Abstract
Melanotic Schwannomas (MS) are rare tumors that share histological features with melanocytic tumors and schwannomas. However, their genetics are poorly understood. To elucidate the genetic characteristics of MS, we performed genome-wide studies in a series of cases. Twelve MS cases were available for the study. Genomic DNAs extracted from formalin-fixed paraffin embedded tumor tissues were subjected to copy number (CN) and allelic imbalance (AI) analysis by Single Nucleotide Polymorphism (SNP)-array and screened for mutations in coding exons of 341 key cancer-associated genes using a hybrid capture-based next-generation sequencing (NGS) assay. Sanger sequencing was used to further verify recurrent mutations detected by NGS study. SNP-array analysis revealed remarkably stereotypic chromosomal abnormalities in MS. Hypodiploidy was common, typically involving monosomies of chromosomes 1, 2, and 17. All 12 samples showed mutations in PRKAR1A gene, including 2 cases with 2 mutations each. The 14 mutations were scattered across PRKAR1A, and most were inactivating mutations. AI on 17q, presenting as loss of heterozygosity with or without CN losses, combined with a PRKAR1A mutation was observed in 9/12 MS cases. The remaining 3 cases included the two samples harboring two mutations in PRKAR1A. MS exhibits a stereotypic pattern of chromosomal losses. In contrast, melanomas are typically characterized by the presence of multiple CN aberrations, without demonstrable differences in the frequency of losses and gains. Inactivation of both alleles of PRKAR1A by “two hits” observed in almost all cases underscores the central role of PRKAR1A in the pathogenesis of this neoplasm.
INTRODUCTION
Melanotic schwannoma is a rare tumor with less than 200 cases reported since its first description in 1932 by Millar (Millar, 1932; Rowlands et al., 1987; Thornton et al. 1992; Ranjan et al., 1995; Akiyoshi et al., 2004; Er et al., 2007; Torres-Mora, et al., 2014). These neuroectodermally derived tumors share histopathological and immunohistochemical features with melanomas (Scheithauer et al,. 2007; Wadasadawala et al., 2010). Additionally, the biological behavior of these tumors is unpredictable with no established clinical and/or pathological criteria for recognition of malignant counterparts. Several theories have been proposed regarding their etiology including melanocytic differentiation of Schwann cells, phagocytosis of melanin by Schwann cells and simultaneous presence of two neoplastic populations of proliferating Schwann cells and melanocytes (Smith et al., 2009). Melanotic schwannomas (MS) can be sporadic or occur in Carney complex (CNC). CNC is an autosomal dominant disorder which was originally described in 1985 as “the complex of myxomas, spotty pigmentation and endocrine overactivity” (Carney et al., 1985). Psammomatous melanotic schwannoma is seen in up to 8% of the individuals with CNC (Espiard and Bertherat, 2013).
MS occurs in adulthood, and tumors arising in association with CNC tend to occur at earlier age than sporadic tumors (a peak incidence in the 3rd vs. 4th decade of life) (Hilton and Hanemann, 2014). The tumors are usually deep seated and commonly occur in the spinal canal, paraspinal regions and rarely in organ systems such as the gastrointestinal tract. Their potential for aggressive behavior is underscored by reports of metastases ranging from 13 to 26% (Vallat-Decouvelaere et al., 1999). Their morphological overlap with melanomas poses additional diagnostic challenges. The genetic characteristics of MS are largely unknown except in the context of CNC. In about two-third of CNC cases, germline mutations in PRKAR1A gene on 17q24 encoding protein kinase A regulatory subunit 1-α (RIα) have been reported (Salpea and Stratakis, 2014). To delineate the genetics of apparently sporadic MS, we utilized a genome-wide high-resolution Single Nucleotide Polymorphism (SNP)-array and next-generation sequencing (NGS)-based molecular profiling platform to analyze CN changes, allelic imbalances (AIs) as well as somatic gene mutations. Furthermore, to better distinguish MS from melanomas, we compared the genome-wide DNA CN changes of MS to previously obtained data in melanomas.
MATERIALS AND METHODS
Tumor Collection
This study was conducted with the approval of the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board (IRB Waiver Number: WA0256-13). We collected formalin-fixed paraffin embedded (FFPE) tumor blocks and slides of 12 well-characterized MS cases (SCH1–12) and one tumor of conventional schwannoma with melanin pigment from the case archives of the Department of Pathology at MSKCC. The histopathologic and immunohistochemical findings of all cases were further reviewed by experienced bone and soft tissue pathologists and neuropathologists (MH, MR, and NA). Tumor-rich areas were circled to enrich tumor pure population to enhance sensitivity of genome-wide SNP-array and next generation sequencing analysis. Clinical follow-up was obtained from medical records.
