Abstract
During extracellular dehydration neural systems that sense deficits in body fluid homeostasis operate in tandem with those that mediate motivation and reward in order to promote ingestive behaviors that restore fluid balance. We hypothesized that hypothalamic orexin (Ox) neurons act as an interface to couple brain regions sensing and processing information about body fluid status with CNS motivation and reward systems. An initial set of anterograde and retrograde tracing experiments suggested that structures along the lamina terminalis (LT), a region of the forebrain that serves to monitor and integrate information reflecting body fluid balance, project to hypothalamic Ox neurons which, in turn, project to dopamine neurons in the VTA. A second set of experiments determined whether Ox neuron activation is associated with extracellular dehydration and the seeking out and consumption of water and saline. An elevation of Fos-like immunoreactivity (FLI) in Ox neurons was observed in fluid depleted rats that were allowed to ingest water and sodium. A final experiment was conducted to determine whether Ox release in the ventral tegmental area (VTA) promotes thirst and salt appetite. Bilateral microinjection of the Ox type 1 receptor antagonist SB-408124 into the VTA prior to acute extracellular dehydration attenuated fluid intake. Together, these studies support the hypothesis that structures along the LT modulate activity in the VTA through actions of orexinergic neurons that have cell bodies in the hypothalamus. This pathway may function to facilitate sustained consumption of fluids necessary for restoration of fluid balance.
Keywords: Thirst, Salt appetite, Orexin, Motivation, Homeostasis
Introduction
When animals are depleted of water and sodium they manifest the motivated states of thirst and salt appetite, which are associated with behaviors involved in obtaining and ingesting water and salt. It is likely that there are important interactions between brain regions involved in sensing deficits in water and sodium (Johnson & Thunhorst, 2007) and the neural circuitry that promotes motivation and reward (Hurley & Johnson, 2014; Loriaux, Roitman, & Roitman, 2011) associated with thirst and salt appetite.
A set of forebrain nuclei located along the rostral wall of the third ventricle, or the lamina terminalis (LT), sense and process information related to deficits in body fluid homeostasis (Johnson & Thunhorst, 2007; McKinley & Johnson, 2004). These structures include the subfornical organ (SFO), median preoptic nucleus (MnPO), and organum vasculosum of the lamina terminalis (OVLT). Importantly, these LT structures are capable of modulating the activity of downstream brain areas in order to initiate behaviors associated with thirst and sodium appetite (Swanson & Lind, 1986).
The mesolimbic dopamine system consists of dopaminergic cell bodies located in the ventral tegmental area (VTA) that project to the NAc, and its integrity is critical for the performance of most appetitive motivated behaviors (Kelley & Berridge, 2002; Mogenson, Jones, & Yim, 1980). Information related to body fluid status is likely to be relayed from LT structures to key motivation and reward systems in order to generate the behaviors associated with thirst and salt appetite and signal the resolution of these states (Hurley & Johnson, 2015). In the present experiments we sought to determine whether there is a neural pathway that allows for information processed in LT structures to modulate mesolimbic activity. Specifically, it was hypothesized that hypothalamic neurons that synthesize orexin (Ox) aid in coupling information related to deficits in fluid balance sensed and processed in LT structures with the mesolimbic dopamine system.
Ox is a neuropeptide that is distributed along the caudal half of the hypothalamus in an arc that extends from the dorsomedial hypothalamus (DMH) to the dorsal region of the lateral hypothalamus (LHAd; Swanson, Sanchez-Watts, & Watts, 2005). Ox neurons have been partialed into 3 cell-clusters: one cell-cluster lies in the DMH, a second in the perifornical area (PeF), and a third in the LHAd (Harris & Aston-Jones, 2006). Ox is present in two forms – orexin A (Ox-A; also known as hypocretin-1) and orexin B (Ox-B; also known as hypocretin-2). Ox-A primarily binds to Ox type 1 receptors while both Ox-A and Ox-B bind to Ox type 2 receptors (Ebrahim, Howard, Kopelman, Sharief, & Williams, 2002). Ox neurons promote arousal and mobilization of motived behaviors (i.e., motivational activation) in the presence of highly salient stimuli and physiological need states (e.g., states of hunger and thirst or exposure to a threat; Mahler, Moorman, Smith, James, & Aston-Jones, 2014; Thompson & Borgland, 2011). It is worth noting that infusions of Ox into the cerebral ventricles produces a robust drinking response (Kunii et al., 1999). One mechanism by which Ox neurons may promote the consumption of salient rewards is through the action of Ox on the VTA (Fadel & Deutch, 2002; Korotkova, Sergeeva, Eriksson, Haas, & Brown, 2003; Peyron et al., 1998; Zheng, Patterson, & Berthoud, 2007).
