Abstract
Background:
Major Depressive Disorder (MDD) is characterized by aberrant resting-state functional connectivity (FC) in anterior cingulate regions (e.g., subgenual anterior cingulate [sgACC]) and by negative emotional functioning that is inflexible or resistant to change.
Methods:
MDD (N=33) and control (CTL; N=31) adults completed a resting-state scan, followed by a smartphone-based Experience Sampling Methodology (ESM) protocol surveying 10 positive and negative emotions 5 times per day for 21 days. We used multilevel modeling to assess moment-to-moment emotional inflexibility (i.e., strong temporal connections between emotions). We examined group differences in whole-brain FC analysis of bilateral sgACC, and then examined associations between emotional experiences and the extracted FC values within each group.
Results:
As predicted, MDDs had inflexibility in sadness and avoidance (p<.001, FDR-corrected p<.05), indicating that these emotional experiences persist in depression. MDDs showed weaker FC between the right sgACC and pregenual/dorsal anterior cingulate (pg/dACC) than did CTLs (FWE-corrected, voxelwise p=.01). Importantly, sgACC–pg/dACC FC predicted sadness inflexibility in both MDDs (p=.046) and CTLs (p=.033), suggesting that sgACC FC is associated with day-to-day negative emotions.
Limitations:
Other maladaptive behaviors likely also affect the flexibility of negative emotions. We cannot generalize our finding of a positive relation between sgACC FC and inflexibility of sadness to individuals with more chronic depression or who have recovered from depression.
Conclusions:
Our preliminary findings suggest that connections between portions of the ACC contribute to the persistence of negative emotions and are important in identifying a brain mechanism that may underlie the maintenance of sadness in daily life.
Keywords: MDD, resting-state functional connectivity, experience sampling methodology, subgenual anterior cingulate cortex, dorsal anterior cingulate cortex, emotion
Introduction
Major Depressive Disorder (MDD) has significant adverse personal and public health consequences; it is the leading contributor to the global burden of disease (Ferrari et al., 2013) and a primary cause of years of life lived with disability (Lopez et al., 2006). In identifying factors that might perpetuate depressive episodes, researchers have focused on the high levels of negative affect reported by depressed individuals. In this context, individuals with MDD have been characterized as being “stuck” in negative emotional cycles, perseverating on feelings and thoughts of sadness and engaging in avoidance behaviors that both perpetuate negative affect and adversely impact their daily functioning (Koval et al., 2012; Trew, 2011). Recently, investigators have used the Experience Sampling Method (ESM) to examine the nature of the day-to-day emotional difficulties experienced by depressed individuals (Csikszentmihalyi and Larson, 1987; Höhn et al., 2013; Wichers et al., 2010). Most laboratory assessments rely on reported symptoms, which can introduce recall bias. Recall biases, such as under- or over-reporting of symptoms, could contribute to ineffective treatment plans. Particularly in depression, individuals commonly experience remission and relapse, which can often be difficult to report. Under-reporting can lead to reductions in seeking treatment and lack of adherence to treatment (Wang et al., 2005). ESM allows investigators to gain a more comprehensive understanding of moment-to-moment experiences in individuals’ daily lives outside of the laboratory.
Researchers have used ESM to assess the temporal dynamics of emotion in depressed individuals, examining how emotions at one moment in time predict subsequent levels of emotion within the same day (Pe et al., 2015). For example, investigators have assessed the inflexibility of negative emotions by examining emotional inertia, or how an emotion at the current time point is predicted by the same emotion at the previous time point (i.e., the autocorrelation or persistence of a particular emotion) (Kuppens et al., 2010; Pe et al., 2015; Suls et al., 1998; Thompson et al., 2012). In contrast to examining inertia of a single emotion, other research focuses on connections among a wide range of emotions. Pe et al. (2015) took a “network density approach” to assess resistance to changing emotion in MDD, examining how a current emotion (e.g., sadness at time t) is predicted by all emotions (e.g., sadness, anxiety, hopefulness) at the previous time point (i.e., at time t-1). This approach yields a more comprehensive characterization depression than does examining the persistence of a single emotion. A dense network of emotions, operationalized as emotions that are strongly associated over time, can reflect inflexibility or a resistance to emotional change. Using multilevel modeling of both positive and negative emotions, Pe et al. (2015) found that depressed individuals have a denser overall emotion network and, more specifically, a denser network of negative, but not positive, emotions than do their nondepressed counterparts. Thus, dense negative emotional networks may underlie the pervasive negative emotional state that characterizes MDD. In contrast, less dense emotional networks might allow contextual events or internal regulatory strategies (Kuppens et al., 2010) to ameliorate negative emotional states; indeed, investigators have posited that more flexible emotional networks are adaptive (Kashdan and Rottenberg, 2010; Kuppens et al., 2010; Pe et al., 2015).
Investigators have also begun to examine neural foundations of emotional functioning in MDD, which can elucidate mechanisms involved in the maintenance of negative affect in this disorder. In a recent Bayesian computational model of depression, Smith et al., (2018) describe a feedback loop in which strong expectations for negative emotional experiences (e.g., negative view of the self or pessimism) facilitate neural and behavioral changes that, in turn, increase the probability of experiencing negative emotions, thereby maintaining depressive episodes. This feedback-loop model implicates the anterior cingulate cortex (ACC), a region of the brain involved in cognitive control, the experience of negative affect, and the resolution of emotional conflict (Etkin et al., 2006; Shackman et al., 2011). Smith et al. posit that negative self-referential biases lead to aberrant patterns of connectivity of the subgenual ACC (sgACC), a structure within the ventral portion of the ACC that is associated with negative self-referential processing, integrating physiological responses to external stimuli, and generating phenomenological experiences (Cooney et al., 2010; Dedovic et al., 2013; Kross et al., 2009; Price and Drevets, 2012). This abnormal sgACC connectivity, in turn, may engage dorsal regions of the ACC to increase the individual’s attention to negative information and perpetuate avoidant behaviors, such as social withdrawal and rejection (Masten et al., 2011; Smith et al., 2018; Smith and Lane, 2015).