SNP-Array Analysis of the Tumor Genome
Genomic DNA was extracted from FFPE tumor tissues using Qiagen DNeasy Tissue & Blood kit. Genome-wide DNA CN alterations and AIs were analyzed by SNP-array using Affymetrix Onco-Scan FFPE Assay (Affymetrix, CA). We used 80 ng of genomic DNA for each sample. Processing of samples was performed according to the manufacturer’s guidelines (Affymetrix). OncoScan SNP-array data were analyzed by the software couple of OncoScan Console (Affymetrix) and Nexus Express (BioDiscovery, CA) using Affymetrix TuScan algorithm. All array data were also manually reviewed for subtle alterations not automatically called by the software.
NGS-Targeted Sequencing
The tumor DNA samples used for SNP-array analysis were also screened for gene mutations in 341 key cancer-associated genes (gene list is available in Supporting Information Table 1) using solution-phase exon capture and next generation sequencing MSK-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). MSK-IMPACT was designed to focus on somatic mutation detection by filtering out alterations also present in matched normal samples. Matching normal with enough material for MSK-IMPACT study was available for 3 out of 12 MS cases (SCH2, SCH5, and SCH12), and the normal DNAs were sequenced in parallel with the corresponding tumor DNAs. For the remaining 9 MS tumor samples, a mixture of pooled FFPE normal DNA was used as unmatched normal control for mutation calling. Briefly, barcoded sequences are prepared and captured by hybridization with custom biotinylated DNA probes for all exons and selected introns of 341 oncogenes and tumor suppressor genes using 100–250 ng of input DNA. Captured libraries are sequenced on an Illumina HiSeq (2 × 100 bp paired-end reads). Bioinformatics analysis included alignment of reads to the human genome (hg19) using BWA-MEM; duplicate read removal, base recalibration and indel realignment using GATK (v 2.6–5) following best practices; variant calling using MuTect (v 1.1.4) for single nucleotide variants and Somatic Indel Detector (GATK 2.3–9) for indels. Annovar was used to annotate the variants for cDNA and amino acid changes as well as presence in dbSNP database (v137) and COSMIC database (v68) and 1,000 Genomes minor allele frequencies.
PCR-Based Confirmatory Tests for PRKAR1A Gene Mutations
Somatic mutations in PRKAR1A detected by MSK-IMPACT were further verified by Sanger sequencing following PCR (exonic mutations) or RT-PCR (intronic mutations which interrupted splicing). RNA was extracted from SCH1, SCH7, and SCH8 for RT-PCR verification of splicing mutations. Sequences of primers are available in Supporting Information Table 2.
RESULTS
Clinical and Pathologic Features of Melanotic Schwannoma Cases
Tumor sites of the 12 MS cases included sacrum (1), cervical spine (1), paraspinal (3), chest wall (2), thoracic spine (1), spine not specified (1), orbital metastases (1), metacarpal bone (1), and lung (1). The additional case of schwannoma with melanin pigment arose in the presacral space. Histological diagnoses for MS cases were epithelioid melanotic schwannoma (6), psammomatous melanotic schwannoma (2), and the rest 4 samples were spindle or spindle and epithelioid melanotic schwannoma. Four patients (SCH3, 6, 7, and 12) developed metastatic disease (33%), of which two ultimately died of the disease. In all cases mitoses, necrosis, results of immunohistochemical stains included Ki-67 when available were recorded and summarized in Table 1. For genomic analysis, 10 samples were from the primary tumor, SCH6 was from a recurrent lesion, and SCH12 was from an orbital metastases.
TABLE 1.
Clinical and Histological Data of All 12 Melanotic Schwannoma (MS) Cases
| Case # | Age/Sex | Presenting Tumor | Site | MS Histology | Mitoses/HPF | Necrosis | KI-67 | S100 & HMB45 | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| SCH1 | 48/F | Melanotic Schwannoma | Paraspinal | Epithelioid | <1/10 | Present | 5% | pos | NA |
| SCH2 | 36/F | Melanotic Schwannoma | Paraspinal | Epithelioid | None | Present | ND | ND | NA |
| SCH3 | 44/M | Melanotic Schwannoma | Chest wall | Epithelioid | 3/10 | present | ND | ND | Mets/DOD |
| SCH4 | 25/F | Melanotic Schwannoma | metacarpal | Spindle | None | None | ND | pos | NED-3 years-lost to follow-up |
| SCH5 | 44/F | Melanotic Schwannoma | Chest wall | Epithelioid | <1/10 | None | 1% | pos | NED-8 years |
| SCH6 | 53/M | Melanotic Schwannoma | Cervical Spine/recurrent | Psammomatous | None | None | 5–10% | ND | Mets/DOD |
| SCH7 | 72/M | Melanotic Schwannoma | Thoracic spine | Spindle and epithelioid | None | None | 5–10% | Pos | Alive with mets |
| SCH8 | 51/M | Melanotic Schwannoma | Sacrum | Epithelioid | None | None | 1% | pos | NED-2 years |
| SCH9 | 19/M | Atrial Myxoma followed by Melanotic Schwannoma | Paraspinal | Epithelioid | <1/10 | None | <5% | ND | NA |
| SCH10 | 33/M | Melanotic Schwannoma | Spine | Psammomatous | <1/10 | None | ND | Pos | NA |
| SCH11 | 31/M | Melanotic Schwannoma | Lung | Spindle | none | None | 2% | Pos | NED-6 months |
| SCH12 | 51/F | Melanotic Schwannoma | Orbital met | Spindle and epithelioid | 10/10 | Present | ND | ND | Alive with mets |
DOD, died of disease; NED, no evidence of disease; ND, not done; NA, not available, Met: metastasis.