The aim of the present study was to determine whether Ox neurons are likely to integrate information from forebrain systems that sense the status of body fluid homeostasis with motivation and reward systems in order to promote water and sodium intake in depleted animals. The first set of experiments employed anterograde and retrograde tracing to identify whether hypothalamic Ox-A neurons are positioned to convey information from the LT to VTA dopaminergic neurons. In a second experiment, Fos-like immunoreactivity (FLI) and Ox-A double-labeling were assessed to determine whether orexin neurons are activated during thirst and sodium appetite. In a final experiment we tested whether antagonism of Ox type 1 receptors within the VTA would attenuate extracellular dehydration-induced fluid intake. Together, the results support the presence of a LT-Ox-VTA neural pathway that may operate to promote salt and water intake.
Methods
Subjects
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by The University of Iowa Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan Teklad, Indianapolis, IN) weighing between 275–300 grams upon arrival were used as subjects. Rats were maintained on a 12/12 light/dark cycle and housed in translucent cages (28.5 × 28.5 ×17.5 cm) or suspended wire mesh cages (24 × 17.2 × 17.0 cm) in a temperature and humidity controlled room. Unless noted otherwise, the animals had ad libitum access to filtered tap water and NIH-31 irradiated modified open formula mouse/rat diet. Rats that received extracellular dehydration had at least 3 days of 1.8% W/V NaCl access prior to experimentation. Hypertonic saline (1.8%) and deionized water were used to assess sodium appetite and thirst.
Extracellular dehydration
Water and sodium depletion was induced using an acute model of extracellular dehydration, which involves co-administration of the diuretic and natriuretic drug furosemide (furo; 10 mg/kg, SC; Hospira Inc. Lake Forest, IL) along with a low dose of the antihypertensive drug captopril (cap; 5 mg/kg, SC, Sigma Aldrich). When these drugs are administered together, they induce water and sodium depletion (i.e., extracellular dehydration) and slightly reduce blood pressure (Thunhorst & Johnson, 1994). The combination of extracellular dehydration, along with modest hypotension, evokes the intake of water and a rapid onset of salt intake (~1 hour after injections; Fitts & Masson, 1989, 1990).
Retrograde and anterograde tracing experiments
The first set of experiments tested whether a LT-Ox-VTA neural pathway is present in the rat brain by using anterograde and retrograde tracing in combination with immunohistochemistry for Ox-A and tyrosine hydroxylase. In the first anterograde tracing experiment, rats were anesthetized with Nembutal (60 mg/kg), and 10,000 molecular weight biotinylated dextran amine (BDA; 10% in physiological saline) was iontophoresed (+5 μA 7s on/off for 15 minutes) into the SFO (coordinates: −1.1 AP, −5.2 DV from skull) or dorsal MnPO (coordinates: −.4 AP, −6.7 DV). One rat also received a bolus (100 nl) injection of 5% BDA into the SFO. In a second retrograde tracing experiment, Fluorogold (FG; 2% in physiological saline) was iontophoresed ipsilaterally into the PeF (coordinates: −3.2 AP, +1.4 ML, −.8.8 DV). In order to determine whether PeF Ox neurons receive inputs from the LT and to ascertain if PeF neurons project to dopamine neurons in the VTA, a third experiment was conducted where a co-injection of 1.3% FG and 2.5% BDA was iontophoresed into the PeF (coordinates −3.0 AP, +1.2 AP, −8.8 DV). The PeF was selected as the region of interest in the hypothalamus because a relatively large portion of neurons in the PeF project to the VTA (Fadel & Deutch, 2002) and we observed the largest increase in Fos-positive Ox neurons in the PeF (see c-Fos results below). Seven to eight days after tracer injections, rats were perfused and brains were collected.
c-Fos expression during water and sodium consumption
A second experiment examined whether FLI in Ox neurons is associated with the consumption of water and sodium. Rats received vehicle or furo/cap treatment and 90 minutes later were given access to water and 1.8% hypertonic saline (n=3 per group). Rats were allowed to drink for 90 minutes, were then anesthetized and perfused, after which brains were collected for immunohistochemistry.
Orexin type 1 receptor antagonism in the VTA
The final experiment aimed to determine whether Ox type 1 receptor antagonism in the VTA attenuates extracellular dehydration-induced water or sodium intake. At the beginning of the experiment, 13 rats were assigned to the vehicle group and 13 were assigned to the SB-408124 group. Rats were anesthetized with Nembutal® (60 mg/kg) and implanted bilaterally with a 26-gauge bilateral guide cannula aimed at the VTA (coordinates: −5.2 AP, +1.8 ML, −6.2 DV). One week after surgery, a 32-gauge injector that extended 2mm beyond the guide cannula was inserted and vehicle (DMSO) or 300 ng/hemisphere of the Ox type 1 receptor antagonist SB-408124 (Sigma Aldrich, St. Louis, Missouri) was microinjected in a volume of 300 nl per side over 1 minute. Ten minutes after VTA microinjection, rats received furo/cap treatment. In an effort to dissociate water drinking from hypertonic saline intake a water-first/saline-second presentation of fluids after furo/cap administration was used (Hurley & Johnson, 2013): rats were immediately provided water after furo/cap treatment and 90 minutes later were also given access to hypertonic saline. Water and sodium intakes were recorded at 0, 60, 90, 105, 120, and 180 minutes after fluid access. At the end of behavioral testing, rats were anesthetized and 300 nl/hemisphere of India Ink waterproof dye (Dick Blick, Galesburg, IL) was microinjected to identify cannula placements. Rats with placements outside of the VTA were removed from analysis.
