Abstract
Context
The impact of Hashimoto thyroiditis (HT) on the risk of thyroid cancer and its accurate detection remains unclear. The presence of a chronic lymphocytic infiltration imparts a logical mechanism potentially altering neoplastic transformation, while also influencing the accuracy of diagnostic evaluation.
Methods
We performed a prospective, cohort analysis of 9851 consecutive patients with 21,397 nodules ≥1 cm who underwent nodule evaluation between 1995 and 2017. The definition of HT included (i) elevated thyroid peroxidase antibody (TPOAb) level and/or (ii) findings of diffuse heterogeneity on ultrasound, and/or (iii) the finding of diffuse lymphocytic thyroiditis on histopathology. The impact of HT on the distribution of cytology and, ultimately, on malignancy risk was determined.
Results
A total of 2651 patients (27%) were diagnosed with HT, and 3895 HT nodules and 10,168 non-HT nodules were biopsied. The prevalence of indeterminate and malignant cytology was higher in the HT vs non-HT group (indeterminate: 26.3% vs 21.8%, respectively, P < 0.001; malignant: 10.0% vs 6.4%, respectively, P < 0.001). Ultimately, the risk of any nodule proving malignant was significantly elevated in the setting of HT (relative risk, 1.6; 95% CI, 1.44 to 1.79; P < 0.001), and was maintained when patients with solitary or multiple nodules were analyzed separately (HT vs non-HT: 24.5% vs 16.3% solitary; 22.1% vs 15.4% multinodular; P < 0.01).
Conclusion
HT increases the risk of thyroid malignancy in any patient presenting for nodule evaluation. Diffuse sonographic heterogeneity and/or TPOAb positivity should be used for risk assessment at time of evaluation.
Keywords: thyroid nodule, Hashimoto disease, FNA, thyroid cancer
Hashimoto thyroiditis (HT) is the most common autoimmune disease and the most frequent cause of hypothyroidism, affecting between 2% and 15% of the global population, depending on their age [1–4]. The pathogenesis of HT involves a chronic inflammatory infiltrate in the thyroid gland as a consequence of a breakdown in immune tolerance. This leads to activation of cellular and humoral immune responses [5]. Histologically, HT is characterized by diffuse lymphocytic infiltration of the gland, with numerous lymphoid follicles and germinal centers, fibrosis, and, ultimately, parenchymal atrophy [6, 7]. Genetic and environmental factors, such as dietary iodine uptake, have been shown to contribute to the development of HT [8].
In 1893, Rudolf Virchow first proposed an association between chronic inflammation and the formation of cancer. Over the ensuing century, this hypothesis has been verified through our understanding of a multitude of human illnesses. Classic examples of this adverse effect of inflammation on malignancy risk include the predisposition of patients with ulcerative colitis to adenocarcinoma of the colon and the impact of chronic hepatitis on development of hepatocellular carcinoma [9, 10].
Given the well-established cause-and-effect relationship between chronic inflammation and risk of malignancy, it has long been postulated that HT, a chronic thyroid inflammatory disease, would also be associated with an increased risk of thyroid cancer. Although numerous studies have since investigated this hypothesis, nearly all have been confounded by substantial selection bias, imprecise metrics, and/or retrospective analysis [11–16]. For these reasons, the association between HT and papillary thyroid cancer continues to remain controversial. To date, no large, unbiased, and prospective analysis has been performed to examine this important question, to our knowledge.
By definition, HT is a histological diagnosis. However, it is clinically impractical to necessitate surgical intervention when other means of preoperative diagnosis have proven highly predictive of the disease. These include the presence of antibodies to thyroid peroxidase (TPOAbs), as well as the identification of a diffusely heterogeneous parenchyma upon sonographic imaging of the gland [17]. It follows, therefore, that all three means of identifying HT (i.e., histopathologic, biologic, and sonographic) should be applied to any investigation seeking the broadest inclusion of patients with evidence of inflammation.
Thus, using a large, prospectively tracked database of consecutive patients seen for nodule evaluation, we evaluated the association between HT and thyroid cancer. Separately, we also sought to determine the impact of HT on diagnostic nodule evaluation, specifically on the distribution of preoperative cytology.