All patients were adults with a median age of 40. Medical record was not available for patient SCH10. As for the other patients, there was no clinical documentation of CNC for anyone, and MS was their presenting tumor except case SCH9. SCH9 was a 19-years old male with a history of atrial myxoma (presenting sign) which had been removed elsewhere and presented to our institution with a back mass that was diagnosed as melanotic schwannoma. Although this patient had a history of atrial myxoma, there was no clinical documentation of CNC in the patient’s medical record.
Copy Number and Allelic Imbalance Analysis by SNP-Array
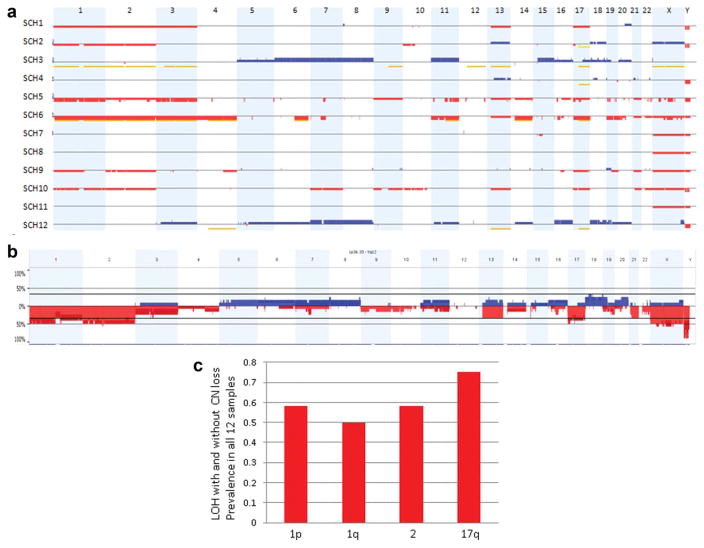
SNP-array analysis showed predominantly CN losses across the genome in MS tumor samples. In contrast, unbalanced genomic aberrations were not detected in the individual case diagnosed with conventional Schwannoma with melanin pigment. CN changes and loss of heterozygosity (LOH) without CN change are displayed for each individual MS case in Figure 1a. The aggregated DNA CN analyses (Fig. 1b) revealed several recurrent unbalanced genomic aberrations. The most frequent changes included losses of 1p (50%), 1q (42%), 2 (50%), and 17 (42%). When integrating LOH with CN alterations, we noticed that unbalanced genomic alterations (AI with or without CN changes) involving chromosomes 1, 2, and 17 were even more dominant in MS (Fig. 1c), and the most frequent recurrent aberration was AI of chromosome arm 17q observed in nine samples (75%), five of which presented as monosomy of chromosome 17, and four had LOH of 17q without DNA CN loss. In the latter group, three cases (SCH2, SCH4, and SCH12) had copy neutral LOH (CN-LOH) of 17q and one case (SCH3) had a trisomy 17q with LOH (uniparental trisomy [UPT]). It is worth noting that cases SCH3 and SCH12 showed multiple chromosomal gains rather than stereotypic pattern of chromosomal losses across the genome, however, LOH without CN loss was also identified in multiple chromosomes typically affected in MS, including chromosome 17, in both samples. Therefore, the combined CN and AI analyses of SCH3 and SCH12 indicated that the distinct tumor genome of each sample may result from doubling of a hypodiploid clone, respectively.
Figure 1.
