Abstract
Open label trials suggest that escitalopram (up to 20mg/d) is an effective treatment for some, but not all posttraumatic stress disorder (PTSD) patients. Higher doses of escitalopram effectively reduced major depression symptoms in patients who had not responded to regular doses. The current study examines the efficacy, tolerability, and adherence to high-dose escitalopram in PTSD. 45 PTSD patients received 12 weeks of gradually increasing doses of escitalopram reaching 40mg daily at four weeks. Among those, 12 participants received regular doses of antidepressants at study onset including escitalopram (n=7). The Clinician Administered PTSD Scale (CAPS) evaluated PTSD symptoms severity before treatment, at 3 months (upon treatment termination), and at six months (maintenance effect). A 20% reduction in CAPS scores was deemed clinically significant. Adverse events and medication adherence were monitored at each clinical session. Linear mixed models analysis showed a significant reduction of mean CAPS scores (11.5±18.1 points) at 3 months and maintenance of gains by 6 months (F(2, 34.56) = 8.15, p = 0.001). Eleven participants (34.3%) showed clinically significant improvement at 3 months. Only nine participants (20%) left the study. There were no serious adverse events and few mild ones with only two (diarrhea, 11.1%; drowsiness, 11.1%) reported by more than 10% of participants. High doses of escitalopram are tolerable and well adhered to in PTSD. Their beneficial effect at a group level is due to a particularly good response in a subset of patients. Variability in prior pharmacological treatment precludes a definite attribution of the results to high doses of escitalopram.
Keywords: PTSD, SSRI, escitalopram, civilian trauma
Introduction
Selective serotonin reuptake inhibitors (SSRIs) are a commonly pharmacological used in posttraumatic stress disorder (PTSD). Clinical evidence shows that treatment with SSRIs (e.g., paroxetine, sertraline) leads to a reduction in PTSD symptoms, with small to medium effect sizes 1. Open-label trials suggest that escitalopram, another commonly prescribed SSRI (off-label use in treating PTSD), may also be effective in treating PTSD2–4, in some patients. Previous escitalopram studies followed a recommended daily dose of up to 20 mg. However, in obsessive-compulsive disorder 5 and major depression6 higher doses were effective in patients who did not respond to a regular doses. The use of higher doses of escitalopram has not been systematically evaluated in PTSD.
This study examined the efficacy, adherence to and tolerance of high-dose escitalopram (up to 40mg/d) in the treatment of PTSD. The study examined escitalopram’s immediate effect after three months of treatment and its role as maintenance treatment after six months. We pre-defined efficacy as statistically significant reduction of PTSD symptoms at group level and a clinically significant effect as a 20% reduction in PTSD symptoms at the level of the individual. We used the proportion of prescribed drug taken, and the three and six months completion rate as a measure of adherence, and the prevalence and severity of adverse effects as indices of tolerability.
