Abstract
HIV stigma and fears surrounding the disease pose a challenge for public health interventions, particularly those that target pregnant women. In order to reduce stigma and improve the lives of vulnerable populations, researchers have recognized a need to integrate different types of support at various levels. To better inform HIV interventions, the current study draws on social-ecological and evolutionary theories of reproduction to predict stigma and fear of contracting HIV among pregnant women in South India. The aims of this study were twofold: compare the social-ecological model to a modified maternal-fetal protection model and test a combined model that included strong predictors from each model. The study took place in 2008–2011 in Mysore District, Karnataka, India. Using data from a cross-sectional survey and biological indicators of health, we statistically modeled social-ecological variables representing individual, interpersonal, and community/institutional levels. Participants were 645 pregnant women. The social-ecological and combined models were the best-fitting models for HIV-related stigma, and the combined model was the best fit for HIV-related fear. Our findings suggest that combining reproductive life history factors along with individual, interpersonal, and community/institutional factors are significant indicators of HIV-related stigma and fear. Results of this study support a multifaceted approach to intervention development for HIV-related stigma and fear. The combined model in this study can be used as a predictive model for future research focused on HIV stigma and fear, with the intent that dual consideration of social-ecological and evolutionary theories will improve public health communication efforts.
Keywords: social-ecological model, maternal-fetal protection, HIV/AIDS stigma, pregnancy, India
HIV stigma and fears surrounding the disease pose a challenge for public health efforts that aim to prevent new infections from arising, treat existing infections, and motivate people to get tested. Because of these barriers, research has found only moderate support for interventions (Brown et al. 2003; Sengupta et al. 2011; Stangl et al. 2013). In order to improve the lives of vulnerable populations, current research emphasizes the need to investigate barriers to HIV testing and treatment at various levels, including individual factors, peer networks, the community, and surrounding organizations (Stangl et al. 2013). One potential way to improve HIV intervention strategies is to consider how local social-ecological conditions and humans’ evolved psychology shape perceptions of pathogen infection. The current study draws on these perspectives to model HIV stigma and fear among pregnant women in South India. Pregnancy is a particularly salient period for investigating perceptions of HIV because pathogen-avoidant behaviors purportedly increase at this time (Fessler et al. 2005; Flaxman and Sherman 2000; Navarrete et al. 2007), and women have a higher risk of HIV because of biological factors that are unique to pregnancy (Aagaard et al. 2012; Drake et al. 2014; Gray et al. 2005; Taha et al. 1998).
Stigma, broadly speaking, is a complex phenomenon with social and evolutionary underpinnings (Goffman 1963; Kurzban and Leary 2001; Parker and Aggleton 2003). Goffman (1963) originally defined stigma as having three primary components that focus on attributes of the target individual, such as physical deformities, character defects, or problems associated with group identity. Since this conception, the meaning of stigma in public health is now considered a complex social process that perpetuates cycles of power and control (Parker and Aggleton 2003), or as the process of devaluing individuals who publicly live with character traits that others deem unacceptable in society (UNAIDS 2003). From an evolutionary perspective, stigma is a fear-generated avoidance strategy that exists because it solved particular adaptive problems that threatened fitness throughout the course of human evolution (Kurzban and Leary 2001). In this light, stigma can arise from two scenarios: when individuals present cues of pathogen infection, or when they pose a threat in social exchanges (Kurzban and Leary 2001). Altogether, stigma can be defined by a few key features, including fear, prejudice, and stereotypes, leading to a gap in how one views oneself socially versus how one is viewed by others in society (Stangl et al. 2013).
HIV stigma, specifically, can lead to adverse health outcomes, particularly during pregnancy. Mother-to-child transmission (PMTCT) interventions, which provide antenatal care, HIV testing, and antiretroviral treatment, face the issue of pregnant women avoiding care because they are often the first members of their families to get tested for HIV, therefore increasing their risk of being blamed for bringing the virus into the family (Turan and Nyblade 2013). Furthermore, pregnant women who report higher levels of HIV stigma are less likely to deliver in a health facility with a skilled attendant owing to negative community perceptions (Steward et al. 2008). These familial and social barriers ultimately lead to a greater risk of negative maternal and infant health outcomes. Framing HIV stigma within the context of both social-ecological theory and evolutionary theory could be useful because these approaches can help identify the social, environmental, and individual layers that predict HIV stigma and fear during such a critical life stage.
The social-ecological model was originally developed as a transdisciplinary approach to understand the multiple dimensions that influence health spanning beyond individual characteristics, with the goal of designing community-based interventions that can lead to behavioral change (McLeroy et al. 1988; Stokols 1996). As the term “ecology” implies, this model aims to describe the relationship between humans and their physical and social environments as it pertains to health (Stokols 1996). The strength of this framework is that it considers the following levels of influence on human health and behavior: individual, interpersonal, institutional, community, and policy (McLeroy et al. 1988).