CN changes and AIs detected in 12 MS tumor samples by OnsoScan SNP-array analysis. A. Heat map of SNP-array results. CN gains (blue) and losses (red) as well as LOH without copy loss (yellow) are displayed for each individual MS case (rows) with chromosomes organized in columns and indicated by labels on the top. B: Genome-wide frequency plot of DNA CN gains (blue) and losses (red) for all 12 melanotic schwannoma samples. C. Frequency plot of unbalanced genomic alterations (AI with or without CN changes) of 1p/1q, 2 and 17 for all 12 MS samples. The most frequent recurrent aberration was AI on chromosome arm 17q observed in nine samples (75%). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Mutation Screening by NGS-Targeted Sequencing
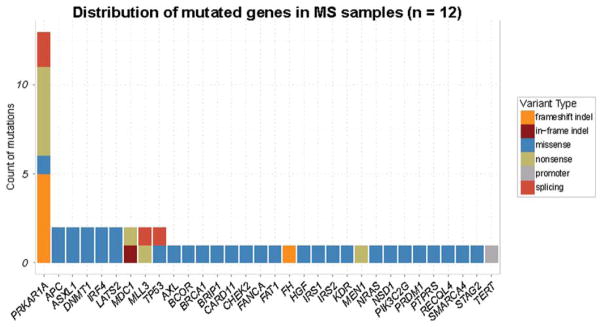
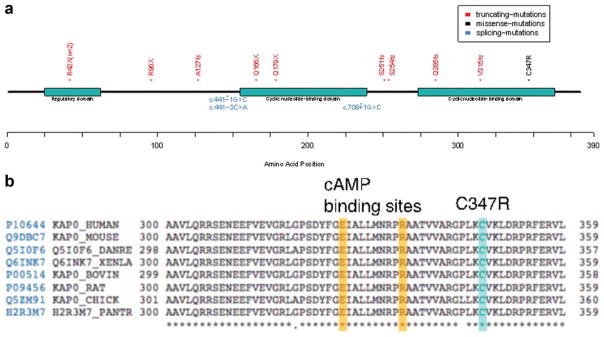
Analysis of sequence variant data showed an average of 4.3 mutations per sample for cases without a matched normal and 1.67 for the three cases with matched normal. All the mutations detected in each MS tumor sample are listed in Supporting Information Table 2 and the distribution of mutated genes in 12 MS samples is illustrated in Figure 2. Interestingly, the targeted sequencing revealed PRKAR1A gene mutations in all MS tumor samples. In contrast, the case of conventional schwannoma with melanin pigment did not show PRKAR1A mutation. SCH7 and SCH8 harbored two mutations in PRKAR1A gene, and the others had one mutation for each sample. The two mutations in SCH8 were occurring in-trans (Supporting Information Figure 1). A schematic presentation of the type and the approximate location of the 14 mutations in PRKAR1A identified in this study are illustrated in Figure 3a. The 14 mutations were scattered across the gene, and most were overtly inactivating mutations which included 5 nonsense mutations and 4 indels that resulted in frame shifts and premature stop codons. Other PRKAR1A mutations included 3 intronic mutations and one missense mutation. Two intronic mutations altered the acceptor splicing site (gt/ag) and the remaining one was a possible splicing mutation (c.441-3C>A in SCH1). The only missense mutation identified in this study, p.C347R (c.1039T>C) in SCH6, is within the cyclic nucleotide binding domain, being adjacent to the cAMP binding sites and affects a residue that is well conserved across different species (Fig. 3b). Therefore, the substitution C347R may also impair PRKAR1A function. In addition, PRKAR1A mutations were verified to be somatic in three cases in which matched normal sample was available (SCH2, SCH5, and SCH12).
Figure 2.
Mutation screening of 12 MS tumor samples by MSK-IMPACT NGS-based analysis. Count of different mutation types in the genes sequenced by MSK-IMPACT. Counts are color coded for the different mutation types [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
PRKAR1A gene mutations detected. A. Protein domain structure of PRKAR1A along with mutations observed in the study. Different mutation types are color coded along the protein axis. B. Multiple alignment of PRKAR1A gene sequence from different organisms showing the highly conserved nature of the site of missense mutation C347R. The alignment also shows the close proximity of the missense mutation to the cAMP binding sites. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
A comprehensive summary of all the PRKAR1A gene mutations identified in this study as well as a comparison to the PRKAR1A mutation database (http://prkar1a.nichd.nih.gov) is presented in Table 2.
TABLE 2.