Perfusion and sectioning
Rats were heavily anesthetized with Nembutal® (50 mg) and perfused transcardially with PBS followed by 4% paraformaldehyde. Brains were post-fixed in paraformaldehyde for 4–6 hours and then transferred to 20% sucrose dissolved in phosphate buffer and stored at 4°C overnight. The next day brains were sectioned at 30 μm (tracing experiments) or 40 μm (FLI and Ox labeling) with a cryostat set at −20°C. A series of 2 non-consecutive sections were obtained for all tract tracing studies and a series of 3 non-consecutive sections were collected for FLI and Ox double-labeling. Tissue was either stained immediately after sectioning or stored in cryoprotectant at −20°C and stained at a later time.
Immunohistochemistry, tract tracing, and FLI quantification
To ensure reliability of histological procedures, all tissue staining was performed in cohorts such that each batch of processed tissue contained an equal number of rats from each experimental and control group. Tissue was blocked with normal goat serum prior to all staining. FLI was examined by using a rabbit-raised anti-c-Fos antibody (1:2000, Santa Cruz Biotechnology, Dallas, Texas) and Ox-A was labeled with a rabbit-raised anti-orexin-A antibody (1:8000, Phoenix Peptides, Burlingame, CA). This Ox-A antibody does not cross-react with other known hypothalamic peptides and cell body staining was not observed outside of the hypothalamus. Tissue was incubated with the primary antibody for 20 hours at room temperature and then incubated with a biotinylated goat-raised anti-rabbit antibody (1:200, Vector Laboratories, Burlingame, CA) for 1 hour. Tissue was then exposed to avidin-biotin complex for 1 hour (Vector Laboratories, Burlingame, CA). To dissociate FLI from Ox staining in the double-labeling experiment, tissue was stained for FLI first. FLI was visualized using a DAB peroxidase reaction in the presence of nickel and cobalt chloride, which yielded a dark nuclear stain. Afterwards, tissue was incubated with an Ox-A antibody that was visualized with a DAB peroxidase reaction in the absence of nickel and cobalt chloride which produced a light brown cell body stain.
For retrograde and anterograde tracing studies a similar staining protocol was employed, however anti-rabbit Alexa Fluor 647 was used to visualize Ox neurons (1:1000, Jackson ImmunoResearch Laboratories Inc., West Grove, PA) except in one case where DAB was used to visualize anterograde projections (in the presence of nickel and cobalt chloride) and Ox-A expression (in the absence of nickel and cobalt chloride). In all other cases, anterograde tracing was visualized through the use of an Alexa Fluor 555 Streptavidin conjugate (1:1000, Life Technologies, Grand Island, NY). For the BDA and FG co-injection experiment, dopamine neurons were visualized with a rabbit-raised anti-tyrosine hydroxylase antibody (1:1000, AB152; EMD Millipore, Billerica, MA) and Alexa Fluor 647. Fluorescence was captured using TRITC and Cy5 (far red) filters and images were pseudo-colored. No cross expression was observed between Alexa Fluor 555 and Alexa Fluor 647.
Digital images of Ox neurons, the VTA, and the LT were taken using an Olympus IX81 microscope. To improve identification of FLI positive Ox neurons, a stack of digital images were taken at a range of Z-coordinates. Ox and FLI positive neurons were manually counted by an experimenter blind to the treatment conditions using ImageJ (version 1.48, National Institutes of Health). In the experiment where rats were depleted and allowed access to water and 1.8% saline, 3 images of the anterior portion of the VTA were captured and examined for FLI immunoreactivity. The anterior VTA was chosen based upon the coordinates used in the SB-408124 experiment.
Statistics
Student’s t-tests were used to analyze data from all FLI and intake experiments.
Results
Retrograde and anterograde tracing studies
Iontophoretic infusions of BDA into the SFO (n=2), dorsal MnPO (n=2), and a bolus microinjection of BDA into the SFO (n=1) revealed labeling of anterograde projections with varicosities and axon terminals in apposition with Ox neurons (Figure 1). Similarly, the bolus injection resulted in robust hypothalamic labeling. Projections that were in proximity of Ox neurons were observed in all Ox cell-clusters. Iontophoretic application of FG and the combination of FG and BDA into the PeF Ox cell-cluster (n=2 for FG alone, n=1 for the co-injection) found that retrograde labeling occurred across all LT structures (Figure 2). Missed FG injections that were dorsal to Ox neuron clusters either failed to retrogradely label structures along the LT or exhibited sparse retrograde labeling. BDA and FG co-injection also revealed varicosities and axon terminals in apposition to VTA dopamine neurons (Figure 2, panels G-J).
Figure 1 -.