Applications of the social-ecological model to HIV stigma are rare; instead, research typically focuses on high-risk behaviors that lead to HIV. In reference to HIV stigma, social-ecological research often focuses on individual factors and perceptions of interpersonal relationships. To illustrate, Sandelowski et al. (2004) found that the experience of stigma translated into fear of social rejection, discrimination, and violence when HIV-positive individuals chose to disclose their status (Sandelowski et al. 2004). This effect is often pronounced among women; they have described feeling dirty, worthless, or deficient because of an HIV-positive diagnosis (Sandelowski et al. 2004). Cases such as these are concerning because prior research conducted among Indian women found that isolation and HIV are correlated with increased risk for other negative health outcomes such as intimate partner violence (IPV) (Go et al. 2003; Silverman et al. 2008; Stephenson 2007). In reference to high-risk sexual behavior, Larios et al. (2009) used multiple levels to explain condom use among sex workers in Tijuana, Mexico. In this study, female sex workers who were employed in bars were more likely to engage in protected sex compared with sex workers on the streets (Larios et al. 2009). The social-ecological model could explain differences in condom use, such that sex workers in bars reported higher self-efficacy (individual factor) and had better access to condoms because of their working conditions (institutional factor), whereas street workers used condoms less often because they were given economic incentives for not using them (interpersonal factor). This study, among others, points to the importance of considering a multilevel approach when investigating the factors that shape HIV risk (Kerrigan et al. 2006; Larios et al. 2009). One pitfall of the social-ecological model, however, is that it does not account for the evolutionary factors that shape human health and behavior.
An evolutionary perspective is necessary for research on HIV stigma because humans have co-evolved with pathogens and must continuously adapt to novel threats as they emerge. Researchers speculate that this constant selection pressure contributes to the development of specific traits that function as a second line of defense for individuals faced with heightened risk of infection (Parker et al. 2011; Schaller and Park 2011). “Risk of infection” can include environmental factors, such as the emergence of a novel pathogen, but also an individual’s capacity to mount a robust immune response, which varies across the life course. Regardless of the underlying cause, these protective mechanisms, or “counter-adaptations,” can consist of psychological mechanisms, such as disgust, stigma, and ethnocentrism (Schaller and Park 2011), and behavioral traits, such as the avoidance of certain foods perceived to cause harm (Placek and Hagen 2015; Placek et al. 2017).
Pregnancy, the stage relevant to the current study, is a period of increased vulnerability to pathogens. In the first trimester, the maternal adaptive immune system partially down-regulates to support placentation and fetal organogenesis, leading both mothers and fetuses to be more vulnerable to certain extracellular pathogens, such as HIV, herpes simplex virus, and hepatitis E virus (Gray et al. 2005; Racicot et al. 2014). Studies show that women often engage in behaviors geared toward protecting both themselves and their developing fetuses from infections (Henrich and Henrich 2010; Placek et al. 2017). Evidence of such “maternal-fetal protection” include women reporting aversions to animal products (Fessler 2002; Flaxman and Sherman 2000) and showing heightened ethnocentrism and disgust during the first trimester (Fessler et al. 2005; Navarrete et al. 2007). In a study conducted in the United States, for example, pregnant women in the first trimester reported higher ethnocentrism than women in their second and third trimesters (Navarrete et al. 2007).
Women’s immunocompetence is further influenced by their life histories, including early developmental exposures to pathogens, nutritional stress, and reproductive histories, such as age at first birth and number of pregnancies (Georgiev et al. 2016; Gibbs et al. 2012; Miller 2010). Multiple births and short interbirth intervals, for example, are associated with higher rates of anemia in subsequent pregnancies (Miller 2016); and higher rates of iron-deficiency anemia, specifically, are associated with impaired immune function (Kumar and Choudhry 2010). These life history factors, however, are not typically linked with the classic maternal-fetal protection hypothesis, which is surprising given that maternal-fetal protection mechanisms hypothetically evolved prior to the advent of agriculture, when Homo sapiens had unpredictable access to resources (Profet 1988). The current study incorporates these factors because emerging evidence shows that resource scarcity and fertility rates play a role in hypothesized pathogen avoidant strategies and therefore warrant further consideration (Placek and Hagen 2013; Young and Pike 2012).
The goals of this study were to (1) explore the predictive abilities of the social-ecological and the maternal-fetal protection models in predicting stigma and fear associated with HIV among a population of pregnant women and (2) test if a combined model could better predict HIV stigma and fear than each model could separately. To do this, significant predictors from each initial model were combined into one. The three models are then compared to see if combining the models is a useful framework to study HIV stigma and fear.