Methods
Study participants
Participants
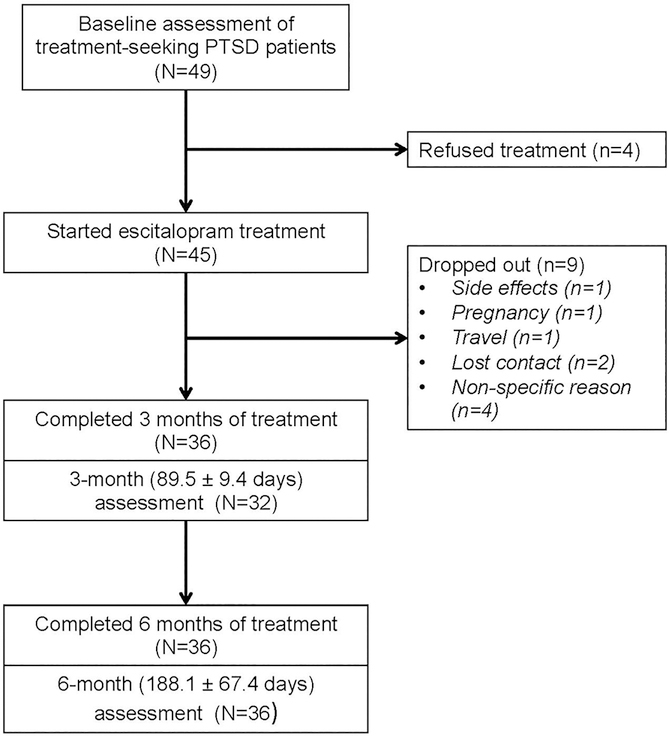
(N=45) were treatment seeking civilian PTSD patients recruited from two psychiatric outpatient clinics in Jerusalem, Israel. Eligible participant were not included if they had prior adverse reactions to SSRIs, current neurological, endocrine, renal or liver disease disorders, medication counter-indicating the use of SSRIs or lifetime history of psychosis or manic episodes. Prior treatment with SSRIs was not used as an exclusion criterion. The Clinician Administered PTSD Scale (CAPS) for DSM-IV measured PTSD symptom severity and provided a PTSD diagnosis. Thirty six participants had at least two efficacy assessments (see study flow chart in Figure 1). The study was approved and monitored by the Hadassah University Hospital IRB. Written informed consent was obtained after explaining the study’s procedures and possible side effects. The study was registered at ClinicalTrials.gov (identifier: NCT00736021).
Figure 1:
Study Flow diagram
Intervention and procedure
Escitalopram (Lexapro; Lundbeck Pharmaceuticals, Copenhagen, Denmark) treatment was started with an initial oral dose of 10–20mg daily, and gradually increased to the target dose of 40mg daily over the first month. For medication at study onset (n=12), SSRIs other than escitalopram were discontinued during a two-week washout period prior to study onset and existing escitalopram dose (n=6) was progressively augmented to 40 mg daily. Escitalopram efficacy, adherence and tolerability were sanctioned after 12 weeks (three months) of treatment, during which time only supportive counseling and non-psychiatric medication were allowed. Treatment was extended by 3 additional months to explore a longer-term (maintenance) effect and sustained adherence and tolerability (Figure 1). Participants were seen a psychiatrist every week during the first month, biweekly during the second and third months, and monthly after that. Leftover medication tablets were counted and recorded at each visit to indicate dose actually taken, and side effects were evaluated using a checklist.
Instruments
PTSD symptom severity:
The CAPS is a clinical interview considered the golden standard for diagnosing PTSD. The main outcome measure in this study was the total severity score (range 0–136), a summation of frequency and intensity scores for the 17 DSM-IV defined symptoms of PTSD7.
Depression:
The severity of depression was measured with the Beck Depression Inventory (BDI), a 21-item self-report scale (range 0–63).8
Adverse Events
Emerging adverse events were evaluated by a 14-item checklist of common adverse events of antidepressants, which also included an other-category for participants to report possible adverse medication effects which were not on the list.
Outcome measures
The study’s main outcome measure was the severity of PTSD symptoms at three and six months as recorded by the CAPS. Following a previous study4, we used a pre-defined threshold of 20% reduction in symptom to infer clinically significant improvement. Additionally, tolerability is expressed as as percentage of participants reporting (a) serious adverse events, (b) mild adverse events at multiple assessments, and (c) any adverse event during the study. Indices of adherence include (a) proportion of participants who completed three and six months of treatment and (b) the proportion of prescribed dose actually taken by the participants.
Data analysis
We used data from participants who at least completed one follow-up CAPS assessment (N = 36) for analysis. Baseline characteristics were compared between completers (n=36) and dropouts (n=9) using t-test and chi-square test. To test the effect of treatment on symptoms, we used SPSS 20.0 MIXED MODELS procedure with maximum likelihood estimation, an autoregressive covariance structure and a random intercept, separately for PTSD symptoms and depression. Time was entered as a fixed factor in these analyses. We also compared symptom differences between two adjacent time points using post-hoc pairwise comparisons. Across all analyses, p-values under 0.05 (two-tailed) were considered statistically significant.
