Abstract
Aims:
There is a critical need to reduce infectious disease transmission among individuals with opioid use disorder (OUD). Here we examine the ability of a novel, automated educational intervention, delivered via iPad in a single visit, to improve human immunodeficiency virus (HIV) and Hepatitis C (HCV) knowledge among adults with OUD.
Methods:
Participants were 25 adults enrolled in a 12-week trial evaluating the efficacy of an Interim Buprenorphine Treatment for reducing illicit opioid use and other risk behaviors during delays to opioid treatment. Participants completed baseline HIV and HCV knowledge assessments with corrective feedback. They then completed an interactive HIV flipbook and HCV video followed by a second administration of the knowledge assessments. The knowledge assessments were repeated at post-intake Weeks 4 and 12.
Results:
At baseline, participants answered 69% and 65% of items correctly on the HIV and HCV assessments, respectively. The educational intervention was associated with significant increases in knowledge (86% and 86% correct on the HIV and HCV assessments, respectively; p’s < .001). These improvements persisted throughout the study, with scores at Week 4 and 12 significantly greater than baseline (p’s < .001).
Conclusion:
This HIV + Hepatitis Education intervention was associated with significant and sustained improvements in knowledge of HIV + HCV transmission and risk behaviors in this vulnerable group of individuals with OUD. Given the continuing opioid epidemic, efforts are urgently needed to reduce HIV and HCV contraction and transmission among individuals with OUD. Mobile health educational interventions may offer a time- and cost-effective approach for addressing these risks.
Keywords: Opioid use disorder, HIV, Hepatitis C, Opioid agonist treatment, mobile health, Technology
1. Introduction
Rates of opioid use disorder (OUD) have reached epidemic proportions in the US (Birnbaum et al., 2011; Clausen et al., 2009; Rudd et al., 2016). Of particular concern is the disproportionate prevalence of human immunodeficiency virus (HIV) and Hepatitis C (HCV) among individuals with OUD. Untreated OUD has been associated with unprecedented recent outbreaks of HIV and HCV (CDC, 2015, 2016; Dunn et al., 2016; Wang et al., 2011). Infectious disease risks among individuals with OUD and other substance use disorders stems from engaging in risky drug use and sexual behaviors (e.g., sharing injection equipment, having unprotected sex, trading sex for drugs). Efforts to improve HIV and HCV knowledge in this population are critical for reducing the individual and societal consequences associated with infectious disease. Educational interventions are a widely-used approach and have been associated with improvements in HIV- and HCV-related knowledge (Arain et al., 2016), decreasing self-reported risk behaviors (Copenhaver et al., 2006; Meader et al., 2010), and improved utilization of HIV and HCV screening and treatment services (Lubega et al., 2013; Marinho et al., 2016). Despite this, several features have limited their widespread use. The interventions have often been delivered across multiple lengthy sessions and relied on delivery by trained peer, staff, or health care professionals (Shah and Abu-Amara, 2013). These factors can increase cost and time burdens associated with education delivery. Staff-delivered assessments and interventions may also be less appealing to some individuals due to potential concerns regarding confidentiality or perceived judgment around the sensitive behaviors being assessed.
The recent development of mobile health (mHealth) platforms may hold promise for overcoming this limitation. mHealth interventions use portable computerized devices to extend the reach of health care by permitting delivery of monitoring, education, point-of-care diagnostics and treatment beyond the confines of the medical office (Boyer et al., 2010). The limited studies to date examining the utility of mHealth approaches for improving HIV and HCV knowledge suggest this approach may be promising (Aronson et al., 2017; Catalani et al., 2013; Festinger et al., 2016; Niakan et al., 2017). We recently adapted a single-visit, therapist-delivered educational intervention, which was developed and shown by our group in prior studies to improve HIV and HCV knowledge in illicit drug abusers (Dunn et al., 2013; Heil et al., 2005; Herrmann et al., 2013), for automated delivery using an iPad platform. We report here on our initial examination of this novel mHealth application for improving HIV and HCV knowledge among individuals seeking but waitlisted for opioid agonist maintenance.