Study Population
The study took place from 2008 to 2011 in rural Mysore, Karnataka, approximately 10 km outside the city (Kojima et al. 2017). Mysore is located in southwest India at 12.30° N, 76.65° E. The urban area of Mysore district has a population of more than 1.6 million people, and more than 900,000 reside in the district’s rural villages (Census of India 2011). During pregnancy, women residing on rural farms are expected to follow social norms that are geared toward protecting the developing fetus from external threats (Placek et al. 2017). Women also report limited knowledge of the causes of pregnancy loss, which highlights a need for more public health efforts that focus on maternal-child health (Placek and Madhivanan 2017). A previous study conducted with this population of pregnant women found the rate of HIV to be 0.6% (Kojima et al. 2017). Women also experienced low rates of hepatitis B (0.5%), syphilis (0.1%), bacterial vaginosis (7.1%), and hypertension (4.3%). Rates of anemia, however, were high, with 49.5% cases of moderate to severe anemia (Kojima et al. 2017).
Focusing on India for this study provides a unique opportunity to systematically explore the multiple layers that potentially impact HIV stigma and fear during pregnancy. Prior research on Indian women has shown that the ability to engage in health decision-making processes and negotiate health outcomes result from the interplay between the individual characteristics of the person and the culture with which she interacts (D’Souza et al. 2013; Osamor and Grady 2016; Senarath and Nalika Sepali Gunawardena 2009; Van Hollen 2007). For example, Van Hollen (2007) found that HIV positive women in Tamil Nadu reported that their status and desire to become pregnant required navigating cultural constructions of gender, international and state agency priorities, and social networks beyond their familial unit. From the perspective of maternal-fetal protection, accumulating evidence is showing a much more complex relationship between hypothesized protective behaviors, social and environmental factors, and pregnancy status among South Indian women (Placek and Hagen 2013, 2015; Placek et al. 2017).
Methods
The Public Health Research Institute of India (PHRII) carried out the present study as part of a larger research program that established a mobile medical clinic in rural Mysore. The program, called the Kisalaya project, educated rural communities about maternal-child health and trained community health workers about safe birthing practices. In addition, PHRII provided antenatal care and education on preventing maternal-to-child transmission of HIV. Pregnant women were tested for infections, and nurses assessed blood sugar, urine albumin, and anemia. Counselors from PHRII administered questionnaires in the local language of Kannada. Questions included sociodemographic information as well as medical and obstetric history. In total, 1675 (76%) of the 2211 pregnancies received antenatal care. Of this sample, 1639 (97.85%) women consented to counseling and testing for HIV along with all the other routine tests. See Kojima et al (2017) for additional details of the program procedures. This project was approved by the Independent Ethics Committee of Vikram Hospital in Mysore, India (Protocol number 2008–04-12–01) and by the Institutional Review Board at Florida International University.
Outcome Measures
HIV Stigma, or stigma toward HIV-infected individuals, was assessed using five scenarios of contact that one might have with an HIV-infected person. Questions included: How worried would you feel if you were to (1) eat lunch with someone with HIV/AIDS? (2) buy food at the market from someone with HIV/AIDS? (3) get a haircut from someone with HIV/AIDS? (4) let your child play with children with HIV/AIDS? and (5) work with someone with HIV/AIDS? Answers were formatted according to a four-point Likert scale from one (not at all worried) to four (extremely worried). A total “stigma” score was computed for each participant.
Fear of contracting HIV was assessed with seven questions: How worried would you feel that: (1) you will get the HIV virus; (2) you will infect others with the HIV virus if you have the virus; (3) your family will not treat you as a full family member if the family knows you have the HIV virus; (4) your friends will ostracize you if they know you have the HIV virus; (5) your community will treat you like a social outcast if any of the community members know you have the HIV virus; (6) your family will not care for you to the end if you have HIV/AIDS; and (7) there will be no one to take care of your children if you die of AIDS. Answers were formatted according to a four-point Likert scale from one (not at all worried) to four (extremely worried). A total “fear” score was computed for each participant.
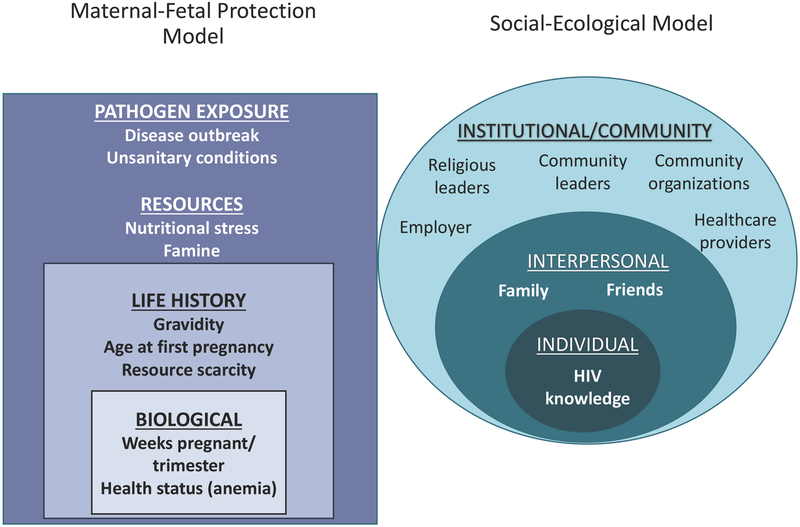
Maternal-Fetal Protection Model
The maternal-fetal protection model predicts that because of immunological changes that occur during the first trimester of pregnancy, women are more likely to report psychological and behavioral patterns that are hypothesized to provide protection from pathogens (Flaxman and Sherman 2000; Hook 1978; Profet 1995). In addition to testing the main tenets of this hypothesis, we also included several measures known to contribute to impaired immunological functioning.
