicuousness and toxicity have been identified in a number of species [e.g. ladybird beetles (Bezzerides et al., 2007; Blount et al., 2012; Arenas et al., 2015) and paper wasps (Vidal‐Cordero et al., 2012)]. However, the association is not universally positive, with negative correlations between levels of signal and defence identified across sexes or populations of the same species (Daly & Myers, 1967; Wang, 2011; Blount et al., 2012). Furthermore, in some groups it seems that related species can reach approximately equal protection from predators with multiple different signal–toxin strategies (Darst, Cummings & Cannatella, 2006). A number of theories have been proposed to explain these differences (comprehensively reviewed by Summers et al., 2015). Research on the availability of resources, those used for coloration and preventing autotoxicity (antioxidants), has provided a feasible mechanism: the ‘resource‐allocation hypothesis’ (Blount et al., 2009). In this model, coloration and chemical defence both utilise antioxidants which are commonly acquired from the diet. Thus, individuals have to balance investments in the signal and defence, or deal with a trade‐off between investing in the signal or the defence (Blount et al., 2009). The model predicts that when resources are low individuals will signal honestly, whereas under high resource conditions quantitative honesty would degrade as individuals would preferentially invest in defence over warning coloration. These predictions indicate that the influence of spatial or temporal variation in resource availability upon warning‐signal conspicuousness is unlikely to be consistently linear.
Empirical tests of the resource‐allocation theory are scarce however, and results equivocal in their support, showing that resource variation can lead to both positive and negative relationships between signal and defence (Blount et al., 2012). The predictions of theoretical models investigating how individuals might invest in each component of an aposematic signal when resources vary also differ, depending on whether the model assumes that individual conspicuousness is an intrinsic component of the defensive signal or can act as a stand‐alone defensive trait (Blount et al., 2009; Holen & Svennungsen, 2012; Summers et al., 2015). The latter refers to a scenario where the warning coloration alone elicits wariness or acts as a deterrent against predators through its conspicuousness or novelty (Guilford, 1994). Alternatively, other work has suggested that the honesty of aposematic signals is not mediated by the cost of production, but instead by costs imposed by predators, because predators are able to determine levels of protection rapidly while sampling potential prey (Guilford & Dawkins, 1995; Hurd & Enquist, 2005). It is therefore clear that whether individuals respond to increased resource availability with a concomitant increase in warning‐signal conspicuousness will depend on the mechanisms of honesty enforcement at work. Further work to clarify the mechanisms determining the honesty of signalling in aposematic species (Summers et al., 2015) will therefore aid predictions about how spatial and temporal variation in resources will influence within‐ and between‐population variation in conspicuousness.
(2). Biotic selection pressures
(a). Disease and parasite load
As the influence of resource availability on warning signals demonstrates, animal coloration is strongly influenced by factors that affect an individual's condition (Griffith, Parker & Olson, 2006). Disease and parasite load both negatively influence condition and consequently can lead to trade‐offs between immune function and signal expression (McGraw & Hill, 2000). For example, increased parasite load leads to generally duller coloration in fish and birds of both sexes (Martínez‐Padilla et al., 2011; Ciccotto, Dresser & Mendelson, 2014). Currently it is uncertain how such factors may influence aposematic signals specifically, but based on the shared physiological basis of aposematic and non‐aposematic coloration (e.g. sexual signals), it is possible that a similar ‘condition‐dependent’ relationship may occur (Blount et al., 2009).
How the melanic component of aposematic coloration will be influenced by parasites and disease is unlikely to be clear cut. In common with coloured parts of warning signals, the production of melanin has various associated costs which may lead to trade‐offs between the production of melanin for pigmentation and immune responses (Guindre‐Parker & Love, 2014). Under such a scenario, a negative relationship between melanin pigmentation and disease or parasite load can occur (Cotter et al., 2008; Gangoso et al., 2011) and may result in polytypisms if these loads vary spatially. However the association between melanisation and resistance to pathogens is not straightforward; for example, in invertebrates, cuticle melanisation acts directly in the protection of individuals from pathogens (Dubovskiy et al., 2013). Melanic pigmentation is also highly heritable in both invertebrates and vertebrates (Roff & Fairbairn, 2013; Roulin & Ducrest, 2013). It has been suggested that in many species the association between melanic coloration and a suite of disease‐resistance characteristics is a consequence of linkage disequilibrium and/or pleiotropy (Roulin, 2016). The result is differential life‐history strategies between more‐ and less‐pigmented individuals of the same species, the associated fitness of which is environmentally dependent (Emaresi et al., 2014).
The preference of parasites, particularly ovipositing parasites such as parasitic wasps, for specific colour morphs and for within‐morph conspicuousness (or traits correlated with within‐morph conspicuousness) may act as alternative selection pressures on warning coloration. Parasites may prefer one colour morph over another, as is the case for the aphid parasitoid wasp Aphidius ervi which preferentially lays eggs in pea aphids, Acyrthosiphon pisum, of a colour morph not favoured by predators (Losey et al., 1997). As discussed previously, coloration in aposematic species may be quantitatively linked to chemical defence (Summers et al., 2015), levels of which have been linked to decreased (Weldon et al., 2006) and increased (Zvereva & Kozlov, 2016) parasitism risk, the latter being especially prevalent for specialist parasites (Al Abassi et al., 2001). Chemical defences have even been hypothesised to arise as a mechanism of preventing parasitism, with subsequent predator avoidance a secondary benefit (Weldon et al., 2006). This relationship between colour and defence may be further complicated by the fact that some chemical defences can also have antimicrobial properties (Mina et al., 2015). It is therefore conceivable that in areas with high risk of parasitism, colour morphs or levels of conspicuousness less attractive to parasitoids may be selected for, either through the parasites' direct response to colour or their response to levels of the strongly associated chemical defence. This may be especially important if infection dramatically reduces host survival (e.g. Dinocampus coccinellae; Maure et al., 2014).
In summary, disease has the potential to cause continuous variation in the chromatic and achromatic parts of an aposematic signal due to current infection, plastic changes at the individual level where infection stimulates increase in melanisation, and local adaptation via correlated trait responses if coloration is linked to factors such as immunocompetence and if the level of infection risk varies spatially. Pathogens may also cause local extinctions, or repeated bottlenecks, which can disrupt purifying selection and maintain colour variation (Gordon, 2013; Idris & Hassan, 2013). Meanwhile, parasitism is likely to influence both morph abundance and within‐morph conspicuousness in populations of aposematic species. These areas are ripe for exploration, and have huge potential for contributing to the understanding of diversity in aposematic coloration and the life‐history trade‐offs involved in its determination.
(b). Interspecific interactions
Although predator–prey relationships dominate the study of interspecies interactions, other forms can and do occur. One such example is reproductive interference, i.e. sexual interactions between members of different species (Gröning & Hochkirch, 2008; Burdfield‐Steel & Shuker, 2011). Since this is, by definition, costly, mate discrimination and avoidance of reproductive interference could constrain warning signals, particularly in cases of mimicry, where effective mimicry could have consequences for mate discrimination (Estrada & Jiggins, 2008; but see Llaurens, Joron & Théry, 2014). Thus, the purifying selection on colour and pattern imposed by predators could be counteracted by the costs of sexual or territorial harassment by heterospecifics. While this phenomenon has not been investigated in aposematic species, such harassment has been suggested to play an important role in the maintenance of female colour polymorphisms in odonates (Fincke, 2004, and references therein). Because avoidance of conspecific harassment has been shown to influence female colour in Batesian mimics (Cook et al., 1994) this phenomenon may be worthy of further research.
(c). Intraspecific interactions
(i). Mate choice and parental effects
Mate choice can act either to reinforce or to disrupt the selection imposed on warning coloration by predators. The interaction between warning signals and sexual selection can occur when aposematic traits play a function in mate choice and recognition, or when there is a trade‐off between traits used in mate acquisition and those involved in predator defence. As anti‐predator defence is a key survival trait, we would expect that natural and sexual selection would work in tandem, with better protected individuals also gaining advantages during mating and reproduction, thus enforcing purifying selection on warning coloration. However, when this is not the case sexual selection may act to counter the effect of selection imposed by predators, allowing for polymorphism and other forms of warning‐signal variation to arise (e.g. Cummings & Crothers, 2013).
Sexual selection could also lead to sex‐specific differences in warning coloration. For example, increased brightness in male poison frogs could be the result of female preference for brighter males (Maan & Cummings, 2009; but see Meuche et al., 2013). Whether such selection would lead to true polymorphism in the eyes of predators depends on the strength of the respective pressures, as well as the sensitivity of the signalling system itself to evolutionary inputs. In the case of poison frogs, the colour cues selected for by females (i.e. brightness) may be different from those selected by predators (i.e. hue). Indeed there is evidence that Heliconius and Melinaea co‐mimics show increased interspecies variation in colour combinations that are less visible to their avian predators, allowing for ‘cryptic’ signalling of species identity (Llaurens et al., 2014) and similar patterns may exist for within‐species signalling. Alternatively, if the visual conspicuousness of both sexes is already very high, any increases as a result of sexual selection may have no effect on predator learning (Maan & Cummings, 2009; Crothers & Cummings, 2013). Variation in the strength of female preferences among populations (e.g. Maan & Cummings, 2009) may create divergent evolutionary trajectories in different populations, causing polytypisms to arise, whilst assortative mating or local morph preference can enable their persistence and even lead to the exaggeration of morph differences. In such cases, warning signals may constitute so‐called ‘magic’ traits, as they are both subject to ecological pressures from predators and contribute to non‐random mating, as is the case for many Heliconius species (Merrill, Chia & Nadeau, 2014). Assortative mating by morph may also occur if individuals in a population have different anti‐predator strategies. For example, associating with a conspicuous individual may increase your own risk of attack if you are cryptic (Segami Marzal et al., 2017).
In addition to assortative mating, disassortative mating, where individuals prefer to mate with colour morphs different from themselves, can also occur. For example, in the polymorphic Heliconius numata females show a strong aversion to mating with males of their own morph, preferring instead males of a different morph to themselves. Thus, while males of rare morphs may suffer increased predation risk, they will also have a higher mating success with females of the common morph (Chouteau et al., 2017). This may be the result of heterozygote advantage, which has been suggested as a possible mechanism for the maintenance of many polymorphisms (Hedrick, 2012).
However, warning signals may not always be indicators of mate quality. Instead, there may be trade‐offs between traits related to predator defence and those that grant higher mating success. In addition, while selection on aposematic‐signal colour patterns is expected to be positively frequency dependent (see Section II), it could potentially be opposed if negative frequency‐dependent sexual selection also acts on the signals. Evidence for both phenomena has been found in the wood tiger moth. In this species white males have less‐effective warning signals (Nokelainen et al., 2012) than the yellow morph they coexist with, but gain higher mating success in white‐biased populations, despite showing no advantage over yellow males in offspring hatching success (Gordon et al., 2015). This could be due to differences in flying behaviour and mating effort (Rojas, Gordon & Mappes, 2015). Thus, frequency‐dependent selection could allow yellow and white males to co‐exist, as whites compensate for their higher predation rate through increased mating success. Sexual selection may therefore allow for the maintenance of polymorphism within populations, in particular if it leads to, or is a consequence of, a trade‐off between anti‐predator defence and mating success/fecundity.
While we naturally expect mate choice to influence offspring aposematic phenotype via genetic inheritance, transgenerational non‐genetic effects are also likely to play a role, especially in determining continuous within‐morph variation in warning coloration (Winters et al., 2014). It is increasingly clear that offspring phenotype is influenced non‐genetically via maternal investment in response to a multitude of abiotic and biotic variables present in the offspring environment (Wolf & Wade, 2009; Day & Bonduriansky, 2011). Mothers can alter offspring phenotype in response to mate ‘quality’ (‘differential allocation’; Ratikainen & Kokko, 2010) and reliable cues of the offspring environment (‘anticipatory maternal effects’; Marshall & Uller, 2007). In terms of mate choice, as mentioned above, male warning coloration has been shown to be influential in female mate choice in aposematic species (Nokelainen et al., 2012; Mishra & Omkar, 2014). Theoretical and empirical work suggest two likely responses, that females will either increase investment in response to the brightness or colour of male warning coloration (‘positive differential allocation’; Sheldon, 2000; Horváthová, Nakagawa & Uller, 2012) or decrease it (‘negative differential allocation’; Saino et al., 2002; Bolund, Schielzeth & Forstmeier, 2009). Recent work provides the first evidence that such a phenomenon may take place, with female Adalia bipunctata laying brighter eggs when mated with brighter males (Paul et al., 2018). This is important as offspring aposematic phenotype in early life can persist into adulthood (Winters et al., 2014) and such differential allocation could therefore facilitate the perpetuation of variation in male warning coloration through subsequent generations. However, it is worth noting that work on differential allocation has also shown that a female's reproductive response to signals of male ‘quality’ varies with female age and phenotype (Sheppard et al., 2013). Combined with other effects linked to maternal phenotype, condition, or maternal response to the offspring environment, this differential maternal investment in response to male phenotype is likely to lead to a mosaic of continuous colour variation within and among populations of aposematic species.
(ii). Social signals and competition
If warning signals can be used as signals during mate choice, and there is evidence that they can, they may also play a role in other forms of intraspecific interactions such as male–male competition, dominance and territorial disputes. While there are many studies that show the importance of visual signals in such interactions [see Shreeve, 1987, Setchell & Wickings, 2005, López‐Idiáquez et al., 2016 for examples], few have considered aposematic species. One example comes from work on male–male competition in the frog O. pumilio; male brightness affected both their own behaviour and the behaviour of other males towards them. Brighter focal males were more likely to approach intruders to their territory, and brighter intruders elicited more calls and approaches than dull ones (Crothers, Gering & Cummings, 2011). This suggests that continuous variation in male brightness may be a conditional signal in this species, and that male–male aggression may play a role in its maintenance within populations.
Intraspecific warning signals may also occur when conspecifics can benefit from signalling their presence to others, and by heeding such signals. This may arise when competition between conspecifics is particularly costly. Possible examples include larval coloration signalling the presence of existing larvae on potential host plants to ovipositing females in order to reduce larval cannibalism in the pipevine swallowtail butterfly Battus philenor (Papaj & Newsom, 2005) and bright colours in male damselflies signalling their presence to other males in order to reduce male–male mating attempts during scramble competition (Sherratt & Forbes, 2001). While intriguing, honest communication of this sort seems unlikely to result in signal polymorphism, as it should also favour a single signal. If however, signals are dishonest, with signallers attempting to deceive the conspecific receivers, then there is the potential for red queen/chase away selection dynamics to unfold, similar to those that may occur during Batesian mimicry. Dishonest intrasexual signalling has been suggested as a potential reason for females displaying male‐like coloration, as this deceives mate‐searching males and reduces male harassment in butterflies (Cook et al., 1994) and damselflies (Hammers & Van Gossum, 2008).
As well as increased competition for resources, high conspecific density is linked to increased detectability by predators (Riipi et al., 2001). In aposematic species such aggregations actually augment signal strength and thus predator deterrence (Finkbeiner, Briscoe & Reed, 2012; Rowland, Ruxton & Skelhorn, 2013), changing the costs and benefits of large conspecific densities. This is reflected in the developmental ‘phase shift’ of the desert locust (Schistocerca gregaria). Coloration in these toxic locusts changes from a cryptic phenotype to an aposematic yellow and black when raised at high conspecific densities (Sword, 1999). This shift in anti‐predator strategy is a response to the increased likelihood of detection by predators when the cryptic locusts are in large aggregations. Predators learn to associate prey with toxicity more quickly when they are conspicuous rather than cryptic (Sword et al., 2000) and the detection costs of warning coloration are diminished by gregariousness (Gamberale & Tullberg, 1998; Riipi et al., 2001). Conspecifics can therefore influence not only within‐ but between‐morph variation in aposematic signalling.
