Abstract
Aims/hypothesis:
Excess adiposity is commonly associated with insulin resistance, which can increase the risk of cardiovascular disease. However, the exact molecular mechanisms by which obesity results in insulin resistance are yet to be clearly understood. The intracellular nutrient sensing protein, mechanistic target of rapamycin (mTOR), is a critical signaling component in the development of obesity-associated insulin resistance. Since increased tissue activation of mTOR complex-1 (mTORC1) occurs in obesity, diabetes, and aging, we hypothesized that pharmacologic inhibition of mTORC1 would improve metabolic dysregulation in diet-induced obesity.
Methods:
We administered continuous rapamycin, a specific mTORC1 inhibitor, orally to C57BL/6J mice concurrently with a high fat, high sucrose (HFHS) diet for 20 weeks. The control group received placebo microcapsules.
Results:
Rapamycin-treated mice showed significantly reduced weight gain and adiposity (33.6 ± 4.9 vs 40.4 ± 3.0 % body fat, p<0.001, n=8 mice/group), despite increased or equivalent food intake compared to placebo. The rapamycin-fed mice also demonstrated reduced plasma glucose (252 ± 57 vs 297 ± 67 mg/dL, p<0.001) and improved insulin sensitivity during insulin and glucose tolerance testing. Rapamycin-treated mice also had lower plasma triglycerides (48 ± 13 vs 67 ± 11, p<0.01) and hepatic triglyceride content (89 ± 15 vs 110 ± 19, p<0.05) compared to placebo.
Conclusions/interpretation:
A moderately low dose of rapamycin decreased adiposity and improved the metabolic profile in a model of diet-induced obesity. These data suggest that low-grade chronic mTORC1 inhibition may be a potential strategy for anti-obesity therapies.
Keywords: insulin sensitivity, mTORC1, white adipose tissue, browning
INTRODUCTION
Obesity, driven by chronic caloric excess and sedentary lifestyles, presents a major public health issue in most societies. Excess adiposity is accompanied by a chronic inflammatory state with alterations in the adipose tissue immune milieu. Metabolic derangements that are commonly associated with obesity include insulin resistance, type 2 diabetes, fatty liver disease and cardiovascular disease (Olefsky & Glass, 2010).
Mechanistic target of rapamycin (mTOR) is a highly conserved, ubiquitous serine-threonine protein kinase that functions to integrate signals from growth factors and nutrients (Sarbassov et al., 2005; Wullschleger et al., 2006; Sengupta et al., 2010). Abnormal, increased mTOR-complex1 (mTORC1) signaling has been demonstrated in metabolic diseases, cancers and neurological disorders (Dann et al., 2007; Tsang et al., 2007). In rodent models of diet-induced obesity and insulin resistance, sustained mTORC1 activation in liver and skeletal muscle has been observed (Khamzina et al., 2005; Korsheninnikova et al., 2006). Deletion of adipocyte specific mTORC1 protein Raptor results in mice that are lean and insulin sensitive with enhanced fatty acid oxidation (Polak et al., 2008); similarly whole body deletion of S6K1, the downstream substrate of mTORC1, improves insulin sensitivity and extends lifespan in mice (Um et al., 2004; Selman et al., 2009). These findings suggest that mTORC1 plays an important role in regulation of insulin sensitivity. Thus it would be predicted that inhibition of mTORC1 pharmacologically may have important implications for glucose homeostasis, and that rapamycin, an mTORC1 inhibitor, would act in a similar manner.
Since mTORC1 activity is increased in obesity and diabetes, its inhibition in chronic metabolic disease offers an attractive therapeutic opportunity. Rapamycin (sirolimus), a widely used immunosuppressant (Cruzado, 2008), is primarily a specific mTORC1 inhibitor and could be anticipated to mimic the beneficial metabolic effects of longevity. However, the clinical use of rapamycin in transplantation is plagued with significant metabolic dysregulation including hyperlipidemia and hyperglycemia and its effects on the immune system are quite complex (Morrisett et al., 2002; Teutonico et al., 2005). In rodent models, rapamycin has been utilized to evaluate effects on glucose tolerance with conflicting results so far. Many published studies have clearly demonstrated that rapamycin administration leads to the development of hyperglycemia, increased insulin levels, and glucose intolerance (Aggarwal et al., 2006; Chang et al., 2009b; Cunningham et al., 2007; Fraenkel et al., 2008; Houde et al., 2010; Paschoal et al., 2017; Yang et al., 2012). Similarly, it is well established that transplant subjects receiving rapamycin develop abnormal metabolic profiles (Teutonico et al., 2005; Johnston et al., 2008). However, a handful of studies have shown that rapamycin improves glucose metabolism in mice (Deepa et al., 2013; Makki et al., 2014; Reifsnyder et al., 2016). Nevertheless, low-dose rapamycin has been shown to consistently extend life span in older mice in several studies (Harrison et al., 2009; Anisimov et al., 2011; Miller et al., 2011; Neff et al., 2013), and to abolish cognitive defects in a mouse model of Alzheimer’s disease (Spilman et al., 2010; Lin et al., 2017), suggesting that mTORC1 inhibition may be beneficial in the appropriate setting.
Nutrient overabundance is associated with increased mTORC1 activity in insulin-sensitive tissues including liver and muscle (Korsheninnikova et al., 2006). Thus, we hypothesized that the metabolic consequences of overnutrition can be improved by mild suppression of mTORC1 activity. Therefore, in the present study, we examined the effects of chronic pharmacologic inhibition of mTORC1 using oral rapamycin administered at a low-moderate dose concurrently with a high fat high sucrose diet. We utilized C57BL/6 mice which are susceptible to excessive weight gain when fed diets rich in refined sugar and saturated fat. We show here that chronic oral rapamycin does not induce hyperglycemia or hyperlipidemia, but in fact has beneficial effects on the metabolic abnormalities that accompany obesity in this model.
METHODS
Ethical approval:
All experimental procedures in mice were undertaken with approval from the Institution Animal Care and Use Committee of the University of Washington (protocol #3104–01 03/15/13 – 02/28/19) and followed the guidelines of the National Institutes of Health guide for the care and use of laboratory animals (NIH publications No. 8023, revised 1978). Pain and suffering were kept to a minumim whenever possible. The work described herein complies with the animal ethics principles maintained by the journal.
Animals and diet:
C57BL/6J male mice were obtained commercially (Jackson Laboratories, Sacramento CA, USA), and were fed a high fat, high sucrose diet (HFHS, Bioserv F1850, Frenchtown, NJ, USA) ad libitum starting at age 10 weeks for 20 weeks. This diet provides 36% fat as lard and 36% carbohydrates as sucrose and thus 60% total calories from fat. Microencapsulated oral rapamycin (obtained from Dr. Randy Strong, University of Texas, San Antonio), at the dose of 14ppm (2.24 mg/kg/day) as described by Harrison et al (Harrison et al., 2009) or empty microcapsules for placebo (n=10 mice per group) were incorporated in the diets (Bio-Serv). Mice were group housed (4–5 mice/cage), randomized into treatment groups, and maintained in a temperature- and light-controlled specific pathogen-free facility with ad libitum access to food and water on a 12-hour standard light/dark cycle. Body weights were measured weekly. Food intake was recorded after 5, 9 and 13 weeks of diet and calculated as an average of three sequential days from a known amount of food given. The food was reweighed daily and the amount of food consumed was calculated. At sacrifice, mice were anesthetized by continuous isoflurane inhalation, approximately 1 mL of blood was collected through the retro-orbital sinus, and mice were euthanized by cervical dislocation while still under continuous isoflurane anesthesia. Following perfusion of phosphate-buffered saline through the left ventricle, harvested epididymal white adipose tissue, inguinal white adipose tissue, and liver were snap-frozen in liquid nitrogen and stored at −80°C, or were fixed with 10% neutral-buffered formalin and embedded in paraffin wax.