2. Methods
2.1. Parent study
The HIV + HCV educational intervention was delivered as part of a 12-week randomized trial investigating the initial efficacy of interim buprenorphine dosing for reducing illicit opioid use and other risk behaviors during delays to community treatment. To be eligible for the study, participants had to be > 18 years old, meet Diagnostic and Statistical Manual criteria for OUD, provide an opioid-positive urine specimen, and be waitlisted for opioid agonist treatment. The study was approved by the University of Vermont Institutional Review Board and all participants provided written informed consent prior to participating.
Participants were randomized 1:1 using a balanced allocation procedure to Interim Buprenorphine Treatment (IBT; n = 25) or a continued waitlist control condition (WLC; n = 25), while stratifying on variables that may be associated with treatment outcome (e.g., duration on waiting list, primary opioid, amount of opioids used per day, past-month cocaine use, lifetime IV use). IBT participants visited the clinic bimonthly for staff-observed medication ingestion and urinalysis, with the remaining doses dispensed via computerized device (Med-O-Wheel Secure; Addoz, Finland). They also received daily calls assessing drug use, craving and withdrawal via an Interactive Voice Response (IVR) phone system, as well as IVR-generated random callbacks and HIV + HCV Education. Waitlist control participants remained on the waitlist of their local clinic and did not receive these services. The primary outcomes from this study demonstrated the efficacy of IBT and have been reported previously (Sigmon et al., 2016). As only IBT participants received the HIV + HCV education described below, our analyses focused on those individuals.
2.2. HIV + HCV educational intervention
During Week 1, participants completed a baseline assessment (Pre-Test) of HIV and HCV knowledge and perceived risk using an interactive iPad application developed by us (below), after which the application provided immediate corrective feedback and explanations for any incorrect items. Participants then reviewed an interactive flipbook (“HIV/AIDS Basics,” Aids.gov) and watched a 15-minute video (“What is Hepatitis C and how is it diagnosed?”, amfAR: The Foundation for AIDS Research), both administered via iPad and monitored by study staff. The HIV + HCV knowledge assessment was then administered immediately following delivery of the educational content (Post-Test), with feedback provided for any incorrect answers. At the end of the session, a staff member offered condoms, as well as contact information for free HIV and HCV testing resources. The intervention took approximately an hour to complete. To examine the extent to which changes in HIV and HCV knowledge persisted following the single-visit intervention, participants repeated the Post-Test knowledge assessment at Weeks 4 and 12.
2.3. Measures
The knowledge assessment consisted of a modified version of the HIV/AIDS Knowledge Test (Marsch et al., 2005), a 50-item assessment of HIV knowledge in three areas (i.e., general knowledge, sexual risk behaviors, drug risk behaviors; see supplemental material). Also included was a 17-item HCV knowledge assessment (Dunn et al., 2013; Supplemental material). Both were administered via iPad and included “True,” “False,” or “Don’t know” response options (Herrmann et al., 2013), with correct responses summed to obtain an overall accuracy score (i.e., percent correct) for each questionnaire.
Participants also completed visual analog scale (VAS) items evaluating their perceived risk of infection and disease knowledge, as well as HIV and HCV risk behaviors (Supplemental material). On three additional VAS items, participants rated the helpfulness of the HIV iPad flipbook, the helpfulness of the HCV video, and their comfort with the iPad application more generally. Scores for each VAS item ranged from 0 (Not at all) to 100 (Extremely).
