Abstract
Context
Variants in bone morphogenetic protein 7 (BMP7) have been reported in patients with hypospadias. Here we report and analyze two variants in the BMP7 prodomain in monozygotic twins with hypospadias.
Materials and Methods
Patients with hypospadias were prospectively recruited. After informed consent was obtained, DNA was extracted from blood. The coding regions of 1034 genes [including 64 known diagnostic genes and candidate genes for disorder/difference of sex development (DSD)] were sequenced using a targeted capture approach (HaloPlex, Agilent, Santa Clara, CA), combined with massively parallel sequencing. The resulting variants were filtered for rarity in the general population (<1%) and in our screen. Quality, depth of the reads, and predicted pathogenicity were also considered. The consequences of the identified mutations on BMP7 expression was determined by Western blot analysis on culture media from transfected cells, and activity measured using a SMAD 1/5-responsiveness luciferase assay.
Results
We analyzed DNA from 46 patients with hypospadias. Two variants in BMP7 were identified in two pairs of monozygotic concordant twins exhibiting proximal hypospadias. Both variants are heterozygous, nonsynonymous, and affect highly conserved amino acids in the prodomain of BMP7 in regions predicted to be important for BMP7 assembly/folding. Functional analyses demonstrated that both variants disrupt BMP7 synthesis or secretion.
Conclusion
Through our targeted DSD panel we have identified two variants in the prodomain of BMP7 in hypospadias. By decreasing BMP7 synthesis, these variants are likely to limit BMP7 bioavailability during closure of the urethral plate.Further analysis of patients with hypospadias may uncover additional variants that cause this DSD.
Keywords: hypospadias, disorders of sex development, massively parallel sequencing, bone morphogenetic protein
Hypospadias is the second most common malformation of the external genitalia in males after cryptorchidism, affecting 1 in 200 to 300 boys [1]. Hypospadias is defined as the misplacement of the urethral meatus along the shaft of the penis. It is believed to arise from disrupted development of the genital tubercle (GT) and it is considered to be a form of 46,XY disorder/difference of sex development (DSD) [2].
The etiology of this defect is still a matter of debate although it is currently believed to be caused by a combination of genetic and environmental factors [3]. Forty-eight genes have been associated with hypospadias in either mice or human [4], including bone morphogenetic protein 7 (BMP7).
BMP7 is a member of the TGF-β superfamily of secreted signaling molecules and has been implicated in the development of the GT [5]. In mice, Bmp7 expression is detected in the urethral plate and the adjacent mesenchyme of the genital swelling at embryonic day 11.5 (E11.5). Following the sex-specific differentiation of the external genitalia, Bmp7 is expressed in the urethra of male embryos (E17.5), thus Bmp7 may also play a role later in penile development. Consistent with these roles, Bmp7 null mice exhibited a 50% shorter urethral plate with formation of a large urethral groove and these mice develop severe hypospadias [6] (Fig. 1).
Figure 1.
BMP7 expression and function in the developing genital tubercle. When BMP7 is expressed in the urethral epithelium of the GT, it triggers mesenchymal proliferation and epithelial apoptosis to result in the closure of the urethral plate and formation of the median raphe. In the absence of BMP7 expression, there is no mesenchymal proliferation and no epithelial apoptosis. This results in a hypospadias phenotype with enlarged capillaries in the mesenchyme.
Human BMP7 is synthetized intracellularly as a 431 amino acid precursor [7] that then undergoes proteolytic cleavage at an R-S-I-R furin cleavage consensus site by proprotein convertases. Cleavage releases the 263 amino acid N-terminal propeptide from the 139amino acid C-terminal mature protein [8]. Following separation, it is believed that the propeptide remains noncovalently associated with the mature disulfide cross-linked BMP7 homodimer [9]. Although it is the mature domain that carries the receptor binding activity, the prodomain is essential for the correct folding and dimerization of BMP7. Additionally, upon cellular secretion, the prodomain targets the mature BMP7 dimer to fibrillin microfibrils in the extracellular matrix [8].
Seventy-nine single nucleotide polymorphisms (SNPs) in BMP7 have been reported in Exome Sequencing Projects, in both the European American (54 SNPs) and African American (42 SNPs) populations. Of those, 23 are missense variants including 9 benign, 2 possibly damaging, and 12 probably damaging as per PolyPhen 2. Eighteen are located in the prodomain, including 10 predicted as probably damaging.