Weeks pregnant Women reported their number of weeks pregnant. Early pregnancy is a critical period of development when the mother faces increased risk of pathogen infection; this shift in immunity is associated with a cascade of physiological, behavioral, and psychological changes that function to protect the mother and the fetus from harm (Fessler 2002; Flaxman and Sherman 2000; Hook 1978; Profet 1995). Therefore, early pregnancy is predicted to increase HIV-related stigma and fear.
Hemoglobin Hemoglobin levels were assessed with blood samples using the Hemoglobin (Cyanomethemoglobin) Beacon Test (Beacon Diagnostics Pvt. Ltd. Kabilpore, Gujarat, India). Since the purpose of this study was not to diagnose anemia status, this variable remained continuous. Higher levels of anemia deficiency are predicted to increase HIV-related stigma and fear since iron deficiency impairs immunity, rendering individuals more susceptible to pathogenic infections (Kumar and Choudhry 2010).
Age at first pregnancy In life history research, age at first pregnancy is a response to ecological conditions, such as stressful life events that occur throughout development (Chisholm et al. 2005; Nettle et al. 2011). Other research shows that the timing of first pregnancy is negatively associated with pro-inflammatory cytokines in subsequent pregnancies (Blackmore et al. 2011). We predict that if earlier age at first pregnancy functions as a developmental indicator of adverse ecological conditions, then women with earlier ages at first pregnancies will report higher HIV stigma and fear.
Number of pregnancies (gravidity) Research is mixed regarding the relationship between gravidity and infection risk and appears to depend on the virus in question (Cumberland et al. 2007; Rastogi et al. 1999; Saute et al. 2002; Shulman et al. 2001). Other research suggests that higher numbers of pregnancies are associated with greater risk of anemia, particularly in resource-scarce environments (Miller 2016). We hypothesize that higher numbers of pregnancies are associated with heightened HIV stigma and fear for additional protection from pathogens.
Weight in kilograms was included as an indicator of nutritional stress. Prior research shows that maternal undernutrition, often a result of nutrition deprivation throughout the developmental lifespan, is associated with increased infection risk (Steketee 2003).
Social-Ecological Model
The social-ecological model is divided by levels that typically consist of individual, interpersonal, institutional, community, and policy-related factors (McLeroy et al. 1988). In the current study, we focus on the individual, interpersonal, and institutional/community levels. The latter two categories are combined because in rural regions in India these categories can easily overlap. For example, physicians could represent the institutional level, since this individual provides care, but might also live in the same community.
Individual level: Knowledge of HIV transmission Previous research has shown that correct knowledge of HIV transmission is an important indicator of the willingness to avoid or stigmatize HIV-infected individuals (Herek and Capitanio 1994; Herek et al. 2002; Price and Hsu 1992). HIV knowledge was therefore assessed using twelve questions including four correct pathways of HIV transmission: drug injection with syringes used by someone with HIV, an HIV-infected mother’s transmission to an infant, having sexual intercourse without a condom, and a baby breastfed by his/her mother who has the HIV virus. There were eight incorrect modes of transmission. A total score was computed for the number of correct responses reported by a participant for each question, for a possible range of 0 to 12.
Interpersonal level: HIV-positive disclosure Social-ecological research has shown that the person to whom an individual discloses their HIV-positive status is associated with perceived reactions from interpersonal relationships (Qiao et al. 2015). Respondents were asked “If you test positive for HIV, would you tell any of the following individuals about your HIV test result?” (yes=1, no=0). At the interpersonal level the options were spouse, brothers, sisters, relatives, and friends. A total score was computed, for a possible range of 0 to 5.
Interpersonal level: Perceived reactions to HIV-positive status was measured by asking participants to respond to 13 scenarios that began with the question: “If you test positive for HIV and choose to disclose, would the following happen to you?” (yes=1, no=0). Positive scenarios were reverse-coded and, similar to the disclosure variable, composite scores were created based on the social-ecological model categories. Scenarios that were not relevant for the majority of participants (e.g., scenarios related to work) were left out of the total score so as to limit missing data. The following eight scenarios were included in the total score for a possible range of 0 to 8: breakup of marriage, physical abuse by spouse, neglect from family, disowned by family, breakup of sexual relationships, increased emotional support from family, increased emotional support from peers, and strengthened relationship with partner.