2.4. Data analyses
Descriptive statistics were used to characterize participants’ baseline demographics and drug use history. The percentage of items correct on the HIV and HCV assessments were calculated for each participant at the Pre-Test, Post-Test, and Week 4 and 12 follow-up assessments, with higher scores indicating greater knowledge accuracy. The significance associated with temporal changes in mean scores was evaluated using a linear mixed model for repeated measures data (SAS, PROC MIXED). Pairwise comparisons among timepoints were performed using a Fisher’s LSD procedure. McNemar’s tests were used to evaluate changes in accuracy on individual items. Analyses of temporal changes on the VAS items paralleled those described above, with the exception of the three VAS items assessing the perceived helpfulness of the intervention components which were descriptively examined at Post-Test. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC), with significance determined based on α=.05.
3. Results
3.1. Participant characteristics
At study intake, 64% and 36% of participants reported heroin or prescription opioids as their primary opioid of abuse, respectively (see supplementary material). Fifty-six percent of participants endorsed the intravenous (IV) route as their primary route of opioid administration, 80% endorsed a lifetime history of IV drug use, and 40% reported a history of opioid overdose. Of those reporting a lifetime history of overdose, 89% had experienced multiple overdoses. Among participants with a history of IV drug use, 30% reported using a syringe or needle after someone, and 15% had used an unsterilized syringe or needle (see supplementary material2). Twelve percent of participants’ partners were also opioid users, and 67% of those partners were IV drug users. Twenty-two percent of participants had received a diagnosis of HCV, and 14%, 6%, 2%, and 2% had been previously diagnosed with chlamydia, gonorrhea, herpes, and viral warts, respectively.
3.2. HIV knowledge
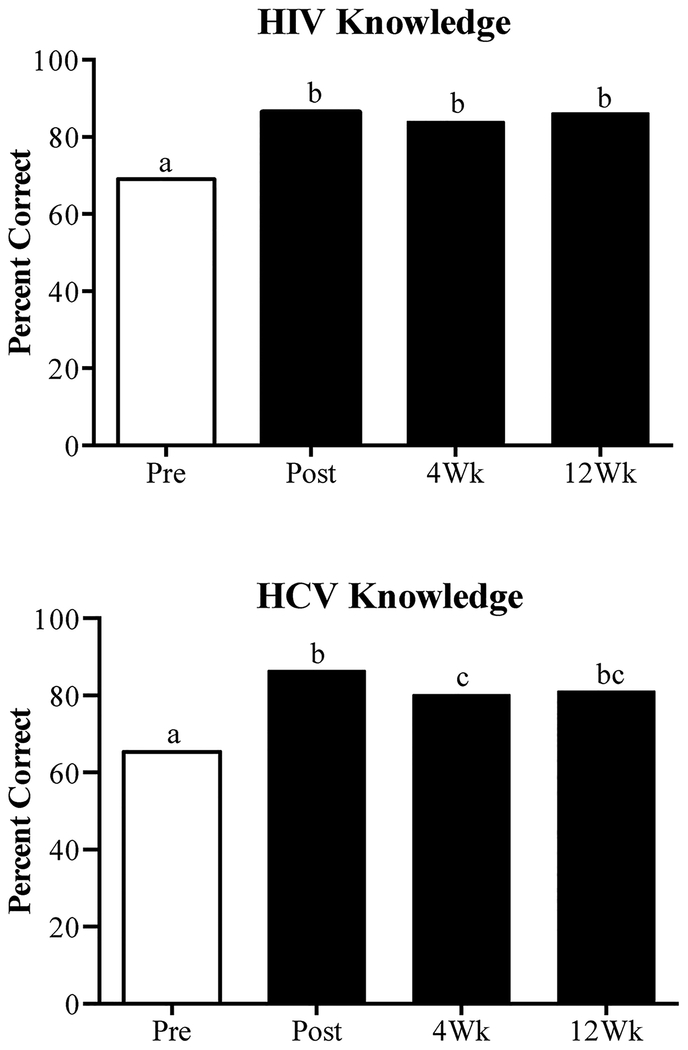
On the baseline (Pre-test) HIV knowledge assessment, participants answered an average of 69% of items correctly (Table 1). Following delivery of the intervention, this increased to 86% (t(66)=−9.49, p < .001) (Fig. 1, top). Examination of individual items showed that significant increases were seen on 13 of the 50 (26%) items. At Week 4 and 12 follow-ups, participants answered 84% and 86% of HIV items correctly, respectively. These scores were significantly greater than baseline (p’s < .001) and did not differ from one another.