1. Materials and Methods
We performed a prospective cohort analysis studying 10,054 consecutive adult patients (age ≥18 years) with thyroid nodules ≥1 cm who underwent nodule evaluation between 1995 and 2017 at the Brigham and Women’s Hospital Thyroid Nodule Clinic.
All patients were referred for evaluation of a clinically relevant thyroid nodule and then underwent sonographic and clinical evaluation. Sonographic evaluation was performed by one of five radiologists with expertise in thyroid imaging, using a 5- to 17-mHz transducer. All thyroid nodules were evaluated as previously described [18], and the background thyroid parenchyma was separately assessed. From the ultrasound report, HT was considered present if a diffusely heterogeneous parenchyma or the presence of HT was reported [19]. Clinical evaluation typically included a full medical history and physical examination, and assessment of serum TSH level. When the TSH level was elevated, or at the discretion of the clinician, measurement of serum TPOAbs was performed (n = 2551 patients). For the purposes of this study, a TPOAb test was considered positive when TPOAb level was elevated above the reference range at the time of blood sampling, as defined by the test manufacturer. For most patients in this study, one of three different assays was used over 20 years.
Thyroid nodules were treated per the clinical guidelines applicable to the time, typically performing fine-needle aspiration (FNA) on solid or partially cystic nodules ≥1 cm. FNA was performed by a thyroidologist under ultrasound guidance using a 25-gauge needle after local anesthesia was administered. Typically, three passes from different areas of the nodule constituted a single aspiration. Patients were submitted for surgery on the basis of abnormal cytologic findings concerning for, or supportive of, malignancy. Separately, a minority of patients had their thyroid surgically removed owing to large size, obstructive symptoms, or cosmetic reasons.
For each patient, age at the time of the first FNA and the total number of nodules ≥1 cm were documented. Aspiration specimens were processed using ThinPrep liquid-based cytology preparation (Hologic Corp., Marlborough, MA) and were examined by a cytopathologist with thyroid expertise. Although the period of study partially predates The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC), cytologic classification through the entire study period uniformly used the same criteria and terminology later adopted by the TBSRTC [20]. Thyroid FNA cytology was reported as nondiagnostic, negative for malignant cells (benign), atypia of undetermined significance, suspicious for follicular or Hürthle cell neoplasm, suspicious for malignancy, or malignant. For patients with more than one nodule evaluated by ultrasound-guided FNA, the primary cytology was defined as the cytologic result that carried the highest risk of malignancy. Indeterminate cytology collective included those with atypia of undetermined significance, suspicious for follicular or Hürthle cell neoplasm, or suspicious for malignancy results. When a thyroidectomy was performed (n = 3186 patients), histopathologic data were also obtained, specifically documenting malignant or benign disease, as well as the histologic evidence of HT. A histologic diagnosis of HT was based on the presence of a diffuse lymphoplasmacytic infiltrate with germinal center formation, oncocytic change, and areas of atrophy or fibrosis.
To avoid selection bias while simultaneously applying the most translatable, real-world protocol, we used a holistic definition of HT that included (i) elevated TPOAb level, and/or (ii) findings of diffuse heterogeneity or HT on ultrasound, and/or (iii) diffuse lymphocytic thyroiditis on histopathology. The HT-negative population, therefore, included all other patients without a single positive finding. Given the decreased accuracy of ultrasound in assessing background thyroid parenchyma in the presence of extensive multinodularity, we excluded from our analysis those patients with more than six nodules each ≥1 cm. If sonographic reporting was unclear, images were reviewed by a blinded expert. Multinodularity was defined as two or more nodules each ≥1 cm. Incidental thyroid microcarcinoma (<1 cm) identified separately from the clinically relevant nodule(s) was not considered malignant nor was it included in our analysis.
Summary statistics are provided as mean ± SD for continuous, normally distributed variables; median with range and interquartile range for nonnormally distributed, continuous variables; or numbers and percentages for categorical variables. Comparisons were made using χ2 or Fisher exact test for categorical variables and with the Student t or Mann-Whitney tests for continuous variables, according to the data distribution as evaluated by Kolmogorov-Smirnov test. For analysis, we calculated the relative risks (RRs), the 95% CIs, and the pooled effects. A two-sided P value < 0.05 was considered significant. All calculations were performed using SPSS, version 25 (IBM, Armonk, NY). Permission for this study was granted by the Brigham and Women’s Hospital institutional review board.