It is unlikely that one brain region in isolation underlies these maladaptive behaviors; instead, the persistence of depression likely involves aberrant functioning of different brain regions. The hypothesized involvement of the sgACC in the inflexibility of negative emotions in depression underscores the importance of examining the functional connectivity (FC) of this brain region with other regions in the context of understanding the temporal dynamics of daily emotional functioning in MDD. Researchers focusing on the sgACC in MDD have assessed both task-based and resting-state patterns of this brain structure. Although patterns of FC during task-based and resting-state assessments are correlated (Fox and Raichle, 2007), assessing intrinsic FC during rest (i.e., unconstrained by a task) allows investigators to examine stable patterns of neural connectivity among brain regions that are related to complex behaviors (Fox and Greicius, 2010). Indeed, using a resting-state fMRI paradigm, researchers have consistently documented differences between depressed and nondepressed individuals in sgACC FC (Davey et al., 2012; Greicius et al., 2007; Mulders et al., 2015); however, the directionality of the association between depressive symptoms and strength of connectivity between the sgACC and other canonical emotional processing regions of the brain has been less consistent (Wang et al., 2012). For instance, Sheline et al., (2010) found greater connectivity between the sgACC and dorsal medial prefrontal cortex (PFC) in depressed than in nondepressed individuals. Importantly, aberrant FC between the sgACC and medial prefrontal regions has been found to be associated with self-generated sadness, negative self-referential processing, rumination, and impaired emotion regulation (Davey et al., 2012; Drevets et al., 2008; Hamilton et al., 2015, 2013). In contrast, Wu et al., (2016) found weaker FC of the sgACC with the posterior insula and middle and inferior temporal gyrus in depressed than in nondepressed individuals.
Although investigators have not examined the relation between intrinsic FC and daily functioning in currently depressed adults, they have assessed the association between resting-state FC and daily functioning in adults who had recovered from a depressive episode (Servaas et al., 2017) (see Forbes et al. 2010, 2009), for similar examples using task-based fMRI in depressed adolescents). Specifically, Servaas et al., (2017) found that greater fluctuations in ESM-assessed negative mood were associated with reduced FC between networks of brain regions involved in reward processing and attention, identified using graph theory.
The present study was designed to explore the relation between resting-state sgACC FC and the temporal dynamics of depression-related emotional functioning, assessed several times each day over three weeks using smartphone-based ESM, in currently depressed and never-depressed adults. Findings from this study may provide insight into biological factors that contribute to the persistence of depression as manifested in naturalistic settings. Given evidence of stronger associations among emotions in depressed than in nondepressed individuals (Pe et al., 2015), we hypothesized, first, that depressed individuals would exhibit stronger temporal connections in their overall emotional experience than would nondepressed participants, reflecting a greater emotional inflexibility, or resistance to change. Second, we hypothesized that MDD participants would have stronger temporal connections among negative emotions, reflecting the persistence of these emotions in depression. Finally, given the posited role of the sgACC in MDD (Hamilton et al., 2015, 2013), we predicted that sgACC FC would be associated with the emotions that exhibited stronger temporal connections in MDDs than in CTLs.
Method
Participants and procedures
Forty-one individuals with current MDD (n=26 female) and 41 CTL (n=23 female) individuals ages 18–35 years were recruited from the community to participate in this study. Trained interviewers administered the Structured Clinical Interview for DSM-IV-TR (SCID) (First et al., 1996) to establish a diagnosis of MDD for the depressed participants and to ensure that the CTL participants did not meet diagnostic criteria for any current or past DSM-IV-TR Axis-I disorder. Potential participants were excluded if they had a history of psychosis, substance/alcohol abuse within the past six months, had impaired mental status, had history of traumatic brain injury, or were taking medication that influenced blood flow. Individuals with MDD were not excluded on the basis of comorbid anxiety (n=14) or use of psychotropic medications (n=10). All participants were scheduled for a subsequent neuroimaging scan session conducted within 2 weeks of the administration of the SCID. This study was approved by the Stanford University Institutional Review Board and all participants provided informed consent.
Measures
At the scan session, participants completed the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996) and the Beck Anxiety Inventory (BAI) (Beck et al., 1988). The BDI-II is a 21-item self-report measure of the severity of depressive symptoms over the last two weeks; it is reliable and has high construct validity (Steer et al., 1997). The BAI is a reliable and valid 21-item self-report measure of the severity of anxious symptoms over the last two weeks (Beck et al., 1988). After completing these self-report measures, individuals underwent an MRI scan session to acquire structural and functional MRI data.
Following the MRI scan, participants were asked to complete a 21-day ESM protocol in which they were prompted with questions about their current emotions and behaviors on their smartphone several times a day. Nine (n=4 MDD and n=5 CTL) participants elected not to proceed with the ESM protocol, leaving 37 MDD and 36 CTL participants. Participants were offered monetary incentives for completing each day of prompts and received a bonus if they completed 90% of all prompts. Participants used the MetricWire application (MetricWire, Inc) on their phone to respond to 18 questions 5 times per day (9am, 12pm, 3pm, 6pm, 9pm), yielding 105 prompts total per participant. Ten questions were directly related to depression symptoms and asked about participants’ feelings and thoughts (e.g., “How sad do you feel?” “How nervous or anxious do you feel?” “How much are you avoiding people, places, or activities?”). These ten items assess features of depression that are directly relevant to emotional states; thus, we refer to these items collectively as ‘emotional experience’. Full text of all prompts is presented in the Appendix. Participants responded to each question on a 7-point Likert rating scale (0=“not at all,” 6=“very much”). Of the other eight questions given at each prompt, one was binary and asked about social context, one was associated with appetite and thus influenced by additional factors, such as time of day (e.g., timing of meals), and six asked about positive and negative life events that may have occurred since the last prompt; these items had a low frequency of responses. Because these items were not central to our research question about temporal dynamics of emotional experiences, they were excluded from further analysis.
fMRI data acquisition and preprocessing
MRI scans were conducted on a GE Discovery MR750 scanner (GE Medical Systems, Milwaukee, WI) equipped with a 32-channel head coil (Nova Medical). We collected spoiled gradient echo (SPGR) T1-weighted sagittal anatomical images (repetition time [TR]= 7.24 ms, echo time [TE]= 2.78 ms, flip angle=12°, FOV=232×232mm, matrix=256×256, voxel size=.90mm3, scan time=4:50) to be used for alignment and registration of functional images and for segmenting tissue types for facilitating resting-state fMRI preprocessing. Resting-state BOLD fMRI data were acquired using T2*-weighted oblique slices aligned to the anterior and posterior commissure (repetition time [TR]= 2.0 s, echo time [TE]=30 ms, flip angle=77°, 200 volumes, FOV=232×232mm, matrix=80×80, voxel size=2.9mm3, total scan time=6:40). During the resting-state scan, participants were instructed to “close your eyes and relax, but try not to fall asleep.” Physiological signal was collected via a photoplethysmograph attached to the right hallux. Higher-order shims were applied prior to our resting-state scans, which has been shown to reduce geometric distortions (Kim et al., 2002). Structural and functional data were visually inspected to ensure data integrity was not compromised by ghosting, magnetic field inhomogeneities, and scanner spiking. Two MDD participants and five CTL participants did not have usable scan data (mostly related to scanner acquisition issues), and two MDD participants were excluded for having no signal recorded, as we were therefore unable to adequately account for physiological noise in the preprocessing pipeline. Thus, we report all results from a final sample of 64 participants (33 MDD and 31 CTL). See Supplementary Tables 1–3 for clinical and demographic characteristics of the final sample.