Community/Institutional: HIV-positive disclosure To whom respondents would disclose their HIV-positive status at the community/institutional level included neighbors, physicians, religious leaders, and community leaders. A total score was computed for a possible range of 0 to 4.
Covariates Each model included age, years of education, and income (rupees per month). The predictive models tested herein are presented in Fig. 1.
Figure 1.
The modified maternal fetal protection and social-ecological models for predicting HIV related fear and stigma among pregnant women
Analysis
Data were analyzed using R version 3.4.1 for Macintosh. Summary statistics and graphical techniques were generated to explore the distributions of variables. For the outcome variables, correlation statistics were computed to determine the degree of similarity between measures, and Cronbach’s alpha was computed to test for internal consistency within each measure. Since total stigma was positively skewed and bounded, and total fear was negatively skewed and bounded, we tested for overdispersion for each measure using the “dispersiontest” function from the AER package (Cameron and Trivedi 2005). The variance was significantly greater than the mean for both outcome measures, indicating that a negative binomial was the most appropriate multivariate modeling technique. All predictor variables were scaled and centered. Models were ranked according to the Akaike information criterion (AIC) to determine if the final combined model outperformed the social-ecological and maternal-fetal protection models.
Participants initially totaled 1675 pregnant women, but the sample was ultimately reduced because only 645 responded to all prompts included in this study. Although data were missing across all study measures, the majority of cases came from the HIV knowledge measure (missing cases=603). To determine if missing cases presented meaningful differences in findings, models were run without HIV knowledge; those data are reported in the ESM. Summary statistics and results presented below are therefore for the final sample of 645 women.
Results
On average, women were 20.92 years old and had completed 7.93 years of education. Only two women tested positive for HIV in this sample of participants. Women were an average of 24.71 weeks pregnant, had experienced their first birth around age 19.21, and averaged 0.72 pregnancies. Summary statistics for remaining variables are provided in Table 1.
Table 1.
Descriptive statistics of the participants and model variables
| Variable | N | Min | Max | Mean | Median | SD |
|---|---|---|---|---|---|---|
| Total stigma | 645 | 5.00 | 20.00 | 8.73 | 5.00 | 5.01 |
| Total fear | 645 | 7.00 | 28.00 | 23.89 | 28.00 | 6.47 |
| Gestation weeks | 645 | 6.00 | 41.00 | 24.71 | 26.00 | 8.26 |
| Hemoglobin | 645 | 5.10 | 15.20 | 10.11 | 10.10 | 1.66 |
| Age at first pregnancy (in years) | 645 | 13.00 | 35.00 | 19.21 | 19.00 | 2.32 |
| Pregnancy number (gravidity) | 645 | 0.00 | 5.00 | 0.72 | 1.00 | 0.80 |
| Weight (kg) | 645 | 30.00 | 80.00 | 49.64 | 49.00 | 7.56 |
| HIV correct knowledge | 645 | 4.00 | 12.00 | 9.27 | 11.00 | 3.03 |
| Disclosure: Interpersonal | 645 | 0.00 | 5.00 | 1.68 | 1.00 | 1.24 |
| Disclosure: | 645 | 0.00 | 4.00 | 0.98 | 1.00 | 0.49 |
| Community/Institutional | ||||||
| Reaction: Interpersonal negative | 645 | 0.00 | 8.00 | 3.12 | 3.00 | 2.22 |
| Age (in years) | 645 | 15.00 | 36.00 | 20.92 | 20.00 | 2.83 |
| Education (in years) | 645 | 0.00 | 17.00 | 7.93 | 9.00 | 3.50 |
| Rupees per month | 645 | 0.00 | 40000.00 | 5269.00 | 4000.00 | 3925.73 |
Stigma indicators were highly correlated with each other, but were negatively and weakly correlated with fear indicators (Fig. 1a in the ESM). The same was true for fear indicators; the independent indicators for fear were highly correlated, providing support that these items were measuring separate constructs. Furthermore, the Cronbach’s alpha for total stigma was 0.971 and for total fear it was 0.967, indicating high internal consistency for both measures.
Regarding knowledge of HIV transmission, 18.0% of participants answered all questions incorrectly, whereas 33.33% answered all questions correctly. Only one question was answered incorrectly by the majority of participants (51.0%), and this was the belief that mosquitoes or other insects could transmit the virus by biting a person. The other question answered incorrectly in higher proportions was the belief that HIV could be transmitted from being coughed or sneezed on by a person who has the virus (39.0%). The remaining questions generated fewer incorrect responses, and the “correct” modes of transmission were known by the majority of respondents: injecting drugs with syringes by someone who has the HIV virus (correct = 70.0%), an infant whose mother has the HIV virus (correct = 96.74%), having sex without a condom with someone who has the HIV virus (correct = 60.62%), and a baby breast-fed by his/her mother who has the HIV virus (correct = 98.30%).