2. Results
Our final study population included 9851 patients with 21,397 relevant nodules. As expected, the population was predominantly female (83.9%) and had a mean age of 52.2 years. Within the evaluable cohort, 14,063 (66%) nodules were aspirated. The remaining nonaspirated nodules were generally cystic, small, had sonographically benign characteristics, or were resected in conjunction with a separate index nodule prompting concern in the same gland. Baseline patient and nodules characteristics are summarized in Table 1.
Table 1.
Baseline Patient and Nodule Characteristics
| Patient Characteristics | |
|---|---|
| No. of patients | 9851 |
| Sex | |
| Female | 8263 (83.9) |
| Male | 1588 (16.1) |
| Age, y | |
| Mean ± SD | 52.2 ± 15.0 |
| Range | 18–95 |
| Multinodular gland | |
| Yes | 4495 (45.6) |
| No | 5356 (54.4) |
| Thyroidectomy | 3186 (32.3) |
| Hashimoto thyroiditisa | |
| Yes | 2651 (26.9) |
| No | 7200 (73.1) |
| Nodule characteristics | |
| No. of nodules | 21,397 |
| Largest dimension, cm | |
| Mean ± SD | 2.6 ± 1.3 |
| Range | 1.0–12.8 |
| Nodules biopsied | 14,063 (65.7) |
Data reported as no. (%) unless otherwise indicated.
HT criteria: chronic lymphocytic thyroiditis on histopathology and/or elevated thyroperoxidase antibodies and/or diffuse heterogeneity on ultrasound.
Evidence (i.e., serological, sonographic or histologic) of HT was confirmed in 2651 patients (27%). A total of 3895 nodules were evaluated in patients with HT; the remaining 10,168 nodules were evaluated in patients without evidence of HT.
The influence of HT on the diagnostic evaluation and cytology classification is described by the data in Table 2. The proportion of nodules with indeterminate and malignant cytology was higher in the HT group than in the non-HT group. Indeterminate cytology was obtained in 20.6% of patients with nodules in the setting of HT, compared with 17.1% of patients with nodules in a gland not affected by HT (indeterminate cytology: RR, 1.3, 95% CI, 1.17 to 1.44, P < 0.01). An increase in malignant cytology was similarly identified in patients with HT (RR, 1.7; 95% CI, 1.44 to 1.99; P < 0.01; Fig. 1).
Table 2.
Influence of HT on Nodule Cytology Classification According to TBSRTC
| HT | Non-HT | P Valuea | |
|---|---|---|---|
| No. of nodules biopsied | 3895 | 10,168 | |
| Nodule cytology by TBSRTC, no. (%) | <0.01 | ||
| Nondiagnostic | 168 (4.3) | 728 (7.2) | |
| No malignant cells | 2652 (68.1) | 7217 (71.0) | |
| Indeterminate | 791 (20.3) | 1750 (17.2) | |
| AUS, FLUS | 307 (7.9) | 635 (6.2) | |
| SFN | 249 (6.4) | 626 (6.2) | |
| SUSP | 235 (6.0) | 489 (4.8) | |
| Positive for malignancy | 284 (7.3) | 473 (4.7) |
Abbreviations: AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; SFN, suspicious for follicular or Hürthle cell neoplasm; SUSP, suspicious for malignancy.
P value for 2 × 6 χ2 analysis of six TBSRTC categories.
Figure 1.
Relative risk of indeterminate or malignant cytology vs benign cytology, given HT. Indeterminate cytology includes TBSRTC categories 3, 4, and 5. Malignant cytology includes TBSRTC category 6.