Data were preprocessed using conservative motion correction and regression of physiological noise based on tools from Freesurfer (Fischl et al., 2004), FSL (Smith et al., 2004), and AFNI (Cox, 1996) and according to well-validated protocols (Ordaz et al., 2017). See Supplemental Information for details on preprocessing.
Analytic Plan
Demographic Characteristics
We used two-tailed t-tests and χ2 tests to test differences between the MDD and CTL groups with respect to age, severity of depressive and anxious symptoms reported on the BDI-II and BAI, and sex distribution. We also examined whether the two groups differed in ESM response rate (see Table 1).
Table 1.
Characteristics of MDD and CTL Groups
| Group |
|||
|---|---|---|---|
| Variable | MDD (N=33) | CTL (N=31) | Group Comparison |
| BDI-II (M,SD) | (27.00,10.33) | (1.39,2.78) | t(62)=13.35, p<.001 |
| BAI (M,SD) | (35.39,12.33) | (25.26,7.77) | t(62)=3.91, p<.001 |
| Sex (# F) | 23 | 18 | χ2(1)=.94, p=.33 |
| Age (M,SD) | (26.01,3.71) | (25.18,4.16) | t(62)=0.84, p=.41 |
| ESM response rate (%) | 78.42 | 80.61 | t(62)=−0.55, p=.59 |
Note. BDI-II = Beck Depression Inventory-II; BAI = Beck Anxiety Inventory; CTL = nondepressed control group with no past or current psychiatric disorder; MDD = Major Depressive Disorder; ESM = Experience Sampling Methodology
ESM: Emotional Inflexibility
All ESM data preparation and analyses were conducted using R (version 1.1.383) (R Core Team, 2014) and hierarchical linear modeling (HLM 7) (Raudenbush, S.W., Bryk, A.S., Congdon, 2011). To prepare the ESM data for HLM modeling, we first created lagged (t −1) variables in R using tidyverse packages (Wickham, 2016) for each ESM item. To calculate an overall emotional inflexibility, we estimated the average temporal strength in connection among the ten emotional experience ESM items (Bringmann et al., 2013; Pe et al., 2015). Based on methods by Pe et al., (2015), we used HLM to conduct multilevel analyses with each of the ten items at time t predicted by all items, including the target, at t-1, where t-1 and t are consecutive prompts. Multilevel modeling is well suited for a nested data structure (prompts nested within participants) and is also appropriate when accounting for missing data (Snijders and Bosker, 1999). By predicting each item with all ESM items using this time-lagged model, we can examine how well each emotional experience at the previous time point explains the current emotional experience. All Level 1 predictors were group-centered, in which predictors are centered around the group (in this case, participant) mean. We did not include a Level 2 variable because diagnostic group effects were planned to be estimated in R outside of HLM. Therefore, random intercepts and slopes at Level 2 across the whole sample come from each emotion regression model. For example:
Level-1 Model: Sadnessti = β0j + β1j(Sadnesst-1)+ β2j(Interestt-1) + β3j(Avoidt-1) … + etj Each slope indicates the strength of the temporal connection between the current emotion and each emotion at the previous time point, including the same emotion at the previous time point.
Second, using R, we extracted each participant’s slope for each item. We then averaged the absolute value of the slopes for each item being predicted to obtain a measure of inflexibility for each emotion, and then averaged these ten slopes to yield a measure of overall emotional inflexibility for each participant. We conducted independent-sample t-tests comparing MDDs and CTLs on the inflexibility of each emotion and overall emotional inflexibility (i.e., the mean of the ten item-specific slopes).
Resting-State Functional Connectivity
To examine group differences in sgACC FC, we first defined bilateral seed regions with 3mm radius (MNI RAI coordinates: x=+5, y=−25, z=−10; k=19, 463 mm3) based on previous resting-state FC mappings of the sgACC (Margulies et al., 2007). See Figures S1, S2 and Supplemental Information for visualization of bilateral sgACC. We performed a series of steps to constrain our whole-brain search. First, we conducted a regression analysis with the full sample (with AFNI’s 3dttest++). To identify a cluster-size threshold, we computed noise from our data (within a gray matter mask so as to eliminate spurious white matter signal) by applying 3dClustSim with the Autocorrelation Function (ACF) estimates from 3dFWHMx. 3dClustSim uses a FWE correction to control Type 1 error rate, which is the most updated threshold approach (Cox et al., 2017). We set a voxel-wise and cluster-wise threshold at p=.05, which yielded a cluster-size threshold of 2669 voxels. Next, we constrained our analyses within gray matter voxels that had significant FC with sgACC. Finally, we compared sgACC FC between MDDs and CTLs using a voxelwise t-test (AFNI’s 3dttest++) within the aforementioned mask (i.e., gray matter voxels that had significant FC with sgACC). To identify a cluster-size threshold, we used 3dClustSim with the ACF estimates from 3dFWHMx. We set a voxel-wise threshold at p=.01 and the probability of identifying a significant cluster at p=.05, which yielded a cluster-size threshold of 317 voxels. We used this p=.01 threshold instead of the more conservative p=.001 voxel-wise threshold to detect significant group differences in FC in order to examine relations between FC and temporal patterns of mood outside of the lab in depressed adults.
Association between Functional Connectivity and Emotional Inflexibility
Within each diagnostic group, we used linear regression modeling in R to add the Fisher’s z-transformed connectivity correlation coefficients from the group difference test as a predictor of the emotions of interest. Age (linear and quadratic terms), sex, and BAI were not significantly related to the emotion items that showed significant group differences in inflexibility or to sgACC connectivity; nevertheless, we followed formal model-fitting procedures in our linear regressions in order to test for the use of covariates of age, sex, and BAI. We began with a model with only the FC coefficient as our predictor of interest. We compared this model with each model that included the covariates noted above; however, including these covariates did not improve model fit. There were also no significant associations between medication use and the emotional items that showed significant group differences in inflexibility within the MDD group (dummy coded) or between medication use and FC within the MDD group.
Results
Demographic Characteristics
As shown in Table 1, the MDD and CTL groups did not differ in age, sex composition, or number of missed ESM prompts; in fact, both groups had overall high response rates (~80%). As expected, the MDD participants obtained significantly higher scores than did the CTL participants on both the BDI-II (Beck et al., 1996) and the BAI (Beck et al., 1988). The mean BDI-II score of the MDD group was in the moderate to severe range, and the mean BDI-II score of the CTL group was well below the cutoff score of 9 for mild depression. Similarly, the mean BAI score of the MDD participants was in the moderate to severe range, and the mean BAI score of the CTLs was in the mild to moderate range.