Results
Baseline characteristics
Baseline characteristics are presented in Table 1. Dropouts did not differ from followed participants in any baseline features, including age, gender, time from trauma, trauma type, BDI total score, and CAPS total score (Table 1).
Table 1.
Baseline characteristics
| Followed (N= 36) | Dropout (N = 9) | p | |||||
|---|---|---|---|---|---|---|---|
| Age, mean±SD, years | 43.5 | ± | 13.4 | 41.6 | ± | 13.1 | 0.695 |
| Time from trauma mean±SD, days | 988.7 | ± | 630.0 | 795.8 | ± | 367.7 | 0.245 |
| Gender, n, % | 0.530 | ||||||
| Male | 18 | 50.0 | 4 | 44.6 | |||
| Female | 18 | 50.0 | 5 | 55.4 | |||
| Trauma type n, % | 0.320 | ||||||
| Motor Vehicle Accident | 29 | 80.6 | 7 | 77.8 | |||
| Terrorist Attack | 3 | 8.3 | 0 | 0 | |||
| Work Accident | 2 | 5.6 | 2 | 22.2 | |||
| Other | 2 | 5.6 | 0 | 0 | |||
| BDI, mean±SD | 25.7 | ± | 10.7 | 29.6 | ± | 11.5 | 0.400 |
| CAPS, mean±SD | 71.8 | ± | 14.3 | 77.8 | ± | 11.6 | 0.208 |
High-dose escitalopram treatment was started an average of 2.7 years (SD = 1.73) after the traumatic event that triggered current PTSD. One-third of the participants were on stable treatment with SSRIs at study onset, including six participants (16.7%) treated with escitalopram (10–20mg/d), three (8.3%) with citalopram (20–40mg/d), and two (5.6%) with paroxetine (20mg/d).
Efficacy
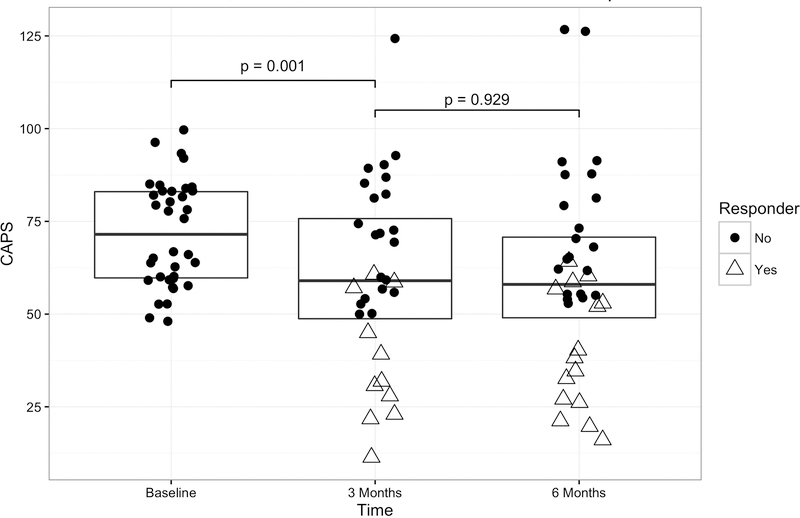
At three months, the mean CAPS scores for study participants were significantly lower than those recorded at baseline (F(2, 34.56) = 8.15, p = 0.001). The average decrease in PTSD symptoms for the entire group was 11.5 points (SD = 18.1). These results were sustained at the six months assessment (Table 2, Figure 2). All PTSD symptom clusters decreased by a similar degree (Table 2), with a mean decrease after 3 months of treatment of 3.87 points (18.5%) in intrusion, 3.97 points (14.1%) in avoidance, and 4.63 points (19.4%) in hyperarousal. BDI scores showed similar and significant reduction at three months (F(2, 35.25) = 4.28, p = 0.022), decreasing by an average of 4.2 points. The results were sustained at six months (Table 2).