Analytic procedures:
Metabolic variables were measured in blood samples (50–100 μL), collected every 4 weeks and during oral glucose tolerance tests, that were obtained from the retro-orbital sinus after a 4h fast during the light cycle in mice that had been anesthetized using isoflurane inhalation. Whole blood rapamycin levels were measured from 200 uL of blood by high performance liquid chromatography (HPLC) through the Nutrition and Obesity Research Center of the University of Washington. Whole blood glucose was measured using a One Touch Ultra glucometer (Life Scan, Milpitas, CA, USA). Cholesterol, triglycerides and free fatty acids in plasma were measured using colorimetric assay kits within 24h of collection. Plasma insulin and serum amyloid A (SAA) levels were measured using ELISA as previously described (Subramanian et al., 2008). Hepatic triglyceride content was determined following lipid extraction using the Folch method (Folch et al., 1957). Intra-peritoneal glucose (1 mg/kg) and insulin (0.75 U/kg) tolerance tests were performed after a 4h fast as described previously (Subramanian et al., 2008). Body composition was performed noninvasively on conscious immobilized mice (n=7–8 mice/group) using quantitative magnetic resonance (EchoMRI whole body composition analyzer, Echo Medical Systems, Houston, TX, USA) by the University of Washington Nutrition Obesity Research Center Energy Balance Core, as described previously (den Hartigh et al., 2017). All analyses were performed in a blinded manner.
Real time PCR:
Total RNA was extracted from ~100mg of whole adipose or liver using a commercially available RNA extraction kit according to the manufacturer’s protocol (Agilent Technologies, Santa Clara, CA, USA). After spectroscopic quantification, 2 μg of RNA was reverse-transcribed and cDNA thus obtained was analyzed by real-time quantitative PCR by standard protocols using an ABI 7900HT instrument. Primer and probe sets for individual genes were purchased from Applied Biosystems (Assay-on-Demand, Life Technologies, Carlsbad, CA, USA). GAPDH was used as the control housekeeping gene. Relative amounts of the target gene were calculated using the ΔΔCt formula.
Histology and immunohistochemistry:
Formalin-fixed, paraffin-embedded adipose tissues were used. Macrophages in adipose tissue were detected using a rat monoclonal antibody (Mac2; titer 1:2500, Cedarlane Laboratories, Burlington, NC, USA). Liver sections were stained with Masson’s Trichrome staining using standard protocols. Adipocyte cross-sectional area was measured as previously described (Subramanian et al., 2008).
Immunoblotting:
Mice were fasted overnight and injected with 1 U/kg body weight insulin or vehicle (normal saline) intraperitoneally and sacrificed 10 minutes later. Tissues harvested were snap frozen at −8°C until further analysis. Tissues were homogenized in cold RIPA buffer supplemented with protease and kinase inhibitors, incubated on ice and centrifuged. Protein concentration was measured using the BCA Protein Assay (Thermoscientific, Rockford, IL, USA). Western blotting analysis was performed on equal amounts of protein using standard protocols with antibodies against phospho-Akt (Ser 473, Cell Signaling #4060), total Akt (Cell Signaling #9272), phospho-p70 S6 Kinase 1 (Thr 389, Cell Signaling #9234), total p70 S6 Kinase 1 (Cell Signaling #9202), phospho-S6 (Ser 235/236, Cell Signaling #4857), total S6 (Cell Signaling #2212), phospho-GSK3β (Cell Signaling #9336), total GSK3β (BD Transduction #610202), or Rictor (Cell Signaling #2140), normalized to Tubulin (Sigma (DMIA) #T9026). All antibodies were used at a 1:1000 dilution according to recommendations by the manufacturers. Band density was quantified from three replicate blots using NIH ImageJ software.
Statistical analyses:
Sample sizes were determined using power calculations from previous studies evaluating weight gain in C57Bl/6 mice fed a HFHS diet. In order to see >2-fold significant differences at 90% power with α=0.05, we used 10 mice/group. Data were analyzed using the GraphPad Prism 6 program (GraphPad Software Inc., La Jolla, CA, USA) and are represented as means ± standard deviations. Student’s t-test (two-tailed) or two-way ANOVA were used to detect differences between groups and across multiple time points, as appropriate. A p value <0.05 was considered as statistically significant.
RESULTS
Rapamycin administered chronically in a high fat, high sucrose diet decreases weight gain and adiposity
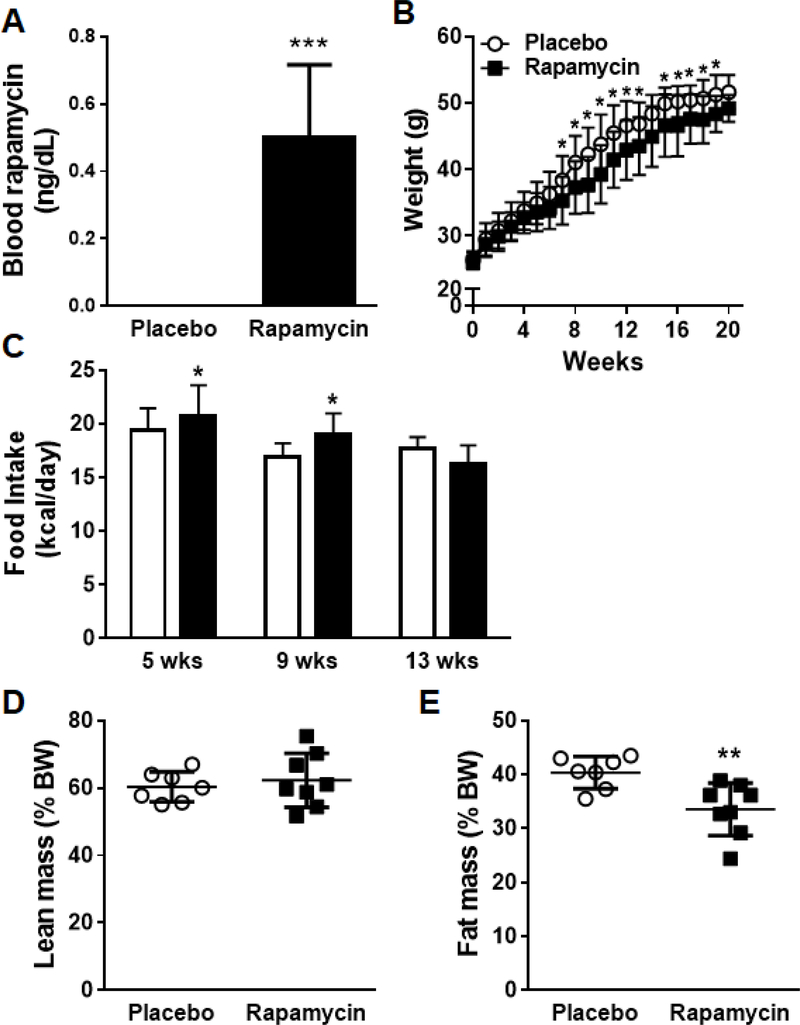
We first evaluated the effects of a low-moderate dose of oral rapamycin on weight gain. C57BL/6J mice were placed on a HFHS diet admixed with rapamycin or placebo for 20 weeks. The dose of rapamycin utilized was that which has been shown to prolong life span in mice (Harrison et al., 2009; Miller et al., 2014). Mice appeared healthy and tolerated rapamycin. We confirmed that rapamycin levels were detectable in the blood of mice which received the drug at 2–4 weeks (Figure 1A). Mice which received dietary rapamycin gained less weight compared to placebo-fed HFHS fed animals (Figure 1B). The lesser degree of weight gain was noticeable at around 8–10 weeks on diets and was despite increased or equivalent food intake in the animals that received rapamycin (Figure 1C). Body composition analysis revealed decreased generalized body fat distribution in rapamycin-fed mice without differences in lean body mass (Figure 1D, E). The difference in body fat was not due to due to changes in epididymal fat pad size which were not different between the two groups (not shown), suggesting that the difference in adiposity was likely due to differences in other fat depots.