ESM: Emotional Inflexibility
Consistent with prior literature and our hypothesis, the MDD and CTL groups differed in their inflexibility of emotions. MDDs had significantly stronger connections among their overall emotional experience than did CTLs, t(62)=2.52, p=.014, indicating that MDDs exhibited greater inflexibility in their overall emotional experience. Specifically, compared with CTLs, MDDs had stronger temporal connections among emotions predicting sadness (t(62)=3.68, p<.001) and avoidance (t(62)=4.29, p<.001). For depressed individuals, both low ratings of positive items and high ratings of negative items were strongly associated with sadness, and high ratings of negative items were strongly associated with avoidance. However, for CTLs, only high ratings of negative items were associated with sadness, and only high ratings of previous avoidance were associated with current avoidance. See Table 2 for emotions related to inflexibility of sadness and avoidance for each group. The MDD and CTL groups did not differ significantly on any other specific emotion. See Table 3 (includes uncorrected and FDR corrected p values) and Figure 1 for group comparisons of each emotion.
Table 2.
Emotions Temporally Connected to Sadness and Avoidance in MDDs and CTLs
| Group | |||
|---|---|---|---|
| Current Emotion | Emotion at t-1 | MDD (N=33) | CTL (N=31) |
| Sadness | Sadness | b=0.13, SE=0.04, p=.003 | b=0.22, SE=0.05, p<.001 |
| Interest | b=−0.08, SE=0.03, p=.009 | b=−0.04, SE=0.02, p=.102 | |
| Fatigue | b=0.00, SE=0.03, p=.864 | b=−0.00, SE=0.02, p=.903 | |
| Self-Esteem | b=−0.01, SE=0.04, p=.869 | b=0.03, SE=0.03, p=.312 | |
| Difficulty in Concentration | b=0.03, SE=0.03, p=.300 | b=−0.01, SE=0.02, p=.473 | |
| Hope | b=−0.16, SE=0.04, p=.001 | b=−0.02, SE=0.03, p=.556 | |
| Anxiety | b=0.07, SE=0.04, p=.067 | b=−0.03, SE=0.03, p=.205 | |
| Worry | b=0.03, SE=0.03, p=.206 | b=0.05, SE=0.03, p=.119 | |
| Rumination | b=0.01, SE=0.04, p=.829 | b=0.04, SE=0.02, p=.028 | |
| Avoidance | b=0.06, SE=0.02, p=.005 | b=−0.01, SE=0.04,p=.745 | |
| Avoidance | Sadness | b=0.09, SE=0.03, p=.017 | b=0.00, SE=0.03, p=.912 |
| Interest | b=0.00, SE=0.03, p=.881 | b=−0.02, SE=0.03, p=.290 | |
| Fatigue | b=−0.05, SE=0.03, p=.086 | b=0.02, SE=0.02, p=.268 | |
| Self-Esteem | b=−0.02, SE=0.03, p=.567 | b=−0.01, SE=0.02, p=.638 | |
| Difficulty in Concentration | b=0.08, SE=0.03, p=.006 | b=0.03, SE=0.02, p=.135 | |
| Hope | b=0.03, SE=0.03, p=.392 | b=−0.02, SE=0.04, p=.513 | |
| Anxiety | b=0.02, SE=0.03, p=.442 | b=−0.01, SE=0.03, p=.688 | |
| Worry | b=0.09, SE=0.03, p=.007 | b=0.07, SE=0.04, p=.105 | |
| Rumination | b=0.01, SE=0.03, p=.778 | b=0.00, SE=0.03, p=.892 | |
| Avoidance | b=0.17, SE=0.03, p<.001 | b=0.21, SE=0.04, p<.001 | |
Note. Table of slopes for lagged ESM items associated with current sadness and current avoidance. Slopes are from the full models for sadness and avoidance.
Values in bold indicated significant association between Emotion at t-1 and Current Emotion.
MDD = Major Depressive Disorder; CTL = nondepressed control
Table 3.
Emotional Inflexibility by Diagnostic Group
| Group | Group Comparison | |||||
|---|---|---|---|---|---|---|
| Emotion | MDD (N=33) | CTL (N=31) | t(62) | p | Effect size (Cohen’s d) | p (FDR corrected) |
| Overall Emotional Experience | 0.08 (0.02) | 0.07 (0.01) | 2.52 | 0.01 | 0.63 | 0.05 |
| Sadness | 0.09(0.03) | 0.06 (0.03) | 3.68 | < .001 | 0.92 | < .05 |
| Interest | 0.08 (0.02) | 0.08 (0.02) | ‒1.14 | 0.26 | 0.29 | 0.41 |
| Fatigue | 0.08 (0.02) | 0.08 (0.02) | ‒0.76 | 0.45 | 0.19 | 0.55 |
| Self-Esteem | 0.06 (0.02) | 0.06 (0.01) | 0.55 | 0.59 | 0.14 | 0.59 |
| Difficulty in Concentration | 0.08 (0.03) | 0.06 (0.02) | 1.91 | 0.06 | 0.48 | 0.17 |
| Hope | 0.07 (0.03) | 0.06 (0.02) | 0.99 | 0.33 | 0.25 | 0.45 |
| Anxiety | 0.08 (0.03) | 0.06 (0.03) | 1.60 | 0.11 | 0.40 | 0.25 |
| Worry | 0.08 (0.03) | 0.07 (0.03) | 1.47 | 0.15 | 0.37 | 0.27 |
| Rumination | 0.08 (0.03) | 0.07 (0.02) | 0.61 | 0.54 | 0.15 | 0.59 |
| Avoidance | 0.08 (0.03) | 0.05 (0.02) | 4.29 | < .001 | 1.07 | < .05 |
Note. Table of means for each emotion.
Standard deviations are shown in parentheses.
MDD = Major Depressive Disorder; CTL = nondepressed control
Figure 1. Emotion-Inflexibility of MDDs and CTLs.
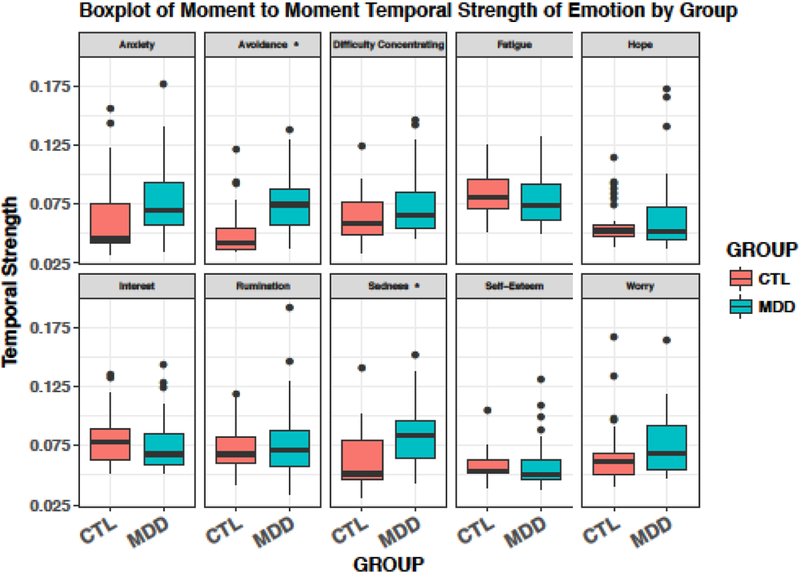
* indicates a significant group difference (p <.001).