Table 2.
Treatment dosage, adverse events, and symptom severity over time
| Before treatment | 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dosage | |||||||||
| Prescribed, mean±SD, mg | 38.33 | ± | 5.07 | 38.61 | ± | 4.87 | |||
| Taken, mean±SD, mg | 36.53 | ± | 6.64 | 37.78 | ± | 5.40 | |||
| Adverse events, n, % | 21 | 58.3% | 6 | 16.7% | |||||
| Change in appetite | 2 | 5.6% | 0 | 0% | |||||
| Weight loss/gain | 2 | 5.6% | 1 | 2.7% | |||||
| Abdominal pain | 3 | 8.3% | 2 | 5.6% | |||||
| Diarrhea | 4 | 11.1% | 0 | 0% | |||||
| Constipation | 1 | 2.8% | 0 | 0% | |||||
| Dry mouth | 2 | 5.6% | 1 | 2.8% | |||||
| Restlessness | 0 | 0% | 0 | 0% | |||||
| Drowsiness | 4 | 11.1% | 1 | 2.8% | |||||
| Insomnia | 1 | 2.8% | 0 | 0% | |||||
| Headache | 3 | 8.3% | 0 | 0% | |||||
| Vertigo | 3 | 8.3% | 0 | 0% | |||||
| Sweating | 2 | 5.6% | 0 | 0% | |||||
| Palpitations | 1 | 2.8% | 0 | 0% | |||||
| Tremor | 0 | 0% | 0 | 0% | |||||
| Sexual dysfunction | 0 | 0% | 0 | 0% | |||||
| Other | 15 | 42% | 3 | 8.3% | |||||
| CAPS total, mean±SD | 71.80 | ± | 14.29 | 60.53 | ± | 24.60 | 60.08 | ± | 25.86 |
| Intrusion | 20.93 | ± | 4.41 | 17.06 | ± | 7.34 | 16.89 | ± | 7.79 |
| Avoidance | 28.16 | ± | 8.08 | 24.19 | ± | 11.96 | 23.36 | ± | 12.31 |
| Hyperarousal | 23.91 | ± | 3.84 | 19.28 | ± | 6.80 | 19.83 | ± | 6.91 |
| BDI, mean±SD | 25.71 | ± | 10.71 | 21.52 | ± | 11.84 | 21.86 | ± | 13.53 |
Figure 2: Posttraumatic stress disorder symptom scores before, after 3 months, and after 6 months treatment with escitalopram.
Box plots including individual observations of post traumatic stress disorder (PTSD) symptom scores measured with the Clinician Administered PTSD Scale (CAPS, y-axis, higher scores mean more symptoms) before the start of high-dose escitalopram treatment (baseline, n=36), after initial treatment (3 months, n=32) and after sustained treatment (6 months, n=36). Individual observations are color coded to reflect treatment response (blue, >20% decrease in symptoms relative to individual baseline) or non-response (red) at that time point. The p-values show the significance level in pairwise comparison of CAPS total scores between the adjacent two time points.
Individual differences
Eleven (34.4%) participants showed clinically significant improvement at 3 months (Figure 2), including three out of ten participants who received escitalopram or citalopram at study onset and one of two participants on paroxetine.
At 6 months, 15 participants (41.7%) showed clinically significant improvement including all 11 subjects who improved by three months, three who improved between three and six months and one who had not been assessed at three months. Importantly, more than half of participants did not show clinically relevant improvement during the trial.
There were no significant differences in age, gender, trauma type, days since trauma, and baseline PTSD symptoms between participants with significant responses (responders) and others (non-responders) at 3 months. Responders, however, had a lower mean BDI score at baseline (responders’ mean = 19.64, SD = 9.78 and non-responders’ mean = 27.90, SD = 10.30; p = 0.037). Responders also showed larger decrease in depression symptoms at three months (respectively for responders and non-responders BDI mean = 13.67, SD = 7.86 vs. BDI mean = 25.05, SD = 12.08).
