Summary
In this issue, Maertens and colleagues demonstrate that HDAC3 inhibition potentiates the effects of MAPK pathway inhibitors in melanoma, including difficult-to-treat NRAS- and NF1-driven tumors, with MGMT expression serving as a biomarker for responsiveness to the BRAF/MEK/HDAC inhibitor combination. Mechanistically, this triple cocktail suppresses expression of genes involved in DNA repair, leading to enhanced killing of melanoma cells.
Cutaneous melanoma remains by far the most lethal skin cancer. In 2018, there were an estimated 91,270 new melanoma cases and 9,320 melanoma-related deaths in the US (1), and the incidence of this malignancy has risen steadily. Advanced melanoma is notoriously aggressive and resists traditional forms of cancer therapy. Melanomas are characterized by upregulation of MAPK pathway signaling, typically caused by mutations in BRAF, NRAS, NF1, or KIT. The identification of activating mutations in BRAF in roughly half of all cutaneous melanomas provided the rationale for clinical development of BRAF inhibitors, heralding the dawn of targeted therapy for this cancer. Clinically, these drugs are combined with MEK inhibitors to maximize efficacy and reduce side effects (2). Similarly, immune checkpoint inhibitors (ICIs) have proven highly efficacious in melanoma treatment, with some patients experiencing long-term remissions. Although these treatments have revolutionized the therapeutic landscape for patients with advanced melanoma, the sobering fact is that the majority of these patients will still ultimately succumb to their disease. Clinical responses to targeted therapies are usually short-lived. No highly effective targeted therapy exists for melanoma patients with oncogenic drivers besides BRAF. Many patients show primary or acquired resistance to ICIs, and these agents are associated with autoimmune side effects, particularly when administered in combinations. Thus, elucidation of new targets and treatment strategies for advanced melanoma still represents an important unmet oncologic need.
In this issue, Maertens and colleagues explore combining HDAC inhibitors with BRAF and/or MEK inhibitors in melanoma models harboring mutations in BRAF, NRAS, PTEN or NF1 (3). HDAC inhibitors had previously been shown to suppress expression of MITF, an oncogenic transcription factor amplified in a subset of melanomas (4,5). Maertens et al. reasoned that these compounds might potentiate the therapeutic effect of BRAF and MEK inhibitors. Indeed, the pan-HDAC inhibitor vorinostat demonstrated synergy when combined with the MEK inhibitor trametinib, to suppress growth of a subset of melanoma cell lines with varied driver mutations. The authors then showed that simultaneous application of vorinostat with combined BRAF and MEK inhibitors (dabrafenib and trametinib) induced apoptosis in BRAF mutant melanoma cells. Similarly, markedly reduced cell viability was observed upon combining HDAC and MEK inhibitors in a subset of NRAS mutant melanoma cells. Surprisingly, sensitivity to combined HDAC and BRAF/MEK inhibition was independent of MITF expression levels. Via pharmacologic and genetic approaches, the authors showed that silencing HDAC3 alone is sufficient to kill melanoma cells in combination with trametinib.
Next, the authors combined entinostat, the most selective HDAC3 inhibitor, currently in Phase II clinical trials in breast cancer, with BRAF/MEK inhibitors in vivo. Importantly, entinostat enhanced the in vivo response to BRAF/MEK inhibitors, resulting in substantial tumor regression in two BRAF mutant melanoma xenograft models. They then applied entinostat together with BRAF/MEK inhibitors in a BRAF mutant model harboring cooperating mutations in NF1 or PTEN, associated with resistance to BRAF inhibition. Remarkably, this triple combination potentiated the effects of BRAF/MEK inhibitors and triggered substantial tumor regression in genetically engineered allograft models of melanoma harboring Braf/Nf1 or Braf/Pten mutants. Overall, the authors demonstrated that entinostat can enhance the therapeutic effect of BRAF/MEK inhibitors in BRAF mutant models with distinct cooperating mutations and varying sensitivities to standard of care targeted therapy for melanoma. Similarly, combining entinostat with trametinib enhanced tumor regression in an NRAS mutant xenograft model. This represents a very important finding, as currently there are no effective targeted treatments for NRAS mutant tumors.
To identify markers of sensitivity to BRAF/MEK/HDAC inhibitors, the authors compared sensitive and resistant melanoma cell lines, and found that expression of O6-methylguanine DNA methyltransferase (MGMT) was elevated in sensitive cells. This suggests that MGMT expression might serve as a predictive biomarker of sensitivity to the BRAF/MEK/HDAC drug combination. Interestingly, the authors discovered that methylation of the MGMT promoter was substantially lower in sensitive versus resistant melanoma cell lines, and the DNA demethylating agent 5-azacitidine restored MGMT expression in MGMT-low melanoma cells. These results suggest that MGMT promoter methylation testing, currently used in glioblastoma (6), could help to identify melanoma patients responsive to the BRAF/MEK/HDAC inhibitor combination. On the other hand, the authors demonstrate that MGMT does not play a functional role in mediating sensitivity to this drug combination, but rather serves as a marker of sensitivity. Nevertheless, as the MGMT promoter is methylated in <26% of human metastatic melanomas (7), this suggests that majority of melanoma patients might benefit from combination treatment with BRAF/MEK/HDAC3 inhibitors.
