Advances in Computed Tomographic (CT) image acquisition, reconstruction and post-processing have led to an increasing number of quantitative features that can be used to detect pulmonary vascular disease, stratify severity, define subpopulations with homogeneous characteristics and monitor response to therapeutic intervention1,2,3. The goal of this investigation was to develop and examine techniques for the quantification of the pulmonary vascular response to pharmacologic vasodilation using clinically available non-contrast CT imaging. We examined the effect of inhaled nitric oxide (iNO) on the pulmonary arterial and venous vasculature in healthy subjects. Specifically, we examined changes in distal vessel volume in aggregate, and by specific vessel segments. We hypothesized that inhaled nitric oxide would lead to detectable and quantifiable arterial vasodilation on both aggregate and vessel specific analyses.
After obtaining informed consent (IRB# 2011P001880), supine CT scans were obtained in five healthy subjects prior to and while breathing 30ppm iNO and 80% oxygen at relaxed exhalation (Siemens Biograph 64, 160mA, 120 kVp, Convolution Kernel B31f; Total Radiation Exposure ~6.4 mSv). Automated lung segmentation and 3D reconstruction of the vasculature using scale-space particles were performed1. The minimum spanning tree was used to identify individual vessel segments2 using a maximum angle of 20 degrees and a maximum gap distance of 2mm. Arterial/venous segmentation was performed using an automated validated Deep Learning approach4 and validation of the results were performed in three cases by manual tracing of the arteries and veins to the origin. The distribution of vessel volume as a function of cross-sectional area was used to compute the vascular volume in vessels with cross sectional area of less than 5mm2 (BV5)1,2 as well as total vessel volume (TBV)
Each image pair was registered by performing an initial alignment of the centers of intensity followed by a three stage sequential scheme: rigid, affine and finally a diffeomorphic Bspline registration5. This registration was used to align pre and post iNO arterial and venous vascular trees. The local matching of vessel segments used a combination of subsegment angle and location. Vascular segments present on the iNO but not pre iNO scans were labeled “newly detected”. For matched segments, change in vessel radius was computed and used to color code the vascular reconstructions to create the vascular activity map for each participant.
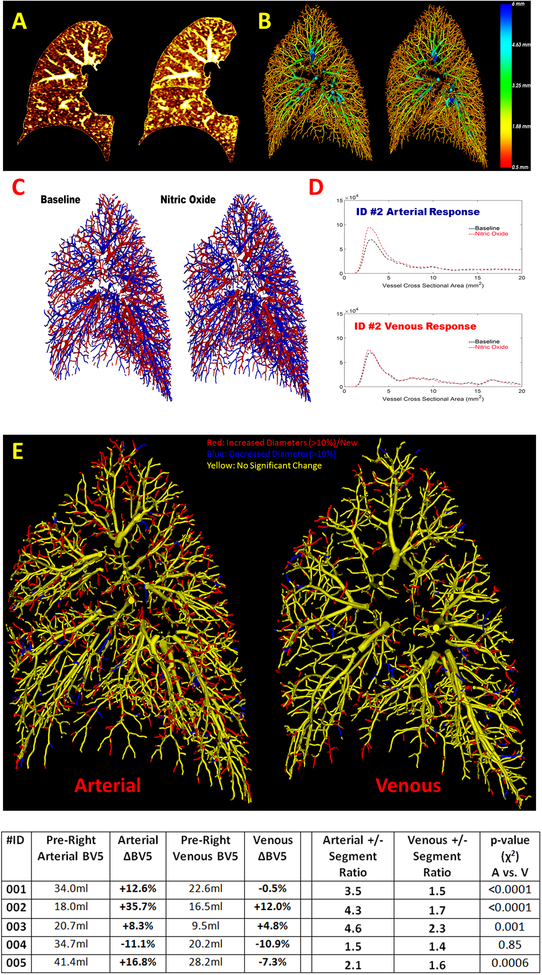
Examples of CT response and vascular reconstruction is shown in Figure 1(A&B). Overall, there was an increase in both the small vessel volume (BV5: 5.4%±13.5%) and total vessel volume (TBV: 7.2%±7.4%). Arterial and venous trees were labeled in the right lung as shown in Figure 1(C&D). There was an increase in the right lung arterial volumes (aBV5: 12.5%±16.8 Figure 1; aTBV: 12.0%±11.7). The venous volumes showed no average increase in BV5 (vBV5: −0.4%±9.2% Figure 1) and an increase in TBV (vTBV: 3.9%±5.1)
Figure 1:
Two axial cross sections prior to and during the administration of inhaled nitric oxide (iNO) demonstrating increased vascular caliber and parencymal density(A). A 3D vascular reconstruction of the right lung prior to and during iNO with the color of the vessels denoting diameter (red being small vessels) (B). An arterial-venous labeled vascular reconstruction is shown prior to and after iNO administration (C). The accompanying vascular response profile as a function of cross sectional area shows an increase in the arterial volume (D). An arterial and venous vascular activity map in which post iNO vessel segments are matched with the baseline vasculature (E). Red segments represent segments with increase in diameter or newly detected segments whereas blue segments represent those with decrease in diameter. The table at the bottom shows measurements of the vasculature for each subject.