1. Mice fed rapamycin in conjunction with a high fat, high sucrose (HFHS) diet show reduced adiposity.
(A) Rapamycin levels in the blood after 2–4 weeks on placebo or rapamycin-containing diets, (B) Body weight gain was measured weekly for the duration of the study, (C) Food intake measured at 5, 9 and 13 weeks, (D) Whole body fat mass and (E) lean body mass measured by body composition analysis. White bars: placebo-fed mice; black bars: rapamycin-fed mice, n=7–10 per group, *p<0.05, **p<0.01 vs placebo.
Chronic rapamycin treatment with a HFHS diet improves metabolic abnormalities associated with obesity
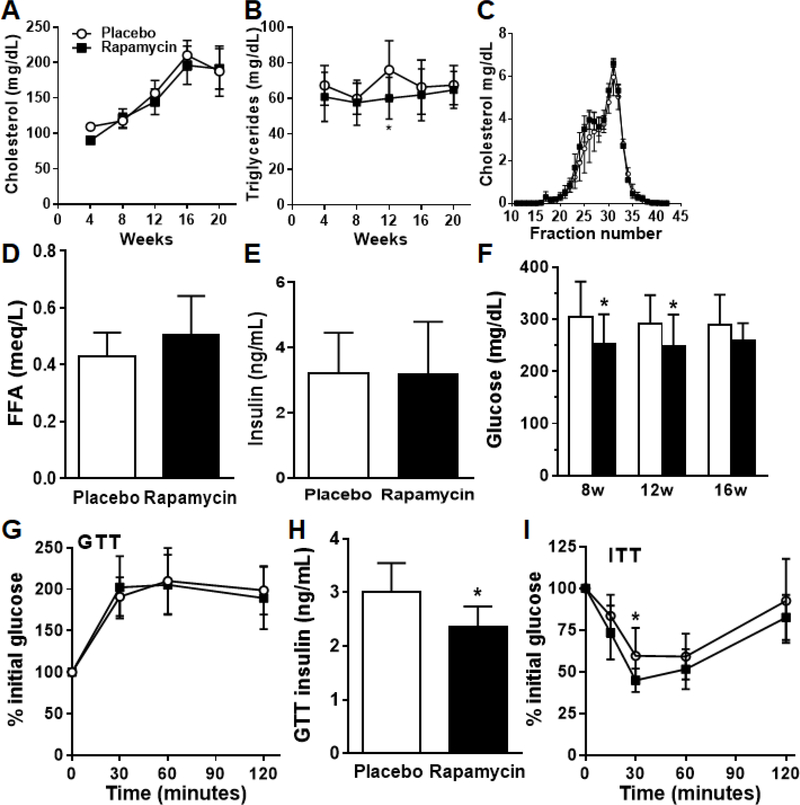
Since rapamycin causes lipid abnormalities when given in the clinical setting (Kasiske et al., 2008), we measured plasma lipid levels. Interestingly, rapamycin-fed animals did not demonstrate worsening of hypercholesterolemia (Figure 2A). Hypertriglyceridemia is a common side effect of rapamycin in lean mice and organ transplanted humans; however, in our study we observed no increases in triglyceride levels above the placebo mice and in fact observed a reduction in levels at the 12-week time point (Figure 2B). There were no differences in plasma lipoprotein fractions on FPLC (Figure 2C). Circulating free fatty acids and insulin levels were not different between the two groups (Figure 2D–E). Fasting hyperglycemia observed in the HFHS placebo animals was significantly reduced in the rapamycin fed animals across all measured time points on diets (Figure 2F). Intraperitoneal glucose tolerance testing revealed no differences in glucose excursions in the rapamycin compared to placebo HFHS-fed control mice (Figure 2G). However, mice given rapamycin exhibited lower plasma insulin levels at the 30 minute time point during the GTT, suggesting improved insulin sensitivity (Figure 2H). In line with this, insulin tolerance testing revealed an improvement in the rapamycin fed mice (Figure 2I). Collectively, these data suggest that administration of rapamycin in conjunction with a HFHS diet improved insulin sensitivity.
2. Chronic rapamycin treatment in a HFHS diet does not adversely affect dyslipidemia and improves hyperglycemia.
(A) Plasma cholesterol and (B) triglycerides over the course of 20 weeks in mice fed rapamycin or placebo. (C) Lipoprotein cholesterol distribution, (D) free fatty acids, (E) fasting plasma insulin, and (F) fasting blood glucose at 20 weeks on diets. (G) Intra-peritoneal glucose tolerance test (GTT) after a 5h fast at week 14 on diets. (H) Plasma insulin measured at the 30 minute time point during the GTT. (I) Insulin tolerance testing performed 1 week after GTT in the same mice. White bars: placebo-fed mice; black bars: rapamycin-fed mice, n=8–10 per group, *p<0.05, **p<0.01 vs placebo.