(d). Age‐structured populations and senescence
Although not an alternative selection pressure per se, the conspicuousness of warning signals might also be expected to change over the lifetime of an individual, closely tracking major physiological changes resulting from processes such as reproduction and senescence (Booth, 1990b). For example, when warning coloration has a dual role as a predator deterrent and mate attractant, there might be an increase in signal strength during the mating season (Örnborg et al., 2002; Pérez‐Rodríguez, 2008). Reproduction is also costly and adult condition is often poorer post‐, relative to pre‐breeding (Stearns, 1992; Monaghan, Metcalfe & Torres, 2009). Such a decrease in condition may potentially have consequences for the conspicuousness or ‘quality’ of an individual's aposematic signal, especially in longer‐lived species with multiple reproductive bouts (Velando, Drummond & Torres, 2010). Senescence, on the other hand, is strongly linked to a general decline in phenotype (Rose, 1991), and the strength of aposematic signals may become less effective with age in the same way as other colour signals, for example the blue feet of male blue‐footed boobies Sula nebouxii (Torres & Velando, 2007), the yellow bibs of the common yellowthroat Geothlypis trichas (Freeman‐Gallant et al., 2011), and the wings of the orange sulphur butterfly Colias eurytheme (Kemp, 2006).
The effects of reproduction on coloration also vary according to an individual's age and sex, leading to a mosaic of colour expression within a population resulting from individual variation in sex, age class, and reproductive status (Evans, Gustafsson & Sheldon, 2011; Grunst, Rotenberry & Grunst, 2014). Furthermore, holometabolous insects such as Lepidoptera naturally show dramatic changes in form as well as coloration throughout their lifetime (Booth, 1990b), and more subtle changes in coloration are common in hemimetabolous insects, such as true bugs, between different nymphs and adult forms. Incorporate the aforementioned genetic correlations between warning signals at different life stages, or warning signals and other life‐history traits, and the picture becomes even more complex (Lindstedt et al., 2016). An explicit test of the effects of reproduction and senescence on warning coloration variation and efficiency, also taking into consideration possible genetic correlations, is therefore needed. This would then enable us to ascertain whether similar patterns occur in populations of aposematic species and therefore if variation in age class within a population contributes to individual variation in conspicuousness.
It is clear therefore that there are myriad different selective pressures with the potential to influence the warning signals of aposematic species, some of which have already been empirically demonstrated to be important, and others worthy of further research. These sources of influence may act in ways that can be diametrically opposed to, or act synergistically with, predation pressure (Table 1). These ‘alternative’ selection pressures are most likely to produce and maintain warning signal diversity if they: (i) produce negative frequency‐dependent selection favouring rare morphs (for example those described in Heliconius by Chouteau et al., 2017), or (ii) act in combination with heterogeneous selection imposed by predators (as described in Section IV) to produce different optimal phenotypes either within or among populations. In the latter case, the resulting selection landscape and associated phenotypic optima are also likely to vary over space and time, further slowing phenotypic convergence and allowing within‐population signal diversity to persist for longer. For example, temperatures will vary not only among habitats but also depending on climatic and seasonal timescales, and selection pressures associated with mate choice will vary in intensity throughout the year, especially in those species with distinct breeding seasons. It is vitally important that future work investigating the role of these selection pressures in producing warning‐signal variation incorporates their potential interaction, both with each other and with predators (e.g. temperature and either predator motivation or intraspecific aggression). It seems only rational to predict that such a movement away from the consideration of selection pressures on warning coloration in isolation is likely to result in a better understanding of the complex patterns of signal variation seen in nature.
Table 1.
Summary of key factors facilitating the maintenance of different levels of variation within and among aposematic species.
| Factor | Effect | Predicted form of signal variation |
|---|---|---|
| Variation among predators | (1) Broad‐scale differences in physiology (differences in sensory capacities, toxin tolerance and cognition) and behaviour among species and populations of predators | Polytypism; polymorphism if predators are structured across microhabitats; continuous variation; seasonal variation |
| (2) Differences in predator experience among species, populations, and temporally within populations | Polytypism; polymorphism if predators are structured across microhabitats; seasonal variation | |
| (3) Small‐scale differences in physiology and behaviour among individuals, linked to motivation or individual experience | Would relax purifying selection, potentially allowing polymorphism or continuous variation | |
| Temperature | Lower temperatures favour melanic components of warning signals, whereas predation selects against melanic morphs | Polytypism; polymorphism; continuous variation across populations along altitudinal or latitudinal gradients; continuous variation within populations (linked to microclimate during development); polyphenism/seasonal variation |
| UV damage | Increased UV risk favours melanic components of warning signals, whereas predation selects against melanic morphs | Polytypism; polymorphism; continuous variation across populations along altitudinal or latitudinal gradients; continuous variation within populations (linked to microclimate during development); polyphenism/seasonal variation |
| Desiccation | Increased desiccation risk favours melanic components of warning signals, whereas predation selects against melanic morphs | Polytypism; polymorphism; continuous variation across populations along altitudinal or latitudinal gradients; continuous variation within populations (linked to microclimate during development); polyphenism/seasonal variation |
| Resource availability | Availability of resources influences investment in warning coloration, often via effect on signalling honesty | Continuous variation within or among populations; polytypism |
| Disease and parasite load | (1) Effect of infection on individual condition | Continuous variation within or among populations |
| (2) Stimulation of melanisation by infection or trade‐offs between use of melanin for pigmentation or infection resistance | Continuous variation within or among populations | |
| (3) Correlated trait responses if coloration is linked to factors such as immunocompetence or parasitism risk | Polytypism; polymorphism; polyphenism | |
| (4) Pathogen‐driven local extinctions, or repeated bottlenecks, which disrupt purifying selection and maintain colour variation | Polytypism; polymorphism | |
| Intraspecific signalling | Warning colours may also serve as social cues, for example of quality or social status | Polymorphism; sexual dichromatism; continuous variation within populations |
| Density and aggregation | Density of aposematic species can alter selective landscapes, particularly the influence of frequency‐dependent selection imposed by predators. Aggregation of aposematic species can have a similar effect (and play into predator psychology to decrease the likelihood of an attack). | Polytypism; polymorphism; polyphenism |
VI. TAXONOMIC OCCURRENCE OF WARNING‐SIGNAL POLYMORPHISM
In our search for variation in aposematic signals, we found examples in nearly every taxon in which we find aposematism (see online Table S1), suggesting that variation in warning signals is far more widespread than previously appreciated. Despite this taxonomic diversity, a disproportionate amount of research effort has focused on a limited number of taxa, most notably Lepidoptera (especially the Neotropical Heliconius) and, to a lesser extent, the dendrobatid poison frogs. While this has enabled researchers to delve deeply into the various mechanisms producing patterns of variation within these species, it is unclear whether their findings generalise to other taxonomic groups.
Aposematic research has, for the past century, focused predominantly on terrestrial insects and their avian predators, possibly due to the tractability of these systems. However, examples of aposematic colour and pattern variation in other taxa such as birds (Dumbacher et al., 1992, 2008) and mammals (Hunter, 2009; Stankowich, Caro & Cox, 2011; Caro et al., 2013) have more recently been revealed. Warning‐signal variation in several marine species has also begun to be investigated (Hanlon & Messenger, 1998; Cortesi & Cheney, 2010; Winters et al., 2017), although the aposematic function of conspicuous coloration in aquatic environments has been questioned. This is due to limited light availability, poorly known predator visual systems and predator–prey interactions, and the lack of known defence mechanisms for many brightly coloured organisms in the marine environment (Pawlik, 2012). It is probably in the non‐animal kingdoms where aposematism has received the least attention, despite reported examples in plants (e.g. Cahn & Harper, 1976; Karageorgou, Buschmann & Manetas, 2008; Lev‐Yadun, 2009) and even fungi (Sherratt, Wilkinson & Bain, 2005). There is therefore a need for more comparative studies on different taxa with robust phylogenies and detailed ecological information in order to address the ultimate causes of signal variation across taxa. Furthermore, utilising other systems parallel to the traditional terrestrial avian–insect interactions, for example, terrestrial plant–herbivore or marine food webs, may well provide new insights into the selective pressures and commonalities creating aposematic variation.
VII. CONCLUSIONS
(1) While predator‐enforced selection on aposematic species appears to favour signal monomorphy in some cases, a growing appreciation of animal sensory systems and of the complexity of predator psychology in particular is challenging the concept of the predator community as a single invariant selective agent.
(2) Investigations of continuous variation or polymorphisms in aposematic species should first assess whether and to what extent the differences between individual signals actually impact predation risk. The perceptual abilities and responses of relevant predators, natural conditions and the microhabitat structure shaping encounter rates between predators and prey are especially important considerations.
(3) Equally as important, a variety of biotic and abiotic selection pressures experienced by aposematic species can contribute to warning signal variation within and among populations, and may potentially act antagonistically or synergistically with predator selection (summary in Table 1). Testing the relevance of visual signals to other behaviours, such as mate choice or thermoregulation, as informed by the natural history of the study species, will help piece together a more complex picture of the selective landscape driving signal variation.
(4) Moving forward, the field of aposematism should step away from the paradigm that warning signals are entirely determined by a uniform class of predators (generally birds), and instead consider both the strength of selection imposed by predators and alternative selective forces. Future work on aposematic species should adopt a more holistic approach to understanding colour and pattern, applying the tools of behavioural ecology, physiology and genetics to assess the relative power of predation versus other selective pressures in producing specific phenotypes.
(5) Broadening the taxonomic spread of research on warning signals and focusing on less well‐studied systems, encompassing different types of predators, would also help build a more comprehensive picture of the selective pressures determining variation in aposematism.
(6) Despite an overwhelmingly narrow research focus on predation pressures as the primary determinant of warning coloration, aposematism is affected by a range of forces, of which predation may not necessarily always be the most important. At the outset of this review we asked whether variation in warning coloration is a paradox or if it is the norm. It appears to be both; it is a paradox from the historical perspective that defines aposematic pressures via purifying selection enforced by predators, and the norm if we consider the empirical data and alternative selective pressures facing these species.
VIII. ACKNOWLEDGEMENTS
We thank J. Mappes, K. Summers, M. Stevens, the organisers (Sensory Ecology Group) and attendees of the Anti‐Predator Coloration Symposium in Cornwall 2016 for discussions and inspiration. We would also like to thank K. Summers and J. Mappes for support and comments, as well as Mathieu Chouteau and an anonymous reviewer for their valuable input. Publication was funded by a BBSRC SWBio DTP studentship (award ref. 1355867). Data access statement: This is a review article and did not generate any new data; all data underlying this study is cited in the references.
Supporting information
Appendix S1. Methods for compiling Table S1.
Table S1. Examples of warning‐colour variation described in existing literature.
IX. REFERENCES
References marked with asterisk have been cited within the supporting information
- Abram, P. K. , Guerra‐Grenier, E. , Després‐Einspenner, M. L. , Ito, S. , Wakamatsu, K. , Boivin, G. & Brodeur, J. (2015). An insect with selective control of egg coloration. Current Biology 25, 2007–2011. [DOI] [PubMed] [Google Scholar]
- Al Abassi, S. , Birkett, M. A. , Pettersson, J. , Pickett, J. A. , Wadhams, L. J. & Woodcock, C. M. (2001). Response of the ladybird parasitoid Dinocampus coccinellae to toxic alkaloids from the seven‐spot ladybird, Coccinella septempunctata . Journal of Chemical Ecology 27, 33–43. [DOI] [PubMed] [Google Scholar]
- Amézquita, A. , Castro, L. , Arias, M. , González, M. & Esquivel, C. (2013). Field but not lab paradigms support generalisation by predators of aposematic polymorphic prey: the Oophaga histrionica complex. Evolutionary Ecology 27, 769–782. [Google Scholar]
- * Amézquita, A. , Ramos, Ó. , González, M. C. , Rodríguez, C. , Medina, I. , Simões, P. I. & Lima, A. P. (2017). Conspicuousness, colour resemblance, and toxicity in geographically diverging mimicry: the pan‐Amazonian frog Allobates femoralis . Evolution 71, 1039–1050. [DOI] [PubMed] [Google Scholar]
- Arenas, L. M. & Stevens, M. (2017). Diversity in warning coloration is easily recognised by avian predators. Journal of Evolutionary Biology 30, 1288–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas, L. M. , Troscianko, J. & Stevens, M. (2014). Colour contrast and stability as key elements for effective warning signals. Frontiers in Ecology and Evolution 2, 1–12. [Google Scholar]