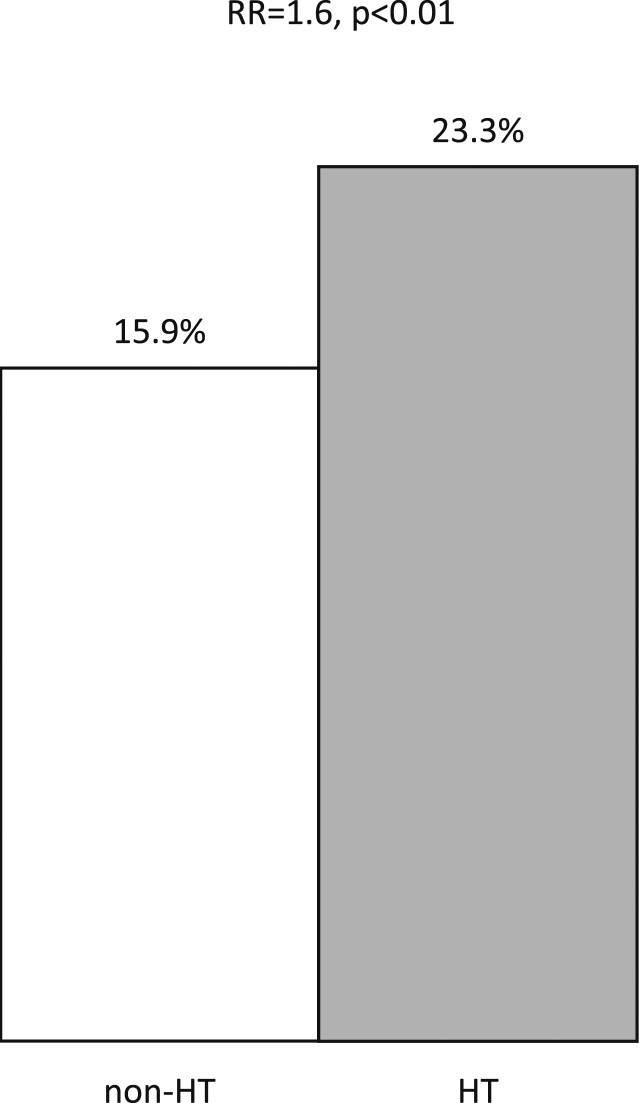
Finally, we evaluated the association between HT and the risk of malignancy in the setting of clinically relevant thyroid nodular disease. The frequency of a nodule proving cancerous was higher in patients with coexistent HT compared with those without (23.3% vs 15.9%; RR, 1.6; 95% CI, 1.44 to 1.79; P < 0.01; Fig. 2). This increased cancer prevalence was maintained when patients with solitary or multiple nodules were analyzed separately, suggesting a field effect of HT itself (HT vs non-HT groups: 24.5% vs 16.3% in solitary nodules; 22.1% vs 15.4% in multinodular glands; P < 0.01). The types and proportions of thyroid cancer in both groups are listed in Table 3. The frequency of malignancy was also higher in the setting of HT when we evaluated only patients with a final indeterminate cytology (HT vs non-HT: 43.1% vs 38.7%; P = 0.05). The association between HT and thyroid cancer according to the final cytological diagnosis is reported in Table 4.
Figure 2.
Relative risk of having one or more malignant nodules vs no malignant nodules given HT.
Table 3.
Association Between HT and Thyroid Cancer
| Total (n = 9851) | HT (n = 2651) | Non-HT (n = 7200) | P Value | |
|---|---|---|---|---|
| Benign disease, no. (%) | 8140 (82.7) | 2045 (77.2) | 6095 (84.6) | |
| Thyroid cancer, no. (%) | ||||
| All subtypes | 1711 (17.3) | 606 (22.8) | 1105 (15.4) | <0.01 |
| PTC | 1521 (88.9) | 547 (90.3) | 974(88.1) | |
| FTC | 118 (6.8) | 39 (6.4) | 79 (7.1) | |
| MTC | 14 (0.8) | 5 (0.8) | 9 (0.8) | |
| Anaplastic | 18 (1.1) | 3 (0.5) | 15 (1.4) | |
| Poorly differentiated | 18 (1.1) | 4 (0.7) | 14 (1.3) | |
| Nonthyroid malignancy | 22(1.3) | 8 (1.3) | 14 (1.3) |
Abbreviations: FTC, follicular thyroid cancer; MTC, medullary thyroid cancer; PTC, papillary thyroid cancer.
Table 4.