Summary of All PRKAR1A Mutations Identified in This Study, Correlation with 17q CN Status and Comparison to PRKAR1A Mutation Database (http://prkar1a.nichd.nih.gov)
| SCH # | PRKAR1A Exon | PRKAR1A cDNA | Effect of the mutation | Sanger confirmation | Reported in PRKAR1A mutation database | 17q Status by SNP-array | Both Alleles of PRKAR1A Inactivated |
|---|---|---|---|---|---|---|---|
| SCH1 | 5 | c.441-3C>A | Likely alters the splice acceptor site | NA | No | monosomy 17 | Yes |
| SCH2 | 6 | c.535C>T | p.Q179X | NA | No | CN-LOH 17q | Yes |
| SCH3 | 3 | c.286C>T | p.R96X | Confirmed | Yes | trisomy of 17q21.3-25.3-LOH (UPT) | Yes |
| SCH4 | 8 | c.761_762delCT | p.S254Yfs×15 | Confirmed | No | CN-LOH 17q | Yes |
| SCH5 | 2 | c.124C>T | p.R42X | Confirmed | Yes | Monosomy 17 | Yes |
| SCH6 | 11 | c.1039T>C | p.C347R | Confirmed | No | Monosomy 17 | Yes |
| SCH7 | 2 | c.124C>T | p.R42X | Confirmed | Yes | Normal | Yes |
| 7 | c.708+1G>C | exon 7 skipped (shorter PRKAR1A protein) | Confirmed | Yes | |||
| SCH8 | 5 | c.441-1G>C | Alters the splice acceptor site | Confirmed | No | Normal | Yes |
| 5 | c.496C>T | p.Q166X | Confirmed | Yes | |||
| SCH9 | 9 | c.851dupT | p.Q285Afs×7 | NA | No | Monosomy 17 | Yes |
| SCH10 | 4 | c.380_402del23 | p.A127Efs×8 | Confirmed | No | Monosomy 17 | Yes |
| SCH11 | 10 | c.944delT | p.V315Gfs×16 | Confirmed | No | Normal | No |
| SCH12 | 8 | c.750_761delinsG | p.S251Yfs×15 | Confirmed | No | CN-LOH of 17 | Yes |
NA: not available, confirmatory Sanger sequencing could not be performed due to sample insufficiency CN-LOH, copy neutral-loss of heterozygosity; UPT, uniparental trisomy.
Confirmatory PCR
All exonic mutations except c.535C>T in SCH2 and c.851dupT in SCH9 were confirmed by PCR-Sanger sequencing (see Table 2 for a summary). Among the three intronic splicing mutations, one (c.708+1G>C in SCH7) was confirmed by RT-PCR demonstrating a variant transcript with exon 7 skipped (Supporting Information Figure 2); another one (c.441-1G>C in SCH8) was confirmed at the DNA level. RT-PCR failed in SCH1 and SCH8 due to the very poor quality of FFPE-RNA. PCR-Sanger sequencing data are available in Supporting Information Figure 3. Mutations identified by MSK-IMPACT that could not be evaluated by Sanger sequencing due to unavailability of tumor materials are presented in Supporting Information Figure 4.
DISCUSSION
MS are uncommon pigmented Schwann cell tumors. These tumors show ultrastructural evidence of melanosome formation and are immunoreactive for melanocytic proteins. Therefore, MS often cannot be distinguished from primary melanocytic tumors (Mooi and Krausz, 2007). The genomic aberrations of benign melanocytic tumors and melanomas have been well investigated in the past several years and the CN signature of melanomas are well-established and are used in the diagnostic setting (Curtin et al., 2005; Wang et al., 2013). The genomic characteristics of MS have not been studied so far.
Using the Affymetrix genome-wide SNP-array, we found remarkably stereotypic chromosomal abnormalities in MS. Hypodiploidy was common, and typically involves monosomies of chromosomes 1, 2, and 17. In contrast, melanomas were characterized by the presence of multiple genomic CN aberrations, without differences in the frequency of losses and gains. Recurrent unbalanced genomic aberrations known to occur in association with melanomas are CN increases of 1q, 6p, 7, 8q, 17q, and 20q and frequent losses of 6q, 8p, 9p, 10, and 21q (Curtin et al., 2005; Wang et al., 2013). Therefore, a genome-wide CN analysis can aid in distinguishing melanotic schwannoma and melanoma in histologically ambiguous cases. Additionally, melanocytomas which arise from leptomeninges can pose diagnostic challenges due to morphological and immunohistochemical similarities with melanotic schwannoma. However it has been shown that over 50% of melanocytomas harbor GNAQ and/or GNA11 mutations which can serve as a marker to distinguish these neoplasms (Küsters-Vandevelde et al., 2010a; Koelsche et al., 2014). As reported previously (Küsters-Vandevelde et al., 2010b), none of the MS in our series showed GNAQ or GNA11 mutation.