The authors then mined TCGA data to characterize differences in gene expression between MGMT-high and -low tumors. They found that expression of genes involved in double-strand break (DSB) repair were suppressed in MGMT-high tumors. DSBs represent a particularly toxic class of DNA lesions, and cells have evolved multiple repair mechanisms to fix them, including non-homologous end-joining (NHEJ) and homologous recombination (HR) (8). Indeed, expression of HR genes was markedly reduced in MGMT-high melanomas. Strikingly, in gene expression profiling studies, the authors found that melanoma cells treated with BRAF/MEK inhibitors also showed reduced HR gene expression. Addition of entinostat suppressed a much larger group of DNA repair genes, including NHEJ factors. These data suggest that combining BRAF/MEK inhibitors with entinostat is efficacious by taking advantage of a preexisting HR defect in a subset of melanomas, inhibiting expression of many DSB repair genes. Functionally, sensitive melanoma cells showed reduced formation of RAD51 foci in response to irradiation, a marker of HR function. Likewise, sensitive, but not resistant cells showed induction of ɣH2AX, a marker of unrepaired DSBs, in response to the BRAF/MEK/HDAC inhibitor combination. Conversely, overexpression of RAD51, or the NHEJ protein LIG4, suppressed cell death in response to the triple drug cocktail.
To further understand how inhibition of MAPK signaling and HDAC3 impairs DNA repair, the authors analyzed genes suppressed upon BRAF/MEK/HDAC inhibitor treatment. This led to the observation that these genes are enriched in binding sites for ETS family transcription factors, particularly ELK1 and ELK3. They showed that treatment with either dabrafenib/trametinib or entinostat inhibited the ELK1-driven transcription program, an effect that was potentiated when the drugs were used in combination. Unexpectedly, simultaneous inhibition of the MAPK pathway and HDAC3 strongly suppressed levels of ELK1 and ELK3. Silencing of ELK genes cooperated with dabrafenib/trametinib or entinostat in suppression of melanoma cell growth. Overall, these studies link inhibition of MAPK signaling and HDAC3 to suppression of ELK transcription factors and subsequent disruption of DSB repair.
In summary, Maertens et al. demonstrate that HDAC inhibitors potentiate the effect of MAPK pathway inhibitors -- the standard of care targeted therapy in BRAF mutant melanomas -- in BRAF, NRAS, PTEN and NF1 mutant melanoma (Fig. 1). This study raises the exciting possibility that combining epigenetic-based therapy, such as HDAC3 inhibition, with BRAF/MEK inhibitors may provide a novel strategy to improve the depth and durability of therapeutic responses to targeted therapy in melanoma patients with different oncogenic drivers. Since entinostat is currently in phase II clinical trials for breast cancer, clinical translation of the BRAF/MEK/HDAC inhibitor cocktail could be readily explored. There are, however, important questions remaining for future studies. If indeed the major effects of combined BRAF/MEK/HDAC3 inhibition occur via suppression of DSB repair, then it may be possible to enhance the potency of this regimen still further by adding a genotoxic therapy, e.g. irradiation or temolozomide -- the latter is an alkylating agent previously used in the treatment of melanoma. Notably, the authors identified oxidative phosphorylation as another gene signature suppressed by BRAF/MEK/HDAC3 inhibition. BRAF inhibitors enhance oxidative metabolism in melanoma cells, rendering them more susceptible to respiratory chain inhibition (9). Thus it may be worth exploring potential synergies between BRAF/MEK/HDAC inhibitors and mitochondrial inhibitors. In this regard, a recent publication showed that HDAC inhibitors are toxic to melanomas that have developed resistance to MAPK inhibition, via a mechanism involving increased reactive oxygen species (ROS) (10). Maertens et al. rule out ROS as the primary driver of effects they observe. It is likely that MAPK pathway and HDAC inhibition interact with each other via multiple mechanisms in melanoma, including impairing DSB repair, suppressing MITF expression, and altering cellular redox.