HIV Stigma
In the maternal-fetal protection model (Table 2, model 1), HIV stigma was positively and significantly associated with number of weeks pregnant (Est. = 0.05, p < 0.02; 95% CI = 1.01, 1.10) and negatively and significantly associated with weight (Est. = −0.05, p < 0.042; 95% CI = 0.91, 1.00). However, the confidence interval included 1.00, so this result should be interpreted with caution. Significant covariates included age (Est. = −0.10, p < 0.01; 95% CI = 0.84, 0.98) and education (Est. = −0.13, p < 0.0001; 95% CI = 0.84, 0.92).
Table 2.
Model results from the maternal fetal-protection (MFP) model, social-ecological (S-E) model, and the combined models
| Est. | SE | Z | P>|z| | 95% CI | Model Stats | AIC | ||
|---|---|---|---|---|---|---|---|---|
| MODEL 1 | θ = 6.51, SE = 0.60 | 3670.60 | ||||||
| Stigma: MFP | ||||||||
| Weeks Pregnant | 0.05 | 0.02 | 2.32 | 0.02 | * | 1.01, 1.10 | ||
| Weight | −0.05 | 0.02 | −2.03 | 0.04 | * | 0.91, 1.00 | ||
| Age at first pregnancy | 0.06 | 0.04 | 1.56 | 0.12 | 0.99, 1.13 | |||
| Hemoglobin | 0.01 | 0.02 | 0.35 | 0.72 | 0.97, 1.05 | |||
| Gravidity | 0.04 | 0.03 | 1.36 | 0.17 | 0.98, 1.10 | |||
| Age | −0.10 | 0.04 | −2.45 | 0.01 | ** | 0.84, 0.98 | ||
| Education | −0.13 | 0.02 | −5.98 | 0.0001 | *** | 0.84, 0.92 | ||
| Income | −0.02 | 0.02 | −1.11 | 0.27 | 0.94, 1.02 | |||
| (Intercept) | 2.16 | 0.02 | 105.23 | 0.001 | *** | 8.29, 8.99 | ||
| MODEL 2 | θ =10.96, SE= 1.31 | 3485.74 | ||||||
| Stigma: S-E | ||||||||
| HIV correct | −0.23 | 0.02 | −13.29 | 0.0001 | *** | 0.76,0.81 | ||
| Disclosure interpersonal | −0.05 | 0.02 | −2.45 | 0.01 | ** | 0.91,0.99 | ||
| Disclosure Community/Institutional | −0.01 | 0.02 | −0.68 | 0.49 | 0.95, 1.03 | |||
| Reactions interpersonal | 0.09 | 0.02 | 5.02 | 0.0001 | *** | 1.06, 1.13 | ||
| Age | −0.00 | 0.02 | −0.27 | 0.79 | 0.96, 1.03 | |||
| Education | −0.06 | 0.02 | −3.07 | 0.002 | ** | 0.91,0.98 | ||
| Income | −0.00 | 0.02 | −0.17 | 0.87 | 0.96, 1.03 | |||
| (Intercept) | 2.12 | 0.02 | 116.12 | 0.001 | *** | 8.06, 8.66 | ||
| MODEL 3 | θ = 11.02, SE= 1.32 | 3484.10 | ||||||
| Stigma: Combined | ||||||||
| Weeks pregnant | 0.03 | 0.02 | 1.47 | 0.14 | 0.99, 1.07 | |||
| Weight | −0.01 | 0.02 | −0.33 | 0.74 | 0.96, 1.03 | |||
| HIV correct | −0.24 | 0.02 | −13.20 | 0.0001 | *** | 0.76, 0.82 | ||
| Disclosure interpersonal | −0.05 | 0.02 | −2.95 | 0.003 | ** | 0.91,0.98 | ||
| Reactions interpersonal | 0.09 | 0.02 | 5.00 | 0.0001 | *** | 1.06, 1.13 | ||
| Age | −0.01 | 0.02 | −0.27 | 0.79 | 0.96, 1.03 | |||
| Education | −0.06 | 0.02 | −3.02 | 0.003 | ** | 0.91,0.98 | ||
| MODEL 4 | θ = 21.95, SE = 2.66 | 4419.17 | ||||||
| Fear: MFP | ||||||||
| Weeks pregnant | 0.00 | 0.01 | 0.18 | 0.86 | 0.98, 1.03 | |||
| Weight | −0.02 | 0.01 | −1.46 | 0.14 | 0.96, 1.01 | |||
| Age at first pregnancy | −0.06 | 0.02 | −2.95 | 0.003 | ** | 0.91,0.98 | ||
| Gravidity | −0.02 | 0.02 | −1.22 | 0.26 | 0.95, 1.01 | |||
| Hemoglobin | 0.13 | 0.01 | 1.15 | 0.25 | 0.99, 1.04 | |||
| Age | 0.04 | 0.02 | 1.71 | 0.09 | † | 0.99, 1.09 | ||
| Education | 0.01 | 0.01 | 0.46 | 0.64 | 0.98, 1.03 | |||
| Income | 0.00 | 0.01 | 0.29 | 0.77 | 0.98, 1.03 | |||
| (Intercept) | 3.17 | 0.01 | 272.39 | 0.0001 | *** | 23.33, 24.42 | ||
| MODEL 5 | θ = 26.94, SE = 3.59 | 4353.68 | ||||||
| Fear: S-E | ||||||||
| HIV correct | −0.03 | 0.01 | −3.04 | 0.002 | ** | 0.94, 0.99 | ||
| Disclosure interpersonal | −0.03 | 0.01 | −2.10 | 0.04 | * | 0.95, 1.00 | ||
