Nair and Wirtz preview work from Mason et al. showing that YAP/TAZ signaling regulates a transcriptional feedback pathway necessary for migration persistence.
Abstract
Persistent cell migration plays a crucial role in physiological processes, but its underlying mechanisms of regulation remain unclear. Mason et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201806065) show that YAP/TAZ limit cytoskeletal tension, which is essential for persistent (but not initiation of) cell migration. Potential implications of this study range from embryonic development to metastasis.
Cell migration is a ubiquitous physiological process (1). Healing of wounds requires the proliferation and migration of fibroblasts toward the center of the wound to close it. To mount an immune response, immune cells must migrate toward those immunogenic stimuli and infiltrate the region of inflammation. Thus, cell migration is essential for normal physiological function of an organism. However, cell migration is also critical for metastasis, the spread of cancer cells from the primary site to colonize distant organs (1). Cancer cells in the primary tumor need to migrate toward a blood vessel or lymph node before using the vasculature to spread to distant sites. Of significance, metastasis accounts for the vast majority (∼90%) of cancer-related deaths. Hence, gaining a better understanding of cell migration is critical to gaining better insight into these normal and neoplastic processes.
Yes-associated protein (YAP) and transcriptional coactivator with PDZ-motif (TAZ) are transcriptional coactivators that also play important roles in normal and neoplastic processes. After translocation to the nucleus, they interact with other transcription factors, such as TEAD, to drive the expression of target genes (2). YAP/TAZ have also been implicated in cell migration and metastatic invasion (3), but the underlying mechanism and any role of YAP/TAZ in controlling cytoskeletal processes has remained unclear. In this issue, Mason et al. show that YAP/TAZ play a critical role in enabling persistent cell migration (4). They report that YAP/TAZ control cytoskeletal tension and focal adhesion (FA) maturation via a negative feedback loop. Their first key result was that cells undergo delayed migration arrest following inhibition of transcription or translation, indicating that continued transcription/translation was required for persistent cell migration. The impact on cell migration was not evident in the first 2 h. This suggested that continued synthesis of the cytoskeleton machinery might be essential for cells to migrate for extended periods of time. However, the time required for migration to cease (2 h) was much shorter than the degradation rate of the cytoskeleton machinery (tens of hours to days). Hence, the migratory arrest observed after inhibiting transcription could not have been due to depletion of essential proteins.
Mason et al. then turned their attention to the cytoskeleton itself. They observed that transcription-inhibited cells had increased cytoskeletal tension (4), indicated by prominent stress fibers, and larger FAs. FAs are large complexes that permit attachment of the cell to its surrounding matrix and are themselves connected to the actin cytoskeleton (5). As cells move, new FAs form at the leading edge, enabling cell–matrix attachment. Tethering of the actin cytoskeleton to FAs permits the cytoskeleton to exert forces, which is essential for cell migration. This force is generated by myosin II, whose phosphorylation and activation are controlled by an interplay between Rho-associated protein kinase and myosin light chain phosphatase (6). However, as cells continue their motion, it is critical for the FAs at the trailing edge to dissociate (4), detaching the cell from the matrix. This is contrary to the observed “over-maturation” of FAs and increased cytoskeletal tension, explaining the migratory arrest.
Depletion of YAP/TAZ by siRNA phenocopied the loss of continued migration observed after transcription inhibition. Additionally, loss of YAP/TAZ also led to reduced directionality in cell movement. The involvement of YAP/TAZ in microtubule organizing center polarization and Golgi reorientation, both essential for cell migration, was also parsed out. YAP/TAZ depletion was found to increase myosin II phosphorylation, but not the total content in myosin itself. Reducing the levels of phosphorylated myosin using small-molecule inhibitors partially rescued cell migration. This result suggested a role for YAP/TAZ in negatively regulating the phosphorylation of myosin, which enabled persistent motility. To identify the downstream target of YAP/TAZ that mediates this effect, analysis of available chromatin immunoprecipitation sequencing data, which shows locations in the genomes that YAP/TAZ bind to, was performed. Three proteins, including NUAK2 and two Rho GTPase activating proteins, were identified as possible targets of YAP/TAZ. NUAK2 has been reported to prevent the dephosphorylation of myosin II by sequestering myosin phosphatase. YAP/TAZ deletion increased the expression of NUAK2 several-fold. When NUAK2 was depleted in addition to YAP/TAZ depletion, cells exhibited increased migration, smaller FAs, and reduced stress fiber formation. NUAK2-depleted cells also exhibited reduced phospho-myosin II.