Adipose tissue inflammation is not altered in obese mice with chronic rapamycin treatment
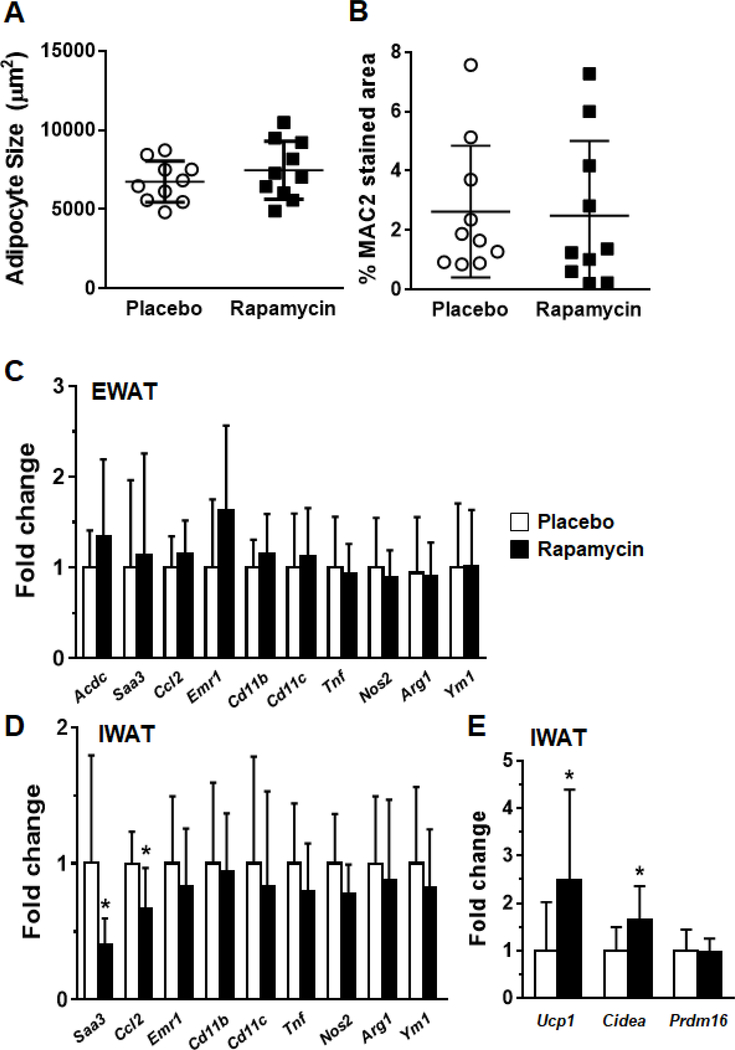
Since adipose tissue macrophage accumulation is a critical feature in obesity, we examined whether rapamycin treatment influenced macrophage recruitment to adipose tissue. Adipocyte size was not different between the rapamycin and placebo groups in epididymal fat pads (Figure 3A). No differences were observed in adipose tissue macrophage immunostaining with the macrophage specific protein MAC2 (Figure 3B). Expression of the adipokine adiponectin was increased in whole epididymal adipose tissue, but no differences were observed in macrophage or chemokine gene expression patterns in epididymal fat pads (Figure 3C). However, in inguinal fat we observed a decrease in the chemotactic genes Ccl2 and Saa3, as well as increased expression of Ucp1 and Cidea, genes associated with browning of adipocytes (Figure 3D,E).
3. Rapamycin does not influence adipose tissue inflammation in obese mice.
(A) Adipocyte size in perigonadal fat pads, (B) Quantification of MAC2 staining in adipose tissue sections stained with the macrophage specific antibody, (C) Gene expression in whole epididymal adipose tissue, and (D) inguinal (subcutaneous) tissue, (E) Brown fat specific genes in inguinal adipose tissue. White bars: placebo-fed mice; black bars: rapamycin-fed mice, n=8–10 per group, *p<0.05 vs placebo.
Rapamycin reduces hepatic steatosis without affecting inflammation during diet-induced obesity
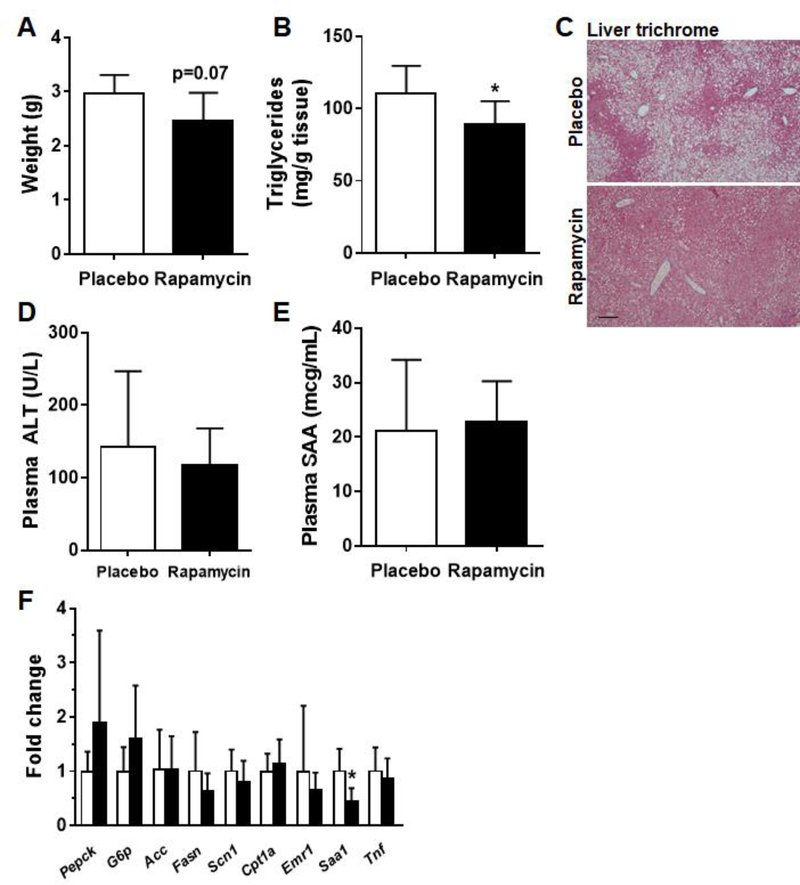
Changes in the liver often accompany those seen in adipose tissue with diet induced obesity. Therefore, we next determined whether rapamycin affects hepatic steatosis and inflammation in diet-induced obesity. Liver weights trended downwards in the rapamycin fed animals but did not reach statistical significance (Figure 4A). Hepatic triglyceride content was decreased in the mice which received rapamycin (p<0.05, Figure 4B). Correspondingly, hepatic steatosis was significantly decreased in the rapamycin-fed animals compared to placebo-fed animals (Figure 4C). There was no evidence of overt liver toxicity in the rapamycin fed animals as evidenced by ALT measurements, which trended downwards in the rapamycin group, but did not reach statistical significance (Figure 4D). Levels of the circulating liver derived inflammatory protein SAA were also unchanged (Figure 4E), and gene expression analysis did not reveal evidence of adverse effects on genes involved in gluconeogenesis, lipogenesis or inflammation (Figure 4F).
4. Rapamycin improves hepatic triglyceride accumulation in obese mice.
(A) Liver weights, (B) Liver triglyceride content (C) Liver sections stained with Masson’s trichrome, 10x magnification (D) plasma ALT levels, (E) plasma SAA levels, (F) Gene expression in liver. White bars: placebo-fed mice; black bars: rapamycin-fed mice, n=8–10, *p<0.05 vs placebo.