Resting-State Functional Connectivity
Seed-based FC analyses indicated weaker connectivity in MDDs than in CTLs between the right sgACC and a right cingulate cluster, encompassing rostral ACC (pregenual ACC subregion; pgACC) and dorsal ACC (dACC) regions (MNI peak RAI coordinates: x=−4, y=−40, z=−2; k=345, 8414 mm3) (see Figure 2A,B). There were no MDD-associated differences in left sgACC FC that reached our defined voxelwise and cluster thresholds.
Figure 2A,B.
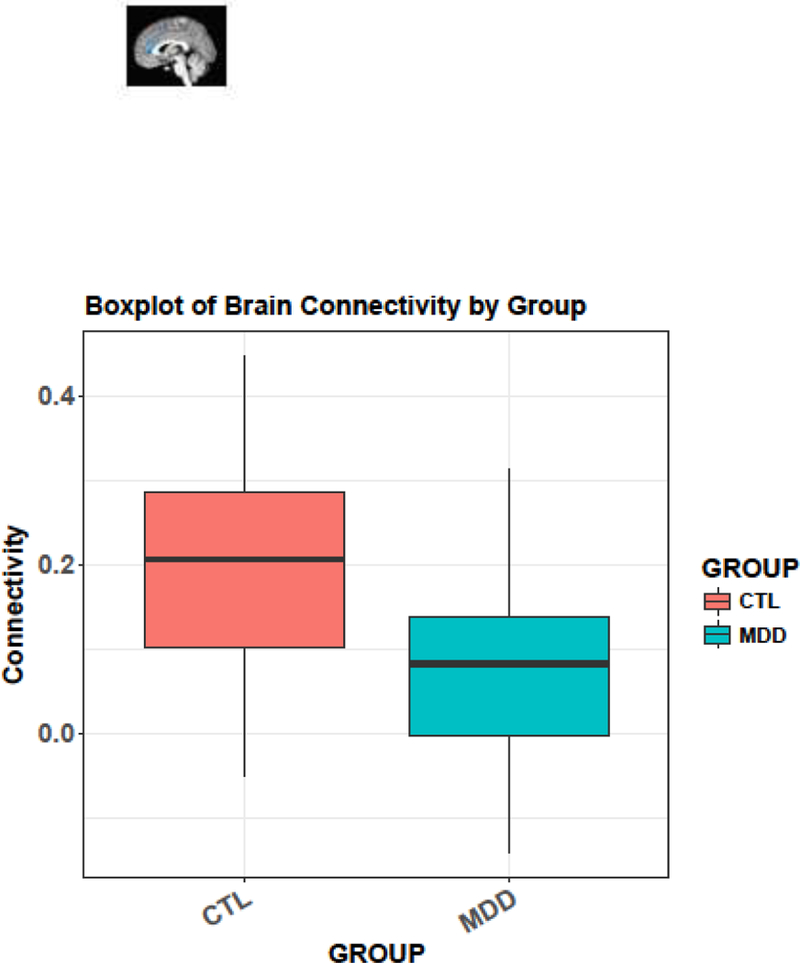
(A) The right sgACC seed (RAI coordinates: x=−5.0, y=−25, z=−10; k=19, 463 mm3) and right cingulate cluster showing reduced connectivity in pregenual anterior cingulate cortex extending to the dorsal anterior cingulate cortex (RAI coordinates: x=−4, y=−40, z=−2; k=345, 8414 mm3) in MDDs compared to CTLs (voxel-wise p=.01). (B) A boxplot showing reduced sgACC connectivity in MDDs compared to CTLs.
Association between Functional Connectivity and Emotional Inflexibility
Given significant group differences in the connections between previous emotions and current sadness and avoidance, we examined the association between right sgACC–right pg/dACC FC and inflexibility of sadness and avoidance. Linear regression analyses yielded a positive association between right sgACC–right pg/dACC FC with sadness inflexibility within both the MDD (β=.35, t(31)=2.08, p=.046) and the CTL (β=.38, t(29)=2.24, p=.033) groups (see Figure 3). To rule out the possibility that sgACC–right pg/dACC FC was not also explained by group differences between overall levels of daily sadness, we covaried for mean levels of sadness within each group. sgACC FC explained inflexibility in sadness above and beyond mean levels of sadness within depressed individuals (β=.35, t(30)=2.09, p=.045); however, the effect of sgACC FC on inflexibility of sadness diminished when covarying for mean level of sadness in CTLs (β=.26, t(28)=1.67, p=.106). FC was not associated significantly with avoidance inflexibility within either group.
Figure 3.
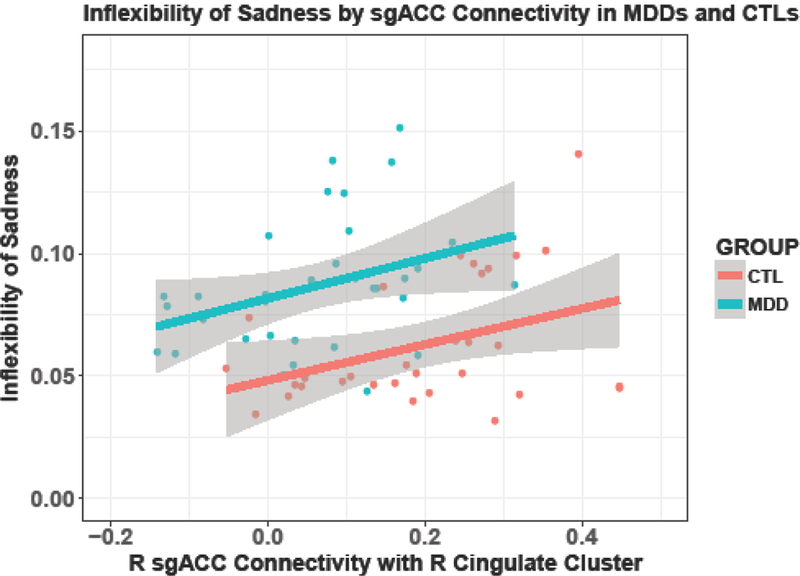
Increased sgACC connectivity associated with increased inflexibility of sadness. Significant association between R sgACC–R pg/dACC connectivity and inflexibility of sadness in MDDs and CTLs, p<.05.