- Arenas, L. M. , Walter, D. & Stevens, M. (2015). Signal honesty and predation risk among a closely related group of aposematic species. Scientific Reports 5, 11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias, M. , Poul, Y. , Chouteau, M. , Boisseau, R. , Rosser, N. , Théry, M. & Llaurens, V. (2016). Crossing fitness valleys: empirical estimation of a fitness landscape associated with polymorphic mimicry. Proceedings of the Royal Society B: Biological Sciences 283, 20160391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubier, T. G. & Sherratt, T. N. (2015). Diversity in Müllerian mimicry: the optimal predator sampling strategy explains both local and regional polymorphism in prey. Evolution 69, 2831–2845. [DOI] [PubMed] [Google Scholar]
- * Ball, E. D. (1930). The toadhoppers of the genus Phylloscelis Germ. (Rhynchota Fulgoridae). The Canadian Entomologist 62, 192–195. [Google Scholar]
- Barnett, C. A. , Bateson, M. & Rowe, C. (2007). State‐dependent decision making: educated predators strategically trade off the costs and benefits of consuming aposematic prey. Behavioral Ecology 18, 645–651. [Google Scholar]
- Barnett, C. A. , Skelhorn, J. , Bateson, M. & Rowe, C. (2012). Educated predators make strategic decisions to eat defended prey according to their toxin content. Behavioral Ecology 23, 418–424. [Google Scholar]
- Barnett, J. B . & Cuthill, I. C . (2014). Distance‐dependent defensive coloration. Current Biology 24, R1157–R1158. [DOI] [PubMed] [Google Scholar]
- Barnett, J. B. , Scott‐Samuel, N. E. & Cuthill, I. C. (2016). Aposematism: balancing salience and camouflage. Biology Letters 12, 20160335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty, C. D. , Beirinckx, K. & Sherratt, T. N. (2004). The evolution of Müllerian mimicry in multispecies communities. Nature 431, 63–67. [DOI] [PubMed] [Google Scholar]
- Beccaloni, G. (1997). Ecology, natural history and behaviour of the Ithomiinae butterflies and their mimics in Ecuador. Tropical Lepidoptera 8, 103–124. [Google Scholar]
- * Beck, D. D. (2005). Biology of Gila Monsters and Beaded Lizards. University of California Press, Berkeley. [Google Scholar]
- Bezzerides, A. L. , McGraw, K. J. , Parker, R. S. & Husseini, J. (2007). Elytra colour as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis . Behavioral Ecology and Sociobiology 61, 1401–1408. [Google Scholar]
- Blount, J. D. , Rowland, H. M. , Drijfhout, F. P. , Endler, J. A. , Inger, R. , Sloggett, J. J. , Hurst, G. D. D. , Hodgson, D. J. & Speed, M. P. (2012). How the ladybird got its spots: effects of resource limitation on the honesty of aposematic signals. Functional Ecology 26, 334–342. [Google Scholar]
- Blount, J. D. , Speed, M. P. , Ruxton, G. D. & Stephens, P. A. (2009). Warning displays may function as honest signals of toxicity. Proceedings of the Royal Society B: Biological Sciences 276, 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloxham, L. , Bateson, M. , Bedford, T. , Brilot, B. & Nettle, D. (2014). The memory of hunger: developmental plasticity of dietary selectivity in the European starling, Sturnus vulgaris . Animal Behaviour 91, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bocek, M. & Bocak, L. (2016). Species limits in polymorphic mimetic Eniclases net‐winged beetles from new Guinean mountains (Coleoptera, Lycidae). ZooKeys 593, 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin, T. , Tullberg, B. S. & Merilaita, S. (2008). The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Animal Behaviour 76, 577–584. [Google Scholar]
- Bolund, E. , Schielzeth, H. & Forstmeier, W. (2009). Compensatory investment in zebra finches: females lay larger eggs when paired to sexually unattractive males. Proceedings of the Royal Society B: Biological Sciences 276, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Bonansea, M. I. & Vaira, M. (2012). Geographic and intrapopulational variation in colour and patterns of an aposematic toad, Melanophryniscus rubriventris (Amphibia, Anura, Bufonidae). Amphibia‐Reptilia 33, 11–24. [Google Scholar]
- Bond, A. B. & Kamil, A. C. (2006). Spatial heterogeneity, predator cognition, and the evolution of colour polymorphism in virtual prey. Proceedings of the National Academy of Sciences of the United States of America 103, 3214–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Booth, C. L. (1990a). Biology of Largus californicus (Hemiptera: Largidae). The Southwestern Naturalist 35, 15–22. [Google Scholar]
- Booth, C. L. (1990b). Evolutionary significance of ontogenetic colour change in animals. Biological Journal of the Linnean Society 40, 125–163. [Google Scholar]
- Borer, M. , Van Noort, T. , Rahier, M. & Naisbit, R. E. (2010). Positive frequency‐dependent selection on warning colour in alpine leaf beetles. Evolution 64, 3629–3633. [DOI] [PubMed] [Google Scholar]
- Brakefield, P. M. (1984). Ecological studies on the polymorphic ladybird Adalia bipunctata in the Netherlands. I. Population biology and geographical variation of melanism. The Journal of Animal Ecology 53, 761–774. [Google Scholar]
- * Brakefield, P. M. (1985). Polymorphic Müllerian mimicry and interactions with thermal melanism in ladybirds and a soldier beetle: a hypothesis. Biological Journal of the Linnean Society 26, 243–267. [Google Scholar]
- * Brakefield, P. M. & Liebert, T. G. (1985). Studies of colour polymorphism in some marginal populations of the aposematic Jersey tiger moth Callimorpha quadripunctaria . Biological Journal of the Linnean Society 26, 225–241. [Google Scholar]
- * Briolat, E. S. , Zagrobelny, M. , Olsen, C. E. , Blount, J. D. & Stevens, M. (2018). Sex differences but no evidence of quantitative honesty in the warning signals of six‐spot burnet moths (Zygaena filipendulae L.). Evolution 72, 1460–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom, M. , Speed, M. P. & Ruxton, G. D. (2005). Evolutionarily stable investment in secondary defences. Functional Ecology 19, 836–843. [Google Scholar]
- * Brower, A. V. Z. & Egan, M. G. (1997). Cladistic analysis of Heliconius butterflies and relatives (Nymphalidae: Heliconiiti): a revised phylogenetic position for Eueides based on sequences from mtDNA and a nuclear gene. Proceedings of the Royal Society B: Biological Sciences 264, 969–977. [Google Scholar]
- Brower, L. P. (1988). Avian predation on the monarch butterfly and its implications for mimicry theory. The American Naturalist 131, S4–S6. [Google Scholar]
- Brower, L. P. , Brower, J. V. Z. & Corvino, J. M. (1967). Plant poisons in a terrestrial food chain. Proceedings of the National Academy of Sciences of the United States of America 57, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower, L. P. , Ryerson, W. N. , Coppinger, L. L. & Glazier, S. C. (1968). Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. [DOI] [PubMed] [Google Scholar]
- * Brown, K. S. (1970). Rediscovery of Heliconius nattereri in eastern Brazil. Entomological News 18, 129–140. [Google Scholar]
- * Brown, K. S. (1972). Heliconians of Brazil (Lepidoptera: Nymphalidae). III. Ecology and biology of Heliconius nattereri, a key primitive species near extinction and comments on the evolutionary development of Heliconius and Euides . Zoologica 57, 41–69. [Google Scholar]
- Brown, K. S. (1979). Ecologia geográfica e evolucao nas florestas neotropicais, 2 vols. Universidade Estadual de Campinas, Campinas. [Google Scholar]
- * Brown, K. S. J. (1981). The biology of Heliconius and related genera. Annual Review of Entomology 26, 427–456. [Google Scholar]
- Brown, K. S. J. & Benson, W. W. (1974). Adaptive polymorphism associated with multiple Müllerian mimicry in Heliconius numata (Lepid. Nymph.). Biotropica 6, 205–228. [Google Scholar]
- * Brown, K. S. & Holzinger, H. (1973). The heliconians of Brazil (Lepidoptera: Nymphalidae). Part IV. Systematics and biology of Eueides tales Cramer, with description of a new subspecies from Venezuela. Arbeitsgemeinschaft Österreichischer Entomologen 24, 44–65. [Google Scholar]
- Brown, K. S. , Sheppard, P. M. & Turner, J. R. G. (1974). Quaternary refugia in tropical America: evidence from race formation in Heliconius butterflies. Proceedings of the Royal Society B: Biological Sciences 187, 369–378. [Google Scholar]
- * Brown, J. L. , Twomey, E. , Amézquita, A. , De Souza, M. B. , Caldwell, J. P. , Lötters, S. , Von May, R. , Melo‐Sampaio, P. R. , Mejía‐Vargas, D. , Perez‐Peña, P. , Pepper, M. , Poelman, E. H. , Sanchez‐Rodriguez, M. & Summers, K. (2011). A taxonomic revision of the Neotropical poison frog genus Ranitomeya (Amphibia: Dendrobatidae). Zootaxa 3083, 1–120. [Google Scholar]
- Bull, J. J. (1987). Evolution of phenotypic variance. Evolution 41, 303–315. [DOI] [PubMed] [Google Scholar]
- Burdfield‐Steel, E. R. & Shuker, D. M. (2011). Reproductive interference. Current Biology 21, R450–R451. [DOI] [PubMed] [Google Scholar]
- Cahn, M. G. & Harper, J. L. (1976). The biology of the leaf mark polymorphism in Trifolium repens L. 2. Evidence for the selection of leaf marks by rumen fistulated sheep. Heredity 37, 327–333. [Google Scholar]
- Calsbeek, B. , Hasselquist, D. & Clobert, J. (2010). Multivariate phenotypes and the potential for alternative phenotypic optima in wall lizard (Podarcis muralis) ventral colour morphs. Journal of Evolutionary Biology 23, 1138–1147. [DOI] [PubMed] [Google Scholar]
- Carle, T. & Rowe, C. (2014). Avian predators change their foraging strategy on defended prey when undefended prey are hard to find. Animal Behaviour 93, 97–103. [Google Scholar]
- Caro, T. , Stankowich, T. , Kiffner, C. & Hunter, J. (2013). Are spotted skunks conspicuous or cryptic? Ethology Ecology & Evolution 25, 144–160. [Google Scholar]
- * Chamberlain, N. L. , Hill, R. I. , Kapan, D. D. , Gilbert, L. E. & Kronforst, M. R. (2009). Polymorphic butterfly reveals the missing link in ecological speciation. Science 326, 847–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth, D. & Charlesworth, B. (1975). Theoretical genetics of Batesian mimicry I. Single‐locus models. Journal of Theoretical Biology 55, 283–303. [DOI] [PubMed] [Google Scholar]
- Chatelain, M. , Halpin, C. G. & Rowe, C. (2013). Ambient temperature influences birds' decisions to eat toxic prey. Animal Behaviour 86, 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chiari, Y. , Vences, M. , Vieites, D. R. , Rabemananjara, F. , Bora, P. , Ramilijaona Ravoahangimalala, O. & Meyer, A. (2004). New evidence for parallel evolution of colour patterns in Malagasy poison frogs (Mantella). Molecular Ecology 13, 3763–3774. [DOI] [PubMed] [Google Scholar]
- Chouteau, M. & Angers, B. (2011). The role of predators in maintaining the geographic organization of aposematic signals. The American Naturalist 178, 810–817. [DOI] [PubMed] [Google Scholar]
- Chouteau, M. & Angers, B. (2012). Wright's shifting balance theory and the diversification of aposematic signals. PLoS One 7, e34028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouteau, M. , Arias, M. & Joron, M. (2016). Warning signals are under positive frequency‐ dependent selection in nature. Proceedings of the National Academy of Sciences of the United States of America 113, 2164–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouteau, M. , Llaurens, V. , Prunier, F. & Joron, M. (2017). Polymorphism at a mimicry supergene maintained by opposing frequency dependent selection pressures. Proceedings of the National Academy of Sciences of the United States of America 114, 8325–8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccotto, P. J. , Dresser, D. J. & Mendelson, T. C. (2014). Association between parasite load and orange, but not blue, male nuptial coloration in Etheostoma caeruleum . Journal of Fish Biology 84, 1590–1598. [DOI] [PubMed] [Google Scholar]
- Clusella‐Trullas, S. , van Wyk, J. H. & Spotila, J. R. (2007). Thermal melanism in ectotherms. Journal of Thermal Biology 32, 235–245. [Google Scholar]
- * Conner, W. E. (2008). Tiger Moths and Woolly Bears: Behavior, Ecology, and Evolution of the Arctiidae. Oxford University Press, New York. [Google Scholar]
- * Conner, W. E. & Weller, S. J. (2004). A quest for alkaloids: the curious relationship between tiger moths and plants containing pyrrolozidine alkaloids In Advances in Insect Chemical Ecology (eds Cardé R. T. and Millar J. G.), pp. 248–282. Cambridge University Press, Cambridge. [Google Scholar]
- Cook, S. E. , Vernon, J. G. , Bateson, M. & Guilford, T. (1994). Mate choice in the polymorphic African swallowtail butterfly, Papilio dardanus: male‐like females may avoid sexual harassment. Animal Behaviour 47, 389–397. [Google Scholar]
- * Cooney, L. J. , van Klink, J. W. , Hughes, N. M. , Perry, N. B. , Schaefer, H. M. , Menzies, I. J. & Gould, K. S. (2012). Red leaf margins indicate increased polygodial content and function as visual signals to reduce herbivory in Pseudowintera colorata . New Phytologist 194, 488–497. [DOI] [PubMed] [Google Scholar]
- Cortesi, F. & Cheney, K. L. (2010). Conspicuousness is correlated with toxicity in marine opisthobranchs. Journal of Evolutionary Biology 23, 1509–1518. [DOI] [PubMed] [Google Scholar]
- Cott, H. B. (1940). Adaptive Coloration in Animals. Methuen, London. [Google Scholar]
- Cotter, S. C. , Myatt, J. P. , Benskin, C. M. H. & Wilson, K. (2008). Selection for cuticular melanism reveals immune function and life‐history trade‐offs in Spodoptera littoralis . Journal of Evolutionary Biology 21, 1744–1754. [DOI] [PubMed] [Google Scholar]
- Crothers, L. & Cummings, M. (2013). Warning signal brightness variation: sexual selection may work under the radar of natural selection in populations of a polymorphic poison frog. The American Naturalist 181, E116–E124. [DOI] [PubMed] [Google Scholar]
- Crothers, L. , Gering, E. & Cummings, M. (2011). Aposematic signal variation predicts male–male interactions in a polymorphic poison frog. Evolution 65, 599–605. [DOI] [PubMed] [Google Scholar]
- Culumber, Z. W. , Bautista‐Hernández, C. E. , Monks, S. , Arias‐Rodriguez, L. & Tobler, M. (2014). Variation in melanism and female preference in proximate but ecologically distinct environments. Ethology 120, 1090–1100. [Google Scholar]
- Cummings, M. E. & Crothers, L. R. (2013). Interacting selection diversifies warning signals in a polytypic frog: an examination with the strawberry poison frog. Evolutionary Ecology 27, 693–710. [Google Scholar]
- * Dafni, J. & Diamant, A. (1984). School‐oriented mimicry, a new type of mimicry in fishes. Marine Ecology Progress Series 20, 45–50. [Google Scholar]
- Daly, J. W. & Myers, C. W. (1967). Toxicity of Panamanian poison frogs (Dendrobates): some biological and chemical aspects. Science 156, 970–973. [DOI] [PubMed] [Google Scholar]
- * Darlington, C. D. & Mather, K. (1949). The Elements of Genetics. George Allen & Unwin, London. [Google Scholar]
- Darst, C. R. , Cummings, M. E. & Cannatella, D. C. (2006). A mechanism for diversity in warning signals: conspicuousness versus toxicity in poison frogs. Proceedings of the National Academy of Sciences of the United States of America 103, 5852–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra, K. K. , Lamas, G. , Simpson, F. & Mallet, J. (2010). The anatomy of a ‘suture zone’ in Amazonian butterflies: a coalescent‐based test for vicariant geographic divergence and speciation. Molecular Ecology 19, 4283–4301. [DOI] [PubMed] [Google Scholar]