Effects of rapamycin on tissue signaling through mTORC1/S6K1
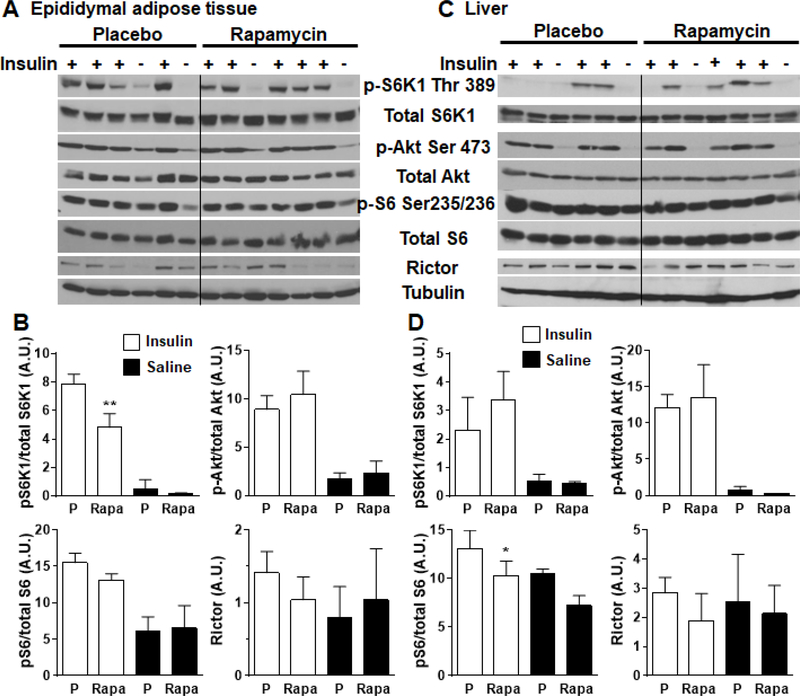
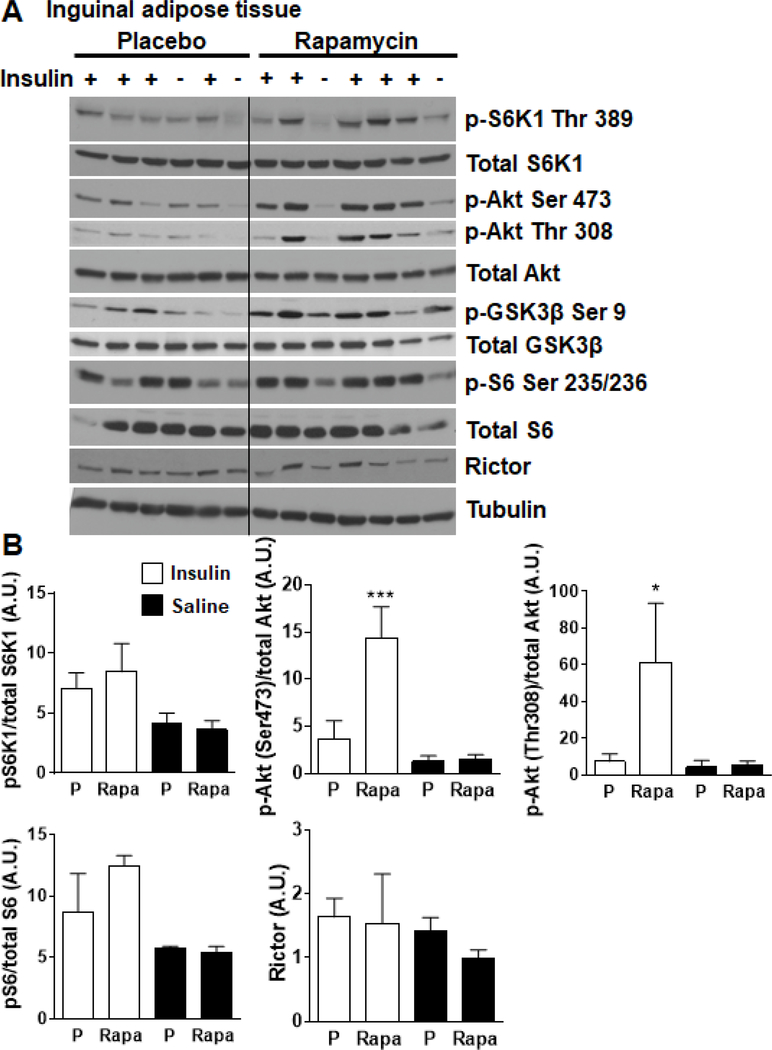
To evaluate the tissue effects of rapamycin in HFHS feeding, we evaluated the phosphorylation status of the mTORC1 effector p70 S6 kinase1 (S6K1) in epididymal white adipose tissue and liver (Figure 5A–D). As expected, mice which received rapamycin had modestly reduced levels of phosphorylated S6K1 and its target S6 in epididymal fat (Figure 5A, B). No differences in phosphorylation of Akt or levels of rictor protein, a component of mTORC2, were observed. Similar changes were also observed in the liver (Figure 5C, D). Further, while inguinal white adipose tissue of rapamycin-treated mice did not show the expected decrease in pS6K1, it did show robust phosphorylation of Akt, indicative of enhanced insulin sensitivity in this tissue (Figure 6A–B). These findings suggest that the observed rapamycin effects on epididymal white adipose tissue may be attributable to signaling through S6K1 without effect on signaling through Akt or rictor, while inguinal white adipose tissue plays a role in the resolution of insulin resistance.
5. Effects of chronic rapamycin on mTORC signaling in adipose and livers of obese mice.
Phosphorylation status of p70S6K1, S6, Akt and total rictor (component of mTORC2) were immunoassayed in tissue lysates prepared from mice consuming microencapsulated rapamycin or placebo containing HFHS diets. Animals were fasted overnight (~16h) and injected with insulin (1U/kg) prior to sacrifice. Epididymal white adipose tissue (EWAT, A-B) and liver (C-D) were harvested 10 minutes after insulin injection. Saline was injected as control in some mice (as depicted in the figures). Tubulin was probed to assess for adequacy of protein loading. White bars: placebo-fed mice; black bars: rapamycin-fed mice, **p<0.01 vs placebo.
6. Effects of chronic rapamycin on mTORC signaling in subcutaneous adipose tissue of obese mice.
(A-B) Phosphorylation status of p70S6K1, S6, Akt and total rictor (component of mTORC2) were immunoassayed in tissue lysates prepared from mice consuming microencapsulated rapamycin (Rapa) or placebo (P) containing HFHS diets. Animals were fasted overnight (~16h) and injected with insulin (1U/kg) prior to sacrifice. Inguinal white adipose tissue (IWAT) was harvested 10 minutes after insulin injection. Saline was injected as control in some mice (as depicted in the figures). Tubulin was probed to assess for adequacy of protein loading. White bars: placebo-fed mice; black bars: rapamycin-fed mice, *p<0.05, ***p<0.001 vs placebo.
DISCUSSION
In this study we show that chronic oral rapamycin when administered at a low-moderate dose with a high fat, high sucrose diet decreased weight gain and had modest beneficial effects on lipid and glucose metabolism. The goal of our study was to evaluate whether rapamycin administered concurrently with a HFHS diet would result in improvement in the metabolic dysregulation that accompanies obesity. Here we found that chronic rapamycin did not result in worsening of metabolic complications as has been demonstrated in other published studies and in fact led to modest improvements in body weight, triglycerides, and glucose metabolism.