Association between HT and Thyroid Cancer According to Primary Cytology Classification Using TBSRTC
| TBSRTC Categorya | HT (n = 2,651) | Non-HT (n = 7,200) | P Value | RR | 95% CI |
|---|---|---|---|---|---|
| Nondiagnostic | 0.08 | 2.34 | 0.877–6.292 | ||
| Total no. | 87 | 458 | |||
| Thyroid cancer, no. (%)b | 6 (6.8) | 14 (3.0) | |||
| No malignant cells | <0.01 | 2.10 | 1.450–3.063 | ||
| Total no. | 1602 | 4707 | |||
| Thyroid cancer, no. (%)b | 48 (2.9) | 68 (1.4) | |||
| Indeterminate | 0.05 | 1.19 | 0.999–1.436 | ||
| Total no. | 696 | 1572 | |||
| Thyroid cancer, no. (%)b | 300 (43.1) | 609 (38.7) | |||
| AUS, FLUS | 0.02 | 1.48 | 1.045–2.095 | ||
| Total no. | 250 | 537 | |||
| Thyroid cancer, no. (%)b | 69 (27.6) | 110 (20.4) | |||
| SFN | 0.50 | 1.11 | 0.808–1.538 | ||
| Total no. | 235 | 589 | |||
| Thyroid cancer, no. (%)b | 79 (33.6) | 184 (31.2) | |||
| SUSP | 0.71 | 1.07 | 0.745–1.541 | ||
| Total no. | 211 | 446 | |||
| Thyroid cancer, no. (%)b | 152 (72.0) | 315 (70.3) | |||
| Positive for malignancy | 0.39 | 2.32 | 0.258–20.907 | ||
| Total no. | 265 | 460 | |||
| Thyroid cancer, no. (%)b | 264 (99.6) | 456 (99.1) |
Abbreviations: AUS, atypia of undetermined significance; FLUS, follicular lesion of undetermined significance; SFN, suspicious for follicular or Hürthle cell neoplasm; SUSP, suspicious for malignancy.
Patients with more than one evaluable nodule were classified according to the highest TBSRTC score of the multiple nodules.
Thyroid cancer was defined using histopathology as well as TBSRTC 6 cytology in cases where surgery did not occur.
Given the increased risk of thyroid cancer in the setting of HT, we also investigated if such malignancy showed signs of increased aggressivity. We investigated the proportion of cancers in both groups showing signs of invasion, local or distant metastasis, or tumor size. Importantly, no significant differences in malignant pathologic characteristics or markers of aggressiveness were identified among patients with or without HT (Table 5).
Table 5.
Differences in Cancer Pathologic Characteristics Between Patients With and Without HT
| HT (n = 2651) | Non-HT (n = 7200) | P Value | |
|---|---|---|---|
| Microinvasion, n (%) | 122 (20.4) | 192 (18.6) | 0.39 |
| Gross invasion, n (%) | 44 (6.7) | 87 (7.2) | 0.70 |
| Lymph node metastasis, n (%) | 108 (22.3) | 177 (26.7) | 0.09 |
| Distant metastasis, n (%) | 23 (5.8) | 41 (6.1) | 0.89 |
| Multifocal, n (%)a | 85 (15.1) | 122(12.5) | 0.16 |
| Tumor size, largest dimension, cm | 0.04 | ||
| ≤2 | 430 (66.0) | 729 (61.9) | |
| >2–4 | 182 (27.9) | 339 (28.8) | |
| >4 | 40 (6.1) | 109 (9.3) |
Data reported as no. (%) unless otherwise indicated.
Multifocal: two or more nodules each ≥1 cm.
Though 13 cases of thyroid lymphoma were documented in the entire study cohort, lymphoma alone was not responsible for the increased prevalence of malignant disease in patients with HT. Of the 13 total cases, nine cases of thyroid lymphoma were documented in the 7200 patients without HT. Four cases of thyroid lymphoma were detected in the 2651 patients with HT.