- Davis Rabosky, A. R. , Cox, C. L. & Rabosky, D. L. (2016a). Unlinked Mendelian inheritance of red and black pigmentation in snakes: implications for Batesian mimicry. Evolution 70, 944–953. [DOI] [PubMed] [Google Scholar]
- Davis Rabosky, A. R. , Cox, C. L. , Rabosky, D. L. , Title, P. O. , Holmes, I. A. , Feldman, A. & McGuire, J. A. (2016b). Coral snakes predict the evolution of mimicry across New World snakes. Nature Communications 7, 11484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Day, M. C. (1984). Male polymorphism in some Old World species of Cryptocheilus Panzer (Hymenoptera: Pompilidae). Zoological Journal of the Linnean Society 80, 83–101. [Google Scholar]
- Day, T. & Bonduriansky, R. (2011). A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. The American Naturalist 178, E18–E36. [DOI] [PubMed] [Google Scholar]
- * Deroe, C. & Pasteels, J. M. (1982). Distribution of adult defense glands in chrysomelids (Coleoptera: Chrysomelidae) and its significance in the evolution of defense mechanisms within the family. Journal of Chemical Ecology 8, 67–82. [DOI] [PubMed] [Google Scholar]
- * Devries, P. J. (1987). The Butterflies of Costa Rica and Their Natural History Volume I: Papilionidae, Pieridae, Nymphalidae Princeton University Press, Princeton. [Google Scholar]
- * Dressler, R. L. (1979). Eulaema bombiformis, E. Meriana, and Müllerian mimicry in related species (Hymenoptera: Apidea). Biotropica 11, 144–151. [Google Scholar]
- * Dubey, A. , Omkar & Mishra, G. (2016). Influence of temperature on reproductive biology and phenotype of a ladybird, Menochilus sexmaculatus (Fabricius) (Coleoptera: Coccinellidae). Journal of Thermal Biology 58, 35–42. [DOI] [PubMed] [Google Scholar]
- Dubovskiy, I. M. , Whitten, M. M. A. , Kryukov, V. Y. , Yaroslavtseva, O. N. , Grizanova, E. V. , Greig, C. , Mukherjee, K. , Vilcinskas, A. , Mitkovets, P. V. , Glupov, V. V. & Butt, T. M. (2013). More than a colour change: insect melanism, disease resistance and fecundity. Proceedings of the Royal Society B: Biological Sciences 280, 20130584–20130584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbacher, J. P. , Beehler, B. M. , Spande, T. F. , Garraffo, H. M. & Daly, J. W. (1992). Homobatrachotoxin in the genus Pitohui: chemical defense in birds? Science 30, 799–801. [DOI] [PubMed] [Google Scholar]
- * Dumbacher, J. P. & Fleischer, R. C. (2001). Phylogenetic evidence for colour pattern convergence in toxic pitohuis: Müllerian mimicry in birds? Proceedings of the Royal Society B: Biological Sciences 268, 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbacher, J. P. , Deiner, K. , Thompson, L. & Fleischer, R. C. (2008). Phylogeny of the avian genus Pitohui and the evolution of toxicity in birds. Molecular Phylogenetics and Evolution 49, 774–781. [DOI] [PubMed] [Google Scholar]
- * Dunn, E. R. (1941). Notes on Dendrobates auratus . Copeia 1941, 88–93. [Google Scholar]
- * Edmunds, M. (1969). Polymorphism in the mimetic butterfly Hypolimnas misippus in Ghana. Heredity 24, 281–301. [DOI] [PubMed] [Google Scholar]
- Elias, M. , Gompert, Z. , Jiggins, C. & Willmott, K. (2008). Mutualistic interactions drive ecological niche convergence in a diverse butterfly community. PLoS Biology 6, 2642–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emaresi, G. , Bize, P. , Altwegg, R. , Henry, I. , van den Brink, V. , Gasparini, J. & Roulin, A. (2014). Melanin‐specific life‐history strategies. The American Naturalist 183, 269–280. [DOI] [PubMed] [Google Scholar]
- Endler, J. A. (1990). On the measurement and classification of colour in studies of animal colour patterns. Biological Journal of the Linnean Society 41, 315–352. [Google Scholar]
- Endler, J. A. (1993). The colour of light in forests and its implications. Ecological Monographs 63, 1–27. [Google Scholar]
- Endler, J. A. & Greenwood, J. J. D. (1988). Frequency‐dependent predation, crypsis and aposematic coloration. Philosophical Transactions of the Royal Society of London B: Biological Sciences 319, 505–523. [DOI] [PubMed] [Google Scholar]
- Endler, J. A. & Mappes, J. (2004). Predator mixes and the conspicuousness of aposematic signals. The American Naturalist 163, 532–547. [DOI] [PubMed] [Google Scholar]
- Endler, J. A. & Rojas, B. (2009). The spatial pattern of natural selection when selection depends on experience. The American Naturalist 173, E62–E78. [DOI] [PubMed] [Google Scholar]
- Estrada, C. & Jiggins, C. D. (2008). Interspecific sexual attraction because of convergence in warning coloration: is there a conflict between natural and sexual selection in mimetic species? Journal of Evolutionary Biology 21, 749–760. [DOI] [PubMed] [Google Scholar]
- Evans, S. R. , Gustafsson, L. & Sheldon, B. C. (2011). Divergent patterns of age‐dependence in ornamental and reproductive traits in the collared flycatcher. Evolution 65, 1623–1636. [DOI] [PubMed] [Google Scholar]
- Exnerová, A. , Landová, E. , Štys, P. , Fuchs, R. , Prokopová, M. & Cehláriková, P. (2003). Reactions of passerine birds to aposematic and non‐aposematic firebugs (Pyrrhocoris apterus; Heteroptera). Biological Journal of the Linnean Society 78, 517–525. [Google Scholar]
- Exnerová, A. , Jezova, D. , Štys, P. , Doktorovova, L. , Rojas, B. & Mappes, J. (2015). Different reactions to aposematic prey in 2 geographically distant populations of great tits. Behavioural Ecology 26, 1361–1370. [Google Scholar]
- Exnerová, A. , Svádová, K. H. , Fucíková, E. , Drent, P. & Štys, P. (2010). Personality matters: individual variation in reactions of naive bird predators to aposematic prey. Proceedings of the Royal Society B: Biological Sciences 277, 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exnerová, A. , Svádová, K. , Štys, P. T. , Barcalová, S. , Landová, E. V. A. , Prokopová, M. , Fuchs, R. & Socha, R. (2006). Importance of colour in the reaction of passerine predators to aposematic prey: experiments with mutants of Pyrrhocoris apterus (Heteroptera). Biological Journal of the Linnean Society 88, 143–153. [Google Scholar]
- Fabricant, S. A. , Exnerová, A. , Ježová, D. & Štys, P. (2014). Scared by shiny? The value of iridescence in aposematic of the hibiscus harlequin bug. Animal Behaviour 90, 315–325. [Google Scholar]
- * Fabricant, S. A. & Herberstein, M. E. (2015). Hidden in plain orange: aposematic coloration is cryptic to a colorblind insect predator. Behavioural Ecology 26, 38–44. [Google Scholar]
- Fabricant, S. A. , Kemp, D. J. , Krajíček, J. , Bosáková, Z. & Herberstein, M. E. (2013). Mechanisms of colour production in a highly variable shield‐back stinkbug, Tectocoris diophthalmus (Heteroptera: Scutelleridae), and why it matters. PLoS One 8, e64082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincke, O. M. (2004). Polymorphic signals of harassed female odonates and the males that learn them support a novel frequency‐dependent model. Animal Behaviour 67, 833–845. [Google Scholar]
- Fink, L. S. (1995). Foodplant effects on colour morphs of Eumorpha fasciata caterpillars (Lepidoptera: Sphingidae). Biological Journal of the Linnean Society 56, 423–437. [Google Scholar]
- Fink, L. S. & Brower, L. P. (1981). Birds can overcome the cardenolide defence of monarch butterflies in Mexico. Nature 291, 67–70. [Google Scholar]
- Finkbeiner, S. D. , Briscoe, A. D. & Reed, R. D. (2012). The benefit of being a social butterfly: communal roosting deters predation. Proceedings of the Royal Society B: Biological Sciences 279, 2769–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores, E. E. , Stevens, M. , Moore, A. J. & Blount, J. D. (2013). Diet, development and the optimization of warning signals in post‐metamorphic green and black poison frogs. Functional Ecology 27, 816–829. [Google Scholar]
- Franks, D. W. & Noble, J. (2004). Warning signals and predator‐prey coevolution. Proceedings of the Royal Society B: Biological Sciences 271, 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks, D. W. & Oxford, G. S. (2009). The evolution of exuberant visible polymorphisms. Evolution 63, 2697–2706. [DOI] [PubMed] [Google Scholar]
- Freeman‐Gallant, C. R. , Amidon, J. , Berdy, B. , Wein, S. , Taff, C. C. & Haussmann, M. F. (2011). Oxidative damage to DNA related to survivorship and carotenoid‐based sexual ornamentation in the common yellowthroat. Biology Letters 7, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván, I. & Alonso‐Alvarez, C. (2008). An intracellular antioxidant determines the expression of a melanin‐based signal in a bird. PLoS One 3, e3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamberale, G. & Tullberg, B. S. (1998). Aposematism and gregariousness: the combined effect of group size and coloration on signal repellence. Proceedings of the Royal Society B: Biological Sciences 265, 889–894. [Google Scholar]
- Gangoso, L. , Grande, J. M. , Ducrest, A. L. , Figuerola, J. , Bortolotti, G. R. , Andrés, J. A. & Roulin, A. (2011). MC1R‐dependent, melanin‐based colour polymorphism is associated with cell‐mediated response in the Eleonora's falcon. Journal of Evolutionary Biology 24, 2055–2063. [DOI] [PubMed] [Google Scholar]
- Gavrilets, S. & Vose, A. (2005). Dynamic patterns of adaptive radiation. Proceedings of the National Academy of the Sciences of the United States of America 102, 18040–18045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffeney, S. (2002). Mechanisms of adaptation in a predator‐prey arms race: TTX‐resistant sodium channels. Science 297, 1336–1339. [DOI] [PubMed] [Google Scholar]
- * van Gelder, R. G. (1968). The genus Conepatus (Mammalia, Mustelidae). American Museum Novitates 2322, 1–37. [Google Scholar]
- * Gibbons, J. W. (1991). Guide to the Reptiles and Amphibians of the Savannah River Site. University of Georgia Press, Athens. [Google Scholar]
- * Gilbert, A. J. (2011). A review and clarification of the alticine genera Hemiphrynus Horn 1889 and Phrynocepha Baly 1861 (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Insecta Mundi 0200, 1–57. [Google Scholar]
- Gilbert, L. E. (2003). Adaptive novelty through introgression in Heliconius wing patterns: evidence for a shared genetic “tool box” from synthetic hybrid zones and a theory of diversification In Ecology and Evolution Taking Flight: Butterflies as Model Systems (eds Boggs C., Watt W. and Ehrlich P.), pp. 281–321. University of Chicago, Chicago. [Google Scholar]
- Gordon, I. J. (2013). Male‐killing and aposematic polymorphism in African butterflies: is there a connection? Ideas in Ecology and Evolution 6, 20–21. [Google Scholar]
- Gordon, S. P. , Kokko, H. , Rojas, B. , Nokelainen, O. & Mappes, J. (2015). Colour polymorphism torn apart by opposing positive frequency‐dependent selection, yet maintained in space. Journal of Animal Ecology 84, 1555–1564. [DOI] [PubMed] [Google Scholar]
- Grant, P. R. , Grant, B. R. & Petren, K. (2005). Hybridization in the recent past. The American Naturalist 166, 56–67. [DOI] [PubMed] [Google Scholar]
- * Grant, T. , Frost, D. R. , Caldwell, J. P. , Gagliardo, R. O. N. , Haddad, C. F. B. , Kok, P. J. R. , Means, D. B. , Noonan, B. P. , Schargel, W. E. & Wheeler, W. C. (2006). Phylogenetic systematics of dart‐poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bulletin of the American Museum of Natural History 299, 1–262. [Google Scholar]
- Greene, E. (1989). A diet‐induced developmental polymorphism in a caterpillar. Science 243, 643–646. [DOI] [PubMed] [Google Scholar]
- Greenwood, J. J. D. , Wood, E. M. & Batchelor, S. (1981). Apostatic selection of distasteful prey. Heredity 47, 27–34. [Google Scholar]
- Griffith, S. C. , Parker, T. H. & Olson, V. A. (2006). Melanin‐ versus carotenoid‐based sexual signals: is the difference really so black and red? Animal Behaviour 71, 749–763. [Google Scholar]
- Grill, C. P. & Moore, A. J. (1998). Effects of a larval antipredator response and larval diet on adult phenotype in an aposematic ladybird beetle. Oecologia 114, 274–282. [DOI] [PubMed] [Google Scholar]
- Gröning, J. & Hochkirch, A. (2008). Reproductive interference between animal species. The Quarterly Review of Biology 83, 257–282. [DOI] [PubMed] [Google Scholar]
- Grunst, A. S. , Rotenberry, J. T. & Grunst, M. L. (2014). Age‐dependent relationships between multiple sexual pigments and condition in males and females. Behavioral Ecology 25, 276–287. [Google Scholar]
- Guilford, T. (1994). Go‐slow signalling and the problem of automimicry. Journal of Theoretical Biology 170, 311–316. [Google Scholar]
- Guilford, T. & Dawkins, M. S. (1991). Receiver psychology and the evolution of animal signals. Animal Behaviour 42, 1–14. [Google Scholar]
- Guilford, T. & Dawkins, M. S. (1995). What are conventional signals? Animal Behaviour 49, 1689–1695. [Google Scholar]
- Guindre‐Parker, S. & Love, O. P. (2014). Revisiting the condition‐dependence of melanin‐based plumage. Journal of Avian Biology 45, 29–33. [Google Scholar]
- Halpin, C. G. , Skelhorn, J. & Rowe, C. (2012). The relationship between sympatric defended species depends upon predators' discriminatory behaviour. PLoS One 7, e44895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin, C. G. , Skelhorn, J. & Rowe, C. (2014). Increased predation of nutrient‐enriched aposematic prey. Proceedings of the Royal Society B: Biological Sciences 281, 20133255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin, C. G. , Skelhorn, J. , Rowe, C. , Ruxton, G. D. & Higginson, A. D. (2017). The impact of detoxification costs and predation risk on foraging: implications for mimicry dynamics. PLoS One 12, e0169043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers, M. & Van Gossum, H. (2008). Variation in female morph frequencies and mating frequencies: random, frequency‐dependent harassment or male mimicry? Animal Behavior 76, 1403–1410. [Google Scholar]
- Hanlon, R. T. & Messenger, J. B. (1998). Cephalopod Behaviour. Cambridge University Press, Cambridge. [Google Scholar]
- Hedrick, P. W. (2012). What is the evidence for heterozygote advantage selection? Trends in Ecology & Evolution 27, 698–704. [DOI] [PubMed] [Google Scholar]
- Hegna, R. H. , Nokelainen, O. , Hegna, J. R. & Mappes, J. (2013). To quiver or to shiver: increased melanization benefits thermoregulation, but reduces warning signal efficacy in the wood tiger moth. Proceedings of the Royal Society B: Biological Sciences 280, 20122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hegna, R. H. , Galarza, J. A. & Mappes, J. (2015). Global phylogeography and geographical variation in warning signals of the wood tiger moth (Parasemia plantaginis). Journal of Biogeography 42, 1469–1481. [Google Scholar]
- Heliconius Genome Consortium (2012). Butterfly genome reveals promiscuous exchange of mimicry adaptations among species. Nature 487, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hensel, J. L. & Brodie, E. D. (1976). An experimental study of aposematic coloration in the salamander Plethodon jordani . Copeia 1, 59–65. [Google Scholar]
- Hetem, R. S. , de Witt, B. A. , Fick, L. G. , Fuller, A. , Kerley, G. I. H. , Meyer, L. C. R. , Mitchell, D. & Maloney, S. K. (2009). Body temperature, thermoregulatory behaviour and pelt characteristics of three colour morphs of springbok (Antidorcas marsupialis). Comparative Biochemistry and Physiology A: Molecular and Integrative Physiology 152, 379–388. [DOI] [PubMed] [Google Scholar]