mTORC1 is highly activated in insulin-sensitive tissues of obese and high fat fed rodents (Um et al., 2004; Khamzina et al., 2005; Tremblay et al., 2005). Thus inhibition of mTORC1 is an attractive possibility for drug targeting to decrease weight gain and its antecedent metabolic deregulation. Rapamycin, an inhibitor of mTORC1, has been evaluated in this context but its effects on glucose and lipid metabolism are conflicting. Clinically, since rapamycin is widely used to prevent graft rejection in transplant recipients, there is an extensive body of literature evaluating its side effects; thus it is well known that metabolic abnormalities such as hyperlipidemia and hyperglycemia often accompany rapamycin treatment. However, in most clinical transplantation settings, rapamycin is administered along with prednisone, an agent which has known dysmetabolic effects. Most animal studies have commonly utilized short-term intraperitoneal rapamycin administered from 11–28 days in the setting of normal chow diets and have demonstrated weight loss, increase in plasma insulin levels, glucose intolerance and dyslipidemia. In a recent study, oral rapamycin administered in conjunction with high fat feeding over the course of 30 days worsened glucose tolerance and adipose tissue inflammation, with no changes in body weight in mice (Paschoal et al., 2017). Similarly, oral rapamycin administered to aged hybrid mice demonstrated impaired glucose tolerance after 4 weeks of rapamycin feeding (Yang et al., 2012). In the same study, intra-peritoneal rapamycin given to 2-month-old C57BL6 mice for 3 weeks worsened glucose tolerance on a chow diet, with a decrease in islet size. On the other hand, in line with our own observations, a study that administered chronic weekly rapamycin injections for 22 weeks improved insulin sensitivity and reduced adipocyte size, but no body weight data were presented (Makki et al., 2014). Similarly, recent studies in genetically obese mice fed rapamycin in a chow diet, at a dose similar to our study, lost significant body weight and fat mass (Deepa et al., 2013), and showed improvements in cardiovascular function (Reifsnyder et al., 2018). In mouse models of type 2 diabetes, including TALLYHO, BKS-db/db, and NcZ10, chronic rapamycin treatment also improved insulin resistance and promoted weight loss (Reifsnyder et al., 2016). Rapamycin has also been evaluated in the setting of high fat diets. High fat-fed KK/HIJ mice given intra-peritoneal rapamycin treatments for 42 days had decreased adiposity with worsened glucose tolerance (Chang et al., 2009b). Despite this, the authors observed a decrease in hepatic steatosis with rapamycin treatment, again similar to our own findings. The same authors also conducted a separate study in C57BL/6 mice and demonstrated that rapamycin decreased adiposity without worsening of hyperglycemia, but they did not evaluate glucose homeostasis (Chang et al., 2009a). In a small study of healthy human subjects, a single, low dose administration of rapamycin improved skeletal muscle glucose uptake after amino acid infusion without affecting lipids or glucose (Krebs et al., 2007). In mice, deletion of whole body S6K1, the downstream mTORC1 target, results in extended life span (Selman et al., 2009), prevents diet-induced obesity (Um et al., 2004) and improves beta cell survival under conditions of chronic hyperglycemia (Pende et al., 2000). However, adipocyte-specific mTORC1 deletion results in resistance to diet-induced obesity, but with worsened hepatic steatosis and glucose metabolism (Xiong et al., 2018). Thus, results from previous studies are quite variable and discrepancies could be attributed to different models utilized, route, dose and duration of drug administration and possibly environmental factors. The present study contributes to accumulating knowledge regarding the beneficial effects of moderate chronic mTORC1 suppression, now for the first time in a mouse model of diet-induced obesity with associated features resembling human metabolic disease.
There are several important findings in our study that are different from the existing literature. We utilized chronic dietary encapsulated rapamycin given at a low-moderate dose, which obviates potential adverse effects on intra-peritoneal administration and therefore is more clinically relevant. We also used the same dose that has unequivocally been shown to prolong life span (Harrison et al., 2009; Miller et al., 2014). Another group has shown that C57Bl/6J mice given the same dose of encapsulated rapamycin admixed into a high fat diet for 4 months showed worsened glucose tolerance and increased adiposity (Liu et al., 2014). We did not observe worsening of glucose or insulin tolerance in rapamycin-fed obese mice over those observed in the placebo-fed animals; it is possible that in the context of higher levels of insulin resistance, as is the case with our mice and is reminiscent of human obesity-associated metabolic disease, rapamycin exerts beneficial effects on glucose metabolism. In fact, we observed an improvement in hyperglycemia as well as insulin sensitivity. This dose also did not inhibit mTORC2, recently implicated in rapamycin-induced insulin resistance (Lamming et al., 2012). Importantly, our findings suggest that in the setting of hyperglycemia and high levels of insulin resistance, rapamycin administration does not adversely disrupt glucose homeostasis.
Dyslipidemia manifested as hypertriglyceridemia and hypercholesterolemia are commonly associated with rapamycin (sirolimus). However, rapamycin is seldom administered as monotherapy but usually in conjunction with other immunosuppressants such as glucocorticoids which also induce insulin resistance and dyslipidemia. Several studies have failed to show worsened lipid abnormalities. Administration of rapamycin by oral gavage three times a week prevented the development of Type 1 diabetes in NOD mice, without causing dyslipidemia (Baeder et al., 1992). In our studies, we did not observe worsening of hypercholesterolemia or free fatty acid levels and demonstrated an improvement in triglyceride levels.
Hepatic steatosis is a common accompaniment in obesity and type 2 diabetes, and mTORC1 plays a known role in hepatic lipogenesis (Porstmann et al., 2008). Liver-specific deletion of S6 kinase-1, the effector protein of mTORC1, resulted in decreased hepatic steatosis (Bae et al., 2012). In our study, rapamycin decreased liver triglyceride accumulation, without detectable differences in lipogenic or fatty acid oxidative genes, although we did observe an upward trend in levels of circulating free fatty acids. Recent work has shown that mTORC1 plays an important role in the regulation of phosphocholine cytidylyltransferase α, the rate-limiting step in phosphatidylcholine synthesis, thus acting as a major regulator of hepatic triglyceride production (Quinn et al., 2017). This suggests that rapamycin could lower plasma triglycerides and improve hepatic steatosis by reducing phospholipid biosynthesis.
In our study, we also observed reduced weight gain and adiposity in rapamycin-fed animals despite equivalent food intake. There were no differences in perigonadal fat pad weights between the groups suggesting that differences in other fat pads such as subcutaneous and retroperitoneal depots may have accounted for the differences. The difference in weight may be related to increased energy expenditure, although we did not perform oxygen consumption studies. Evidence of enhanced expression of Ucp1 and Cidea, genes characteristic of brown fat, suggests that adaptive thermogenesis may be increased in rapamycin-treated animals and could partially account for the differences in weight gain. Recent studies suggest conflicting effects of mTORC1 inhibition on white adipose tissue browning. While the increase in white adipose tissue Ucp1 expression observed herein has previously been suggested in mice deficient in adipocyte mTOR (Shan et al., 2016), several recent studies suggest the opposite. A study of mice fed a chow diet containing 42 ppm encapsulated rapamycin for two weeks exhibited reduced thermogenic gene expression and worsened glucose tolerance (Tran et al., 2016), while studies by Liu et al similarly observed decreased Ucp1 expression with 4–5 mg/kg rapamycin treatment (Liu et al., 2016; Liu et al., 2018). Notably, these studies used acute rapamycin treatment at a dose that was 2–3 times higher than in the current study, suggesting important dose and treatment duration effects of rapamycin that require additional study. We also did not see any effects on adipocyte size, although others have demonstrated decreased adipocyte size with rapamycin treatment (Um et al., 2004; Polak et al., 2008). Macrophages have been implicated as key players in contributing to insulin resistance in obesity. Although rapamycin is known to affect differentiation and trafficking of a variety of immune cells including macrophages (Thomson et al., 2009), we did not see prominent effects on local inflammation in adipose tissue or liver in rapamycin treated mice. These findings suggest that the weight differences observed are not secondary to differences in macrophage infiltration within adipose tissue. However, recent work suggests that genetic inhibition of macrophage mTORC1 leads to improvements in high fat diet-induced insulin resistance and inflammation (Jiang et al., 2014), suggesting a potential role for macrophages in mediating the beneficial effects of chronic low-dose rapamycin. Overall, the phenotype of these rapamycin-fed mice is reminiscent of the phenotype of the adipose-specific raptor knockout mouse (Polak et al., 2008).