| Disclosure Community/Institutional | 0.10 | 0.01 | 8.70 | 0.0001 | *** | 1.10, 1.14 | ||
| Reactions interpersonal | 0.02 | 0.01 | 2.15 | 0.03 | * | 1.00, 1.05 | ||
| Age | 0.00 | 0.01 | −0.39 | 0.70 | 0.97, 1.02 | |||
| Income | 0.00 | 0.01 | 0.26 | 0.79 | 0.98, 1.03 | |||
| Education | 0.01 | 0.01 | 1.16 | 0.24 | 0.99, 1.04 | |||
| (Intercept) | 3.17 | 0.01 | 285.57 | 0.0001 | *** | 23.26, 24.29 | ||
| MODEL 6 | θ = 27.52, SE = 3.72 | 4347.58 | ||||||
| Fear: Combined | ||||||||
| Age at first pregnancy | −0.03 | 0.01 | −2.37 | 0.02 | * | 0.94, 0.99 | ||
| HIV knowledge | −0.03 | 0.01 | −2.79 | 0.005 | ** | 0.95, 0.99 | ||
| Disclosure interpersonal | −0.02 | 0.01 | −1.87 | 0.06 | † | 0.95, 1.00 | ||
| Reaction interpersonal | 0.02 | 0.01 | 2.00 | 0.05 | * | 1.00, 1.04 | ||
| Disclosure Community/Institutional | 0.10 | 0.01 | 8.50 | 0.0001 | *** | 1.10, 1.13 | ||
| Age | 0.01 | 0.01 | 1.05 | 0.29 | 0.99, 1.05 | |||
| (Intercept) | 3.17 | 0.01 | 286.95 | 0.0001 | *** | 23.26, 24.29 |
p ≤ 0.10,
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001
In the social-ecological model (Table 2, model 2), HIV stigma was significantly associated with lower scores on HIV knowledge (Est. = −0.23, p < 0.0001; 95% CI = 0.76, 0.81), less willingness to disclose HIV status to interpersonal relations (Est. = −0.05, p < 0.01; 95% CI = 0.91, 0.99), higher perceived negative reactions from interpersonal relations (Est. = 0.09, p < 0.0001; 95% CI = 1.06, 1.13), and lower education (Est. = −0.06, p < 0.002; 95% CI = 0.91, 0.98). The following variables were not significantly associated with HIV stigma: age, income, and willingness to disclose to community/institutional relations.
The combined model included covariates measuring age and education, along with weeks pregnant, weight, HIV knowledge, disclosure to interpersonal relations, and higher perceived negative reactions from interpersonal relations (Table 2, model 3). The following variables were significantly associated with HIV stigma: lower education (Est. = −0.06, p < 0.003; 95% CI = 0.91, 0.98), lower scores on HIV knowledge (Est. = −0.24, p < 0.0001; 95% CI = 0.76, 0.82), and less willingness to disclose to interpersonal relations (Est. = −0.05, p < 0.003; 95% CI = 0.91, 0.98).
According to AIC, the combined model had the lowest score of 3484.95, but it was not a better fit than the social-ecological model (AIC = 3485.74). Both models outranked the maternal-fetal protection model (AIC = 3670.60).
Fear of HIV
In the maternal-fetal protection model (Table 2, model 4), fear of contracting HIV was negatively and significantly associated with age at first pregnancy (Est. = −0.06, p < 0.003; 95% CI = 0.91, 0.98) and moderately significantly associated with age (Est. = 0.04, p < 0.09; 95% CI = 0.99, 1.09).
In the social-ecological model (Table 2, model 5), the following predictors were significantly associated with the fear of contracting HIV: lower scores on HIV knowledge (Est. = −0.03, p < 0.002; 95% CI = 0.94, 0.99), less willingness to disclose status to interpersonal relations (Est. = −0.03, p < 0.04; 95% CI = 0.95, 1.00), higher willingness to disclose to community and institutional relations (Est. = 0.10, p < 0.0001; 95% CI = 1.10, 1.14), and perceived negative reactions from interpersonal relations (Est. = 0.02, p < 0.03; 95% CI = 0.95, 1.00). Variables measuring age, income, and education were not significantly associated with HIV stigma.