Table 1.
Significant HIV + HCV Pre- to Post-Test Improvements.
| HIV Item | Pre-Test% | Post-Test% | P-value |
|---|---|---|---|
| Total Score | 69% | 86% | < .001 |
| General Knowledge | 72% | 88% | < .001 |
| 15. AIDS is not always fatal. (F) | 28% | 56% | 0.039 |
| 16. An infected mother can give HIV to her infant via breast feeding (milk). (T) |
32% | 88% | <.001 |
| 32. HIV can be transmitted through menstrual blood. (T) |
52% | 96% | 0.001 |
| 34. The HIV virus can be transmitted by mosquitoes or bugs. (F) | 32% | 72% | 0.002 |
| 44. People with AIDS can be cured if they are given very good medical care. (F) | 60% | 92% | 0.022 |
| Sexual Risk Knowledge | 63% | 89% | < .001 |
| 24. The HIV virus is present in vaginal secretions. (T) |
32% | 80% | <.001 |
| 31. Latex condoms are better than natural skin or lambskin condoms in preventing the spread of HIV. (T) |
44% | 88% | 0.003 |
| 36. A person who has had a sexually transmitted disease is at increased risk for HIV. (T) |
24% | 76% | <.001 |
| 41. Condoms that are not long enough to cover the whole penis may not be able to prevent the transmission of HIV. (T) |
52% | 84% | 0.022 |
| 42. Using oil-based lubricants, such as hand lotion, cold cream, food products or baby oil, with a condom will weaken the condom and increase the likelihood that it may break during sex. (T) |
44% | 96% | <.001 |
| Drug Risk Knowledge | 70% | 83% | < .001 |
| 18. Rubbing injection sites with alcohol can lower the risk of getting AIDS. (T) |
20% | 80% | <.001 |
| 27. Needles bought on the street in a sterile wrapper cannot transmit HIV. (F) | 44% | 76% | 0.022 |
| HCV Item | Pre-Test % | Post-Test % | P-value |
| 3. There is a vaccine available for Hepatitis C. (F) |
32% | 64% | 0.022 |
| 5. There is only one test that can be used to diagnose the Hepatitis C infection. (F) | 8% | 80% | <.001 |
| 12. There are 6 different types of Hepatitis C; the main difference between them is that some are easier to cure than others. (T) | 44% | 84% | 0.021 |
| 16. Hepatitis C can be cured in 100 percent of people who begin treatment. (F) | 44% | 92% | <.001 |
Values indicate the percent of participants that provided the correct response. Only items in which changes in accuracy from Pre- to Post-Test were significant (p < .05) are presented; correct answers are denoted in parentheses (T=True, F=False).
Fig. 1.
Mean percent of correct items on the HIV and HCV knowledge assessments in the present study. Data bars sharing a common letter are not significantly different (Fisher’s LSD, p < .05).
3.3. HCV knowledge
At Pre-Test, participants answered 65% of HCV items correctly (Table 1). Following the intervention, this increased to 86% (t(66) =−6.89, p < .001) (Fig. 1, bottom). Significant increases were seen on 4 of the 17 (24%) HCV items. At Weeks 4 and 12, participants answered 80% and 81% of items on the HCV assessment correctly, respectively. These scores were significantly greater than baseline (p’s < .001) and did not differ from one another.