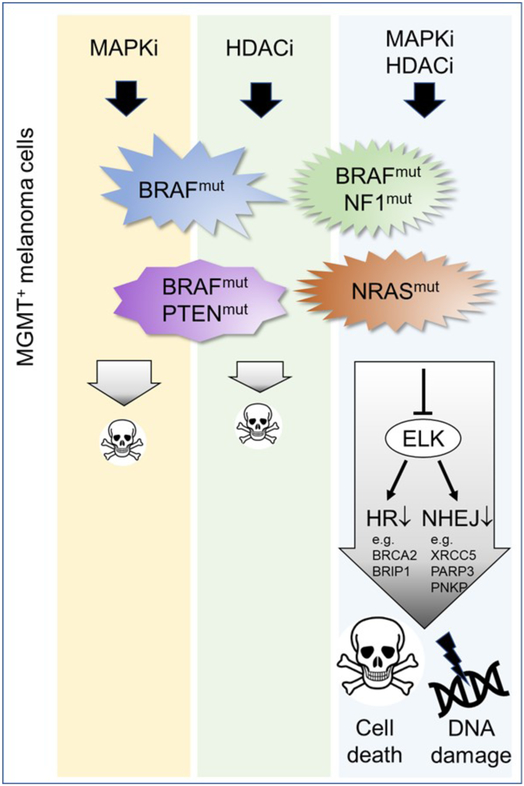
Figure 1.
Combination of MAPK pathway inhibitors dabrafenib/trametinib (MAPKi) with the HDAC3 inhibitor entinostat (HDACi) is highly efficacious against various sub-types of melanoma with BRAF, NRAS, PTEN and NF1 mutations expressing MGMT. Treatment of melanoma cells with the cocktail of MAPKi and HDACi suppresses expression of ELK transcription factors and impairs expression of genes involved in HR and NHEJ DSB repair pathways, potentiating DNA damage and cell death.
Since melanomas are so adept at developing therapeutic resistance, it is important to consider how they might adapt to combined BRAF/MEK/HDAC3 inhibition. A major question is whether the HR-high versus -low states are fixed in any given melanoma patient, or whether melanoma cells might be capable of interconverting between them, potentially permitting emergence of drug-resistant populations. Finally, another key question remains whether the strategy described in this paper would prove efficacious in uveal melanoma, a primary eye tumor typically incurable once metastatic, and/or in difficult-to-treat RAS-driven malignancies, e.g. lung and pancreatic carcinoma.
Acknowledgements:
Drs. Mohammad Fallahi-Sichani and Meenhard Herlyn are gratefully acknowledged for helpful feedback on the manuscript. Support: Melanoma Research Alliance, NIH R01GM101171 (DL); NIH 1R01CA200660 and R01CA201204 (JG); LLS Scholar 1340–17 (TC); DoD CA17068 (DL/JG) and NF17004 (DL); and Rogel Cancer Center support (DL/JG).
Footnotes
Conflict of interest.
The authors declare the following competing financial interest(s): Drs. Grembecka and Cierpicki received research support from Kura Oncology, Inc. They have also an equity ownership in Kura Oncology, Inc. Dr. Lombard has an equity ownership in Abbvie, Giled Sciences, Illumina, Infinity Pharmaceuticals, Lannett Co Inc. and TG Therapeutics Inc.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30 doi 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 2017;14(8):463–82 doi 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 3.Maertens O, Kuzmickas R, Manchester HE, Emerson CE, Gavin AG, Guild CJ, et al. MAPK pathway suppression unmasks latent DNA repair defects and confers a chemical synthetic vulnerability in BRAF, NRAS, and NF1 mutant melanomas. Cancer Discovery 2019. (This issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama S, Feige E, Poling LL, Levy C, Widlund HR, Khaled M, et al. Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res 2008;21(4):457–63 doi 10.1111/j.1755-148X.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannessen CM, Johnson LA, Piccioni F, Townes A, Frederick DT, Donahue MK, et al. A melanocyte lineage program confers resistance to MAP kinase pathway inhibition. Nature 2013;504(7478):138–42 doi 10.1038/nature12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352(10):997–1003 doi 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.Tuominen R, Jewell R, van den Oord JJ, Wolter P, Stierner U, Lindholm C, et al. MGMT promoter methylation is associated with temozolomide response and prolonged progression-free survival in disseminated cutaneous melanoma. Int J Cancer 2015;136(12):2844–53 doi 10.1002/ijc.29332. [DOI] [PubMed] [Google Scholar]
- 8.Her J, Bunting SF. How cells ensure correct repair of DNA double-strand breaks. J Biol Chem 2018;293(27):10502–11 doi 10.1074/jbc.TM118.000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haq R, Shoag J, Andreu-Perez P, Yokoyama S, Edelman H, Rowe GC, et al. Oncogenic BRAF regulates oxidative metabolism via PGC1alpha and MITF. Cancer Cell 2013;23(3):302–15 doi 10.1016/j.ccr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Leite de Oliveira R, Huijberts S, Bosdriesz E, Pencheva N, Brunen D, et al. An Acquired Vulnerability of Drug-Resistant Melanoma with Therapeutic Potential. Cell 2018;173(6):1413–25 e14 doi 10.1016/j.cell.2018.04.012. [DOI] [PubMed] [Google Scholar]