- * Hines, H. M. , Counterman, B. A. , Papa, R. , Albuquerque, P. , Moura, D. & Cardoso, M. Z. (2011). Wing patterning gene redefines the mimetic history of Heliconius butterflies. Proceedings of the National Academy of Sciences of the United States of America 108, 19666–19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hines, H. M. & Williams, P. H. (2012). Mimetic colour pattern evolution in the highly polymorphic Bombus trifasciatus (Hymenoptera: Apidae) species complex and its comimics. Zoological Journal of the Linnean Society 166, 805–826. [Google Scholar]
- Hodek, I. E. , van Emden, H. F. & Honek, A. (2012). Ecology and Behaviour of the Ladybird Beetles (Coccinellidae). John Wiley & Sons, Chichester. [Google Scholar]
- * Hofmann, A. F. & Tremewan, W. G. (2017). The natural history of burnet moths, (Zygaena Fabricius, 1775) (Lepidoptera: Zygaenidae), part I. Proceedings of the Museum Witt Munich 6, 1–631. [Google Scholar]
- Holen, Ø. H. & Svennungsen, T. O. (2012). Aposematism and the handicap principle. The American Naturalist 180, 629–641. [DOI] [PubMed] [Google Scholar]
- Holmes, I. A. , Grundler, M. R. & Davis Rabosky, A. R. (2017). Predator perspective drives geographic variation in frequency‐dependent polymorphism. The American Naturalist 109, E78–E90. [DOI] [PubMed] [Google Scholar]
- * Horn, G. H. (1889). A synopsis of the Halticini of Boreal America. Transactions of the American Entomological Society and Proceedings of the Entomological Section of the Academy of Natural Sciences, 16, 163–320. [Google Scholar]
- Horváthová, T. , Nakagawa, S. & Uller, T. (2012). Strategic female reproductive investment in response to male attractiveness in birds. Proceedings of the Royal Society B: Biological Sciences 279, 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, B. , Whibley, A. , Poul, Y. L. , Navarro, N. , Martin, A. , Baxter, S. , Shah, A. , Gilles, B. , Wirth, T. , McMillan, W. O. & Joron, M. (2015). Conservatism and novelty in the genetic architecture of adaptation in Heliconius butterflies. Heredity 114, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter, J. S. (2009). Familiarity breeds contempt: effects of striped skunk colour, shape, and abundance on wild carnivore behavior. Behavioral Ecology 20, 1315–1322. [Google Scholar]
- Hurd, P. L. & Enquist, M. (2005). A strategic taxonomy of biological communication. Animal Behaviour 70, 1155–1170. [Google Scholar]
- Idris, E. & Hassan, S. S. M. (2013). Biased sex ratios and aposematic polymorphism in African butterflies. Ideas in Ecology and Evolution 6, 5–16. [Google Scholar]
- Ihalainen, E. , Rowland, H. M. , Speed, M. P. , Ruxton, G. D. & Mappes, J. (2012). Prey community structure affects how predators select for Müllerian mimicry. Proceedings of the Royal Society B: Biological Sciences 279, 2099–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Jiggins, C. D. , McMillan, W. O. , King, P. & Mallet, J. (1997). The maintenance of species differences across a Heliconius hybrid zone. Heredity 79, 495–505. [Google Scholar]
- Johansen, A. I. , Exnerová, A. , Svádová, K. H. , Štys, P. , Gamberale‐Stille, G. & Tullberg, B. S. (2010). Adaptive change in protective coloration in adult striated shieldbugs Graphosoma lineatum (Heteroptera: Pentatomidae): test of detectability of two colour forms by avian predators. Ecological Entomology 35, 602–610. [Google Scholar]
- de Jong, P. W. & Brakefield, P. M. (1998). Climate and change in clines for melanism in the two–spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Proceedings of the Royal Society B: Biological Sciences 265, 39–43. [Google Scholar]
- de Jong, P. , Gussekloo, S. & Brakefield, P. (1996). Differences in thermal balance, body temperature and activity between non‐melanic and melanic two‐spot ladybird beetles (Adalia bipunctata). Under controlled conditions. Journal of Experimental Biology 199, 2655–2666. [DOI] [PubMed] [Google Scholar]
- Joron, M. , Frezal, L. , Jones, R. T. , Chamberlain, N. L. , Lee, S. F. , Haag, C. R. , Whibley, A. , Becuwe, M. , Baxter, S. W. , Ferguson, L. , Wilkinson, P. A. , Salazar, C. , Davidson, C. , Clark, R. , Quail, M. A. , Beasley, H. , et al. (2011). Chromosomal rearrangements maintain a polymorphic supergene controlling butterfly mimicry. Nature 477, 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joron, M. & Iwasa, Y. (2005). The evolution of a Müllerian mimic in a spatially distributed community. Journal of Theoretical Biology 237, 87–103. [DOI] [PubMed] [Google Scholar]
- Joron, M. & Mallet, J. L. B. (1998). Diversity in mimicry: paradox or paradigm? Trends in Ecology and Evolution 13, 461–466. [DOI] [PubMed] [Google Scholar]
- Joron, M. , Papa, R. , Beltrán, M. , Chamberlain, N. , Mavárez, J. , Baxter, S. , Abanto, M. , Bermingham, E. , Humphray, S. J. , Rogers, J. , Beasley, H. , Barlow, K. , Ffrench‐Constant, R. H. , Mallet, J. , McMillan, W. O. & Jiggins, C. D. (2006). A conserved supergene locus controls colour pattern diversity in Heliconius butterflies. PLoS Biology 4, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kahn, T. R. , La Marca, E. , Lotters, S. , Brown, J. L. , Twomey, E. & Amézquita, A. (2016). Aposematic Poison Frogs (Dendrobatidae) of the Andean Countries: Bolivia, Colombia, Ecuador, Peru and Venezuela. Conservation International Tropical Field Guide Series, Conservation International, Arlington. [Google Scholar]
- Karageorgou, P. , Buschmann, C. & Manetas, Y. (2008). Red leaf colour as a warning signal against insect herbivory: honest or mimetic? Flora ‐ Morphology, Distribution, Functional Ecology of Plants 203, 648–652. [Google Scholar]
- * Kavanagh, P. H. , Shaw, R. C. & Burns, K. C. (2016). Potential aposematism in an insular tree species: are signals dishonest early in ontogeny? Biological Journal of the Linnean Society 118, 951–958. [Google Scholar]
- * Kawakami, Y. , Yamazaki, K. & Ohashi, K. (2013). Geographical variations of elytral colour polymorphism in Cheilomenes sexmaculata (Fabricius) (Coleoptera: Coccinellidae). Entomological Science 16, 235–242. [Google Scholar]
- Kemp, D. J. (2006). Heightened phenotypic variation and age‐based fading of ultraviolet butterfly wing coloration. Evolutionary Ecology Research 8, 515–527. [Google Scholar]
- Kikuchi, D. W. & Sherratt, T. N. (2015). Costs of learning and the evolution of mimetic signals. The American Naturalist 186, 321–332. [DOI] [PubMed] [Google Scholar]
- King, K. J. & Sinclair, B. J. (2015). Water loss in tree weta (Hemideina): adaptation to the montane environment and a test of the melanisation‐desiccation resistance hypothesis. Journal of Experimental Biology 218, 1995–2004. [DOI] [PubMed] [Google Scholar]
- Kingsolver, J. G. & Wiernasz, D. C. (1991). Seasonal polyphenism in wing‐melanin pattern and thermoregulatory adaptation in Pieris butterflies. The American Naturalist 137, 816–830. [Google Scholar]
- * Klein, A. L. & de Araújo, A. M. (2013). Sexual size dimorphism in the colour pattern elements of two mimetic Heliconius butterflies. Neotropical Entomology 42, 600–606. [DOI] [PubMed] [Google Scholar]
- Knapp, M. & Nedvěd, O. (2013). Gender and timing during ontogeny matter: effects of a temporary high temperature on survival, body size and coloration in Harmonia axyridis . PLoS One 8, e74984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko, H. , Mappes, J. & Lindström, L. (2003). Alternative prey can change model‐mimic dynamics between parasitism and mutualism. Ecology Letters 6, 1068–1076. [Google Scholar]
- Komai, T. (1956). Genetics of ladybeetles. Advances in Genetics 8, 155–188. [Google Scholar]
- * Komai, T. , Chino, M. & Hosino, Y. (1948). Local and chronic variations in some characters of the lady‐beetle, Harmonia axyridis . Memoirs of the College of Science, Kyoto Imperial University, Series B: Biology 19, 47–51. [Google Scholar]
- Kozak, K. M. , Wahlberg, N. , Neild, A. F. E. , Dasmahapatra, K. K. , Mallet, J. & Jiggins, C. D. (2015). Multilocus species trees show the recent adaptive radiation of the mimetic Heliconius butterflies. Systematic Biology 64, 505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Kraemer, A. C. , Serb, J. M. & Adams, D. C. (2015). Batesian mimics influence the evolution of conspicuousness in an aposematic salamander. Journal of Evolutionary Biology 28, 1016–1023. [DOI] [PubMed] [Google Scholar]
- Kronforst, M. R. & Papa, R. (2015). The functional basis of wing patterning in Heliconius butterflies: the molecules behind mimicry. Genetics 200, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst, M. R. , Kapan, D. D. & Gilbert, L. E. (2006a). Parallel genetic architecture of parallel adaptive radiations in mimetic Heliconius butterflies. Genetics 174, 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronforst, M. R. , Young, L. G. , Kapan, D. D. , McNeely, C. , O'Neill, R. J. & Gilbert, L. E. (2006b). Linkage of butterfly mate preference and wing colour preference cue at the genomic location of wingless. Proceedings of the National Academy of Sciences of the United States of America 103, 6575–6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunte, K. , Zhang, W. , Tenger‐Trolander, A. , Palmer, D. H. , Martin, A. , Reed, R. D. , Mullen, S. P. & Kronforst, M. R. (2014). Doublesex is a mimicry supergene. Nature 507, 229–232. [DOI] [PubMed] [Google Scholar]
- Laine, A.‐L. (2009). Role of coevolution in generating biological diversity: spatially divergent selection trajectories. Journal of Experimental Botany 60, 2957–2970. [DOI] [PubMed] [Google Scholar]
- * La Marca, E. , Lips, K. R. , Lötters, S. , Puschendorf, R. , Ibáñez, R. , Rueda‐Almonacid, J. V. , Schulte, R. , Marty, C. , Castro, F. , Manzanilla‐Puppo, J. , Garcia‐Perez, J. E. , Bolanos, F. , Chaves, G. , Pounds, J. A. , Toral, E. & Garcia‐Pérez, J. E. (2005). Catastrophic population declines and extinctions in neotropical harlequin frogs (Bufonidae: Atelopus). Biotropica 37, 190–201. [Google Scholar]
- * Lamas, G. (2004). Checklist: Part 4A. Hesperioidea‐Papilionoidea. Atlas of Neotropical Lepidoptera. Association for Tropical Lepidoptera/Scientific Publishers, Gainesville. [Google Scholar]
- Lee, D. H. , Seo, M. J. , Kang, E. J. , Park, C. R. , Jo, C. W. , Hwang, I. C. , Yu, Y. M. & Youn, Y. N. (2011). Molecular identification of AFLP fragments associated with elytra colour variation of the Asian ladybird beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Journal of Asia‐Pacific Entomology 14, 99–105. [Google Scholar]
- Lev‐Yadun, S. (2009). Aposematic (warning) coloration in plants In Plant‐Environment Interactions (ed. Baluska F.), pp. 167–202. Springer, Berlin, Heidelberg. [Google Scholar]
- Lima, S. L. & Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68, 619–640. [Google Scholar]
- * Lindstedt, C. , Boncoraglio, G. , Cotter, S. , Gilbert, J. & Kilner, R. (2017). Aposematism in the burying beetle? Dual function of anal fluid in parental care and chemical defence. Behavioral Ecology 28, 1414–1422. [Google Scholar]
- Lindstedt, C. , Hendrika, J. , Talsma, R. , Ihalainen, E. , Lindström, L. & Mappes, J. (2009a). Diet quality affects warning coloration indirectly: excretion costs in a generalist herbivore. Evolution 64, 68–78. [DOI] [PubMed] [Google Scholar]
- Lindstedt, C. , Lindström, L. & Mappes, J. (2009b). Thermoregulation constrains effective warning signal expression. Evolution 63, 469–478. [DOI] [PubMed] [Google Scholar]
- Lindstedt, C. , Schroderus, E. , Lindström, L. , Mappes, T. & Mappes, J. (2016). Evolutionary constraints of warning signals: a genetic trade‐off between the efficacy of larval and adult warning coloration can maintain variation in signal expression. Evolution 70, 2562–2572. [DOI] [PubMed] [Google Scholar]
- Lindström, L. , Alatalo, R. V. , Lyytinen, A. & Mappes, J. (2001). Strong antiapostatic selection against novel rare aposematic prey. Proceedings of the National Academy of Sciences of the United States of America 98, 9181–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström, L. , Alatalo, R. V. , Lyytinen, A. & Mappes, J. (2004). The effect of alternative prey on the dynamics of Batesian and Müllerian mimicries. Evolution 58, 1294–1302. [DOI] [PubMed] [Google Scholar]
- Lindström, L. , Alatalo, R. V. & Mappes, J. (1999). Reactions of hand‐reared and wild‐caught predators toward warningly colored, gregarious, and conspicuous prey. Behavioral Ecology 10, 317–322. [Google Scholar]
- Llaurens, V. , Joron, M. & Théry, M. (2014). Cryptic differences in colour among Müllerian mimics: how can the visual capacities of predators and prey shape the evolution of wing colours? Journal of Evolutionary Biology 27, 531–540. [DOI] [PubMed] [Google Scholar]
- López‐Idiáquez, D. , Vergara, P. , Fargallo, J. A. & Martínez‐Padilla, J. (2016). Female plumage coloration signals status to conspecifics. Animal Behaviour 121, 101–106. [Google Scholar]
- Losey, J. E. , Ives, A. R. , Harmon, J. , Ballantyne, F. & Brown, C. (1997). A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388, 269–272. [Google Scholar]
- * Lusis, J. J. (1971). Experimental data on the taxonomical status of three forms of genus Calvia (Coleoptera, Coccinellidae) from Middle Asia. Latvijas Entomology 14, 69–80. [Google Scholar]
- Maan, M. E. & Cummings, M. E. (2009). Sexual dimorphism and directional sexual selection on aposematic signals in a poison frog. Proceedings of the National Academy of Sciences of the United States of America 106, 19072–19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan, M. E. & Cummings, M. E. (2012). Poison frog colors are honest signals of toxicity, particularly for bird predators. The American Naturalist 179, E1–E14. [DOI] [PubMed] [Google Scholar]
- * Machado, V. & Valiati, V. H. (2006). Analysis of the geographical variation of elytral colour polymorphisms in three species of soldier beetles, Chauliognathus Hentz (Cantharidae) in Southern Brazil. Revista Brasileira de Zoologia 23, 1051–1058. [Google Scholar]
- Majerus, M. E. N. (1994). Ladybirds. The New Naturalist, No. 81. HarperCollins, London. [Google Scholar]
- * Mallet, J. (1989). The genetics of warning colour in Peruvian hybrid zones of Heliconius erato and H. melpomene . Proceedings of the Royal Society B: Biological Sciences 236, 163–185. [Google Scholar]
- Mallet, J. (2010). Shift happens! Shifting balance and the evolution of diversity in warning colour and mimicry. Ecological Entomology 35, 90–104. [Google Scholar]
- Mallet, J. & Barton, N. H. (1989). Strong natural selection in a warning‐colour hybrid zone. Evolution 43, 421–431. [DOI] [PubMed] [Google Scholar]
- Mallet, J. , Barton, N. , Lamas, G. , Santisteban, J. , Muedas, M. & Eeley, H. (1990). Estimates of selection and gene flow from measures of cline width and linkage disequilibrium in Heliconius hybrid zones. Genetics 124, 921–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, J. & Gilbert, L. (1995). Why are there so many mimicry rings? Correlations between habitat, behaviour and mimicry in Heliconius butterflies. Biological Journal of the Linnean Society 55, 159–180. [Google Scholar]
- Mallet, J. , Jiggins, C. D. & McMillan, O. W. (1998). Mimicry and warning colour at the boundary between races and species In Endless Forms: Species and Speciation (eds Howard D. J. and Berlocher S. H.), pp. 390–403. Oxford University Press, New York. [Google Scholar]