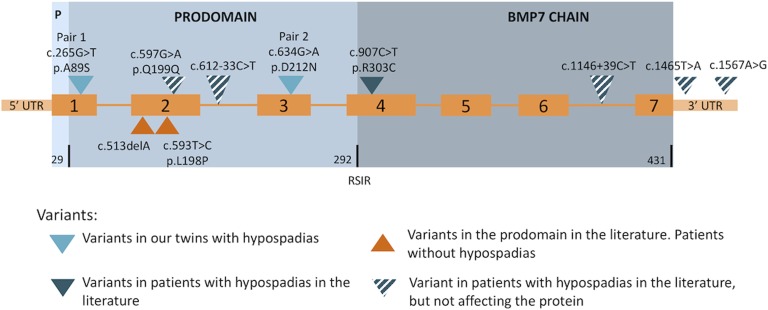
Variants in BMP7 have been associated with human disease [10–12]. Six variants have been reported in children with hypospadias [12]. However, one of them is a silent change, two are within introns, and two are located in the 3′ untranslated region. Therefore, only one nonsynonymous variant, (c.907C>T, p.R303C), is actually associated with hypospadias [12]. Additionally, two nonsynonymous variants within the prodomain have been associated with various forms of facial, ocular, and neurologic malformation [11] but patients were not reported to have genital anomalies. Variants previously reported in humans are shown in Figure 2.
Figure 2.
BMP7 structure and variants reported in humans. Structure: DNA is presented in orange and include 7 exons. Blue represents the protein with its three components: Signal P, Prodomain, and BMP7 chain. Number of amino acids in each part is presented at the bottom line of the protein structure. Note the RSIR furin-like consensus cleavage site between the prodomain and the BMP7 chain.
Using a targeted massively parallel sequencing approach in 46,XY monozygotic twins with hypospadias, we have identified two genetic variants in the prodomain of BMP7. Each variant occurs in a pair of monozygotic concordant twins with hypospadias. The effect of the mutations on both the expression and activity of BMP7 were studied in vitro. These are the first genetic variations in the BMP7 prodomain to be associated with hypospadias or 46,XY DSD.
1. Patients and Methods
This study was approved by the Royal Children’s Hospital and Murdoch Children’s Research Institute ethic committee (HREC 22073) and the Medical Ethic Committee Diponegoro University and Dr. Kariadi Hospital. Patients with hypospadias were recruited by collaborating clinicians. Patients were included after informed consent was obtained, regardless of the severity of the malformation. DNA was extracted from peripheral blood locally and sent to molecular development group at Murdoch Children’s Research Institute. Samples were sent with a phenotypic description sheet including clinical evaluation and hormonal testing, when available.
A. DNA Sequencing
DNA sequencing was carried out as previously described [13].
The panel included 64 diagnostic genes for DSD as well as 967 candidate genes including 41 for hypospadias. The hypospadias candidate genes are detailed in [4].
B. Data Filtering
Variants from sequencing were filtered as described previously [13]. Any interesting remaining variant was further analyzed in ExAC to confirm its rarity (<1%) in the ethnic group of the patient. For concordant monozygotic twins, in which both twins have the same phenotype, only variants present in both twins were considered. Within the list of remaining variants after filtering, priority was given according to the following criteria: (i) variants in DSD diagnostic genes; (ii) variants in hypospadias candidate genes; (iii) homozygous, hemizygous, or compound heterozygous changes; (iv) highly damaging variants (stop codons, frameshift or splice site mutations were considered more deleterious than missense point mutations); and (v) pathogenicity according to four in silico prediction tools (Polyphen 2, SIFT, LRT, and MutationTaster) was also taken into account.
C. Sanger Confirmation
Variants determined to be of interest were confirmed using Sanger sequencing. Primers used are Twin Pair 1 (ex1) forward GTTGGCTCTCTGGACTCCTA, reverse GCGTAACATGGARGGGACTTC. Twin Pair 2 (exon 3) forward GCTCTGCTTCCCATCTGTT, reverse GGAGCACAGGCTGCATTA. PCR was performed using Phusion (Thermo Fischer, Waltham, MA) with HF buffer, three- step program and annealing at 62°C.
D. BMP7 Variant Plasmid Creation
Patient variants were introduced into a mammalian expression vector (pCDNA3.1) containing the human BMP7 open reading frame. Point mutations in the prodomain and mature regions of BMP7 were introduced using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer’s instructions. Primers used for mutagenesis were hBMP7.c.G265T_For ctccaccgccatggagttgtacaggtcca and hBMP7.c.G265T_Rev tggacctgtacaactccatggcggtggag, and hBMP7.c.G634A_For agagggtacggctgttgagcaggaagagatc and hBMP7.c.G634A_Rev gatctcttcctgctcaacagccgtaccctct.