In addition to the stereotypic CN changes, AI on 17q, inactivation of both alleles of PRKAR1A by “two hits,” that is, one mutation coupled with LOH of 17q or by two mutations, was observed in almost all cases. A correlation of PRKAR1A gene mutation status and AI of 17q in all 12 MS cases is summarized in Table 2. In brief, 5 cases showed monosomy of chromosome 17 with one mutation in PRKAR1A; 4 cases showed CN/CG (copy neutral/copy gain)-LOH of 17q with one mutation in PRKAR1A. In the remaining three cases with allelic-balance 17q, two different mutations in PRKAR1A gene were identified in two (SCH7 and SCH8), and MSK-IMPACT data demonstrated that the two mutations in SCH8 presented in-trans which indicated that they occurred in different alleles of PRKAR1A gene, respectively. We were unable to verify whether or not the two mutations identified in SCH7 occurred in two different alleles due to the distance between them. With the combination of SNP-array and mutation screening analyses, we conclude that inactivation of both alleles of PRKAR1A plays a central role in the pathogenesis of this neoplasm.
Inherited mutations in PRKAR1A have been reported to be present in up to 70% of the patients diagnosed with CNC (Kirschner et al., 2000; Bertherat et al., 2009). PRKAR1A (Protein Kinase, cAMP-Dependent, Regulatory, type I, Alpha) gene encodes one of the regulatory subunits (R1-α) of type I protein kinase A (PKA). The inactive holoenzyme PKA is a tetramer composed of two regulatory and two catalytic subunits. Activation occurs when 2 cAMP molecules bind to each regulatory subunit, eliciting a reversible conformational change that releases active catalytic subunits (Horvath et al., 2010). The gene is located in 17q24 and consists of 11 exons. More than 120 pathogenic mutations of PRKAR1A gene have been reported in CNC patients. A review of the PRKAR1A mutation database (http://prkar1a.nichd.nih.gov) shows that the mutations in PRKAR1A are spread along the entire coding sequence, without significant preference for an exon or a domain. Almost all of the mutations reported result in premature stop codons caused by either nonsense mutations or frameshift mutations, and the mutant mRNAs are predicted to be degraded via nonsense-mediated decay (NMD). As such, PRKAR1A haploinsufficiency was hypothesized to be pathogenic. However, studies examining the consequences of Prkar1a haploinsufficiency in mice have demonstrated that Prkar1a haploinsufficiency acts as a relatively weak tumorigenic signal, which can cooperate with other tumor suppressors or tissue-specific factors or even chemicals to induce tumor (Almeida et al., 2010). In our study, only one case (SCH11) harbored only one heterozygous PRKAR1A mutation, and no evidence of inactivation of the other allele. We speculate that other genetic or epigenetic aberrations may synergize with PRKAR1A haploinsufficiency to induce melanotic schwannoma in this case. Mutations in other genes detected in SCH11 by MSK-IMPACT are listed in Supporting Information Table 2.
Another chromosome locus that has been reported to be associated with CNC is 2p16 (Stratakis et al., 1996; Matyakhina et al., 2003); however, the specific gene(s) remains unknown. In our study, 6 out of the 12 MS tumor samples had CN changes involving chromosome 2, including loss of the entire chromosome 2 in 5 cases and CN-LOH of the entire chromosome 2 in the remaining one case (Fig. 1A). Focal CN change on 2p16 was not found. In addition to the CN and AI analysis, somatic mutations in coding exons of genes EPCAM, MSH2, MSH6, and REL in 2p16 were studied by targeted next generation sequencing (MSK-IMPACT). Somatic mutations were not detected in any of these genes. Please note that other genes in 2p16 were not captured in our targeted-sequencing, therefore, were not studied.
Based on the genome-wide CN and AI findings, the MS cases in this study can be classified as two groups. Group I (SCH1, 2, 3, 5, 6, 9, 10, 12) is characterized by the stereotypic multiple losses of chromosomes and or chromosome arms, and Group II (SCH4, 7, 8, 11) with fairly stable genome (Fig. 1A). It is worth noting that, in addition to inactivation of both alleles of PRKAR1A by “two hits,” TP53 haploinsufficiency/inactivation or complete inactivation of a common tumor suppressor gene (most of which are associated with TP53 pathway) was seen in Group I samples (detailed below). SCH1 and 2 had TP53 haploinsufficiency due to deletion of 17p. SCH5 and SCH10 had monosomy of chromosome 17 and TP53 mutations. Others included a missense mutation of BRIP1 associated with a monosomy of 17 (SCH3); a nonsense mutation of MEN1 gene associated with a monosomy of chromosome 11 (SCH6); a missense mutation of BRCA1 gene associated with a monosomy of chromosome 17 (SCH9); and a missense mutation of LATS2 gene associated with CN-LOH of chromosome 13 (SCH12).