Adherence and Tolerability
36 (80%) of the 45 participants continued taking escitalopram for 3 months and all of them further continued treatment until 6 months. Compliance was high, as participants took 95% of prescribed dose (Table 2). No serious adverse effects have been recorded during the study. Only 1 (2.2%) participant listed an adverse reaction as the reason for dropping out (at a dosage of 10mg/d). Other reasons for dropping out included: pregnancy (n = 1, 2.2%), travel (n = 1, 2.2%), loss of contact (n = 2, 4.4%), and unspecified (n = 4, 8.8%). Among those who completed the study (n = 36), 16 participants (44%) did not report any adverse event during 6 months of treatment. 9 participants (25%) reported a mild adverse event in more than one visit, and an additional 11 (30.6%) reported a mild adverse event in one clinical visit only. Diarrhea and drowsiness were most commonly reported (Table 2).
Discussion
This open label study investigated the efficacy, tolerability and treatment adherence of high dosage escitalopram in adult civilians with chronic PTSD. The results show significant reduction in mean PTSD symptom at three months, which was sustained at six months. However, this effect is driven by a subgroup of good responders, while the majority of participants remain unchanged.
Escitalopram treatment also led to a limited reduction in depression symptoms, which could reflect the absence of response of depression symptoms among non-responders. Adverse events were mild and mostly transient, with rates similar to those reported in studies of low dose escitalopram.4 These results are also in line with a study of major depression, in which escitalopram was well tolerated up to 40mg. In that study, however, the tolerance declined with doses over 40mg 6. As indicated by the limited dropout rate, and high compliance with medication, treatment adherence was good.
While the main treatment effect was significant, with a 16% decrease in mean PTSD symptoms, the mean effect was not superior when compared to decreases of 23% - 58% reported in low dose studies 2–4. The reported improvement of four participants on regular doses of SSRI suggests that higher dose escitalopram might be beneficial to some individuals who do not improve on lower doses.
The heterogeneity in responses, in this work, is remarkable: the mean symptom reduction was driven by a subgroup of treatment responders, while over 50% of the participants did not improve. Among those who improved, nine participants reached levels of PTSD symptoms (22–40 total CAPS score) considered as mild. This finding reveals a major limitation of the prevalent use of group average responses in clinical trials, which does not discern responders from non-responders. Reporting response heterogeneities and exploring their sources might lead to better targeting treatment intervention and cost-effective treatments. 9,10
Identifying treatment responders remains a major challenge. In this work, for example, treatment outcome was not predicted by initial symptom severity and trauma type. Yet unexplored genetic and neurobiological features11,12 might help improving such predictions: Genetic polymorphisms of cytochrome P450 enzymes have been shown to influence the metabolism of escitalopram and other antidepressants,13,14 and brain-derived neurotrophic factor levels were found to be associated with greater treatment response to escitalopram in veterans with PTSD.2 Harnessing such information into predictive models requires further work.
Several limitations of this work are worth considering when interpreting its results. Being an open-label-trial without control group our results cannot be definitely attributed to the treatment provided. The study’s small sample did not allow a thorough investigation of uncommon or rare side effects related to higher doses. Because participants were only re-assessed at three months the study does not allow an intent-to-treat analysis. While the sample was all-civilians and quite homogeneous in baseline symptom levels, there were variations in previous treatment that preclude a reliable statement about the effect of increasing the dose of escitalopram. Finally, the study has not revealed specific predictors of improvements and, as such, the sources of better responding remain a future challenge. The study nonetheless shows a potential benefit of high-dose escitalopram in a subset of treatment candidates along with satisfactory tolerability and adherence. It stresses the importance of investigating the mechanisms underlying heterogeneous treatment response.