The mutated region was confirmed by DNA sequencing using plasmid specific primers. Plasmid constructs were expanded in Escherichia coli cultures grown in 300 mL of LB media with ampicillin at 37°C overnight. Plasmid DNA was extracted from the cultures using the NucleoBond® Xtra plasmid purification (Macherey-Nagel, Germany), according to the supplier protocol.
E. Cell Transfection
BMP7 proteins were produced by transient transfection in HEK-293T cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Briefly, HEK-293T cells were seeded at a density of 8 × 105 cells per well onto six-well plates. BMP7 wild-type or mutant DNA (5 µg) was combined with Lipofectamine 2000 for 20 minutes, according to the manufacturer’s instructions. DNA-Lipofectamine complexes were then added directly to cells and incubated in Opti-MEM medium (Invitrogen, Carlsbad, CA) for 24 to 72 hours at 37°C in 5% CO2. Following transfection, conditioned media and was collected for analysis by Western blotting. Cells were lysed with the addition of 0.1% Triton-X-100 in PBS (pH 7.4).
F. Western Blots
To allow analysis of BMP7 production following transient transfection in mammalian cells, conditioned media was concentrated 10-fold using Nanosep microconcentrators (10 kDa; Pall Life Sciences, Port Washington, NY). Concentrated conditioned media was combined with NuPAGE loading dye (Invitrogen, Carlsbad, CA) and nonreduced samples were loaded on 10% SDS-PAGE gels (Bio-Rad, Hercules, CA). Following electrophoresis, proteins were transferred onto ECL Hybond membranes (GE Health Care, Buckinghamshire, UK). Blots were blocked in 1% BSA in TBS-Tween buffer (Tris-buffered saline with 0.05% Tween-20) for a minimum of one hour, and then probed with a BMP7 antibody (MAB3542, R&D Systems, Minneapolis, MN) diluted in TBS-Tw buffer (1:5000) overnight. Bound primary antibody was detected with horseradish peroxidase–conjugated antimouse IgG (Amersham), diluted in TBS-Tw buffer (1:10,000). Western blots were developed using Lumi-light chemiluminescence substrates and detected on a Biorad Chemidoc instrument. The levels of mature BMP7 were quantified by densitometry, as a mean of n = 3 experiments.
Total protein content in the BMP7 conditioned media samples was determined using a PierceTM BCA protein assay (Thermo Scientific, Waltham, MA) according to the manufacturer’s guidelines.
G. Activity Assays
The ability of BMP7 variants to stimulate the BMP-responsive SMAD 1/5 pathway in a luciferase assay in human granulosa tumor cell line (COV434) was determined. In brief, COV434 cells were plated at 75,000 cells per well in 48-well plates in complete medium (DMEM supplemented with 10% fetal calf serum) at 37°C in 5% carbon dioxide. Following overnight incubation, cells were transfected with 250 ng perwell of a BMP response element–luciferase reporter (BRE-reporter as previously described [14]) using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. At 24 hours posttransfection, the COV434 cells were treated with increasing doses of conditioned media containing the BMP7 variants for 16 hours. The medium was then aspirated, the cells were solubilized in solubilization buffer [25 mm glycylglycine (pH 7.8), 15 mm magnesium sulfate, 4 mm EGTA, 1% Triton X-100, and 1 mm dithiothreitol], and luciferase reporter activity was measured, as previously described [14]. Data were analyzed using Graphpad Prism 7 software and expressed as fold of nontreated wells (fold-control). Mean activities were calculated from n = 3 experiments; error is standard deviation.
To support this analysis, the ability of the BMP7 variants to induce endogenous phosphorylation of SMAD 1/5 was determined by Western blot. In brief, COV434 cells were plated at 5 × 105 cells per well in 12-well plates in DMEM media with 10% fetal calf serum. The following day, the media was replaced with low serum media (DMEM, 0.2% fetal calf serum, and 50 mM HEPES) and incubated for 4 hours at 37°C to suppress basal activation of pSMAD 1/5. Cells were then treated with increasing doses of conditioned media containing the BMP7 variants (diluted in low serum media) and incubated for 45 minutes. The treated COV434 cells were then lysed in radioimmunoprecipitation assay buffer (10 mM Tris-Cl, 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM sodium chloride, pH 8.0) on ice, containing complete protease inhibitors and PhosStop phosphatase inhibitors (both Roche). Cell lysates were then loaded onto SDS-PAGE gels and transferred to nitrocellulose membranes as described above. Lysates were probed with antibodies to either pSMAD 1/5 or to loading control protein glyceraldehyde 3phosphate dehydrogenase (both sourced from CST), and developed as described above.