Several mouse and human cancer models have shown that an increase of aneuploid tumor cells was specifically correlated with loss of function of TP53 and/or tumor suppressors such as LATS2 and BRCA1 that synergize with TP53 in ploidy guarding (Aylon and Oren, 2011). Therefore, inactivation of these tumor suppressor genes may lead to a tolerance of aneuploidy, and the aneuploid cell may eventually thrive by accumulating more growth-promoting and transforming genomic alterations. Perhaps reflecting this, clinical follow-up showed that metastasis was seen in three Group I cases and two died of the disease (Table 1). In the remaining cases of Group I, one was disease free at last follow-up (8 years) and no follow-up was available for 4 cases. In contrast, in Group II, three cases were disease free at last follow-up (6 months–3 years) and one is alive with metastasis. Further studies are necessary to verify the correlation between clinical outcome of MS and alterations in the TP53 pathway and other tumor suppressor pathways.
In conclusion, we find that PRKAR1A gene mutation is a recurrent genetic event in MS, which also exhibits a stereotypic pattern of chromosomal losses. In contrast, melanomas are typically characterized by the presence of multiple CN aberrations, without demonstrable differences in the frequency of CN losses and gains. Regarding PRKAR1A mutations, the TCGA data for cutaneous melanoma study at The cBioPortal for Cancer Genomics (Cerami et al., 2012; Gao et al., 2013), showed 3 out of 375 (0.8%) samples bearing PRKAR1A gene mutation. It is worth noting that the PRKAR1A mutations were seen concurrently with BRAF or NRAS mutations in the three samples which had the typical high mutation loads seen in melanoma. This implies that the PRKAR1A mutations likely represent rare secondary genetic events in these cutaneous melanomas. In addition, according to our experience of mutation screening of 34 melanoma samples using MSK-IMPACT, PRKAR1A gene mutation was not identified in any cutaneous melanoma samples. Therefore, we conclude that stereotypic CN changes and recurrent PRKAR1A mutations identified by genome-wide molecular profiling in this study can help distinguish MS from melanomas. Furthermore, inactivation of both alleles of PRKAR1A by “two hits,” that is, either by one mutation coupled with LOH of 17q or by two mutations, was observed in almost all cases, underscoring the central role of PRKAR1A in the pathogenesis of this neoplasm.
Supplementary Material
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Akiyoshi T, Ueda Y, Yanai K, Yamaguchi H, Kawamoto M, Toyoda K, Hayashi T, Ohuchida J. Melanotic schwannoma of the pancreas: report of a case. Surg Today. 2004;34:550–553. doi: 10.1007/s00595-004-2744-2. [DOI] [PubMed] [Google Scholar]
- Almeida MQ, Muchow M, Boikos S, Bauer AJ, Griffin KJ, Tsang KM, Cheadle C, Watkins T, Wen F, Starost MF, Bossis I, Nesterova M, Stratakis CA. Mouse Prkar1a haploinsufficiency leads to an increase in tumors in the Trp53+/− or Rb1+/− backgrounds and chemically induced skin papillomas by dysregulation of the cell cycle and Wnt signaling. Hum Mol Genet. 2010;19:1387–1398. doi: 10.1093/hmg/ddq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Oren M. p53: guardian of ploidy. Mol Oncol. 2011;5:315–323. doi: 10.1016/j.molonc.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, René-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab. 2009;94:2085–2091. doi: 10.1210/jc.2008-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JA, Gordon H, Carpenter PC, Shenoy BV, Go VL. The complex of myxomas, spotty pigmentation, and endocrine overactivity. Medicine (Baltimore) 1985;64:270–283. doi: 10.1097/00005792-198507000-00007. [DOI] [PubMed] [Google Scholar]
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- Er U, Kazanci A, Eyriparmak T, Yigitkanli K, Senveli E. Melanotic schwannoma. J Clin Neurosci. 2007;14:676–678. doi: 10.1016/j.jocn.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Espiard S, Bertherat J. Carney complex. Front Horm Res. 2013;41:50–62. doi: 10.1159/000345669. [DOI] [PubMed] [Google Scholar]