3. Discussion
The impact of coexistent HT on the risk of developing thyroid cancer has been unclear. Building on a sound hypothesis and extensive preliminary data, we performed a large, prospective cohort analysis investigating this question. Using a highly translatable and broad clinical definition of HT, our data confirm a 45% increased risk that a clinically relevant thyroid nodule will prove malignant when in the presence of this chronic inflammatory process. The risk of a nodule being cancerous in the setting of HT was nearly one in four, substantially higher than in patients without the disease. Furthermore, the diagnostic evaluation of thyroid nodules is affected by the presence of HT, even when the nodule is nonmalignant, and a significantly higher risk of indeterminate cytology should be expected. Together, these data provide evidence that HT can be viewed as a risk factor for the development of thyroid cancer. Easily obtainable variables, such as measurement of TPOAb levels and/or identification of a diffusely heterogeneous parenchyma on ultrasound, should be sought at the time of initial thyroid nodule evaluation. The large-scale, prospective nature of this 20-year cohort analysis supports the translatability and durability of these findings, though multivariate analysis is required to better understand if this risk factor remains independent of other variables.
We used the broadest and most practical definition of HT. This proved important to our study, because histopathology alone remains an impractical end point. Patients recommended for surgery typically represent a highly selected group, and routine thyroidectomy of all patients in any study would be deemed unacceptable. The association of an elevated TPOAb level with HT is well established. Furthermore, a diffuse heterogeneous parenchyma on sonographic imaging is highly suggestive of a diffuse inflammatory process, most notable HT [21]. Others have similarly used broad-based, holistic diagnostic criteria for HT, such as in our study [17, 22].
Together, one or more of these findings confirming HT was present in 27% of our population. Although many population estimates of HT (typically using only TPOAb measurement to identify HT) are lower [4, 23, 24], we note that our study cohort was unique. Our population of 9851 consecutive patients were all being evaluated for clinically relevant thyroid nodules. As expected in such a cohort, most were women, and the mean age was older than 52 years. Together, such a group would be expected to have a higher rate of autoimmune thyroid disease than a broad epidemiologic sampling. It is also well documented that a hypoechoic sonographic pattern or irregular echogenic parenchyma may precede TPOAb positivity in autoimmune thyroid disease and thus may not be detected in up to 20% of individuals with HT [25]. Furthermore, our data indicate a higher rate of cancer among patients with HT and known nodules. Stemming from this, it is equally plausible that HT itself may predispose to nodule formation. If so, a much higher rate of HT would be expected in any nodule population subsequently studied. In support of our methodology, we note a separate postmortem histopathologic thyroid analysis on thyroid disease–free individuals that detected evidence of chronic autoimmune thyroiditis in 27% of adult women, a percentage strikingly similar to our findings [1].
Notable to our study were several findings. First, the increased risk of cancer attributable to HT was detected in those with solitary nodules as well as in nodules that were part of a multinodular gland. This supports the rationale that inflammation imparts a field effect through the gland itself. Second, the type, size, and aggressiveness of the cancer detected did not differ from those with or without HT. Thus, although malignant transformation or formation appears affected by HT, papillary thyroid carcinoma (by far the most common cancer subtype) remains generally low risk and indolent. Finally, although an increased RR of thyroid lymphoma has long been associated with the presence of HT, our data speak to an increase in well-differentiated thyroid cancer, and lymphoma is most certainly not responsible for the full increase in RR of malignancy as a whole.
There has been an increase worldwide in differentiated thyroid cancer. According to the Surveillance, Epidemiology, and End Results database, the number of new cases of thyroid cancer was 14.5 per 100,000 men and women per year during the period of 2011 to 2015 [26]. Enhanced thyroid nodule detection has been implicated in this but appears to not fully explain this increase. The concomitant increase in the incidence of HT worldwide (perhaps following iodine supplementation) presents another plausible explanation and reinforces the concept that thyroid chronic inflammation may lead to neoplastic processes [1, 5].
The impact of HT on nodule diagnostic evaluation is also perhaps not surprising. HT may result in reactive atypia that mimics papillary thyroid cancer, such as increased nuclear size and nuclear contour irregularities and grooves, which can result in an indeterminate diagnosis of FNA samples [27]. Also, the differentiation between follicular neoplasms and HT can be difficult because some cytological features, such as hyperplastic follicular cells and Hürthle cells, are encountered in both scenarios [28]. Studies have attempted to link the presence of HT to FNA accuracy, with conflicting results. HT is related to a higher rate of false-negative and false-positive FNA results [27, 29]. Others have demonstrated that the presence of HT significantly decreased the accuracy and increased the indeterminate rate of cytological results of ultrasound-FNA in subcentimeter nodules [30], though data are variable [31].