2. Results
We recruited four pairs of monozygotic twins with hypospadias from Indonesia. This included two pairs of concordant twins (both twins affected) and two pairs of discordant twins (only one twin affected). A rare variant in BMP7 was found in each pair of concordant twins. In both cases, the variant was a heterozygous missense mutation. Additionally, both variants were located in the prodomain of BMP7. Clinical evaluation of the patients and position of the variants are described in Table 1.
Table 1.
Clinical Description and Variants Analysis of the Patients
| Pair | Twin | Age at Diagnosis | Karyotype | Syndromic Features | Scrotum | Testes | Urethral Opening | Stretched Penile Length | Chordee | Variant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variant | ExAC (South Asian) | Freq. in Internal Dataset (278 46,XY) | IGV | cDNA NM_001719 | Protein | |||||||||||||
| Read | WT/Mutant Ratio | GERP | Effect of the Change | |||||||||||||||
| 1 | 1 | 4 y | 46,XY | No | Fused | 2 mL, bilateral descended testes | Penile | 3 cm | Mild | Chr 20: 55840914 C>A | 0 | 0 | 276 | 48/52 | Exon 1c.G265T | p.A89S | 4.06 | Alanine changed to serine. Change in size hydrophobicity of the residue. Likely loss of hydrophobic interaction of the protein. |
| 2 | 46,XY | No | Fused | 2 mL, bilateral descended testes | Penile | 3.3 cm | Mild | 303 | 49/51 | |||||||||
| 2 | 1 | 2 y | 46,XY | No | Fused | 2 mL, bilateral descended testes | Proximal penile | 3 cm | Yes | Chr 20: 55777657 C>T | 6.06e-05 | 0 | 242 | 59/41 | Exon 3 c.G634A | p.D212N | 4.78 | Aspartic acid changed to asparagine. Loss of charge. Likely loss of interaction of the protein. |
| 2 | 46,XY | No | Bifid | 1–2 mL, right descended testis, 1 mL left descended testis | Penoscrotal | 3.5 cm | Mild | 261 | 68/32 | |||||||||
A. Twin Pair 1
Two monozygotic twins were diagnosed at four years of age with proximal hypospadias with chordee and bilateral descended testes. Twin 1 had a fused scrotum with a proximal penile urethral meatus; stretched penile length was 3 cm. Twin 2 had a fused scrotum with a penile meatus; stretched penile length was 3.3 cm. Neither baseline hormonal testing nor human chorionic gonadotropic stimulation test was performed.
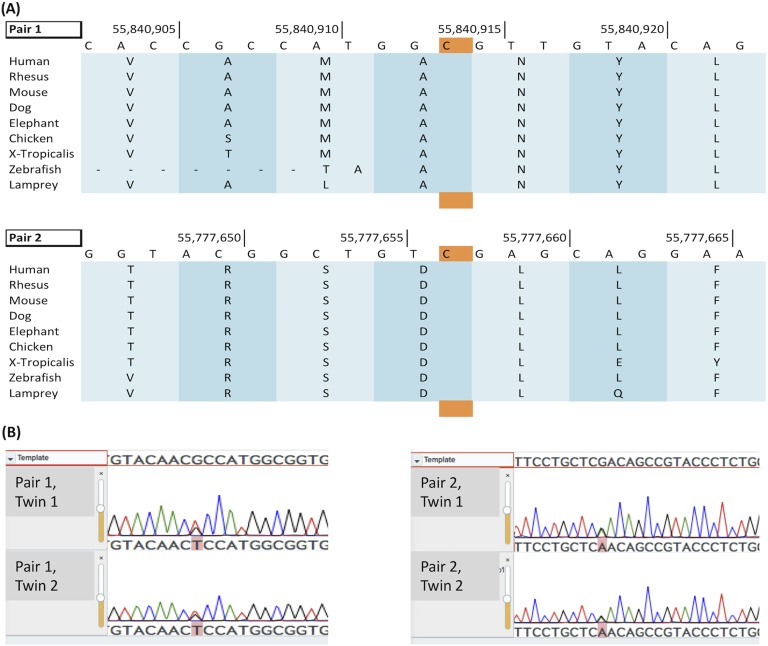
This pair of twins shared 27 rare variants (<1% in ESP6,500 and 1000 Genomes Project) in the 1037 genes targeted by our panel. Three of these genes carrying a variant (BMP7, STAR, and WDR11) have been implicated in DSD but neither STAR nor WDR11 alone are thought to cause hypospadias and BMP7 was the only of those three to be a candidate gene for isolated hypospadias. This variant in BMP7 (NM_001719:exon1:c.G265T:p.A89S) was predicted to be damaging in two out of the four programs. This variant has not been previously reported in online databases (ExAc, ESP6,500). In both cases, quality and depth of the reads were excellent. The twins were the only two patients to exhibit this variant in our whole database (278 additional 46,XY DSD patients, including 97 from Indonesia) and the variant was confirmed by Sanger sequencing (Fig. 3).