The work by Mason et al. unravels a new function of the well-studied transcription factors YAP and TAZ (4), placing it in the midst of the signaling pathways that regulate cell motility (Fig. 1). They found that YAP/TAZ-induced repression of NUAK2 is essential to prevent excessive myosin II phosphorylation and cytoskeletal tension. Besides providing a new piece of the puzzle in the model for cell migration, this new study could have far-reaching consequences on the development of anti-metastatic therapeutics. RHO/ROCK and myosin II have been reported to be essential for motility, and their inhibition has been traditionally thought to impair migration. Higher levels of these proteins have been prognostic of poorer survival (7, 8) and there have been attempts to use their inhibitors as potentially anti-metastatic in vitro (9, 10). Here, Mason et al. show that this might not always be the case (4), which could be relevant for tumors characterized by mutations to YAP/TAZ. The inhibition of these proteins could potentially increase motility depending on the circumstances. However, there are conflicting and conforming reports in literature about the role of YAP/TAZ. Hence, further investigations are warranted to completely clarify the underlying mechanisms. Overall, the signaling network behind cell migration, like most biological processes, can be extremely complex and context dependent. This new work highlights that moderation—in this case, of cytoskeletal tension and myosin phosphorylation—is the key, and inhibiting migration as a phenotype may not be as simple as inhibiting a pathway.
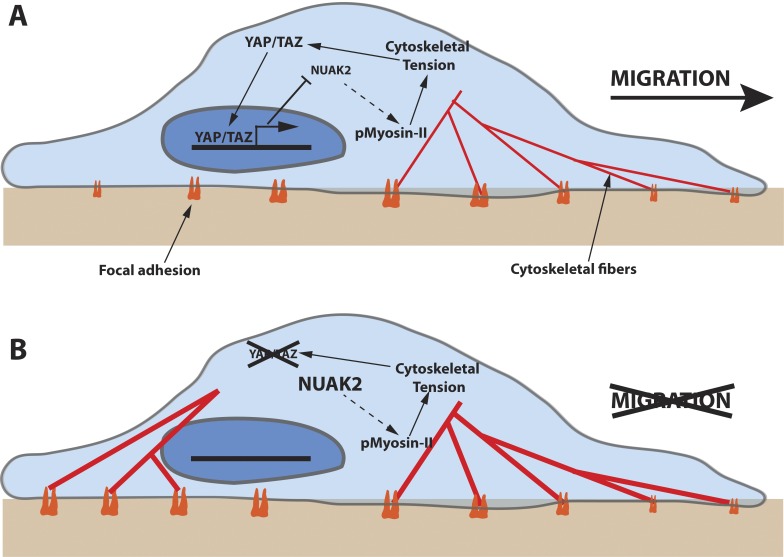
Figure 1.
Schematic illustration depicting the role of YAP/TAZ in regulating cytoskeletal tension. (A) Cytoskeletal tension induces translocation of YAP/TAZ to the nucleus, where it turns on transcriptional programs that moderate phosphorylation of myosin II via NUAK2. (B) Abrogation of this YAP/TAZ regulation leads to excessive cytoskeletal tension and over-maturation of the FAs, leading to an arrest in cell migration.
Acknowledgments
This work was supported by National Institutes of Health grants U54CA210173 and U01AG060903.
The authors declare no competing financial interests.
References
- 1.Horwitz R., and Webb D.. 2003. Curr. Biol. 13:R756–R759. [DOI] [PubMed] [Google Scholar]
- 2.Zanconato F., Cordenonsi M., and Piccolo S.. 2016. Cancer Cell. 29:783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan S.W., et al. . 2008. Cancer Res. 50:717–721. [Google Scholar]
- 4.Mason D., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201806065. [DOI] [Google Scholar]
- 5.Wozniak M.A., et al. . 2004. Biochim. Biophys. Acta. 1692:103–119. [DOI] [PubMed] [Google Scholar]
- 6.Vicente-Manzanares M., et al. . 2009. Nat. Rev. Mol. Cell Biol. 10:778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamai T., et al. . 2003. Clin. Cancer Res. 9:2632–2641. [PubMed] [Google Scholar]
- 8.Minamiya Y., et al. . 2005. Tumour Biol. 26:153–157. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima M., et al. . 2003. Cancer Chemother. Pharmacol. 52:319–324. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko K., et al. . 2002. Pancreas. 24:34–41. [DOI] [PubMed] [Google Scholar]