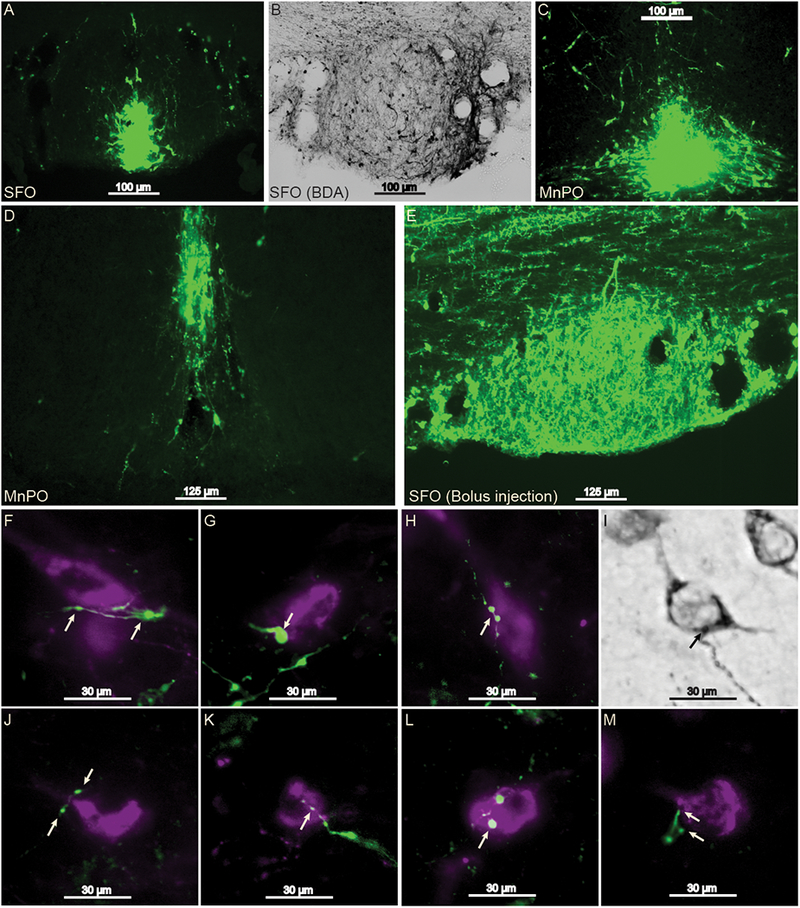
Projections from the SFO and dorsal MnPO to Ox neuron cell bodies in the hypothalamus. 10× magnification of the focal point of iontophoresis injection sites in the SFO (panels A and B; one set of tissue was analyzed with DAB rather than immunofluorescence), dorsal MnPO (C and D), and a bolus injection in the SFO (E). All of the injection sites yielded anterograde labeling that consisted of varicosities or axon terminals in apposition with Ox cell bodies (panels F-M, images taken at 40× magnification). Arrows indicate sites of putative synaptic contact. Green represents BDA labeling and magenta represents Ox labeling. MnPO, median preoptic nucleus; Ox, orexin; SFO, subfornical organ.
Figure 2 –
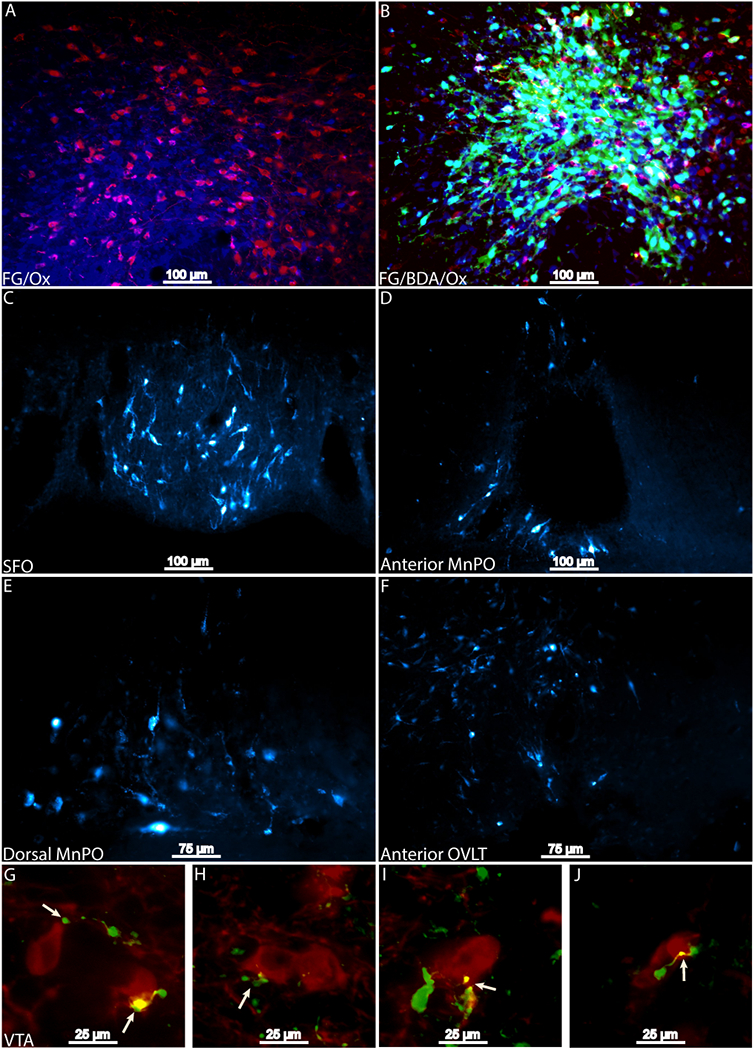
Results from Fluorogold and co-injection of BDA and FG infused into the PeF. Injection sites of 2% FG (blue) into the PeF Ox neurons (red) and a co-injection of 1.33% FG (blue) and 2.5% BDA (green) in PeF Ox neurons (red) are presented in panels A and B, respectively. Retrograde labeling was observed along the entirety of the LT (panels C-F). Axon terminals and varicosities (green) were observed in apposition with tyrosine hydroxylase positive neurons in the VTA (red; panels G-J). Putative synaptic contact is indicated by arrows. BDA, biotinylated dextranamine; FG, Fluorogold; Ox, orexin, PeF, Perifornical Area; SFO, subfornical organ.