While cross-validation (CV) studies with larger sample sizes and a range of psychiatric diagnoses are needed to replicate our finding that right sgACC–right pg/dACC FC is associated with inflexibility of sadness, we conducted CV and bootstrapping analyses within our sample. Specifically, we used a leave-one-out-cross-validation (LOOCV) procedure, which uses all participants except one (n-1) as the training data, and the remaining sample (one data point) as the test data, repeated n times. The goal is to yield an overall model prediction error, which is an average of the mean squared errors (MSE) for each iteration. We conducted LOOCV using the caret package in R (Kuhn, 2018, 2008) for each diagnostic group separately in order to yield estimates of variance explained, error estimates, and beta coefficients. These analyses indicated that sgACC FC alone explains inflexibility in sadness better in depressed individuals than in CTLs, and that adding mean sadness improves performance for CTLs, but not for depressed individuals (see Supplementary Table 4).
Other CV methods, such as split-half and k-fold CV, may be better for minimizing prediction errors; however, we chose to conduct LOOCV due to the relatively small sample size (~ 30 in each group). Although LOOCV minimizes bias, it also increases the variance due to highly similar training samples. Thus, we also performed bootstrapping with 5000 replicates using the boot function in R (Canty and Ripley, 2017; Davison and Hinkley, 1997) to estimate bias corrected statistics. The bootstrapped estimates confirm that sgACC FC in the depressed sample better explains inflexibility of daily sadness than it does in the sample of nondepressed CTLs. Mean sadness explains inflexibility of sadness in nondepressed CTLs above and beyond sgACC FC, whereas sgACC FC explains more variance in inflexibility of sadness than mean sadness in MDDs (See Supplementary Table 5 and Figure S3).
Discussion
Over the past several years, researchers have examined the maladaptive daily emotional functioning of depressed individuals. Investigators have now also begun to elucidate specific patterns of brain activation associated with this disorder. At this point, however, we know little about the nature of the association between intrinsic FC and the daily emotional experiences of depressed adults. The present study was designed to address this gap in our knowledge by exploring patterns of resting-state connectivity that predict specific temporal patterns of the daily emotional functioning of depressed adults.
Consistent with previous work (Pe et al., 2015), we found that MDDs had a significantly greater inflexibility of their overall emotion experience than did CTLs, providing additional evidence of emotional inflexibility in this disorder. Specifically, compared with CTLs, MDDs had stronger temporal connections between current experiences of sadness and avoidance and immediately preceding emotions. In particular, sadness and avoidance appear to be perpetuated by both low positive and high negative emotions in only the group of depressed individuals; the MDD and CTL groups did not differ in the flexibility of any other specific emotion.
We examined whether these group differences in emotional inflexibility were associated with MDD-related differences in patterns of FC of the sgACC. We found that, compared with CTLs, MDDs had weaker connectivity between the right sgACC and a right cingulate cluster (with peak coordinates at the pgACC extending to the dACC region). FC between the sgACC and pregenual and dorsal portions of the ACC is consistent with an affective neural network involved in emotional processing (Bush et al., 2000; Schlösser et al., 2008). Importantly, other investigators examining neural connectivity in MDD have also reported different patterns of connectivity of the dACC in depressed compared to nondepressed individuals (Crowther et al., 2015; Ho et al., 2017). The dACC is a key structure within the salience network (SN) (Uddin, 2015), which is involved in emotional awareness and attention (Hamilton et al., 2012; Menon, 2011). In the context of the feedback-loop computational model proposed by Smith et al., (2018), it is possible that prior experiences of low positive emotions and high negative emotions lead to abnormal activity of the sgACC, which in turn facilitates further negative, self-referential processing. This negative self-bias may lead the dACC to guide individuals’ attention to negative information and perpetuate sadness. The coupling of negative-self bias and disengagement with one’s environment is consistent with findings of aberrant FC patterns of the SN in MDD (Kaiser et al., 2015). Our finding that FC between the right sgACC and right pg/dACC is positively associated with temporal connections of emotions predicting sadness in both MDDs and CTLs may reflect a broad brain mechanism that drives the inflexibility of sadness in both depressed and nondepressed individuals. Depressed individuals, however, experience a significantly more severe inflexibility of sadness than do their nondepressed counterparts, as indicated by the tight temporal connection with prior high ratings of negative emotions and low ratings of positive emotions.
Consistent with our finding of sgACC–pg/dACC FC, researchers have implicated connectivity between the sgACC and other emotional processing regions of the brain in depression; however, the directionality of connectivity has been inconsistent (Veer, 2010; Wang et al., 2012). In the present study we found weaker resting-state sgACC FC in MDDs than in CTLs. This finding is consistent with recent research showing reduced FC of the sgACC in MDDs compared to CTLs (Wu et al., 2016). Other investigators, however, have reported stronger sgACC connectivity in depressed than in nondepressed adults. For example, Zhou et al., (2010) found greater FC between sgACC and the posterior cingulate cortex and precuneus, and Greicius et al., (2007) reported greater sgACC FC to the rest of the default mode network, which is composed of co-activated regions involved in ruminative, negative self-referential processes. Most studies in this area have found greater sgACC FC in depressed than in nondepressed individuals; however, given the heterogeneity of depression, it is critical that we conduct further research in order to elucidate what factors are contributing to findings of depression-associated differences in FC.
It is also important to note that investigators have documented associations between increased sgACC connectivity and clinical characteristics of depression. For example, greater sgACC FC with the dorsomedial frontal cortex has been associated with depression severity (Davey et al., 2012). These studies (e.g., Davey et al., 2012; Zhou et al., 2010) documenting that stronger negative emotional experiences and depressive characteristics are associated with greater sgACC FC are consistent with our finding that greater sgACC FC is associated with stronger inflexibility of sadness. Importantly, however, the opposing directionality of reduced resting-state FC in MDDs is still unclear. Increased sgACC–pg/dACC FC could reflect a brain-based mechanism that underlies this specific temporal dynamic of sadness in depressed and nondepressed individuals. In attempting to understand why nondepressed individuals do not present with the same persistence of sadness as depressed individuals, it may be the case that other functional connections in brain are regulating the perpetuation of sadness in people who are not depressed. If a high level of sadness is maintained by sgACC connectivity, we may not see behavioral inflexibility in nondepressed individuals because they do not have a sufficiently high level of sadness to be perpetuated. In fact, we found that whereas FC was related to emotional inflexibility above and beyond mean levels of daily sadness in MDDs, this was not the case in CTLs, for whom mean levels of sadness were correlated with their emotional inflexibility. Although these findings are preliminary and require replication, they suggest that the strength of sgACC FC with other anterior and dorsal cingulate regions can explain depressed individuals’ resistance to emotional change. Given the heterogeneity of MDD, it is important that this preliminary evidence of an association between sgACC FC and persistence of sadness be examined in a larger sample of depressed individuals who have experienced a more chronic course of disorder in order to characterize more precisely the nature of the association between sgACC FC and inflexibility of sadness in MDD. It will also be important in future studies to relate finer-grained time courses of negative emotion in daily life to more temporally-sensitive approaches, such as magnetoencephalography or electroencephalography, in order to increase our knowledge of the neural mechanisms underlying specific time courses of emotion.