3.4. Visual analog scale items
Significant Pre- to Post-Test increases were also observed on VAS items (range: 0–100) reflecting perceived knowledge of HIV and HCV transmission, including “How much do you know about how HIV is transmitted?” (54 vs. 82, respectively; t(60)=−6.65, p < .001) and “How much do you know about how HCV is transmitted?” (55 vs. 82, respectively; t(60)=−5.73, p < .001). Ratings on these items at Weeks 4 and 12 remained significantly greater than Pre-Test (p’s < .001). Finally, participants’ mean ratings on the perceived helpfulness at Post-Test were 79, 81 and 89 for HIV flipbook, HCV video and iPad, respectively.
4. Discussion
There is a critical need to reduce infectious disease transmission among individuals with OUD. HIV and HCV risk among this sample of waitlisted adults with OUD was considerable with 80% of participants reporting a history of IV drug use, 56% identifying IV as their primary route, 30% having shared a needle or syringe and 56% reporting a history of unprotected sex. Participants’ baseline levels of HIV and HCV knowledge (69% and 65% accuracy, respectively) were generally consistent with prior studies (Arain et al., 2016; Copenhaver et al., 2006; Dunn et al., 2013; Heil et al., 2005; Herrmann et al., 2013; Marinho et al., 2016; Norton et al., 2014; Shah and Abu-Amara, 2013; Zeremski et al., 2016).
The mHealth educational intervention was associated with significant improvements in both HIV and HCV knowledge, with the magnitude gains similar to (Arain et al., 2016; Heil et al., 2005; Herrmann et al., 2013) or greater than (Marinho et al., 2016; Norton et al., 2014; Zeremski et al., 2016) prior studies on this topic. That the improvements in HIV + HCV knowledge persisted over the 12 weeks following the intervention was an unexpected finding. To our knowledge this is the first demonstration of sustained effects of a single-visit, mHealth educational intervention on infectious disease knowledge. The mechanism underlying this persistence in knowledge improvements is unclear but warrants further investigation, as does the duration of improvements that might be expected following a brief mHealth intervention. Finally, participants rated the educational content and iPad platform favorably, providing additional support for the potential acceptance of technology-assisted educational interventions among individuals seeking treatment for OUD (Miller and Himelhoch, 2013; Niakan et al., 2017; Shrestha et al., 2017; Westergaard et al., 2017).
This is the first educational intervention to our knowledge to target HIV and HCV knowledge among the high-risk sample of waitlisted individuals with OUD. It is also the first to demonstrate sustained improvements for several months following the intervention. Several limitations are also worth noting. First, this within-subject evaluation did not include an HIV + HCV education control group. However, our group has previously demonstrated that the improvements in HIV and HCV knowledge are not likely due to a practice effect from repeated exposure to the knowledge assessments (Herrmann et al., 2013). Second, we did not include measures of sexual and drug-use risk behaviors and thus were unable to directly examine the extent to which this educational intervention influenced frequency of HIV + HCV-related risk behaviors. However, prior studies have reported a strong concordance between HIV and HCV education and reductions in high-risk behaviors, as well as increases in protective health behaviors (Copenhaver et al., 2006; Gilchrist et al., 2017; Meader et al., 2010; Shah and Abu-Amara, 2013). Finally, we did not include HIV or HCV screening and thus cannot evaluate whether infectious disease status changed as a function of the educational intervention.
In summary, this mHealth educational intervention was associated with significant and sustained improvements in knowledge of HIV + HCV transmission and risk behaviors in this extremely vulnerable group of individuals with OUD. Using an automated iPad platform for delivery may facilitate its use in settings in which resources are limited. Given the continuing opioid epidemic, efforts are urgently needed to reduce HIV and HCV contraction and transmission among individuals with OUD. Mobile health educational interventions may offer a time- and cost-effective approach for addressing these risks.
Supplementary Material
Acknowledgements
The authors would like to thank Andrew C. Meyer, PhD, Bryce Hruska, PhD, Jacob Pusey, BS, Shoshana Aronowitz, RN, Betsy Bahrenburg, RN, Megan Detweiler, RN, and Theresa Krainz, RN for assistance with the randomized trial.