FLI during the ingestion of water and sodium
Rats receiving furo/cap that ingested fluids exhibited a significant elevation of FLI in Ox neurons (Figures 3 and 4). Specifically, a greater number of FLI expressing Ox neurons were observed in the DMH t(4)=5.164; p<0.01, PeF t(4)=4.014; p<0.05, and LHAd t(4)=3.56; p<0.05. No significant difference between treatment groups was observed in the total number of Ox neurons quantified in each cell-cluster (all p’s>0.30). Additionally, depleted rats with access to fluids exhibited significantly greater FLI in the anterior region of the VTA t(4)=3.899; p<0.05 (Figure 3D).
Figure 3 -.
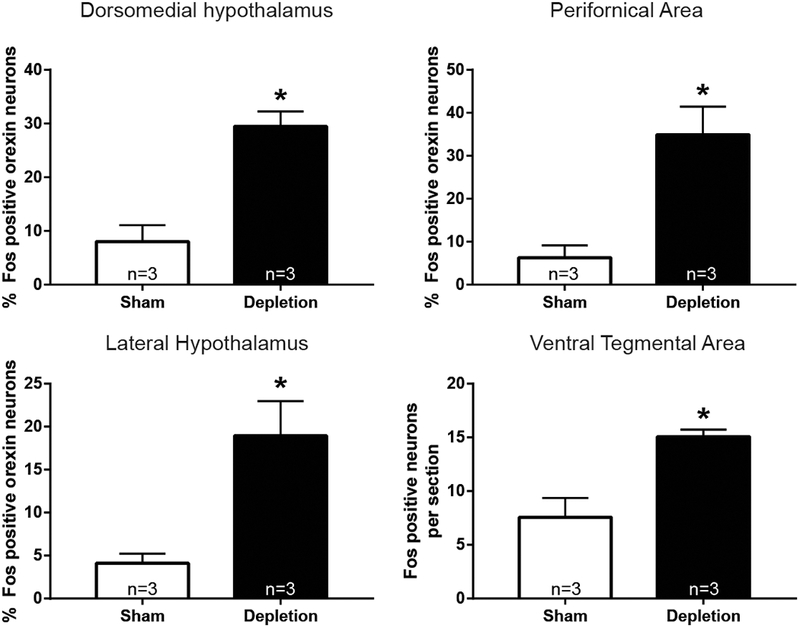
Ingestion of water and salt in depleted rats activates hypothalamic Ox neurons and the VTA. The percentage of Ox neurons that expressed FLI was greater in rats that were depleted and allowed to drink water and sodium across each Ox cluster (*=p<.05). The VTA also exhibited a greater amount of FLI expression in this condition. FLI, Fos-like immunoreactivity; Ox, orexin; VTA, ventral tegmental area.
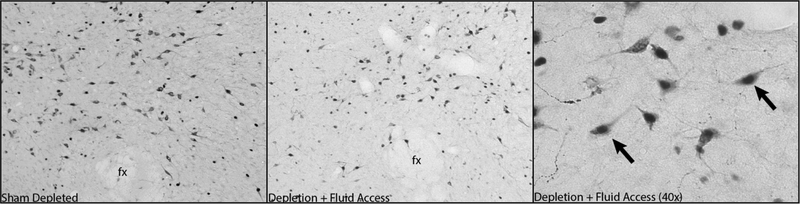
Figure 4 -.
Ox neuron staining in the PeF in rats that were sham depleted and depleted with fluid access. Arrows indicate examples of double-labeled neurons in 40× magnification. fx = fornix; PeF, Perifornical Area.
Orexin type 1 receptor antagonism in the VTA
Analyses revealed that Ox antagonism within the VTA attenuated combined water and sodium intake t(9)=2.78; p<0.05, but a reduction specific to either water or saline intake was not observed (Figure 5).
Figure 5 -.