We should note two limitations of this study. First, it is likely that maladaptive behaviors not assessed in this study also affect the flexibility or maintenance of negative emotions. For example, although sleep problems are transdiagnostic, insomnia in particular is commonly documented in depression (Tsuno et al., 2005). It would be beneficial to understand how poor sleep quality and other maladaptive behaviors affect the temporal dynamics of emotion, particularly in the context of MDD. Second, given the comparison between currently depressed individuals and never-depressed CTLs in this study, we cannot generalize our finding of the relation between greater sgACC FC and a higher inflexibility of sadness to individuals who have recovered from depression. Given the finding that remitted depressed individuals have greater fluctuations of negative emotions than do nondepressed CTLs (Servaas et al., 2017), it will be important to examine whether the same brain mechanism identified in this study as being associated with a more stable pattern of negative emotion is observable following recovery from MDD.
Despite these limitations, the present study is important in being the first to demonstrate the link between resting-state FC and temporal connections among emotions experienced in daily life in depressed adults. We were able to identify a possible neural pathway that appears to be related to inflexibility of sadness in depressed and nondepressed individuals, suggesting that this brain mechanism is broadly related to this temporal pattern in sadness. Future research should examine factors that exacerbate, or increase, the inflexibility of sadness in depressed individuals. In this context, it is important to extend this research by augmenting ESM with the collection of passive data (e.g., mobility, sleep) that are difficult to obtain in the lab; such approaches would increase our understanding of other factors that contribute to maladaptive functioning in MDD. It will be particularly useful if the depression-associated anomalies in FC documented in this study are found to track with symptom course and treatment responses in individuals with MDD.
Supplementary Material
Highlights.
Resting-state functional connectivity (FC) predicts temporal patterns of emotion
Depressed individuals experience more emotional inflexibility than healthy controls
Depressed persons have weaker subgenual anterior cingulate cortex FC than controls
subgenual–pregenual/dorsal anterior cingulate FC predicts inflexibility of sadness
Acknowledgements
We thank Sophie Schouboe, Monica Ellwood, Carly Leininger, Lucinda Sisk, and Anna Cichocki for their help in running participants. We also thank the participants for their time in this research.
Role of Funding Source
This research was supported by NIMH grants R21-MH102696 and R37-MH101495 to IHG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
Declarations of interest: none
Additional Information
Supplementary Information accompanies this paper.
References
- Beck AT, Epstein N, Brown G, Steer RA, 1988. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol 56, 893–897. 10.1037/0022-006X.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck depression inventory-II San Antonio, TX: Psychol. Corp; 1–82. [Google Scholar]
- Bringmann LF, Vissers N, Wichers M, Geschwind N, Kuppens P, Peeters F, Borsboom D, Tuerlinckx F, 2013. A Network Approach to Psychopathology: New Insights into Clinical Longitudinal Data. PLoS One 8 10.1371/journal.pone.0060188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI, 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends.Cogn Sci 4, 215–222. 10.1016/S1364-6613(00)01483-2 [DOI] [PubMed] [Google Scholar]
- Canty A, Ripley BD, 2017. boot: Bootstrap R (S-Plus) Functions
- Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH, 2010. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci 10, 470–478. 10.3758/CABN.10.4.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res 29, 162–73. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. FMRI Clustering in AFNI: False-Positive Rates Redux. Brain Connect 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed]
- Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C, Bizzell J, Schiller CE, Sideris J, Carl H, Dichter GS, 2015. Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology 40, 1659–1663. 10.1038/npp.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, 1987. Validity and Reliability of the Experience-Sampling Method. J. Nerv. Ment. Dis 175, 526–536. 10.1017/CBO9780511663246 [DOI] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yü Cel M, Allen NB, 2012. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol. Med 42, 2071–2081. 10.1017/S0033291712000323 [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV, 1997. Bootstrap Methods and Their Applications Cambridge University Press. [Google Scholar]
- Dedovic K, Duchesne A, Engert V, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC, 2013. Psychological, endocrine and neural responses to social evaluation in subclinical depression. Soc. Cogn. Affect. Neurosci 9, 1632–1644. 10.1093/scan/nst151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M, 2008. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectr 13, 663–681. 10.1038/nature08494.Finding [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J, 2006. Resolving Emotional Conflict: A Role for the Rostral Anterior Cingulate Cortex in Modulating Activity in the Amygdala. Neuron 51, 871–882. 10.1016/j.neuron.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA, 2013. Burden of Depressive Disorders by Country, Sex, Age, and Year: Findings from the Global Burden of Disease Study 2010. PLoS Med 10 10.1371/journal.pmed.1001547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB et, Spitzer RL, Gibbon M, Williams JBW, 1996. Structured clinical interview for DSM-IV for Axis I disorders (SCID-I) clinician version, for DSMIV
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically Parcellating the Human Cerebral Cortex. Cereb. Cortex 14, 11–22. 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE, 2009. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry 166, 64–73. 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE, 2010. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry 49, 162–72.e1–5 10.1177/0300985809358037.Morphologic [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M, 2010. Clinical applications of resting state functional connectivity. Front Syst Neurosci 4, 1–13. 10.3389/fnsys.2010.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME, 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci 8, 700–711. 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF, 2007. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biol. Psychiatry 62, 429–437. 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH, 2013. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiol. Dis 10.1016/j.nbd.2012.01.015 [DOI] [PMC free article] [PubMed]
- Hamilton JP, Etkin A, Furman DJ, Lemus MG, Johnson RF, Gotlib IH, 2012. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am. J. Psychiatry 169, 693–703. 10.1176/appi.ajp.2012.11071105 [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Farmer M, Fogelman P, Gotlib IH, 2015. Depressive Rumination, the Default-Mode Network, and the Dark Matter of Clinical Neuroscience. Biol. Psychiatry 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed]