Figure 3.
Variants affect highly conserved amino-acids in the BMP7 prodomain. (A) Variants in both pairs of twins (nucleic acid highlighted in orange) affect amino acids highly conserved across multiple species. (B) Sanger sequencing confirmation for both variants. Pair 1: exon 1. c.G265T; p.A89S. Pair 2: exon 3 c.G634A; p.D212N.
The genetic variant results in an arginine to serine pronounced change at amino acid 89, thus affecting a highly conserved amino acid in the BMP7 prodomain (Fig. 3). It has a Genomic Evolutionary Rate Profiling (GERP) score of 4.06. The p.Arg89Ser (A89S) substitution lies within a fundamental region of the prodomain (α2-helix) predicted to contact the mature domain during ligand folding [15].
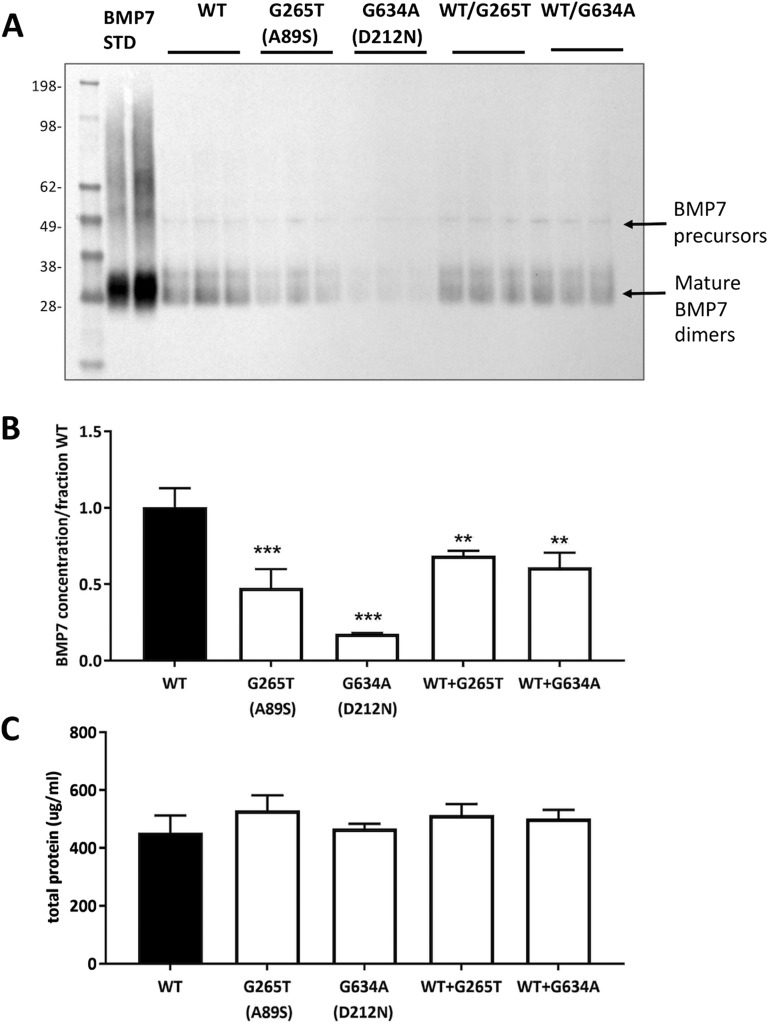
In vitro analysis was undertaken to determine the structural/functional impact of this variant on BMP7 production and activity. Western blot analysis on conditioned media from cells transfected with BMP7 variants revealed that the A89S variant decreased expression of the mature BMP7 dimers by ∼50%, relative to wild-type BMP7 (Fig. 4A and 4B). Additionally, it was found that BMP7 A89S expression could not be fully rescued by the presence of wild-type BMP7 (Fig. 4A and 4B). Moreover, this difference was not attributed to deviations in total protein concentration within these samples (Fig.4C).