Funding
This study was supported by National Institutes of Health research (R34DA037385) and training (T32DA007242) grants. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of interest
The authors have no conflicts to declare.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2018.05. 032.
References
- Arain A, De Sousa J, Corten K, Verrando R, Thijs H, Mathei C, Buntinx F,Robaeys G, 2016. Pilot study: combining formal and peer education with FibroScan to increase HCV screening and treatment in persons who use drugs. J. Subst. Abuse Treat 67, 44–49. 10.1016/j.jsat.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Aronson ID, Bennett A, Marsch LA, Bania TC, 2017. Mobile technology to increase HIV/HCV testing and overdose prevention/response among people who inject drugs. Front. Public Health 5, 217 10.3389/fpubh.2017.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL, 2011. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med 12, 657–667. [DOI] [PubMed] [Google Scholar]
- Boyer EW, Smelson D, Fletcher R, Ziedonis D, Picard RW, 2010. Wireless technologies, ubiquitous computing and mobile health: application to drug abuse treatment and compliance with HIV therapies. J. Med. Toxicol 6, 212–216. 10.1007/s13181-010-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani C, Philbrick W, Fraser H, Mechael P, Israelski DM, 2013. mHealth for HIV treatment and prevention: a systematic review of the literature. Open AIDS J 7, 17–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2015. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—united States and 6 dependent areas—2013. HIV Surveill. Supplement Rep. 20. http://www.cdc.gov/hiv/library/reports/surveillance/. [Google Scholar]
- Centers for Disease Control and Prevention, 2016. HIV Surveillance Report, 2015 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
- Clausen T, Waal H, Thoresen M, Gossop M, 2009. Mortality among opiate users: opioid maintenance therapy, age and causes of death. Addiction 104, 1356–1362. 10.1111/j.1360-0443.2009.02570.x. [DOI] [PubMed] [Google Scholar]
- Copenhaver MM, Johnson BT, Lee I-C, Harman JJ, Carey MP, Team SR, 2006. Behavioral HIV risk reduction among people who inject drugs: meta-analytic evidence of efficacy. J. Subst. Abuse Treat 31, 163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Saulsgiver KA, Patrick ME, Heil SH, Higgins ST, Sigmon SC, 2013. Characterizing and improving HIV and hepatitis knowledge among primary prescription opioid abusers. Drug Alcohol Depend 133, 625–632. 10.1016/j.drugalcdep.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KE, Barrett FS, Herrmann ES, Plebani JG, Sigmon SC, Johnson MW, 2016. Behavioral risk assessment for infectious diseases (BRAID): self-report instrument to assess injection and noninjection risk behaviors in substance users. Drug Alcohol Depend 168, 69–75. 10.1016/j.drugalcdep.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festinger DS, Dugosh KL, Kurth AE, Metzger DS, 2016. Examining the efficacy of a computer facilitated HIV prevention tool in drug court. Drug Alcohol Depend 162, 44–50. 10.1016/j.drugalcdep.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist G, Swan D, Widyaratna K, Marquez-Arrico JE, Hughes E, Mdege ND, Martyn-St James M, Tirado-Munoz J, 2017. A systematic review and meta-analysis of psychosocial interventions to reduce drug and sexual blood borne virus risk behaviours among people who inject drugs. AIDS Behav 21, 1791–1811. 10.1007/s10461-017-1755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Sigmon SC, Mongeon JA, Higgins ST, 2005. Characterizing and improving HIV/AIDS knowledge among cocaine-dependent outpatients. Exp. Clin. Psychopharmacol 13, 238–243. 10.1037/1064-1297.13.3.238. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Heil SH, Sigmon SC, Dunn KE, Washio Y, Higgins ST, 2013. Characterizing and improving HIV/AIDS knowledge among cocaine-dependent outpatients using modified materials. Drug Alcohol Depend 127, 220–225. 