- Mallet, J. & Joron, M. (1999). Evolution of diversity in warning colour and mimicry: polymorphisms, shifting balance, and speciation. Annual Review of Ecology and Systematics 30, 201–233. [Google Scholar]
- Mappes, J. , Marples, N. & Endler, J. A. (2005). The complex business of survival by aposematism. Trends in Ecology & Evolution 20, 598–603. [DOI] [PubMed] [Google Scholar]
- Mappes, J. , Kokko, H. , Ojala, K. & Lindström, L. (2014). Seasonal changes in predator community switch the direction of selection for prey defences. Nature Communications 5, 5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Marek, P. E. & Bond, J. E. (2009). A Müllerian mimicry ring in Appalachian millipedes. Proceedings of the National Academy of Sciences of the United States of America 106, 9755–9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples, N. M. & Mappes, J. (2010). Can the dietary conservatism of predators compensate for positive frequency dependent selection against rare, conspicuous prey? Evolutionary Ecology 25, 737–749. [Google Scholar]
- Marples, N. M. , Roper, T. J. & Harper, D. G. C. (1998). Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83, 161–165. [Google Scholar]
- * Marsh, N. & Rothschild, M. (1974). Aposematic and cryptic Lepidoptera tested on the mouse. Journal of Zoology 174, 89–122. [Google Scholar]
- Marshall, D. J. & Uller, T. (2007). When is a maternal effect adaptive? Oikos 116, 1957–1963. [Google Scholar]
- Martin, A. , Papa, R. , Nadeau, N. J. , Hill, R. I. , Counterman, B. A. , Halder, G. , Jiggins, C. D. , Kronforst, M. R. , Long, A. D. , McMillan, W. O. & Reed, R. D. (2012). Diversification of complex butterfly wing patterns by repeated regulatory evolution of a Wnt ligand. Proceedings of the National Academy of Sciences of the United States of America 109, 12632–12637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐Padilla, J. , Vergara, P. , Pérez‐Rodríguez, L. , Mougeot, F. , Casas, F. , Ludwig, S. C. , Haines, J. A. , Zeineddine, M. & Redpath, S. M. (2011). Condition‐ and parasite‐dependent expression of a male‐like trait in a female bird. Biology Letters 7, 364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Mason, L. G. (1976). Habitat and phenetic variation in Phymata americana Melin (Heteroptera: Phymatidae). II. Climate and temporal variation in colour pattern. Systematic Zoology 25, 123–128. [Google Scholar]
- Maure, F. , Doyon, J. , Thomas, F. & Brodeur, J. (2014). Host behaviour manipulation as an evolutionary route towards attenuation of parasitoid virulence. Journal of Evolutionary Biology 27, 2871–2875. [DOI] [PubMed] [Google Scholar]
- Mavárez, J. , Salazar, C. A. , Bermingham, E. , Salcedo, C. , Jiggins, C. D. & Linares, M. (2006). Speciation by hybridization in Heliconius butterflies. Nature 441, 868–871. [DOI] [PubMed] [Google Scholar]
- Mayr, E. (1963). Animal Species and Evolution. Harvard University Press, Cambridge. [Google Scholar]
- * McCornack, B. P. , Koch, R. L. & Ragsdale, D. W. (2007). A simple method for in‐field sex determination of the multicolored Asian lady beetle Harmonia axyridis . Journal of Insect Science (Online) 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * McGovern, G. M. , Mitchell, J. C. & Knisley, C. B. (1984). Field experiments on prey selection by the whiptail lizard, Cnemidophorus inornatus, in Arizona. Journal of Herpetology 18, 347–349. [Google Scholar]
- McGraw, K. J. (2005). The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Animal Behaviour 69, 757–764. [Google Scholar]
- McGraw, K. J. & Hill, G. E. (2000). Differential effects of endoparasitism on the expression of carotenoid‐ and melanin‐based ornamental coloration. Proceedings of the Royal Society B: Biological Sciences 267, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * McGugan, J. R. , Byrd, G. D. , Roland, A. B. , Caty, S. N. , Kabir, N. , Tapia, E. E. , Trauger, S. A. , Coloma, L. A. & O'Connell, L. A. (2016). Ant and mite diversity drives toxin variation in the little devil poison frog. Journal of Chemical Ecology 42, 537–551. [DOI] [PubMed] [Google Scholar]
- McLean, C. A. & Stuart‐Fox, D. (2014). Geographic variation in animal colour polymorphisms and its role in speciation. Biological Reviews 89, 860–873. [DOI] [PubMed] [Google Scholar]
- * McPherson, K. R. & Wilson, S. W. (1995). The planthopper genus Phylloscelis in the United States (Homoptera: Dictyopharidae). Insecta Mundi 9, 177–188. [Google Scholar]
- Melo, M. C. , Salazar, C. , Jiggins, C. D. & Linares, M. (2009). Assortative mating preferences among hybrids offers a route to hybrid speciation. Evolution 63, 1660–1665. [DOI] [PubMed] [Google Scholar]
- Merrill, R. M. , Chia, A. & Nadeau, N. J. (2014). Divergent warning patterns contribute to assortative mating between incipient Heliconius species. Ecology and Evolution 4, 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill, R. M. , Dasmahapatra, K. K. , Davey, J. W. , Dell'Aglio, D. D. , Hanly, J. J. , Huber, B. , Jiggins, C. D. , Joron, M. , Kozak, K. M. , Llaurens, V. , Martin, S. H. , Montgomery, S. H. , Morris, J. , Nadeau, N. J. , Pinharanda, A. L. , et al. (2015). The diversification of Heliconius butterflies: what have we learned in 150 years? Journal of Evolutionary Biology 28, 1417–1438. [DOI] [PubMed] [Google Scholar]
- Merrill, R. M. , van Schooten, B. , Scott, J. A. & Jiggins, C. D. (2011). Pervasive genetic associations between traits causing reproductive isolation in Heliconius butterflies. Proceedings of the Royal Society B: Biological Sciences 278, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuche, I. , Brusa, O. , Linsenmair, K. E. , Keller, A. & Pröhl, H. (2013). Only distance matters–non‐choosy females in a poison frog population. Frontiers in Zoology 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie, L. J. , Mallard, F. , Majerus, M. E. N. & Jiggins, F. M. (2010). Melanic through nature or nurture: genetic polymorphism and phenotypic plasticity in Harmonia axyridis . Journal of Evolutionary Biology 23, 1699–1707. [DOI] [PubMed] [Google Scholar]
- Michie, L. J. , Masson, A. , Ware, R. L. & Jiggins, F. M. (2011). Seasonal phenotypic plasticity: wild ladybirds are darker at cold temperatures. Evolutionary Ecology 25, 1259–1268. [Google Scholar]
- Mina, A. E. , Ponti, A. K. , Woodcraft, N. L. , Johnson, E. E. & Saporito, R. A. (2015). Variation in alkaloid‐based microbial defenses of the dendrobatid poison frog Oophaga pumilio . Chemoecology 25, 169–178. [Google Scholar]
- Mishra, G. & Omkar (2014). Phenotype‐dependent mate choice in Propylea dissecta and its fitness consequences. Journal of Ethology 32, 165–172. [Google Scholar]
- * Mochida, K. (2009). A parallel geographical mosaic of morphological and behavioural aposematic traits of the newt, Cynops pyrrhogaster (Urodela: Salamandridae). Biological Journal of the Linnean Society 97, 613–622. [Google Scholar]
- Mochida, K. (2011). Combination of local selection pressures drives diversity in aposematic signals. Evolutionary Ecology 25, 1017–1028. [Google Scholar]
- Monaghan, P. (2008). Early growth conditions, phenotypic development and environmental change. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan, P. , Metcalfe, N. B. & Torres, R. (2009). Oxidative stress as a mediator of life history trade‐offs: mechanisms, measurements and interpretation. Ecology Letters 12, 75–92. [DOI] [PubMed] [Google Scholar]
- * Moraes, S. D. S. , Cardoso, L. W. , Silva‐brandão, K. L. & Duarte, M. (2017). Extreme sexual dimorphism and polymorphism in two species of the tiger moth genus Dysschema (Lepidoptera: Erebidae): association between males and females, sexual mimicry and melanism revealed by integrative taxonomy. Systematics and Biodiversity 15, 259–273. [Google Scholar]
- Müller, F. (1879). Ituna and Thyridia; a remarkable case of mimicry in butterflies. Transactions of the Entomological Society of London 1879, 20–29. [Google Scholar]
- * Muñoz‐Ramírez, C. P. , Bitton, P. P. , Doucet, S. M. & Knowles, L. L. (2016). Extreme mimics here and there, but not everywhere: Müllerian mimicry in Ceroglossus ground beetles? Biology Letters 12, 20160429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nabours, R. K. (1929). The Genetics of the Tettigidae (Grouse Locusts). Springer Netherlands, Dordrecht. [Google Scholar]
- Nadeau, N. J. (2016). Genes controlling mimetic colour pattern variation in butterflies. Current Opinion in Insect Science 17, 24–31. [DOI] [PubMed] [Google Scholar]
- Nadeau, N. J. , Pardo‐Diaz, C. , Whibley, A. , Supple, M. A. , Saenko, S. V. , Wallbank, R. W. R. , Wu, G. C. , Maroja, L. , Ferguson, L. , Hanly, J. J. , Hines, H. , Salazar, C. , Merrill, R. M. , Dowling, A. J. , Ffrench‐Constant, R. H. , et al. (2016). The gene cortex controls mimicry and crypsis in butterflies and moths. Nature 534, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Nahirnić, A. & Tarmann, G. (2016). On the early stages of species of the Zygaena purpuralis – complex on the Balkan Peninsula and adjacent regions (Zygaenidae, Zygaeninae) In Abstracts of the XV International Symposium on Zygaenidae (eds Tarmann G. M., Tremewan W. G. and Spalding A.), p. 30 Tarmann G. M. & Bürgergenossenschaft Obervinschgau BGO, Mals. [Google Scholar]
- * Naisbit, R. E. , Jiggins, C. D. & Mallet, J. (2003). Mimicry: developmental genes that contribute to speciation. Evolution and Development 5, 269–280. [DOI] [PubMed] [Google Scholar]
- * Naumann, C. M. , Tarmann, G. M. & Tremewan, W. G. (1999). The Western Palaearctic Zygaenidae. Apollo Books, Stenstrup. [Google Scholar]
- Nelson, B. W. , Ferreira, C. A. C. , da Silva, M. F. & Kawasaki, M. L. (1990). Endemism centres, refugia and botanical collection density in Brazilian Amazonia. Nature 345, 714–716. [Google Scholar]
- * Newman, L. J. & Cannon, L. R. G. (2003). Marine Flatworms: The World of Polyclads. CSIRO Publishing, Collingwood. [Google Scholar]
- * Nielsen, M. E. & Papaj, D. R. (2017). Why have multiple plastic responses? Interactions between colour change and heat avoidance behavior in Battus philenor larvae. The American Naturalist 189(6), 657–666. [DOI] [PubMed] [Google Scholar]
- Nishikawa, H. , Iijima, T. , Kajitani, R. , Yamaguchi, J. , Ando, T. , Suzuki, Y. , Sugano, S. , Fujiyama, A. , Kosugi, S. , Hirakawa, H. , Tabata, S. , Ozaki, K. , Morimoto, H. , Ihara, K. , Obara, M. , Hori, H. , Itoh, T. & Fujiwara, H. (2015). A genetic mechanism for female‐limited Batesian mimicry in Papilio butterfly. Nature Genetics 47, 405–409. [DOI] [PubMed] [Google Scholar]
- Nokelainen, O. , Hegna, R. H. , Reudler, J. H. , Lindstedt, C. & Mappes, J. (2012). Trade‐off between warning signal efficacy and mating success in the wood tiger moth. Proceedings of the Royal Society B: Biological Sciences 279, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nokelainen, O. , Valkonen, J. , Lindstedt, C. & Mappes, J. (2014). Changes in predator community structure shifts the efficacy of two warning signals in Arctiid moths. Journal of Animal Ecology 83, 598–605. [DOI] [PubMed] [Google Scholar]
- Noonan, B. P. & Comeault, A. A. (2009). The role of predator selection on polymorphic aposematic poison frogs. Biology Letters 5, 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * van Noort, T. (2013). Colour polymorphism in the leaf beetle genus Oreina . PhD thesis, Université de Neuchâtel.
- Ogilvy, V. , Preziosi, R. F. & Fidgett, A. L. (2012). A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red‐eye tree frog. Animal Conservation 15, 480–488. [Google Scholar]
- Ojala, K. , Lindström, L. & Mappes, J. (2007). Life‐history constraints and warning signal expression in an arctiid moth. Functional Ecology 21, 1162–1167. [Google Scholar]
- * Okuda, T. , Gomi, T. & Hodek, I. (1997). Effect of temperature on pupal pigmentation and size of the elytral spots in Coccinella septempunctata (Coleoptera: Coccinellidae) from four latitudes in Japan. Applied Entomological Zoology 32, 567–572. [Google Scholar]
- Olendorf, R. , Rodd, F. H. , Punzalan, D. , Houde, A. E. , Hurt, C. , Reznick, D. N. & Hughes, K. A. (2006). Frequency‐dependent survival in natural guppy populations. Nature 441, 633–636. [DOI] [PubMed] [Google Scholar]
- Örnborg, J. , Andersson, S. , Griffith, S. C. & Sheldon, B. C. (2002). Seasonal changes in a ultraviolet structural colour signal in blue tits, Parus caeruleus . Biological Journal of the Linnean Society 76, 237–245. [Google Scholar]
- Ortonne, J. P. (2002). Photoprotective properties of skin melanin. British Journal of Dermatology 146, 7–10. [DOI] [PubMed] [Google Scholar]
- Osorio, D. & Vorobyev, M. (2008). A review of the evolution of animal colour vision and visual communication signals. Vision Research 48, 2042–2051. [DOI] [PubMed] [Google Scholar]
- * Owen, D. F. & Smith, D. A. S. (1991). All‐female broods and mimetic polymorphism in Acraea encedon (L.) (Lepidoptera: Acraeidae) in Tanzania. African Journal of Ecology 29, 241–247. [Google Scholar]
- * Owen, D. F. , Smith, D. A. S. , Gordon, I. J. & Owixy, A. M. (1994). Polymorphic Müllerian mimicry in a group of African butterflies: a re‐assessment of the relationship between Danaus chrysippus, Acraea encedon and Acraea encedana (Lepidoptera: Nymphalidae). Journal of Zoology 232, 93–108. [Google Scholar]
- * Owen, R. E. & Plowright, R. C. (1988). Inheritance of metasomal pile colour variation in the bumble bee Bombus rufocinctus Cresson (Hymenoptera: Apidae). Canadian Journal of Zoology 66, 1172–1178. [Google Scholar]
- Papaj, D. R. & Newsom, G. M. (2005). A within‐species warning function for an aposematic signal. Proceedings of the Royal Society B: Biological Sciences 272, 2519–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo‐Diaz, C. , Salazar, C. , Baxter, S. W. , Merot, C. , Figueiredo‐Ready, W. , Joron, M. , McMillan, W. O. & Jiggins, C. D. (2012). Adaptive introgression across species boundaries in Heliconius butterflies. PLoS Genetics 8, e1002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, S. C. , Stevens, M. , Pell, J. , Birkett, M. A. & Blount, J. D. (2018). Parental phenotype not predator cues influence egg warning coloration and defence levels. Animal Behaviour 140, 177–186. [Google Scholar]
- Pawlik, J. R. (2012). Antipredatory defensive roles of natural products from marine invertebrates In Handbook of Marine Natural Products (eds Fattorusso E., Gerwick W. and Taglialatela‐Scafati O.), pp. 677–710. Springer, Dordrecht. [Google Scholar]
- * Pease, R. W. (1968). Evolution and hybridization in the Utetheisa ornatrix complex (Lepidoptera: Arctiidae). I. Inter‐and intrapopulation variation and its relation to hybridization. Evolution 22, 719–735. [DOI] [PubMed] [Google Scholar]
- Pérez‐Rodríguez, L. (2008). Carotenoid‐based ornamentation as a dynamic but consistent individual trait. Behavioral Ecology and Sociobiology 62, 995–1005. [Google Scholar]
- Perrard, A. , Arca, M. , Rome, Q. , Muller, F. , Tan, J. , Bista, S. , Nugroho, H. , Baudoin, R. , Baylac, M. , Silvain, J. F. , Carpenter, J. M. & Villemant, C. (2014). Geographic variation of melanisation patterns in a hornet species: genetic differences, climatic pressures or aposematic constraints? PLoS One 9, e94162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pilgrim, E. M. , Williams, K. A. , Manley, D. G. & Pitts, J. P. (2009). Addressing the Dasymutilla quadriguttata species‐group and species‐complex (Hymenoptera: Mutillidae): several distinct species or a single, morphologically variable species? Kansas Entomological Society 82, 231–249. [Google Scholar]