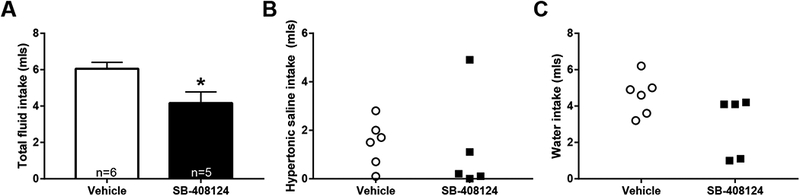
Antagonism of Ox type 1 receptors within the VTA attenuates fluid intake in depleted rats. Compared to vehicle (n=6) pretreated rats, rats pretreated with SB-408124 (n=5) exhibited reduced total fluid intake (A; *=p<.05). Scatterplots display variability in water (B) and sodium (C) intake across treatment conditions. OX, orexin; VTA, ventral tegmental area.
Discussion
The present experiments found evidence for: 1) the presence of a LT-Ox-VTA neural pathway, 2) Ox neuron activation during the ingestion of water and sodium, and 3) the necessity of binding of Ox with its VTA-located receptors for generating a robust deficit-induced thirst and salt appetite. Together, these findings suggest that a LT-Ox-VTA neural pathway operates to enhance the fluid intake exhibited during the consumption of water and sodium. However, it must be cautioned that further work is necessary to establish the functional role of this pathway in fluid intake.
Retrograde and anterograde tracing studies found that LT structures project to Ox neurons and a portion of the PeF that contained Ox neurons projects to VTA dopamine neurons. BDA injections revealed that structures along the LT send projections that are in apposition with Ox neurons. Some of these projections consisted of varicosities while others appeared to be axon terminals. Projections were observed in all Ox neuron cell-clusters. Furthermore, the retrograde tracing studies suggest that Ox neurons located in the PeF receive input from the SFO as well as the MnPO. These findings are consistent with previous reports showing that the SFO innervates the hypothalamus and structures along the LT project to the PeF (Hahn & Swanson, 2010; Swanson & Lind, 1986). Further, a retrograde tracing study employing a genetically-encoded retrograde tracer that specifically labeled Ox-projecting neurons found that the dorsal MnPO sends efferents to Ox neurons, consistent with the present findings (Sakurai et al., 2005). The anterograde and retrograde tracing produced by the co-injection of FG and BDA provides complementary evidence that LT neurons project to the PeF which, in turn, projects to the VTA which contains dopamine neurons. Together, these findings support the hypothesis that the LT is capable of indirectly modulating VTA dopamine neurons through second-order Ox neurons in the hypothalamus.
It should be noted that we also observed projections from the MnPO and SFO distributed throughout the hypothalamus. It is unclear as to what neuronal phenotype these projections were in apposition. For example, they may project to neurons expressing melanin-concentrating hormone (MCH), which is concentrated in the hypothalamus, and MCH stimulates water intake (Clegg et al., 2003). Further, GABAergic neurons in the hypothalamus play a critical role in promoting ingestive behavior (Jennings, Rizzi, Stamatakis, Ung, & Stuber, 2013). Although it was beyond the scope of the current hypothesis that a LT-Ox-VTA pathway promotes fluid intake, future work investigating projections from LT structures to other neuronal phenotypes and the functional role of these projections will likely be fruitful.
When depleted rats were allowed to ingest water and sodium, a significant elevation in the number of FLI positive Ox neurons was observed in the hypothalamus across all Ox neuron cell-clusters. Previous work investigating Ox neuron activation has implicated a role for Ox neurons in promoting intake of salient rewards. For example, intracerebroventricular microinjections of neuropeptide Y, a peptide that induces avid food intake, also induces FLI in Ox neurons (Broberger, De Lecea, Sutcliffe, & Hokfelt, 1998). It has also been shown that Ox neurons exhibit increased firing rates during the consumption of palatable food (Mileykovskiy, Kiyashchenko, & Siegel, 2005). However, Ox neurons are implicated in many coordinated psychological and physiological processes that function to promote motivated behavior. Some of the Fos activity in Ox neurons observed in the present experiments may reflect changes in cardiovascular control (Samson, Bagley, Ferguson, & White, 2007) and psychological arousal, consistent with the motivational activation hypothesis put forth by Mahler and colleagues (Mahler et al., 2014). Finally, others have reported rapid inhibition of Ox neuron activity during eating and have proposed that Ox actually promotes satiety (González et al., 2016). This finding contrasts with our observation that Ox release in the VTA facilitates robust fluid intake. One possibility is that a unique ensemble of Ox neurons induces satiation and it is possible that some of the Fos expression reported here may be consequent of satiation. Future experiments utilizing a gastric fistula and intraoral catheters will allow for fluid delivery directly into the mouth without inducing satiety to determine whether Fos expression in Ox neurons is linked to consumption of fluids independent of satiety.