Figure 4.
BMP7 variants affect protein expression. (A) Homozygous and heterozygous variants’ expression is compared by Western Blot to the wild-type and standard BMP7 (Std). (B) Densitometry results for the two variants, at both homozygous and heterozygous states. Twin pair 1: G265T (A89S). Twin pair 2: G634A (D212N). (C) Total protein concentrations as determined by a BCA assay.
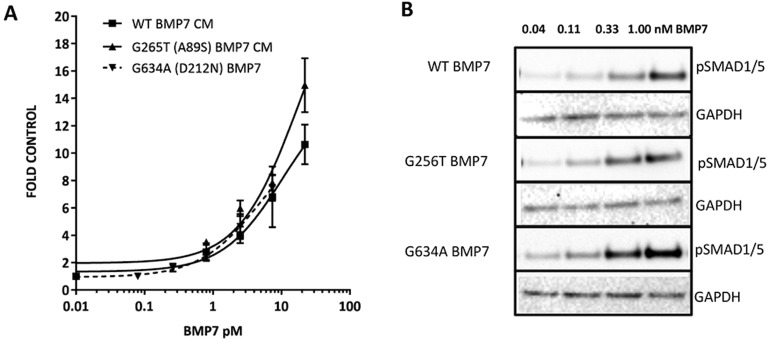
An assessment of the ability of the BMP7 A89S variant to induce a SMAD 1/5 luciferase response in HEK-293 cells revealed that this variant retained wild-type BMP7 activity (Fig. 5). In support of this, equal induction of pSMAD 1/5 was observed for the A89S BMP7 variant following Western blot analysis of endogenous pSMAD 1/5 (Fig. 5B). This analysis suggests that the A89S mutation in BMP7 likely limits the production of bioactive BMP7 in these patients.
Figure 5.
BMP7 bioactivity was not affected in patient variants. The ability of the BMP7 variants to induce pSMAD 1/5 activation was assessed by (A) a luciferase bioassay and (B) analysis of endogenous pSMAD 1/5 levels, both in COV434 cells.
B. Twin Pair 2
Two monozygotic twins were diagnosed at two years of age. Twin 1 had penile hypospadias with mild chordee and bilaterally descended testes into a fused scrotum; penile length was 3 cm. Twin 2 had penoscrotal hypospadias, with bifid scrotum; penile length was 3.5 cm. No baseline hormonal testing nor human chorionic gonadotropic stimulation test was performed. The twins shared 29 rare variants (<1% in ESP6,500 and 1000 Genomes Project) in the 1037 genes of our panel. Of those, two (CHD7 and BMP7) have been implicated in DSD or hypospadias. However, the variant in CHD7 is commonly reported in Southeast Asia in ExAC (3% of the population) and was predicted to be damaging in only two of the four pathogenicity-predicting tools. On the contrary, the variant in BMP7 (NM_001719:exon3:c.G634A:p.D212N) has been previously reported in ExAC with a frequency of 1.68 × 10−5 in the general population and 6.06*10−5 in the Southeast Asian population. It was not reported in ESP6,500. This change was predicted to be damaging in the four structural predictors. The variant was confirmed by Sanger sequencing (Fig. 3).
The identified c.G634A variant was also located in the BMP7 prodomain, at a highly conserved site with a GERP score of 4.78 (Fig. 3). The variant results in a pronounced change from an aspartic acid to asparagine at position 212, toward the C-terminus of the prodomain. The p.Asp212Asn (D212N) variant results in a change in charge within a predicted β-sheet [15], which may hinder intramolecular prodomain contacts and folding.
In vitro mutagenesis revealed that the D212N variant in BMP7 reduced cellular production of the mature BMP7 protein by as much as 75%. The production of BMP7 D212N was partially rescued in the presence of wild-type BMP7 (Fig. 4A and 4B). Again, the impact on BMP7 biosynthesis was not due to deviations in total protein content in the conditioned media (Fig. 4C). BMP7-mediated SMAD 1/5 luciferase activity (Fig. 5A) and endogenous activation of pSMAD 1/5 (Fig. 5B) appeared unhindered for the D212N mutant, suggesting that this variant retains the receptor binding capacity of wild-type BMP7. Rather, the identified D212N mutation appears to limit the biosynthesis of BMP7.