10.1016/j.drugalcdep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubega S, Agbim U, Surjadi M, Mahoney M, Khalili M, 2013. Formal hepatitis C education enhances HCV care coordination, expedites HCV treatment and improves antiviral response. Liver Int 33, 999–1007. 10.1111/liv.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinho RT, Costa A, Pires T, Raposo H, Vasconcelos C, Polónia C, Borges J, Soares M, Vilar G, Nogueira AM, 2016. A multidimensional education program at substance dependence treatment centers improves patient knowledge and hepatitis C care. BMC Infect. Dis. 16 10.1186/s12879-016-1883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Stothart ME, Quesnel KJ, Stanger C,Brooklyn J, 2005. Comparison of pharmacological treatments for opioid-dependent adolescents: a randomized controlled trial. Arch. Gen. Psychiatry 62, 1157–1164. [DOI] [PubMed] [Google Scholar]
- Meader N, Li R, Des Jarlais DC, Pilling S, 2010. Psychosocial interventions for reducing injection and sexual risk behaviour for preventing HIV in drug users. Cochrane Database Syst. Rev 10.1002/14651858.CD007192.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CWT, Himelhoch S, 2013. Acceptability of mobile phone technology for medication adherence interventions among HIV-positive patients at an urban clinic. AIDS Res. Treat 2013, 1–6. 10.1155/2013/670525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niakan S, Mehraeen E, Noori T, Gozali E, 2017. Web and mobile based HIV prevention and intervention programs pros and cons–A review, in: health informatics meets ehealth: digital insight–information-driven health and care Proceedings of the 11th EHealth2017 Conference IOS Press; pp. 319. [PubMed] [Google Scholar]
- Norton BL, Voils CI, Timberlake SH, Hecker EJ, Goswami ND, Huffman KM,Landgraf A, Naggie S, Stout JE, 2014. Community-based HCV screening: knowledge and attitudes in a high risk urban population. BMC Infect. Dis 14, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Aleshire N, Zibbell JE, Gladden RM, 2016. Increases in drug and opioid overdose deaths — united States, 2000–2014. MMWR Morb. Mortal. Wkly. Rep 64, 1378–1382. 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- Shah HA, Abu-Amara M, 2013. Education provides significant benefits to patients with Hepatitis B Virus or Hepatitis C Virus infection: a systematic review. Clin. Gastroenterol. Hepatol 11, 922–933. 10.1016/j.cgh.2013.04.024. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Huedo-Medina TB, Altice FL, Krishnan A, Copenhaver M, 2017Examining the acceptability of mHealth technology in HIV prevention among high-risk drug users in treatment. AIDS Behav 21, 3100–3110. 10.1007/s10461-016-1637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmon SC, Ochalek TA, Meyer AC, Hruska B, Heil SH, 2016. Interim buprenorphine vs. Waiting list for opioid dependence. N. Engl. J. Med 375, 2504–2505. 10.1056/NEJMc1610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang T, Ho W-Z, 2011. Opioids and HIV/HCV infection. J. Neuroimmune Pharmacol 6, 477–489. 10.1007/s11481-011-9296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard RP, Genz A, Panico K, Surkan PJ, Keruly J, Hutton HE, Chang LW, Kirk GD, 2017. Acceptability of a mobile health intervention to enhance HIV care coordination for patients with substance use disorders. Addict. Sci. Clin. Pract 12, 11 10.1186/s13722-017-0076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeremski M, Zavala R, Dimova RB, Chen Y, Kritz S, Sylvester C, Brown LS, Talal AH, 2016. Improvements in HCV-related knowledge among substance users on opioid agonist therapy after an educational intervention. J. Addict. Med 10, 104–109. 10.1097/ADM.0000000000000196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.