Ox is capable of promoting the performance of various motivated behaviors (Mahler et al., 2014; Thompson & Borgland, 2011). A mechanism by which Ox may operate to support motivated behaviors is through its release in the VTA (Zheng et al., 2007), which appears to facilitate activity of the mesolimbic dopamine system (Fadel & Deutch, 2002; Korotkova et al., 2003). Ox neurons have been shown to project to the VTA (Fadel & Deutch, 2002; Peyron et al., 1998) and Ox can depolarize both dopaminergic and non-dopaminergic neurons in this structure (Korotkova et al., 2003). Further, Ox release in the VTA promotes pathological motivated behavior such as cocaine seeking (James et al., 2011; Wang, You, & Wise, 2009). The present results show that administration of the Ox type 1 receptor antagonist SB-408124 into the VTA attenuates extracellular dehydration-induced fluid intake. SB-408124 pretreatment did not appear to selectively reduce water or salt intake. This finding is consistent with the idea that Ox plays a general role in promoting appetitive motivated behaviors (Borgland et al., 2009; Hurley & Johnson, 2014; Mahler et al., 2014; Thompson & Borgland, 2011).
There are limitations to the present findings that must be acknowledged. One is that the Ox antagonism and FLI experiments examined thirst and sodium appetite concurrently. Although we used a model of extracellular dehydration that has been found to dissociate thirst and salt appetite (Hurley & Johnson, 2013), this model may not have been ideal given that Ox appears to play a role in both thirst and sodium appetite. That is, FLI expression observed in the present experiments most likely reflects a combination of the effects of water and sodium intake and extracellular dehydration. Additionally, projections from LT structures were observed in apposition with Ox neurons, indicating putative synaptic contact, however, future work employing electron microscopy is necessary to unequivocally demonstrate synaptic contact. It is worth noting, however, that neurons from the dorsal MnPO have been shown to project to Ox neurons in mice (Sakurai et al., 2005). Further, we did not directly assess whether the LT→Ox→VTA pathway is functionally involved in thirst and sodium appetite. A future experiment using optogenetic activation of the LT→hypothalamus pathway along with Ox receptor antagonism in the VTA can provide a more robust test for the role of this pathway in fluid intake.
Perspectives and significance.
It has been proposed that the hypothalamus functions to integrate information about internal homeostatic state with adaptive physiological and behavioral responses (Stellar, 1954; Swanson & Mogenson, 1981), and Ox neurons appear to contribute to this integrative capacity of the hypothalamus (Berthoud & Munzberg, 2011; Hurley & Johnson, 2014; Zheng, Patterson, & Berthoud, 2005). The present findings provide additional support for this concept by showing that structures along the LT, which function to regulate fluid balance, project to Ox neurons, and Ox receptor blockade in the VTA attenuates fluid intake. Furthermore, Ox neurons expressed FLI when rats were allowed to ingest water and sodium. Based upon this, Ox release in the VTA may function to enhance ongoing ingestion of highly salient rewards. Within this context, it is interesting to note that prior reports have stated that Ox prolongs the motivation to eat (Thorpe, Cleary, Levine, & Kotz, 2005) and delays satiety (Rodgers et al., 2000). Under conditions of deprivation (e.g., food, water, or sodium deficiency) it is important that animals maintain ingestion for a sufficient duration in order to restore homeostasis. An ensemble of Ox neurons that project to the VTA could contribute to the considerable intakes observed under conditions of deficiency. It is also likely that highly salient rewards, such as palatable foods or addictive drugs, can activate Ox neurons to enhance intake independent of inputs from homeostatic systems (e.g., hedonic-driven feeding; James et al., 2011; Wang et al., 2009; Zheng et al., 2007).
Acknowledgements
The authors thank Young In Kim for her excellent technical assistance, Dr. Ralph Johnson and Dr. Jason Radley for advice on performing immunohistochemistry, and Marilyn Dennis for comments on the manuscript. This research was supported by National Institutes of Health grants HL14388, HL098207, MH08241, HL073986, and HL84027. The authors have no disclosures to report.