- Ho TC, Sacchet MD, Connolly CG, Margulies DS, Tymofiyeva O, Paulus MP, Simmons AN, Gotlib IH, Yang TT, 2017. Inflexible functional connectivity of the dorsal anterior cingulate cortex in adolescent major depressive disorder. Neuropsychopharmacology 42, 2434–2445. 10.1038/npp.2017.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhn P, Menne-Lothmann C, Peeters F, Nicolson NA, Jacobs N, Derom C, Thiery E, van Os J, Wichers M, 2013. Moment-to-Moment Transfer of Positive Emotions in Daily Life Predicts Future Course of Depression in Both General Population and Patient Samples. PLoS One 8 10.1371/journal.pone.0075655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale Network Dysfunction in Major Depressive Disorder A Meta-analysis of Resting-State Functional Connectivity. JAMA psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Rottenberg J, 2010. Psychological flexibility as a fundamental aspect of health. Clin. Psychol. Rev 10.1016/j.cpr.2010.03.001 [DOI] [PMC free article] [PubMed]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM, 2002. Regularized higher-order in vivo shimming. Magn. Reson. Med 10.1002/mrm.10267 [DOI] [PubMed]
- Koval P, Kuppens P, Allen NB, Sheeber L, 2012. Getting stuck in depression: The roles of rumination and emotional inertia. Cogn. Emot 26, 1412–1427. 10.1080/02699931.2012.667392 [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K, 2009. Coping with Emotions Past: The Neural Bases of Regulating Affect Associated with Negative Autobiographical Memories. Biol. Psychiatry 65, 361–366. 10.1016/j.biopsych.2008.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, 2018. caret: Classification and Regression Training
- Kuhn M, 2008. Building Predictive Models in R Using the caret Package. J. Stat. Softw 28, 1–26. 10.1053/j.sodo.2009.03.00227774042 [DOI] [Google Scholar]
- Kuppens P, Allen NB, Sheeber LB, 2010. Emotional inertia and psychological maladjustment. Psychol. Sci 21, 984–991. 10.1177/0956797610372634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL, 2006. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet (London, England) 367, 1747–57. 10.1016/S0140-6736(06)68770-9 [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP, 2007. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 37, 579–588. 10.1016/j.neuroimage.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Mcnealy K, Pfeifer JH, Dapretto M, 2011. Subgenual anterior cingulate responses to peer rejection: A marker of adolescents’ risk for depression. Dev. Psychopathol 23, 283–292. 10.1017/S0954579410000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci 10.1016/j.tics.2011.08.003 [DOI] [PubMed]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I, 2015. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev 56, 330–344. 10.1016/j.neubiorev.2015.07.014 [DOI] [PubMed] [Google Scholar]
- Ordaz SJ, LeMoult J, Colich NL, Prasad G, Pollak M, Popolizio M, Gotlib IH, Price A, Greicius M, Gotlib IH, 2017. Ruminative brooding is associated with salience network coherence in early pubertal girls. Cogn. Affect. Behav. Neurosci 298–310. 10.1093/scan/nsw133 [DOI] [PMC free article] [PubMed]
- Pe ML, Kircanski K, Thompson RJ, Bringmann LF, Tuerlinckx F, Mestdagh M, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, Kuppens P, Gotlib IH, 2015. Emotion-Network Density in Major Depressive Disorder. Clin. Psychol. Sci 3, 292–300. 10.1177/2167702614540645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC, 2012. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci 10.1016/j.tics.2011.12.011 [DOI] [PubMed]
- R Core Team, 2014. R: A language and environment for statistical computing R Found. Stat. Comput; Vienna, Austria: 2014. 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- Raudenbush SW, Bryk AS, Congdon R, 2011. HLM 7.00 for Windows [Computer software]
- Schlösser RGM, Wagner G, Koch K, Dahnke R, Reichenbach JR, Sauer H, 2008. Fronto-cingulate effective connectivity in major depression: A study with fMRI and dynamic causal modeling. Neuroimage 43, 645–655. 10.1016/j.neuroimage.2008.08.002 [DOI] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Renken RJ, Wichers M, Bastiaansen JA, Figueroa CA, Geugies H, Mocking RJ, Geerligs L, Marsman J-B, Aleman A, Schene AH, Schoevers RA, Ruhé HG, 2017. Associations between Daily Affective Instability and Connectomics in Functional Subnetworks in Remitted Patients with Recurrent Major Depressive Disorder. Neuropsychopharmacology 1–10. 10.1038/npp.2017.65 [DOI] [PMC free article] [PubMed]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ, 2011. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci 12, 154–167. 10.1038/nrn2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA, 2010. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci 107, 11020–11025. 10.1073/pnas.1000446107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Alkozei A, Killgore WDS, Lane RD, 2018. Nested positive feedback loops in the maintenance of major depression: An integration and extension of previous models. Brain. Behav. Immun 67, 374–397. 10.1016/j.bbi.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Smith R, Lane RD, 2015. The neural basis of one’s own conscious and unconscious emotional states. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2015.08.003 [DOI] [PubMed]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL, in: NeuroImage; 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Snijders TAB, Bosker RJ, 1999. Multilevel analysis: An introduction to basic and advanced multilevel modeling, Comparative and General Pharmacology [Google Scholar]
- Steer R. a, Ball R, Ranieri WF, Beck a T., 1997. Further evidence for the construct validity of the Beck depression Inventory-II with psychiatric outpatients. Psychol. Rep 80, 443–446. 10.2466/pr0.1997.80.2.443 [DOI] [PubMed] [Google Scholar]
- Suls J, Green P, Hillis S, 1998. Emotional reactivity to everyday problems, affective inertia, and neuroticism. Personal. Soc. Psychol. Bull 24, 127–136. 10.1177/0146167298242002 [DOI] [Google Scholar]
- Thompson RJ, Mata J, Jaeggi SM, Buschkuehl M, Jonides J, Gotlib IH, 2012. The everyday emotional experience of adults with major depressive disorder: Examining emotional instability, inertia, and reactivity. J. Abnorm. Psychol 121, 819–29. 10.1037/a0027978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trew JL, 2011. Exploring the roles of approach and avoidance in depression: An integrative model. Clin. Psychol. Rev 31, 1156–1168. 10.1016/j.cpr.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Tsuno N, Besset A, Ritchie K, 2005. Sleep and depression. J. Clin. Psychiatry 10.1016/S0140-6736(72)92514-7 [DOI] [PubMed]
- Uddin LQ, 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 10.1038/nrn3857 [DOI] [PubMed]
- Veer IM, 2010. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci 4 10.3389/fnsys.2010.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hermens DF, Hickie IB, Lagopoulos J, 2012. A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord 10.1016/j.jad.2012.04.013 [DOI] [PubMed]
- Wichers M, Peeters F, Geschwind N, Jacobs N, Simons CJP, Derom C, Thiery E, Delespaul PH, van Os J, 2010. Unveiling patterns of affective responses in daily life may improve outcome prediction in depression: A momentary assessment study. J. Affect. Disord 124, 191–195. 10.1016/j.jad.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Wickham H, 2016. tidyverse: Easily Install and Load “Tidyverse” Packages., R package version 1.0.0
- Wu H, Sun H, Xu J, Wu Y, Wang C, Xiao J, She S, 2016. Changed Hub and Corresponding Functional Connectivity of Subgenual Anterior Cingulate Cortex in Major Depressive Disorder 10, 1–7. 10.3389/fnana.2016.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yu C, Zheng H, Liu Y, Song M, Qin W, Li K, Jiang T, 2010. Increased neural resources recruitment in the intrinsic organization in major depression. J. Affect. Disord 121, 220–230. 10.1016/j.jad.2009.05.029 [DOI] [PubMed] [Google Scholar]
- Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC, 2005. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 629–40. 10.1001/archpsyc.62.6.629 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