3. Discussion
Here we report two variants in BMP7 in two concordant pairs of monozygotic twins presenting with proximal hypospadias. Both variants are located in the prodomain of BMP7. Prodomains control many aspects of TGF-β superfamily biology and alterations in their function are often associated with disease [16]. BMP7 is no exception. Variants in this region have been reported to be associated with several human anomalies [11]. However this is the first report of variants in the prodomain associated with hypospadias or 46,XY DSD.
Mouse studies have conclusively shown that Bmp7 plays an important role in GT development and in hypospadias [17]. Additionally, in humans several mutations have been reported to be associated with hypospadias of different severity. However, all are located within the growth factor domain [12], and the exact function of the prodomain in this developmental processes and hypospadias is unknown. Two variants affecting just this region have been reported in the literature in association with human disorders [11]. One (c.513delA; p.A171Afs264X) was a frameshift mutation resulting in a premature stop codon in a male with bilateral anophthalmia and severe developmental difficulties. The other one was a missense mutation c.593T>C (p.L198P) present in a boy with unilateral microphthalmia, coloboma, and mild learning difficulties. In this latter case, the change from a hydrophobic leucine to a proline, the cyclic structure of which is known to disrupt secondary elements, was suspected to have structural consequences. Moreover, this change affects a highly conserved element of seven residues present in a number of BMPs. However, these patients were not reported to have any genital malformations.
Studies in animal models and in human cell lines have shown that the BMP7 prodomain plays an important role in the function of the BMP7 signaling molecule [18]. Gregory et al. [8] demonstrated that BMP7 is secreted as a highly stable complex that contains both the prodomain and the growth factor domain. They suggested that the prodomain may specifically target the growth factor complex to fibrillins in the extracellular matrix [8]. Moustakas and Heldin [19] demonstrated that the prodomain regulated the stability and processing of the mature ligand and therefore, Wyatt et al. [11] postulated that the L198P variant may result in lower levels of mature BMP7 protein. Both variants reported here decrease the expression of mature BMP7 dimers. Although they do not affect the protein activity, their reduced expression likely causes a lower concentration of active protein in the extracellular matrix, therefore compromising the role of BMP7 in the closure of the urethral plate in our patients. It may be that in vivo BMP7 function relies more heavily on interaction with the extracellular matrix compared with what we observed in our cell culture method. Therefore, although these variants show no decreased bioactivity in a simple cell culture assay, they may also have reduced function in more complex in vivo systems.
4. Conclusion
Using a targeted DSD screen, we identified two variants in the prodomain of BMP7 in two pairs of monozygotic concordant twins with proximal hypospadias. Analysis of the literature revealed that the prodomain of BMP7 is important for the extracellular targeting of the protein. Functional analysis demonstrated that the variant BMP7 proteins showed decreased expression, supporting the hypothesis that variants in this region could lead to hypospadias by restricting BMP7 bioavailability in the developing genital tubercle.
Acknowledgments
Financial Support: The study was part of a grant received by The National Health and Medical Research Council, Australia (Program grant number APP1074258) and Riset Unggulun PNBP Universitas Diponegoro Tahun 2015 (316-01/UN7.5.1/PG/2015).
Disclosure Summary: The authors have nothing to disclose.