References
- Berthoud HR, & Munzberg H (2011). The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav, 104(1), 29–39. doi: 10.1016/j.physbeh.2011.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, … Bonci A (2009). Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci, 29(36), 11215–11225. doi: 10.1523/jneurosci.6096-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, De Lecea L, Sutcliffe JG, & Hokfelt T (1998). Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol, 402(4), 460–474. [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, & Woods SC (2003). Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 284(2), R494–R499. doi: 10.1152/ajpregu.00399.2002 [DOI] [PubMed] [Google Scholar]
- Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, & Williams AJ (2002). The hypocretin/orexin system. J R Soc Med, 95(5), 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, & Deutch AY (2002). Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience, 111(2), 379–387. [DOI] [PubMed] [Google Scholar]
- Fitts DA, & Masson DB (1989). Forebrain sites of action for drinking and salt appetite to angiotensin or captopril. Behav Neurosci, 103(4), 865–872. [DOI] [PubMed] [Google Scholar]
- Fitts DA, & Masson DB (1990). Preoptic angiotensin and salt appetite. Behav Neurosci, 104(4), 643–650. [DOI] [PubMed] [Google Scholar]
- González JA, Jensen Lise T., Iordanidou P, Strom M, Fugger L, & Burdakov D (2016). Inhibitory Interplay between Orexin Neurons and Eating. Current Biology, 26(18), 2486–2491. doi: 10.1016/j.cub.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, & Swanson LW (2010). Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev, 64(1), 14–103. doi: 10.1016/j.brainresrev.2010.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, & Aston-Jones G (2006). Arousal and reward: a dichotomy in orexin function. Trends Neurosci, 29(10), 571–577. doi: 10.1016/j.tins.2006.08.002 [DOI] [PubMed] [Google Scholar]
- Hurley SW, & Johnson AK (2013). Dissociation of thirst and sodium appetite in the furo/cap model of extracellular dehydration and a role for N-methyl-D-aspartate receptors in the sensitization of sodium appetite. Behav Neurosci, 127(6), 890–898. doi: 10.1037/a0034948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SW, & Johnson AK (2014). The role of the lateral hypothalamus and orexin in ingestive behavior: a model for the translation of past experience and sensed deficits into motivated behaviors. Front Syst Neurosci, 8, 216. doi: 10.3389/fnsys.2014.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SW, & Johnson AK (2015). The biopsychology of salt hunger and sodium deficiency. Pflugers Arch, 467(3), 445–456. doi: 10.1007/s00424-014-1676-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, & Dayas CV (2011). Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. International Journal of Neuropsychopharmacology, 14(5), 684–690. doi: 10.1017/S1461145711000423 [DOI] [PubMed] [Google Scholar]
- Jennings JH, Rizzi G, Stamatakis AM, Ung RL, & Stuber GD (2013). The Inhibitory Circuit Architecture of the Lateral Hypothalamus Orchestrates Feeding. Science, 341(6153), 1517–1521. doi: 10.1126/science.1241812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, & Thunhorst R (2007). The neuroendocrinology, neurochemistry and molecular biology of thirst and salt appetite Handbook of Neurochemistry and Molecular Neurobiology (pp. 641–687): Springer. [Google Scholar]
- Kelley AE, & Berridge KC (2002). The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci, 22(9), 3306–3311 doi:20026361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, & Brown RE (2003). Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci, 23(1), 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, & Sakurai T (1999). Orexins/hypocretins regulate drinking behaviour. Brain Res, 842(1), 256–261. [DOI] [PubMed] [Google Scholar]
- Loriaux AL, Roitman JD, & Roitman MF (2011). Nucleus accumbens shell, but not core, tracks motivational value of salt. J Neurophysiol, 106(3), 1537–1544. doi: 10.1152/jn.00153.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, & Aston-Jones G (2014). Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci, 17(10), 1298–1303. doi: 10.1038/nn.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, & Johnson AK (2004). The physiological regulation of thirst and fluid intake. News Physiol Sci, 19, 1–6. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, & Siegel JM (2005). Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron, 46(5), 787–798. doi: 10.1016/j.neuron.2005.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, & Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol, 14(2–3), 69–97. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, & Kilduff TS (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci, 18(23), 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, & Blundell JE (2000). Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept, 96(1–2), 71–84. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, … Yanagisawa M (2005). Input of Orexin/Hypocretin Neurons Revealed by a Genetically Encoded Tracer in Mice. Neuron, 46(2), 297–308. doi: 10.1016/j.neuron.2005.03.010 [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, & White MM (2007). Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 292(1), R382–R387. doi: 10.1152/ajpregu.00496.2006 [DOI] [PubMed] [Google Scholar]
- Stellar E (1954). The physiology of motivation. Psychological review, 61(1), 5–22. [DOI] [PubMed] [Google Scholar]
- Swanson LW, & Lind RW (1986). Neural projections subserving the initiation of a specific motivated behavior in the rat: new projections from the subfornical organ. Brain Res, 379(2), 399–403. [DOI] [PubMed] [Google Scholar]
- Swanson LW, & Mogenson GJ (1981). Neural mechanisms for the functional coupling of autonomic, endocrine and somatomotor responses in adaptive behavior. Brain Res, 228(1), 1–34. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sanchez-Watts G, & Watts AG (2005). Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression patterns in a new parceling scheme of the lateral hypothalamic zone. Neurosci Lett, 387(2), 80–84. doi: 10.1016/j.neulet.2005.06.066 [DOI] [PubMed] [Google Scholar]
- Thompson JL, & Borgland SL (2011). A role for hypocretin/orexin in motivation. Behav Brain Res, 217(2), 446–453. doi: 10.1016/j.bbr.2010.09.028 [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, & Kotz CM (2005). Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl), 182(1), 75–83. doi: 10.1007/s00213-005-0040-5 [DOI] [PubMed] [Google Scholar]
- Thunhorst RL, & Johnson AK (1994). Renin-angiotensin, arterial blood pressure, and salt appetite in rats. Am J Physiol, 266(2 Pt 2), R458–465. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, & Wise RA (2009). Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry, 65(10), 857–862. doi: 10.1016/j.biopsych.2009.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, & Berthoud HR (2005). Orexin-A projections to the caudal medulla and orexin-induced c-Fos expression, food intake, and autonomic function. J Comp Neurol, 485(2), 127–142. doi: 10.1002/cne.20515 [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, & Berthoud HR (2007). Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci, 27(41), 11075–11082. doi: 10.1523/jneurosci.3542-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]