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DA, Hanemann CO. Schwannomas and their pathogenesis. Brain Pathol. 2014;24:205–220. doi: 10.1111/bpa.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath A, Bertherat J, Groussin L, Guillaud-Bataille M, Tsang K, Cazabat L, Libé R, Remmers E, René-Corail F, Faucz FR, Clauser E, Calender A, Bertagna X, Carney JA, Stratakis CA. Mutations and polymorphisms in the gene encoding regulatory subunit type 1-alpha of protein kinase A (PRKAR1A): An update. Hum Mutat. 2010;31:369–379. doi: 10.1002/humu.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- Koelsche C, Hovestadt V, Jones DT, Capper D, Sturm D, Sahm F, Schrimpf D, Adeberg S, Böhmer K, Hagenlocher C, Mechtersheimer G, Kohlhof P, Mühleisen H, Beschorner R, Hartmann C, Braczynski AK, Mittelbronn M, Buslei R, Becker A, Grote A, Urbach H, Staszewski O, Prinz M, Hewer E, Pfister SM, von Deimling A, Reuss DE. Melanotic tumors of the nervous system are characterized by distinct mutational, chromosomal and epigenomic profiles. Brain Pathol. 2014 doi: 10.1111/bpa.12228. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küsters-Vandevelde HV, Klaasen A, Küsters B, Groenen PJ, van Engenvan Grunsven IA, van Dijk MR, Reifenberger G, Wesseling P, Blokx WA. Activating mutations of the GNAQ gene: A frequent event in primary melanocytic neoplasms of the central nervous system. Acta Neuropathol. 2010a;119:317–323. doi: 10.1007/s00401-009-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küsters-Vandevelde HV, van Engenvan Grunsven IA, Küsters B, van Dijk MR, Groenen PJ, Wesseling P, Blokx WA. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 2010b;120:755–764. doi: 10.1007/s00401-010-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matyakhina L, Pack S, Kirschner LS, Pak E, Mannan P, Jaikumar J, Taymans SE, Sandrini F, Carney JA, Stratakis CA. Chromosome 2 (2p16) abnormalities in Carney complex tumours. J Med Genet. 2003;40:268–277. doi: 10.1136/jmg.40.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar WG. A malignant melanotic tumor of ganglion cells arising from thoracic sympathetic ganglion. J Pathol Bacteriol. 1932;35:351–357. [Google Scholar]
- Mooi WJ, Krausz T. Other extracutaneous melanotic tumors. In: Mooi WJ, Krausz T, editors. Pathology of Melanocytic Disorders. 2. London: Hodder Arnold; 2007. pp. 451–454. [Google Scholar]
- Ranjan A, Chacko G, Chandi SM. Intracerebellar melanotic schwannoma: a case report. Br J Neurosurg. 1995;9:687–689. doi: 10.1080/02688699550041007. [DOI] [PubMed] [Google Scholar]
- Rowlands D, Edwards C, Collins F. Malignant melanotic schwannoma of the bronchus. J Clin Pathol. 1987;40:1449–1455. doi: 10.1136/jcp.40.12.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpea P, Stratakis CA. Carney complex and McCune Albright syndrome: An overview of clinical manifestations and human molecular genetics. Mol Cell Endocrinol. 2014;386:85–91. doi: 10.1016/j.mce.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheithauer BW, Louis DN, Hunter S, Woodruff JM, Antonescu CR. Schwannoma. In: Louis DN, Oghaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. 4. Lyon: IARC; 2007. pp. 152–155. [Google Scholar]
- Smith AB, Rushing EJ, Smirniotopoulos JG. Pigmented lesions of the central nervous system: Radiologic-pathologic correlation. Radiographics. 2009;29:1503–1524. doi: 10.1148/rg.295095109. [DOI] [PubMed] [Google Scholar]
- Stratakis CA, Carney JA, Lin JP, Papanicolaou DA, Karl M, Kastner DL, Pras E, Chrousos GP. Carney complex, a familial multiple neoplasia and lentiginosis syndrome. Analysis of 11 kindreds and linkage to the short arm of chromosome 2. J Clin Invest. 1996;97:699–705. doi: 10.1172/JCI118467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton CM, Handley J, Bingham EA, Toner PG, Walsh MY. Psammomatous melanotic schwannoma arising in the dermis in a patient with Carney’s complex. Histopathology. 1992;20:71–73. doi: 10.1111/j.1365-2559.1992.tb00920.x. [DOI] [PubMed] [Google Scholar]
- Torres-Mora J, Dry S, Li X, Binder S, Amin M, Folpe AL. Malignant melanotic schwannian tumor: A clinicopathologic, immunohistochemical, and gene expression profiling study of 40 cases, with a proposal for the reclassification of “melanotic schwannoma”. Am J Surg Pathol. 2014;38:94–105. doi: 10.1097/PAS.0b013e3182a0a150. [DOI] [PubMed] [Google Scholar]
- Vallat-Decouvelaere AV, Wassef M, Lot G, Catala M, Moussalam M, Caruel N, Mikol J. Spinal melanotic schwannoma: a tumour with poor prognosis. Histopathology. 1999;35:558–566. doi: 10.1046/j.1365-2559.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- Wadasadawala T, Trivedi S, Gupta T, Epari S, Jalali R. The diagnostic dilemma of primary central nervous system melanoma. J Clin Neurosci. 2010;17:1014–1017. doi: 10.1016/j.jocn.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Wang L, Rao M, Fang Y, Hameed M, Viale A, Busam K, Jhanwar SC. A genome-wide high-resolution array-CGH analysis of cutaneous melanoma and comparison of array-CGH to FISH in diagnostic evaluation. J Mol Diagn. 2013;15:581–591. doi: 10.1016/j.jmoldx.2013.04.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.