- Plowright, R. C. & Owen, R. E. (1980). The evolutionary significance of bumblebee colour patterns: a mimetic interpretation. Evolution 34, 622–637. [DOI] [PubMed] [Google Scholar]
- Poulton, E. (1890). The Colours of Animals: Their Meaning and Use, Especially Considered in the Case of Insects. Paul, Trench, Trübner & Co Ltd, London. [Google Scholar]
- * Prokopová, M. , Veselý, P. , Fuchs, R. & Zrzavý, J. (2010). The role of size and colour pattern in protection of developmental stages of the red firebug (Pyrrhocoris apterus) against avian predators. Biological Journal of the Linnean Society 100, 890–898. [Google Scholar]
- * Quartau, J. A. & Borges, P. A. V. (1997). On the colour polymorphism of Philaenus spumarius (L.) (Homoptera, Cercopidae) in Portugal. Micellánia Zoológica 20, 19–30. [Google Scholar]
- * Rabemananjara, F. C. E. , Chiari, Y. , Ramilijaona, O. R. & Vences, M. (2007). Evidence for recent gene flow between North‐Eastern and South‐Eastern Madagascan poison frogs from a phylogeography of the Mantella cowani group. Frontiers in Zoology 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratikainen, I. I. & Kokko, H. (2010). Differential allocation and compensation: who deserves the silver spoon? Behavioral Ecology 1, 195–200. [Google Scholar]
- Reed, R. D. , Papa, R. , Martin, A. , Hines, H. M. , Counterman, B. A. , Pardo‐Diaz, C. , Jiggins, C. D. , Chamberlain, N. L. , Kronforst, M. R. , Chen, R. & Halder, G. (2011). Optix drives the repeated convergent evolution of butterfly wing pattern mimicry. Science 333, 1137–1141. [DOI] [PubMed] [Google Scholar]
- Richards‐Zawacki, C. L. , Yeager, J. & Bart, H. P. S. (2013). No evidence for differential survival or predation between sympatric colour morphs of an aposematic poison frog. Evolutionary Ecology 27, 783–795. [Google Scholar]
- Riipi, M. , Alatalo, R. V. , Lindstrom, L. & Mappes, J. (2001). Multiple benefits of gregariousness cover detectability costs in aposematic aggregations. Nature 413, 512–514. [DOI] [PubMed] [Google Scholar]
- * Roberts, H. R. (1947). Revision of the Mexican Melanoplini (Orthoptera: Acrididae: Cyrtacanthacridinae) part I. Proceedings of the Academy of Natural Sciences of Philadelphia 99, 201–230. [Google Scholar]
- * Rodríguez, A. , Alonso, R. , Rodríguez, J. A. & Vences, M. (2012). Geographic distribution, colour variation and molecular diversity of miniature frogs of the Eleutherodactylus limbatus group from Cuba. Salamandra 48, 71–91. [Google Scholar]
- * Rodriguez, J. , Pitts, J. P. , Von Dohlen, C. D. & Wilson, J. S. (2014). Müllerian mimicry as a result of codivergence between velvet ants and spider wasps. PLoS One 9, e112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff, D. A. & Fairbairn, D. J. (2013). The costs of being dark: the genetic basis of melanism and its association with fitness‐related traits in the sand cricket. Journal of Evolutionary Biology 26, 1406–1416. [DOI] [PubMed] [Google Scholar]
- * Rogers, C. E. , Jackson, H. B. , Eikenbary, R. D. & Starks, K. J. (1971). Sex determination in Propylea‐Quatuordecimpunctata (Coleoptera: Coccinellidae), an imported predator of aphids. Annals of the Entomological Society of America 64, 957–959. [Google Scholar]
- Rojas, B. , Devillechabrolle, J. & Endler, J. A. (2014a). Paradox lost: variable colour‐pattern geometry is associated with differences in movement in aposematic frogs. Biology Letters 10, 20140193. [Google Scholar]
- Rojas, B. , Rautiala, P. & Mappes, J. (2014b). Differential detectability of polymorphic warning signals under varying light environments. Behavioural Processes 109, 164–172. [DOI] [PubMed] [Google Scholar]
- * Rojas, B. & Endler, J. A. (2013). Sexual dimorphism and intra‐populational colour pattern variation in the aposematic frog Dendrobates tinctorius . Evolutionary Ecology 27, 739–753. [Google Scholar]
- Rojas, B. , Gordon, S. P. & Mappes, J. (2015). Frequency‐dependent flight activity in the colour polymorphic wood tiger moth. Current Zoology 61, 765–772. [Google Scholar]
- Rönkä, K. , De Pasqual, C. , Mappes, J. , Gordon, S. & Rojas, B. (2018). Colour alone matters: no predator generalisation among morphs of an aposematic moth. Animal Behaviour 135, 153–163. [Google Scholar]
- Rose, M. R. (1991). Evolutionary Biology of Aging. Oxford University Press, New York. [Google Scholar]
- Roulin, A. (2004). The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biological Reviews 79, 815–848. [DOI] [PubMed] [Google Scholar]
- Roulin, A. (2016). Condition‐dependence, pleiotropy and the handicap principle of sexual selection in melanin‐based coloration. Biological Reviews 91, 328–348. [DOI] [PubMed] [Google Scholar]
- Roulin, A. & Ducrest, A. L. (2013). Genetics of coloration in birds. Seminars in Cell and Developmental Biology 24, 594–608. [DOI] [PubMed] [Google Scholar]
- Rowe, C. & Halpin, C. (2013). Why are warning displays multimodal? Behavioral Ecology and Sociobiology 67, 1425–1439. [Google Scholar]
- Rowland, H. M. , Hoogesteger, T. , Ruxton, G. D. , Speed, M. P. & Mappes, J. (2010a). A tale of two signals: signal mimicry between aposematic species enhances predator avoidance learning. Behavioral Ecology 21, 851–860. [Google Scholar]
- Rowland, H. M. , Fulford, J. T. & Ruxton, G. D. (2017). Predator learning differences affect the survival of chemically defended prey. Animal Behaviour 124, 65–74. [Google Scholar]
- Rowland, H. M. , Ihalainen, E. , Lindström, L. , Mappes, J. & Speed, M. P. (2007). Co‐mimics have a mutualistic relationship despite unequal defences. Nature 448, 64–67. [DOI] [PubMed] [Google Scholar]
- Rowland, H. M. , Mappes, J. , Ruxton, G. D. & Speed, M. P. (2010b). Mimicry between unequally defended prey can be parasitic: evidence for quasi‐Batesian mimicry. Ecology Letters 13, 1494–1502. [DOI] [PubMed] [Google Scholar]
- Rowland, H. M. , Ruxton, G. D. & Skelhorn, J. (2013). Bitter taste enhances predatory biases against aggregations of prey with warning coloration. Behavioral Ecology 24, 942–948. [Google Scholar]
- Rowland, H. M. , Wiley, E. , Ruxton, G. D. , Mappes, J. & Speed, M. P. (2010c). When more is less: the fitness consequences of predators attacking more unpalatable prey when more are presented. Biology Letters 6, 732–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rubino, D. L. & Mccarthy, B. C. (2004). Presence of aposematic (warning) coloration in vascular plants of Southeastern Ohio. Torrey Botanical Society 131, 252–256. [Google Scholar]
- * Rudman, W. B. (1983). The Chromodorididae (Opisthobranchia: Mollusca) of the Indo‐West Pacific: Chromodoris splendida, C. aspersa and Hypselodoris placida colour groups. Zoological Journal of the Linnean Society 78, 105–173. [Google Scholar]
- * Rudman, W. B. (1986). The Chromodorididae (Opisthobranchia: Mollusca) of the Indo‐West Pacific: Noumea flava colour group. Zoological Journal of the Linnean Society 88, 377–404. [Google Scholar]
- * Rutowski, R. L. & Rajyaguru, P. K. (2013). Male‐specific iridescent coloration in the pipevine swallowtail (Battus philenor) is used in mate choice by females but not sexual discrimination by males. Journal of Insect Behavior 26, 200–211. [Google Scholar]
- Ruxton, G. D. , Sherratt, T. N. & Speed, M. P. (2004). Avoiding Attack: The Evolutionary Ecology of Crypsis, Warning Signals and Mimicry. Oxford University Press, Oxford, UK. [Google Scholar]
- Saino, N. , Bertacche, V. , Ferrari, R. P. , Martinelli, R. , Møller, A. P. & Stradi, R. (2002). Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proceedings of the Royal Society B: Biological Society 269, 1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino, N. , Romano, M. , Rubolini, D. , Ambrosini, R. , Caprioli, M. , Milzani, A. , Costanzo, A. , Colombo, G. , Canova, L. & Wakamatsu, K. (2013). Viability is associated with melanin‐based coloration in the barn swallow (Hirundo rustica). PLoS One 8, e60426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, C. A. , Baxter, S. W. , Pardo‐Diaz, C. , Wu, G. , Surridge, A. , Linares, M. , Bermingham, E. & Jiggins, C. D. (2010). Genetic evidence for hybrid trait speciation in Heliconius butterflies. PLoS Genetics 6, e1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, C. A. , Jiggins, C. D. , Arias, C. F. , Tobler, A. , Bermingham, E. & Linares, M. (2005). Hybrid incompatibility is consistent with a hybrid origin of Heliconius heurippa Hewitson from its close relatives, Heliconius cydno Doubleday and Heliconius melpomene Linnaeus. Journal of Evolutionary Biology 18, 247–256. [DOI] [PubMed] [Google Scholar]
- Salazar, C. A. , Jiggins, C. D. , Taylor, J. E. , Kronforst, M. R. & Linares, M. (2008). Gene flow and the genealogical history of Heliconius heurippa . BMC Evolutionary Biology 8, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Sanabria, E. A. , Vaira, M. , Quiroga, L. B. , Akmentins, M. S. & Pereyra, L. C. (2014). Variation of thermal parameters in two different colour morphs of a diurnal poison toad, Melanophryniscus rubriventris (Anura: Bufonidae). Journal of Thermal Biology 41, 1–5. [DOI] [PubMed] [Google Scholar]
- Sanders, K. L. , Malhotra, A. & Thorpe, R. S. (2006). Evidence for a Müllerian mimetic radiation in Asian pitvipers. Proceedings of the Royal Society B: Biological Society 273, 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Savage, J. M. (1966). An extraordinary new toad (Bufo) from Costa Rica. Revista de Biologia Tropical 14, 153–167. [PubMed] [Google Scholar]
- * Savage, J. M. (2002). The Amphibians and Reptiles of Costa Rica: A Herpetofauna between Two Continents, between Two Seas. University of Chicago Press, Chicago. [Google Scholar]
- * Sbordoni, V. , Bullini, L. , Scarpelli, G. , Forestiero, S. & Rampini, M. (1979). Mimicry in the burnet moth Zygaena ephialtes: population studies and evidence of a Batesian‐Müllerian situation. Ecological Entomology 4, 83–93. [Google Scholar]
- * Schmidt, B. C. (2009). Taxonomic revision of the genus Grammia Rambur (Lepidoptera: Noctuidae: Arctiinae). Zoological Journal of the Linnean Society 156, 507–597. [Google Scholar]
- Segami Marzal, J. C. , Rudh, A. , Rogell, B. , Ödeen, A. , Løvlie, H. , Rosher, C. & Qvarnström, A. (2017). Cryptic female strawberry poison frogs experience elevated predation risk when associating with an aposematic partner. Ecology and Evolution 7, 74–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell, J. M. & Wickings, E. J. (2005). Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology 111, 25–50. [Google Scholar]
- Sheldon, B. C. (2000). Differential allocation: tests, mechanisms and implications. Trends in Ecology and Evolution 15, 397–402. [DOI] [PubMed] [Google Scholar]
- Sheppard, P. M. , Turner, J. R. G. , Brown, K. S. , Benson, W. W. & Singer, M. C. (1985). Genetics and the evolution of Mullerian mimicry in Heliconius butterflies. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 308, 433–610. [Google Scholar]
- Sheppard, J. L. , Clark, R. G. , Devries, J. H. & Brasher, M. G. (2013). Reproductive effort and success of wild female mallards: does male quality matter? Behavioural Processes 100, 82–90. [DOI] [PubMed] [Google Scholar]
- Sherratt, T. N. (2002). The coevolution of warning signals. Proceedings of the Royal Society B: Biological Sciences 269, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt, T. N. (2003). State‐dependent risk‐taking by predators in systems with defended prey. Oikos 103, 93–100. [Google Scholar]
- Sherratt, T. N. (2006). Spatial mosaic formation through frequency‐dependent selection in Müllerian mimicry complexes. Journal of Theoretical Biology 240, 165–174. [DOI] [PubMed] [Google Scholar]
- Sherratt, T. N. (2008). The evolution of Müllerian mimicry. Naturwissenschaften 95, 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt, T. N. (2011). The optimal sampling strategy for unfamiliar prey. Evolution 65, 2014–2025. [DOI] [PubMed] [Google Scholar]
- Sherratt, T. N. & Forbes, M. R. (2001). Sexual differences in coloration of Coenagrionid damselflies (Odonata): a case of intraspecific aposematism? Animal Behaviour 62, 653–660. [Google Scholar]
- Sherratt, T. N. & Peet‐Paré, C. A. (2017). The perfection of mimicry: an information approach. Philosophical Transactions of the Royal Society B 372, 20160340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherratt, T. N. , Wilkinson, D. M. & Bain, R. S. (2005). Explaining Dioscorides' “double difference”: why are some mushrooms poisonous, and do they signal their unprofitability? The American Naturalist 166, 767–775. [DOI] [PubMed] [Google Scholar]
- Shreeve, T. G. (1987). The mate location behaviour of the male speckled wood butterfly, Pararge aegeria, and the effect of phenotypic differences in hind‐wing spotting. Animal Behaviour 35, 682–690. [Google Scholar]
- * Shull, F. (1944). Inheritance in lady beetles: II. The spotless pattern and its modifiers in Hippodamia convergens and their frequency in several populations. Journal of Heredity 35, 329–339. [Google Scholar]
- * Siddiqi, A. , Cronin, T. W. , Loew, E. R. , Vorobyev, M. & Summers, K. (2004). Interspecific and intraspecific views of colour signals in the strawberry poison frog Dendrobates pumilio . The Journal of Experimental Biology 207, 2471–2485. [DOI] [PubMed] [Google Scholar]
- * Sillen‐Tullberg, B. , Wiklund, C. & Järvi, T. (1982). Aposematic coloration in adults and larvae of Lygaeus equestris and its bearing on Müllerian mimicry: an experimental study on predation on living bugs by the great tit Parus major . Oikos 39, 131–136. [Google Scholar]
- * Singh, H. & Malik, V. S. (1993). Biology of painted bug (Bagrada cruciferaum). Indian Journal of Agricultural Sciences 63, 672–674. [Google Scholar]
- Skelhorn, J. , Halpin, C. G. & Rowe, C. (2016). Behavioral learning about aposematic prey. Behavioural Ecology 27, 955–964. [Google Scholar]
- Skelhorn, J. & Rowe, C. (2006). Taste‐rejection by predators and the evolution of unpalatability in prey. Behavioral Ecology and Sociobiology 60, 550–555. [Google Scholar]
- Skelhorn, J. & Rowe, C. (2007). Automimic frequency influences the foraging decisions of avian predators on aposematic prey. Animal Behaviour 74, 1563–1572. [Google Scholar]
- Skelhorn, J. & Rowe, C. (2009). Distastefulness as an antipredator defence strategy. Animal Behaviour 78, 761–766. [Google Scholar]
- Smith, S. M. (1975). Innate recognition of coral snake pattern by a possible avian predator. Science 187, 759–760. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (1977). Coral‐snake pattern recognition and stimulus generalisation by naive great kiskadees (Aves: Tyrannidae). Nature 265, 535–536. [Google Scholar]
- Smith, K. E. , Halpin, C. G. & Rowe, C. (2016). The benefits of being toxic to deter predators depends on prey body size. Behavioral Ecology 27, 1650–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Souza, G. K. , Pikart, T. G. , Oliveira, H. N. , Serrão, J. E. & Zanuncio, J. C. (2012). Colour polymorphism in Pachycoris torridus (Hemiptera: Scutelleridae) and its taxonomic implications. Revista Chilena de Historia Natural 85, 357–359. [Google Scholar]
- Speed, M. P. (1990). Mimicry and the psychology of predation. Doctoral dissertation, University of Leeds.