We acknowledge limitations to our investigation. First, we studied only those patients with at least one thyroid nodule >1 cm; thus, our findings are not generalizable to a general population nor do they reflect epidemiologic data regarding HT or thyroid cancer in a broad population. Nonetheless, our data are translatable to the real-world clinical environment, allowing preoperative data such as TPOAb level or ultrasound appearance to inform individualized risk assessment during thyroid nodule care. Second, we did not measure TPOAb level in all patients; thus, we cannot assess the overlap of sonographic and serologic findings of HT. Third, ultrasound interpretation was performed by only a single expert radiologist for most patients. This precludes assessment of interrater variability, which is known to exist. And finally, different TPOAb assays were used over the course of 20 years, possibly introducing some variability in defining low-level positive results.
In conclusion, HT adversely affects the diagnostic evaluation of, and increases the risk of thyroid malignancy in, any patient being seen for nodule evaluation. The presence of a diffusely heterogeneous sonographic pattern or of TPOAb positivity may be used for thyroid malignancy risk assessment at the time of nodule evaluation. Future investigations should work to elucidate the relative impact of this finding in relation to other known risk factors such as age and sex [32, 33].
Acknowledgments
Disclosure Summary: J.H.L. has received research support from Bristol-Myers Squibb, Bayer; and Novartis; and consulting fees from Bayer, Genentech, and Eisai. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- FNA
fine-needle aspiration
- HT
Hashimoto thyroiditis
- RR
relative risk
- TBSRTC
The Bethesda System for Reporting Thyroid Cytopathology
- TPOAb
thyroid peroxidase antibody
References and Notes
- 1. Vanderpump MP. The epidemiology of thyroid disease. Br Med Bull. 2011;99(1):39–51. [DOI] [PubMed] [Google Scholar]
- 2. Delemer B, Aubert JP, Nys P, Landron F, Bouée S. An observational study of the initial management of hypothyroidism in France: the ORCHIDÉE study. Eur J Endocrinol. 2012;167(6):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–265. [DOI] [PubMed] [Google Scholar]
- 4. Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, Grimley Evans J, Hasan DM, Rodgers H, Tunbridge F, Young ET. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43(1):55–68. [DOI] [PubMed] [Google Scholar]
- 5. Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: further developments in our understanding. Horm Metab Res. 2015;47(10):702–710. [DOI] [PubMed] [Google Scholar]
- 6. Hiromatsu Y, Satoh H, Amino N. Hashimoto’s thyroiditis: history and future outlook. Hormones (Athens). 2013;12(1):12–18. [DOI] [PubMed] [Google Scholar]
- 7. Caturegli P, De Remigis A, Chuang K, Dembele M, Iwama A, Iwama S. Hashimoto’s thyroiditis: celebrating the centennial through the lens of the Johns Hopkins hospital surgical pathology records. Thyroid. 2013;23(2):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McLeod DS, Caturegli P, Cooper DS, Matos PG, Hutfless S. Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel. JAMA. 2014;311(15):1563–1565. [DOI] [PubMed] [Google Scholar]
- 9. Fung J, Lai CL, Yuen MF. Hepatitis B and C virus-related carcinogenesis. Clin Microbiol Infect. 2009;15(11):964–970. [DOI] [PubMed] [Google Scholar]
- 10. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 11. Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto’s thyroiditis and papillary thyroid carcinoma risk. Oncotarget. 2017;8(37):62414–62424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Resende de Paiva C, Grønhøj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto’s thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. 2017;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98(2):474–482. [DOI] [PubMed] [Google Scholar]
- 14. Boi F, Pani F, Mariotti S. Thyroid autoimmunity and thyroid cancer: review focused on cytological studies. Eur Thyroid J. 2017;6(4):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grani G, Calvanese A, Carbotta G, D’Alessandri M, Nesca A, Bianchini M, Del Sordo M, Vitale M, Fumarola A. Thyroid autoimmunity and risk of malignancy in thyroid nodules submitted to fine-needle aspiration cytology. Head Neck. 2015;37(2):260–264. [DOI] [PubMed] [Google Scholar]