An example of a segment by segment matching is shown in Figure 1(E). In all subjects there were arterial and venous vessels that increased or decreased in diameter. For the distal vasculature (BV5) the ratio of positive to negative change in diameter was uniformly greater than one (ratio: 3.2±1.3 arterial; 1.7±0.3 venous). This ratio was greater for arteries than veins, a difference that reached statistical significance for four subjects (χ2 test p≤0.001, ID #4 p=0.85 Figure 1). New segments were detected in each subject (754±277 Arterial; 364±90 Venous), and there were more newly detected arterial than venous segments for each subject (ratio: 2.0±0.3).
In this study two complimentary approaches were used to quantify the pulmonary vascular response to iNO. In one approach a volume versus cross-sectional area model was used to quantify the aggregate response of the distal and total vascular volume. This analysis demonstrated that the administration of iNO led to an increase in distal and total vascular volume, an increase that was larger in magnitude and consistency in the arteries. In a segment by segment analysis, a larger number of small vessels increased rather than decreased in diameter, and there was an increase in the number of small vessel segments detected. The extent of this response was once again greater in the arteries. The increase in number of segments likely represents the dilation of vessels that were previously below the threshold of detection.
This study is limited by the number of subjects, lack of control subjects, lack of concurrent hemodynamic measurements and reproducibility of mid-expiratory breath-holds.
In conclusion, changes in pulmonary vascular caliber and volume in response to inhaled nitric oxide can be detected and spatially quantified using automated methods deployed on clinically available non-contrast CT scans. Future work will examine response in patients with pulmonary vascular disease and relate these markers to other imaging and physiologic markers.. The combination of pulmonary vasodilation as a perturbation and imaging as a window to the physiologic response represents a unique quantitative method to better understand disease phenotypes and potential response to treatment.
Acknowledgments
Sources of Funding: Collection of this data was made possible by financial support from United Therapeutics Corporation. The authors in this study were supported by NHLBI grants 1R01HL116931 (RSJE) and R01HL116473 (R.S.J.E.and G.R.W), 1K23HL136905 (F.N.R), and 5 T32 HL007633 (S.Y.A.).
Footnotes
Disclosures: None
References:
- 1. Estepar RS, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, Kikinis R, Han MK, Come CE, Diaz AA, Cho MH, Hersh CP, Schroeder JD, Reilly JJ, Lynch DA, Crapo JD, Wells JM, Dransfield MT, Hokanson JE, Washko GR and Study CO. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rahaghi FN, Ross JC, Agarwal M, Gonzalez G, Come CE, Diaz AA, Vegas-Sanchez-Ferrero G, Hunsaker A, San Jose Estepar R, Waxman AB and Washko GR. Pulmonary vascular morphology as an imaging biomarker in chronic thromboembolic pulmonary hypertension. Pulm Circ. 2016;6:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hajian B, De Backer J, Vos W, Van Holsbeke C, Ferreira F, Quinn DA, Hufkens A, Claes R and De Backer W. Pulmonary vascular effects of pulsed inhaled nitric oxide in COPD patients with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis. 2016;11:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nardelli P, Jimenez-Carretero D, Bermejo-Pelaez D, Washko GR, Rahaghi FN, Ledesma-Carbayo MJ and Estepar RSJ. Pulmonary Artery-Vein Classification in CT Images Using Deep Learning. IEEE Trans Med Imaging. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avants BB, Epstein CL, Grossman M and Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]