Rapamycin levels were detectable in the blood in the experimental animals, but at levels lower that what has been reported previously in similar studies, with only partial inhibition of S6K1. The reasons for this are unknown, but may be the result of inhibitory effects of the added dietary sucrose, or alterations to the gut microbiota that modulated rapamycin absorption. Low blood rapamycin levels are likely not due to sub-optimal timing of blood sampling, as the half-life of rapamycin in the blood of mice is approximately 15 hours (Arrioloa Apelo et al., 2016). It is striking that rapamycin could promote metabolic benefits, despite modest inhibition of mTOR. Thus, the notion of optimal mTOR inhibition has been put forth. It is conceivable that a low dose of rapamycin could potentially improve the tissue metabolic milieu by normalizing, but not completely inhibiting, mTORC1 signaling (Laplante & Sabatini, 2012). This dose could also limit the complex feedback mechanisms involved in the activation of IRS1-PI3K-Akt and the inhibition of mTORC2-Akt induced by high dose rapamycin treatment, and still allow for sufficient adaptive thermogenesis. Thus, too much or too little mTOR activity may negatively influence tissue metabolism. Indeed, a recent study showed that intraperitoneal rapamycin administered intermittently at a low dose improved body weight and plasma triglyceride levels with no measureable changes in glucose metabolism, and with minimal evidence of mTOR inhibition (Leontieva et al., 2014), supporting the notion that moderate mTOR inhibition may impart metabolic benefit. Alternatively, in this study, rapamycin delivered orally in microcapsules could have generated byproducts with off-target effects, as evidenced by the lack of inhibition of downstream target S6K1 in inguinal white adipose tissue and liver, or by acting through the gut microbiota. Interestingly, in this study rapamycin inhibited phospho-S6K1 in epididymal white adipose tissue only during acute insulin activation, suggesting mild insulin resistance in this depot, yet improved insulin sensitivity of inguinal white adipose tissue without any measurable changes in phospho-S6K1 in that depot. This suggests a complex interplay between potentially different target and effector tissues of rapamycin that requires further exploration. Future studies could directly compare the oral delivery method utilized herein with the commonly used intraperitoneal or subcutaneous rapamycin delivery to evaluate whether distinct mechanisms of action explain the observed tissue-specific effects.
In summary, we have demonstrated that a chronic low-moderate oral dose of rapamycin does not worsen but instead beneficially modulates metabolic abnormalities during the course of diet-induced obesity. Our study has important clinical implications for the treatment of chronic disease states such as diabetes, Alzheimer’s disease and cancer as well as for healthy life span extension. Although rapamycin itself is plagued with metabolic side effects in the clinical setting, pharmacologic strategies that provide optimal titration of mTORC1 activity have therapeutic potential to improve the tissue metabolic milieu in obesity and diabetes.
NEW FINDINGS.
What is the central question of this study? Whether chronic oral rapamycin promotes beneficial effects on glucose/lipid metabolism and energy balance when administered to mice with an obesogenic diet rich in saturated fat and sucrose has not been explored.
What is the main finding and its importance? Chronic oral rapamycin reduces body weight and fat gain, improves insulin sensitivity, and reduces hepatic steatosis when administered to mice with a high fat high sucrose diet. In addition, we make the novel observation that there appear to be tissue-specific effects of rapamycin. While rapamycin appears to impart its effects mainly on visceral adipose tissue, its effects on insulin sensitivity are mediated by subcutaneous adipose tissue.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the technical assistance of Jinkyu Kim. The authors also acknowledge the University of Washington Nutrition Obesity Research Center Energy Balance Core for body compositional analyses. The authors also gratefully acknowledge Dr. Alan Chait and members of the Chait lab for critical review of the manuscript.
FUNDING
This work was supported in part by grants DK017047, DK035816, AT007177, and HL092969.
Abbreviations:
- HFHS
high fat high sucrose
- HPLC
performance liquid chromatography
- mTOR
mechanistic target of rapamycin
- mTORC1
mTOR complex-1
- SAA
serum amyloid A
Footnotes
Competing Interests Statement: the authors have no conflicts of interests to disclose.
REFERENCES
- Aggarwal D, Fernandez ML & Soliman GA (2006). Rapamycin, an mTOR inhibitor, disrupts triglyceride metabolism in guinea pigs. Metabolism 55, 794–802. https://doi:10.1016/j.metabol.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV & Blagosklonny MV (2011). Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 10, 4230–4236. https://doi:10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- Arrioloa Apelo SI, Neuman JC, Baar EL, Syed FA, Cummings NE, Brar HK, Pumper CP, Kimple ME, Lamming DW (2016). Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 15, 28–38. 10.1111/acel.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EJ, Xu J, Oh da Y, Bandyopadhyay G, Lagakos WS, Keshwani M & Olefsky JM (2012). Liver-specific p70 S6 Kinase Depletion Protects against Hepatic Steatosis and Systemic Insulin Resistance. J Biol Chem 287, 18769–18780. https://doi:10.1074/jbc.M112.365544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeder WL, Sredy J, Sehgal SN, Chang JY & Adams LM (1992). Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol 89, 174–178. https://doi:10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH & Mao FC (2009a). Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci 109, 496–503. https://doi:10.1254/jphs.08215FP. [DOI] [PubMed] [Google Scholar]
- Chang GR, Wu YY, Chiu YS, Chen WY, Liao JW, Hsu HM, Chao TH, Hung SW & Mao FC (2009b). Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol 105, 188–198. https://doi:10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- Cruzado JM (2008). Nonimmunosuppressive effects of mammalian target of rapamycin inhibitors. Transplant Rev (Orlando) 22, 73–81. https://doi:10.1016/j.trre.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK & Puigserver P (2007). mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 450, 736–740. https://doi:10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A & Thomas G (2007). mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13, 252–259. https://doi:10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Walsh ME, Hamilton RT, Pulliam D, Shi Y, Hill S, Li Y & Remmen HV (2013). Rapamycin Modulates Markers of Mitochondrial Biogenesis and Fatty Acid Oxidation in the Adipose Tissue of db/db Mice. J Biochem Pharmacol Res 1, 114–123. [PMC free article] [PubMed] [Google Scholar]
- den Hartigh LJ, Wang S, Goodspeed L, Wietecha T, Houston B, Omer M, Ogimoto K, Subramanian S, Gowda GA, O’Brien KD, Kaiyala KJ, Morton GJ & Chait A (2017). Metabolically distinct weight loss by 10,12 CLA and caloric restriction highlight the importance of subcutaneous white adipose tissue for glucose homeostasis in mice. PLoS One 12, e0172912 https://doi:10.1371/journal.pone.0172912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J, Lees M & Sloane Stanley GH (1957). A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226, 497–509. [PubMed] [Google Scholar]
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N & Leibowitz G (2008). mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57, 945–957. https://doi:10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E & Miller RA (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395. https://doi:10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y & Marette A (2010). Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes 59, 1338–1348. https://doi:10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Westerterp M, Wang C, Zhu Y & Ai D (2014). Macrophage mTORC1 disruption reduces inflammation and insulin resistance in obese mice. Diabetologia 57, 2393–2404. https://doi:10.1007/s00125-014-3350-5. [DOI] [PubMed] [Google Scholar]