- 16. Azizi G, Keller JM, Lewis M, Piper K, Puett D, Rivenbark KM, Malchoff CD. Association of Hashimoto’s thyroiditis with thyroid cancer. Endocr Relat Cancer. 2014;21(6):845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grani G, Carbotta G, Nesca A, D’Alessandri M, Vitale M, Del Sordo M, Fumarola A. A comprehensive score to diagnose Hashimoto’s thyroiditis: a proposal. Endocrine. 2015;49(2):361–365. [DOI] [PubMed] [Google Scholar]
- 18. Angell TE, Vyas CM, Medici M, Wang Z, Barletta JA, Benson CB, Cibas ES, Cho NL, Doherty GM, Doubilet PM, Frates MC, Gawande AA, Heller HT, Kim MI, Krane JF, Marqusee E, Moore FD Jr, Nehs MA, Zavacki AM, Larsen PR, Alexander EK. Differential growth rates of benign vs. malignant thyroid nodules. J Clin Endocrinol Metab. 2017;102(12):4642–4647. [DOI] [PubMed] [Google Scholar]
- 19. Patel S, Giampoli E, Oppenheimer D, Montoya S, Rupasov A, Dogra V.. Sonographic features of diffuse Hashimoto thyroiditis: determining sensitivity of features and predictors of malignancy. Am J Sonogr. 2018;1(6):1–7. [Google Scholar]
- 20. Cibas ES, Ali SZ. The Bethesda System for reporting thyroid cytopathology. Thyroid. 2009;19(11):1159–1165. [DOI] [PubMed] [Google Scholar]
- 21. Pedersen OM, Aardal NP, Larssen TB, Varhaug JE, Myking O, Vik-Mo H. The value of ultrasonography in predicting autoimmune thyroid disease. Thyroid. 2000;10(3):251–259. [DOI] [PubMed] [Google Scholar]
- 22. Castagna MG, Belardini V, Memmo S, Maino F, Di Santo A, Toti P, Carli AF, Caruso G, Pacini F. Nodules in autoimmune thyroiditis are associated with increased risk of thyroid cancer in surgical series but not in cytological series: evidence for selection bias. J Clin Endocrinol Metab. 2014;99(9):3193–3198. [DOI] [PubMed] [Google Scholar]
- 23. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 24. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. [DOI] [PubMed] [Google Scholar]
- 25. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29(1):76–131. [DOI] [PubMed] [Google Scholar]
- 26. Noone AMHN, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2015, National Cancer Institute. https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- 27. Harvey AM, Truong LD, Mody DR. Diagnostic pitfalls of Hashimoto’s/lymphocytic thyroiditis on fine-needle aspirations and strategies to avoid overdiagnosis. Acta Cytol. 2012;56(4):352–360. [DOI] [PubMed] [Google Scholar]
- 28. Kollur SM, El Sayed S, El Hag IA. Follicular thyroid lesions coexisting with Hashimoto’s thyroiditis: incidence and possible sources of diagnostic errors. Diagn Cytopathol. 2003;28(1):35–38. [DOI] [PubMed] [Google Scholar]
- 29. Kapan M, Onder A, Girgin S, Ulger BV, Firat U, Uslukaya O, Oguz A. The reliability of fine-needle aspiration biopsy in terms of malignancy in patients with Hashimoto thyroiditis. Int Surg. 2015;100(2):249–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gao L, Ma B, Zhou L, Wang Y, Yang S, Qu N, Gao Y, Ji Q. The impact of presence of Hashimoto’s thyroiditis on diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy in subcentimeter thyroid nodules: a retrospective study from FUSCC. Cancer Med. 2017;6(5):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baser H, Ozdemir D, Cuhaci N, Aydin C, Ersoy R, Kilicarslan A, Cakir B. Hashimoto’s thyroiditis does not affect ultrasonographical, cytological, and histopathological features in patients with papillary thyroid carcinoma. Endocr Pathol. 2015;26(4):356–364. [DOI] [PubMed] [Google Scholar]
- 32. Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, Krane JF, Barletta JA, Kim MI, Larsen PR, Alexander EK. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab. 2015;100(12):4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frates MC, Benson CB, Doubilet PM, Kunreuther E, Contreras M, Cibas ES, Orcutt J, Moore FD Jr, Larsen PR, Marqusee E, Alexander EK. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91(9):3411–3417. [DOI] [PubMed] [Google Scholar]