- Speed, M. P. (1993). Müllerian mimicry and the psychology of predation. Animal Behaviour 45, 571–580. [Google Scholar]
- Speed, M. P. & Ruxton, G. D. (2007). How bright and how nasty: explaining diversity in warning signal strength. Evolution 61, 623–635. [DOI] [PubMed] [Google Scholar]
- Speed, M. P. , Ruxton, G. D. , Mappes, J. & Sherratt, T. N. (2012). Why are defensive toxins so variable? An evolutionary perspective. Biological Reviews 87, 874–884. [DOI] [PubMed] [Google Scholar]
- Stankowich, T. , Caro, T. & Cox, M. (2011). Bold coloration and the evolution of aposematism in terrestrial carnivores. Evolution 65, 3090–3099. [DOI] [PubMed] [Google Scholar]
- Stearns, S. C. (1992). The Evolution of Life Histories. Oxford University Press, Oxford. [Google Scholar]
- Stevens, M. & Ruxton, G. D. (2012). Linking the evolution and form of warning coloration in nature. Proceedings of the Royal Society B: Biological Sciences 279, 417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckert, A. M. M. , Saporito, R. A. , Venegas, P. & Summers, K. (2014a). Alkaloid defenses of co‐mimics in a putative Müllerian mimetic radiation. BMC Evolutionary Biology 14, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckert, A. M. M. , Venegas, P. J. & Summers, K. (2014b). Experimental evidence for predator learning and Müllerian mimicry in Peruvian poison frogs (Ranitomeya, Dendrobatidae). Evolutionary Ecology 28, 413–426. [Google Scholar]
- * Stuckert, A. M. M. , Saporito, R. A. & Summers, K. (2018). An empirical test indicates only qualitatively honest aposematic signaling within a population of vertebrates. Journal of Herpetology 52, 201–208. [Google Scholar]
- * Summers, K. & Amos, W. (1997). Behavioral, ecological, and molecular genetic analyses of reproductive strategies in the Amazonian dart‐poison frog, Dendrobates ventrimaculatus . Behavioral Ecology 8, 260–267. [Google Scholar]
- Summers, K. , Speed, M. P. , Blount, J. D. & Stuckert, A. M. M. (2015). Are aposematic signals honest? A review. Journal of Evolutionary Biology 28, 1583–1599. [DOI] [PubMed] [Google Scholar]
- Supple, M. A. , Hines, H. M. , Dasmahapatra, K. K. , Lewis, J. J. , Nielsen, D. M. , Lavoie, C. , Ray, D. A. , Salazar, C. , McMillan, W. O. & Counterman, B. A. (2013). Genomic architecture of adaptive colour pattern divergence and convergence in Heliconius butterflies. Genome Research 23, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennungsen, T. O. & Holen, O. H. (2007). The evolutionary stability of automimicry. Proceedings of the Royal Society B: Biological Sciences 274, 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson, E. I. , Abbott, J. & Härdling, R. (2005). Female polymorphism, frequency dependence, and rapid evolutionary dynamics in natural populations. The American Naturalist 165, 567–576. [DOI] [PubMed] [Google Scholar]
- Sword, G. A. (1999). Density‐dependent warning coloration. Nature 397, 217. [Google Scholar]
- Sword, G. A. , Simpson, S. J. , El Hadi, O. T. M. & Wilps, H. (2000). Density–dependent aposematism in the desert locust. Proceedings of the Royal Society B: Biological Sciences 267, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symula, R. , Schulte, R. & Summers, K. (2001). Molecular phylogenetic evidence for a mimetic radiation in Peruvian poison frogs supports a Müllerian mimicry hypothesis. Proceedings of the Royal Society B: Biological Sciences 268, 2415–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symula, R. , Schulte, R. & Summers, K. (2003). Molecular systematics and phylogeography of Amazonian poison frogs of the genus Dendrobates . Molecular Phylogenetics and Evolution 26, 452–475. [DOI] [PubMed] [Google Scholar]
- * Tan, C.‐C. (1946). Mosaic dominance in the inheritance of colour patterns in the lady‐bird beetle, Harmonia axyridis . Genetics 31, 195–210. [PubMed] [Google Scholar]
- Tan, C. C. & Li, J. C. (1934). Inheritance of the elytral colour patterns in the ladybird beetle, Harmonia axyridis Pallas. The American Naturalist 68, 252–265. [Google Scholar]
- Thomas, R. J. , Bartlett, L. A. , Marples, N. M. , Kelly, D. J. & Cuthill, I. C. (2004). Prey selection by wild birds can allow novel and conspicuous colour morphs to spread in prey populations. Oikos 106, 285–294. [Google Scholar]
- Thomas, R. J. , Marples, N. M. , Cuthill, I. C. , Takahashi, M. & Gibson, E. A. (2003). Dietary conservatism may facilitate the initial evolution of aposematism. Oikos 101, 458–466. [Google Scholar]
- * Thompson, V. (1973). Spittlebug polymorphic for warning coloration. Nature 242, 126–128. [Google Scholar]
- * Thompson, V. (1984). Polymorphism under apostatic and aposematic selection. Heredity 53, 677–686. [Google Scholar]
- * Thompson, V. & Carvalho, G. S. (2016). Abrupt geographical transition between aposematic colour forms in the spittlebug Prosapia ignipectus (Fitch) (Hemiptera: Cercopidae). Psyche: A Journal of Entomology 2016, 1–10. [Google Scholar]
- Thompson, M. J. & Jiggins, C. D. (2014). Supergenes and their role in evolution. Heredity 113, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres, R. & Velando, A. (2007). Male reproductive senescence: the price of immune‐induced oxidative damage on sexual attractiveness in the blue‐footed booby. Journal of Animal Ecology 76, 1161–1168. [DOI] [PubMed] [Google Scholar]
- * Tremewan, W. G. (2006). Ecology, Phenotypes and the Mendelian Genetics of Burnet Moths (Zygaena Fabricius, 1775). Gem Publishing Company, Shipton‐under‐Wychwood. [Google Scholar]
- * Tremewan, W. G. (2015). The Mendelian genetics of the two larval morphs of Zygaena (Mesembrynus) corsica Boisduval, [1828] (Lepidoptera : Zygaenidae , Zygaeninae). Entomologist's Gazette 66, 207–215. [Google Scholar]
- * Tseng, H.‐Y. , Lin, C.‐P. , Hsu, J.‐Y. , Pike, D. A. & Huang, W.‐S. (2014). The functional significance of aposematic signals: geographic variation in the responses of widespread lizard predators to colourful invertebrate prey. PLoS One 9, e91777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullberg, B. S. , Gamberale‐Stille, G. , Bohlin, T. & Merilaita, S. (2008). Seasonal ontogenetic colour plasticity in the adult striated shieldbug Graphosoma lineatum (Heteroptera) and its effect on detectability. Behavioral Ecology and Sociobiology 62, 1389–1396. [Google Scholar]
- Tullberg, B. S. , Merilaita, S. & Wiklund, C. (2005). Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proceedings of the Royal Society B: Biological Sciences 272, 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner, J. R. G. (1965). Evolution of complex polymorphism and mimicry in distasteful South American butterflies. In Proceedings International Congress of Entomology, London: 1964, p. 267. [Google Scholar]
- * Turner, J. R. G. (1968). Some new Heliconius pupae: their taxonomic and evolutionary significance in relation to mimicry (Lepidoptera, Nymphalidae). Journal of Zoology 155, 311–325. [Google Scholar]
- * Turner, J. R. G. (1977a). A Bibliography of Heliconius and the Related Genera. Privately published by the author, Stony Brook, New York. [Google Scholar]
- Turner, J. R. G. (1977b). Butterfly mimicry – genetical evolution of an adaptation In Evolutionary Biology (eds Hecht M. K., Steere S. C. and Wallace B.), pp. 163–206. Plenum Press, New York. [Google Scholar]
- Turner, J. R. G. (1983). Mimetic butterflies and punctuated equilibria: some old light on a new paradigm. Biological Journal of the Linnean Society 20, 277–300. [Google Scholar]
- Turner, J. R. G. & Mallet, J. L. B. (1996). Did forest islands drive the diversity of warningly coloured butterflies? Biotic drift and the shifting balance. Philosophical Transactions of the Royal Society B: Biological Sciences 351, 835–845. [Google Scholar]
- Turner, J. R. G. & Speed, M. P. (1999). How weird can mimicry get? Evolutionary Ecology 13, 807–827. [Google Scholar]
- * Twomey, E. , Vestergaard, J. S. & Summers, K. (2014). Reproductive isolation related to mimetic divergence in the poison frog Ranitomeya imitator . Nature Communications 5, 1–8. [DOI] [PubMed] [Google Scholar]
- * Twomey, E. , Vestergaard, J. S. , Venegas, P. J. & Summers, K. (2016). Mimetic divergence and the speciation continuum in the mimic poison frog Ranitomeya imitator . The American Naturalist 187, 205–223. [DOI] [PubMed] [Google Scholar]
- Välimäki, P. , Kivelä, S. M. , Raitanen, J. , Pakanen, V. M. , Vatka, E. , Mäenpää, M. I. , Keret, N. & Tammaru, T. (2015). Larval melanism in a geometrid moth: promoted neither by a thermal nor seasonal adaptation but desiccating environments. Journal of Animal Ecology 84, 817–828. [DOI] [PubMed] [Google Scholar]
- Valkonen, J. K. , Nokelainen, O. , Niskanen, M. , Kilpimaa, J. , Björklund, M. & Mappes, J. (2012). Variation in predator species abundance can cause variable selection pressure on warning signalling prey. Ecology and Evolution 2, 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen, L. (1973). A new evolutionary law. Evolutionary Theory 1, 1–30. [Google Scholar]
- * Vane‐Wright, R. I. (1975). An integrated classification for polymorphism and sexual dimorphism in butterflies. Journal of Zoology 177, 329–337. [Google Scholar]
- Velando, A. , Drummond, H. & Torres, R. (2010). Senescing sexual ornaments recover after a sabbatical. Biology Letters 6, 194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Venesky, M. D. & Anthony, C. D. (2007). Antipredator adaptations and predator avoidance by two colour morphs of the eastern red‐backed salamander, Plethodon cinereus . Herpetologica 63, 450–458. [Google Scholar]
- * Verts, B. J. (1967). Biology of the Striped Skunk. University of Illinois Press, Urbana. [Google Scholar]
- * Veselý, P. , Veselá, S. , Fuchs, R. & Zrzavý, J. (2006). Are gregarious red‐black shieldbugs, Graphosoma lineatum (Hemiptera: Pentatomidae), really aposematic? An experimental approach. Evolutionary Ecology Research 8, 881–890. [Google Scholar]
- Vestergaard, J. S. , Twomey, E. , Larsen, R. , Summers, K. & Nielsen, R. (2015). Number of genes controlling a quantitative trait in a hybrid zone of the aposematic frog Ranitomeya imitator . Proceedings of the Royal Society B: Biological Sciences 282, 20141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal‐Cordero, J. M. , Moreno‐Rueda, G. , López‐Orta, A. , Marfil‐Daza, C. , Ros‐Santaella, J. L. & Ortiz‐Sánchez, F. J. (2012). Brighter‐colored paper wasps (Polistes dominula) have larger poison glands. Frontiers in Zoology 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallbank, R. W. R. , Baxter, S. W. , Pardo‐Diaz, C. , Hanly, J. J. , Martin, S. H. , Mallet, J. , Dasmahapatra, K. K. , Salazar, C. , Joron, M. , Nadeau, N. , McMillan, W. O. & Jiggins, C. D. (2016). Evolutionary novelty in a butterfly wing pattern through enhancer shuffling. PLoS Biology 14, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, I. J. (2011). Inversely related aposematic traits: reduced conspicuousness evolves with increased toxicity in a polymorphic poison‐dart frog. Evolution 65, 1637–1649. [DOI] [PubMed] [Google Scholar]
- * Weisrock, D. W. , Kozak, K. H. & Larson, A. (2005). Phylogeographic analysis of mitochondrial gene flow and introgression in the salamander, Plethodon shermani . Molecular Ecology 14, 1457–1472. [DOI] [PubMed] [Google Scholar]
- Weldon, P. J. , Kramer, M. , Gordon, S. , Spande, T. F. & Daly, J. W. (2006). A common pumiliotoxin from poison frogs exhibits enantioselective toxicity against mosquitoes. Proceedings of the National Academy of Sciences of the United States of America 10, 17818–17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whinnett, A. , Zimmermann, M. , Willmott, K. R. , Herrera, N. , Mallarino, R. , Simpson, F. , Joron, M. , Lamas, G. & Mallet, J. (2005). Strikingly variable divergence times inferred across an Amazonian butterfly ‘suture zone’. Proceedings of the Royal Society B: Biological Sciences 272, 2525–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Williams, C. R. , Brodie, E. D. , Tyler, M. J. & Walker, S. J. (2000). Antipredator mechanisms of Australian frogs. Journal of Herpetology 34, 431–443. [Google Scholar]
- Willink, B. , Brenes‐Mora, E. , Bolaños, F. & Pröhl, H. (2013). Not everything is black and white: colour and behavioral variation reveal a continuum between cryptic and aposematic strategies in a polymorphic poison frog. Evolution 67, 2783–2794. [DOI] [PubMed] [Google Scholar]
- Willink, B. , García‐Rodríguez, A. , Bolaños, F. & Pröhl, H. (2014). The interplay between multiple predators and prey colour divergence. Biological Journal of the Linnean Society 113, 580–589. [Google Scholar]
- Willmott, K. R. , Robinson Willmott, J. C. , Elias, M. & Jiggins, C. D. (2017). Maintaining mimicry diversity: optimal warning colour patterns differ among microhabitats in Amazonian clearwing butterflies. Proceedings of the Royal Society B: Biological Sciences 284, 20170744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, K. , Cotter, S. C. , Reeson, A. F. & Pell, J. K. (2001). Melanization and disease resistance in insects. Ecology Letters 4, 637–649. [Google Scholar]
- * Wilson, J. S. , Clark, S. L. , Williams, K. A. & Pitts, J. P. (2012). Historical biogeography of the arid‐adapted velvet ant Sphaeropthalma arota (Hymenoptera: Mutillidae) reveals cryptic species. Journal of Biogeography 39, 336–352. [Google Scholar]
- Winters, A. E. , Green, N. F. , Wilson, N. G. , How, M. J. , Garson, M. J. , Marshall, N. J. & Cheney, K. L. (2017). Stabilizing selection on individual pattern elements of aposematic signals. Proceedings of the Royal Society B: Biological Sciences 284, 20170926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters, A. E. , Stevens, M. , Mitchell, C. , Blomberg, S. P. & Blount, J. D. (2014). Maternal effects and warning signal honesty in eggs and offspring of an aposematic ladybird beetle. Functional Ecology 28, 1187–1196. [Google Scholar]
- Wolf, J. B. & Wade, M. J. (2009). What are maternal effects (and what are they not)? Philosophical Transactions of the Royal Society B: Biological Sciences 364, 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Yen, S. H. , Robinson, G. S. & Quicke, D. L. J. (2005). Phylogeny, systematics and evolution of mimetic wing patterns of Eterusia moths (Lepidoptera, Zygaenidae, Chalcosiinae). Systematic Entomology 30, 358–397. [Google Scholar]
- * Zimmermann, K. (1931). Wirkung von Selektion und Temperatur auf die Pigmentierung von Epilachna chrysomelina F. Naturwissenschaften 19, 768–781. [Google Scholar]
- * Zrzavý, J. & Nedvěd, O. (1999). Evolution of mimicry in the New World Dysdercus (Hemiptera: Pyrrhocoridae). Journal of Evolutionary Biology 12, 956–969. [Google Scholar]
- Zvereva, E. L. & Kozlov, M. V. (2016). The costs and effectiveness of chemical defenses in herbivorous insects: a meta‐analysis. Ecological Monographs 86, 107–124. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methods for compiling Table S1.
Table S1. Examples of warning‐colour variation described in existing literature.