- Johnston O, Rose CL, Webster AC & Gill JS (2008). Sirolimus is associated with new-onset diabetes in kidney transplant recipients. J Am Soc Nephrol 19, 1411–1418. https://doi:10.1681/ASN.2007111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske BL, de Mattos A, Flechner SM, Gallon L, Meier-Kriesche HU, Weir MR & Wilkinson A (2008). Mammalian target of rapamycin inhibitor dyslipidemia in kidney transplant recipients. Am J Transplant 8, 1384–1392. https://doi:10.1111/j.1600-6143.2008.02272.x. [DOI] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S & Marette A (2005). Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146, 1473–1481. https://doi:10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Korsheninnikova E, van der Zon GC, Voshol PJ, Janssen GM, Havekes LM, Grefhorst A, Kuipers F, Reijngoud DJ, Romijn JA, Ouwens DM & Maassen JA (2006). Sustained activation of the mammalian target of rapamycin nutrient sensing pathway is associated with hepatic insulin resistance, but not with steatosis, in mice. Diabetologia 49, 3049–3057. https://doi:10.1007/s00125-006-0439-5. [DOI] [PubMed] [Google Scholar]
- Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, Roth E, Furnsinn C, Promintzer M, Anderwald C, Bischof M & Roden M (2007). The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 56, 1600–1607. https://doi:10.2337/db06-1016. [DOI] [PubMed] [Google Scholar]
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, Guertin DA, Sabatini DM & Baur JA (2012). Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643. https://doi:10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M & Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149, 274–293. https://doi:10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontieva OV, Paszkiewicz GM & Blagosklonny MV (2014). Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 13, 616–622. 10.1111/acel.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AL, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, Galvan V, Richardson A (2017). Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab 37, 217–226. 10.1177/0271678X15621575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL, Guilherme A, Guntur K, Czech MP & Collins S (2016). Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Invest 126, 1704–1716. https://doi:10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Ceddia RP & Collins S (2018). Cardiac natriuretic peptides promote adipose ‘browning’ through mTOR complex-1. Mol Metab 9, 192–198. https://doi:10.1016/j.molmet.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Diaz V, Fernandez E, Strong R, Ye L, Baur JA, Lamming DW, Richardson A & Salmon AB (2014). Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY) 6, 742–754. https://doi:10.18632/aging.100688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki K, Taront S, Molendi-Coste O, Bouchaert E, Neve B, Eury E, Lobbens S, Labalette M, Duez H, Staels B, Dombrowicz D, Froguel P & Wolowczuk I (2014). Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One 9, e92684 https://doi:10.1371/journal.pone.0092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL & Strong R (2011). Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66, 191–201. https://doi:10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, Javors MA, Li X, Nadon NL, Nelson JF, Pletcher S, Salmon AB, Sharp ZD, Van Roekel S, Winkleman L & Strong R (2014). Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477. https://doi:10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S & Kahan BD (2002). Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res 43, 1170–1180. https://doi:10.1194/jlr.M100392-JLR200. [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schroder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Holter SM, Moreth K, Prehn C, Puk O, Racz I, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski J, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Hofler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M & Ehninger D (2013). Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest 123, 3272–3291. https://doi:10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM & Glass CK (2010). Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72, 219–246. https://doi:10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Paschoal VA, Amano MT, Belchior T, Magdalon J, Chimin P, Andrade ML, Ortiz-Silva M, Castro É, Yamashita AS, Rosa Neto JC, Câmara NO & Festuccia WT (2017). mTORC1 inhibition with rapamycin exacerbates adipose tissue inflammation in obese mice and dissociates macrophage phenotype from function. Immunobiology 222, 261–271. https://doi:10.1016/j.imbio.2016.09.014. [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B & Thomas G (2000). Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 408, 994–997. https://doi:10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA & Hall MN (2008). Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8, 399–410. https://doi:10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL & Schulze A (2008). SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab 8, 224–236. https://doi:10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WJ, Wan M, Shewale SV, Gelfer R, Rader DJ, Birnbaum MJ & Titchenell PM (2017). mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J Clin Invest 127, 4207–4215. https://doi:10.1172/JCI96036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder PC, Flurkey K, Te A & Harrison DE (2016). Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY) 8, 3120–3130. https://doi:10.18632/aging.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifsnyder PC, Ryzhov S, Flurkey K, Anunciado-Koza RP, Mills I, Harrison DE & Koza RA (2018). Cardioprotective effects of dietary rapamycin on adult female C57BLKS/J-Lepr. Ann N Y Acad Sci 1418, 106–117. https://doi:10.1111/nyas.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM & Sabatini DM (2005). Growing roles for the mTOR pathway. Curr Opin Cell Biol 17, 596–603. https://doi:10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D & Withers DJ (2009). Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144. https://doi:10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Peterson TR & Sabatini DM (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40, 310–322. https://doi:10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan T, Zhang P, Jiang Q, Xiong Y, Wang Y & Kuang S (2016). Adipocyte-specific deletion of mTOR inhibits adipose tissue development and causes insulin resistance in mice. Diabetologia 59, 1995–2004. https://doi:10.1007/s00125-016-4006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R & Galvan V (2010). Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One 5, e9979 https://doi:10.1371/annotation/05c1b976-7eab-4154-808d-0526e604b8eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A 3rd, Kirk EA, O’Brien KD & Chait A (2008). Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol 28, 685–691. https://doi:10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teutonico A, Schena PF & Di Paolo S (2005). Glucose metabolism in renal transplant recipients: effect of calcineurin inhibitor withdrawal and conversion to sirolimus. J Am Soc Nephrol 16, 3128–3135. https://doi:10.1681/ASN.2005050487. [DOI] [PubMed] [Google Scholar]
- Thomson AW, Turnquist HR & Raimondi G (2009). Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9, 324–337. https://doi:10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran CM, Mukherjee S, Ye L, Frederick DW, Kissig M, Davis JG, Lamming DW, Seale P & Baur JA (2016). Rapamycin Blocks Induction of the Thermogenic Program in White Adipose Tissue. Diabetes 65, 927–941. https://doi:10.2337/db15-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A & Roden M (2005). Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes 54, 2674–2684. 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF & Zheng XF (2007). Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today 12, 112–124. https://doi:10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J & Thomas G (2004). Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205. https://doi:10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R & Hall MN (2006). TOR signaling in growth and metabolism. Cell 124, 471–484. https://doi:10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Xu Z, Wang Y, Kuang S & Shan T (2018). Adipocyte-specific DKO of Lkb1 and mTOR protects mice against HFD-induced obesity, but results in insulin resistance. J Lipid Res 59, 974–981. https://doi:10.1194/jlr.M081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SB, Lee HY, Young DM, Tien AC, Rowson-Baldwin A, Shu YY, Jan YN & Jan LY (2012). Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med (Berl) 90, 575–585. https://doi:10.1007/s00109-0110-834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]