Acknowledgement:
The following members of the Center for Traumatic Stress at Hadassah University Hospital, Jerusalem provided support to conduct the study: Yevgeny Levin (study psychiatrist), Yael Ankri and Yossi Israeli-Shalev (admin and data entry). Lundbeck (Israel) personnel Orna Dolberg, M.D., and Doron Ben Ami facilitated the study’s investigator-initiated submission and funding.
Funding/support: Funding for this study was received from Lundbeck Pharmaceuticals via Investigator Initiated Trial Mechanism.
Role of the sponsor: The funding sources had no role in the design and conduct of the study, in collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript and decision to submit the manuscript for publication.
Footnotes
Potential conflicts of interest: Dr. Shalev received an investigator initiated grant from Lundbeck Pharmaceuticals Ltd for this study and for a multi-center study (Joseph Zohar, MD, principal investigator) entitled “Prevention of PTSD by Escitalopram.” The other coauthors have no conflicts of interest to declare.
References
- 1.Jonas DE, Cusack K, Forneris CA, et al. Psychological and pharmacological treatments for adults with posttraumatic stress disorder (PTSD). Comparative Effectiveness Reviews. 2013;92. [PubMed] [Google Scholar]
- 2.Berger W, Mehra A, Lenoci M, et al. Serum brain-derived neurotrophic factor predicts responses to escitalopram in chronic posttraumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramaswamy S, Selvaraj V, Driscoll D, et al. Effects of Escitalopram on Autonomic Function in Posttraumatic Stress Disorder Among Veterans of Operations Enduring Freedom and Iraqi Freedom (OEF/OIF). Innov Clin Neurosci. 2014;12:13–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Robert S, Hamner MB, Ulmer HG, et al. Open-label trial of escitalopram in the treatment of posttraumatic stress disorder. J. Clin Psychiatry. 2006;67:1522–1526. [DOI] [PubMed] [Google Scholar]
- 5.Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15:850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade AG, Crawford GM, Yellowlees A. Efficacy, safety and tolerability of escitalopram in doses up to 50 mg in Major Depressive Disorder (MDD): an open-label, pilot study. BMC psychiatry. 2011;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake DD, Weathers FW, Nagy LM, et al. Clinician-administered PTSD scale for DSM-IV. Boston: National Center for Posttraumatic Stress Disorder; 1998. [Google Scholar]
- 8.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 9.Steenkamp MM. TRue evidence-based care for posttraumatic stress disorder in military personnel and veterans. JAMA Psychiatry. 2016;Published online doi: 10.1001/jamapsychiatry.2015.2879. [DOI] [PubMed] [Google Scholar]
- 10.Yehuda R, Hoge CW. The meaning of evidence-based treatments for veterans with posttraumatic stress disorder. JAMA Psychiatry. 2016;Published online. doi: 10.1001/jamapsychiatry.2015.2878. [DOI] [PubMed] [Google Scholar]
- 11.Evans KC, Dougherty DD, Pollack MH, et al. Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry. 2006;18:33–42. [DOI] [PubMed] [Google Scholar]
- 12.Lawford BR, Young RM, Noble EP, et al. D2 dopamine receptor gene polymorphism: paroxetine and social functioning in posttraumatic stress disorder. Eur Neuropsychopharmacol. 2003;13:313–320. [DOI] [PubMed] [Google Scholar]
- 13.Porcelli S, Fabbri C, Spina E, et al. Genetic polymorphisms of cytochrome P450 enzymes and antidepressant metabolism. Expert Opin Drug Metab Toxicol. 2011;7:1101–1115. [DOI] [PubMed] [Google Scholar]
- 14.Tsai M-H, Lin K-M, Hsiao M-C, et al. Genetic polymorphisms of cytochrome P450 enzymes influence metabolism of the antidepressant escitalopram and treatment response. Pharmacogenomics. 2010;11:537–546. [DOI] [PubMed] [Google Scholar]