Glossary
- Abrreviations: BMP7
bone morphogenetic protein 7
- DSD
disorder/difference of sex development
- GERP
Genomic Evolutionary Rate Profiling
- GT
genital tubercle
- SNP
single nucleotide protein
References and Notes
- 1. Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2012;84(3):261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes IA, Houk C, Ahmed SF, Lee PA; Lawson Wilkins Pediatric Endocrine Society/European Society for Paediatric Endocrinology Consensus Group.Consensus statement on management of intersex disorders. J Pediatr Urol. 2006;2(3):148–162. [DOI] [PubMed] [Google Scholar]
- 3. van der Zanden LFM, van Rooij IALM, Feitz WFJ, Franke B, Knoers NVAM, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18(3):260–283. [DOI] [PubMed] [Google Scholar]
- 4. Bouty A, Ayers KL, Pask A, Heloury Y, Sinclair AH. The genetic and environmental factors underlying hypospadias. Sex Dev. 2015;9(5):239–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suzuki K, Haraguchi R, Ogata T, Barbieri O, Alegria O, Vieux-Rochas M, Nakagata N, Ito M, Mills AA, Kurita T, Levi G, Yamada G. Abnormal urethra formation in mouse models of split-hand/split-foot malformation type 1 and type 4. Eur J Hum Genet. 2008;16(1):36–44. [DOI] [PubMed] [Google Scholar]
- 6. Wu X, Ferrara C, Shapiro E, Grishina I. Bmp7 expression and null phenotype in the urogenital system suggest a role in re-organization of the urethral epithelium. Gene Expr Patterns. 2009;9(4):224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swencki-Underwood B, Mills JK, Vennarini J, Boakye K, Luo J, Pomerantz S, Cunningham MR, Farrell FX, Naso MF, Amegadzie B. Expression and characterization of a human BMP-7 variant with improved biochemical properties. Protein Expr Purif. 2008;57(2):312–319. [DOI] [PubMed] [Google Scholar]
- 8. Gregory KE, Ono RN, Charbonneau NL, Kuo CL, Keene DR, Bächinger HP, Sakai LY. The prodomain of BMP-7 targets the BMP-7 complex to the extracellular matrix. J Biol Chem. 2005;280(30):27970–27980. [DOI] [PubMed] [Google Scholar]
- 9. Sengle G, Ono RN, Lyons KM, Bächinger HP, Sakai LY. A new model for growth factor activation: type II receptors compete with the prodomain for BMP-7. J Mol Biol. 2008;381(4):1025–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F. Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int. 2014;85(6):1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wyatt AW, Osborne RJ, Stewart H, Ragge NK. Bone morphogenetic protein 7 (BMP7) mutations are associated with variable ocular, brain, ear, palate, and skeletal anomalies. Hum Mutat. 2010;31(7):781–787. [DOI] [PubMed] [Google Scholar]
- 12. Chen T, Li Q, Xu J, Ding K, Wang Y, Wang W, Li S, Shen Y. Mutation screening of BMP4, BMP7, HOXA4 and HOXB6 genes in Chinese patients with hypospadias. Eur J Hum Genet. 2007;15(1):23–28. [DOI] [PubMed] [Google Scholar]
- 13. Eggers S, Sadedin S, van den Bergen JA, Robevska G, Ohnesorg T, Hewitt J, Lambeth L, Bouty A, Knarston IM, Tan TY, Cameron F, Werther G, Hutson J, O’Connell M, Grover SR, Heloury Y, Zacharin M, Bergman P, Kimber C, Brown J, Webb N, Hunter MF, Srinivasan S, Titmuss A, Verge CF, Mowat D, Smith G, Smith J, Ewans L, Shalhoub C, Crock P, Cowell C, Leong GM, Ono M, Lafferty AR, Huynh T, Visser U, Choong CS, McKenzie F, Pachter N, Thompson EM, Couper J, Baxendale A, Gecz J, Wheeler BJ, Jefferies C, MacKenzie K, Hofman P, Carter P, King RI, Krausz C, van Ravenswaaij-Arts CMA, Looijenga L, Drop S, Riedl S, Cools M, Dawson A, Juniarto AZ, Khadilkar V, Khadilkar A, Bhatia V, Dũng VC, Atta I, Raza J, Thi Diem Chi N, Hao TK, Harley V, Koopman P, Warne G, Faradz S, Oshlack A, Ayers KL, Sinclair AH. Disorders of sex development: insights from targeted gene sequencing of a large international patient cohort. Genome Biol. 2016;17(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patiño LC, Walton KL, Mueller TD, Johnson KE, Stocker W, Richani D, Agapiou D, Gilchrist RB, Laissue P, Harrison CA. BMP15 mutations associated with primary ovarian insufficiency reduce expression, activity, or synergy with gdf9. J Clin Endocrinol Metab. 2017;102(3):1009–1019. [DOI] [PubMed] [Google Scholar]
- 15. Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-β structure and activation. Nature. 2011;474(7351):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. 2011;29(5):174–186. [DOI] [PubMed] [Google Scholar]
- 17. Morgan EA, Nguyen SB, Scott V, Stadler HS. Loss of Bmp7 and Fgf8 signaling in Hoxa13-mutant mice causes hypospadia. Development. 2003;130(14):3095–3109. [DOI] [PubMed] [Google Scholar]
- 18. Sengle G, Charbonneau NL, Ono RN, Sasaki T, Alvarez J, Keene DR, Bächinger HP, Sakai LY. Targeting of bone morphogenetic protein growth factor complexes to fibrillin. J Biol Chem. 2008;283(20):13874–13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–3714. [DOI] [PubMed] [Google Scholar]