The combined model included age at first pregnancy, HIV knowledge, disclosure to interpersonal relations, disclosure to community and institutional relations, negative perceived reactions from interpersonal relations, and age (Table 2, model 6). All predictors except for age were significantly associated with higher fear of HIV: lower age at first pregnancy (Est. = −0.03, p < 0.02; 95% CI = 0.94, 0.99), lower scores on HIV knowledge (Est. = −0.03, p < 0.005; 95% CI = 0.95, 0.99), less willingness to disclose status to interpersonal relations (Est. = −0.02, p < 0.06; 95% CI = 0.95, 1.00), higher willingness to disclose to community and institutional relations (Est. = 0.10, p < 0.0001; 95% CI = 1.10, 1.13), and negative perceived reactions from interpersonal relations (Est. = 0.02, p < 0.05; 95% CI = 1.00, 1.04).
According to AIC, the combined model (4347.58) outperformed the social-ecological model (4353.68) and the maternal-fetal protection model (4419.17).
Discussion
The overall goal of this study was to test the extent to which a combined social-ecological and modified maternal-fetal protection model could predict barriers that prevent women from getting HIV testing and treatment. Existing studies that use social-ecological theory have shown positive trends in HIV-related stigma reduction and have employed methods such as information-based approaches, skill building, counseling, and contact with HIV-affected groups (Brown et al. 2003; Sengupta et al. 2011). PMTCT efforts, however, continue to struggle with individual, interpersonal, and community biases against pregnant women receiving antenatal care and HIV testing (Turan and Nyblade 2013). The current study addressed this issue by considering the dual role of maternal-fetal protection and social-ecological factors in predicting HIV-related stigma and fear. Our results found support for three levels of social-ecological theory (individual, interpersonal, and community/institutional) and indicated that aspects of women’s reproductive histories and nutritional stressors were predictive of HIV stigma and fear. These findings are significant because they contribute to a growing literature that points to additional factors that influence protection mechanisms in pregnancy that span beyond the first trimester (Placek 2017; Placek and Hagen 2013; Placek et al. 2017; Young and Pike 2012). More importantly, public health efforts can use this information by considering women’s reproductive life histories and the social contexts that surround them.
Maternal-Fetal Protection Models
The positive relationship between weeks pregnant and HIV stigma was in the opposite direction as predicted by the classic maternal-fetal protection hypothesis for other protective psychological mechanisms (Fessler et al. 2005; Navarrete et al. 2007). Our result could reflect underlying cultural perceptions of fetal development and vulnerability that do not overlap with biological changes during gestation. Prior research in this region has found that women perceive fetuses to be vulnerable to certain threats throughout the course of pregnancy (Placek and Madhivanan 2017), which opens the possibility that diseases, such as HIV, are viewed in a similar light. Alternatively, this finding could challenge the validity of maternal-fetal protection and related prophylactic theories since recent evidence suggests that maternal susceptibility to pathogens is not always limited to the first trimester (Kourtis et al. 2014), and indicators of immunosuppression do not consistently predict hypothesized prophylactic behaviors both during pregnancy and across the menstrual cycle (Jones et al. 2018; Mikolić 2016; Placek 2017; Placek and Hagen 2013; Timmers et al. 2018).
The inverse relationship between maternal weight and HIV stigma supported our prediction that nutritional stress, which can make women more vulnerable to certain infections (Steketee 2003), predicted higher HIV stigma. In South India, women who suffered from undernutrition reported cravings for uncooked rice and pica substances (i.e., nonfood items such as clay and chalk) (Placek and Hagen 2013). The link between human nutritional deprivation and the immune system requires more research, however (Norgan 1997). Nonetheless, our study provides a unique view for the role nutritional stress has on evolved psychological mechanisms.
Age at first pregnancy was the only significant predictor of HIV fear. In contrast to the measure of weight, an indicator of current nutritional stress, age at first pregnancy is indicative of early stressful conditions (Chisholm et al. 2005; Nettle et al. 2011). Although research shows that timing of first pregnancy is associated with immune function in subsequent pregnancies (Blackmore et al. 2011), this is the first study to establish a link between age at first pregnancy and pathogen-avoidant perceptions. Women who start reproduction at a young age spend more time investing in reproductive effort, which might require them to rely more on secondary lines of pathogen defense, particularly if they suffer from additional environmental stressors that impact immune function.
Although this study did not find strong support for all measures from the maternal-fetal protection hypothesis, the significant findings regarding women’s reproductive life histories and current nutritional stress point to the need to consider these factors in shaping stigma and discrimination. Not only is this relevant for efforts that aim to improve antenatal care, but this is also relevant in cases where individuals make “false-positive” errors. “False positive” errors can involve the avoidance of harmless individuals who display ambiguous signs of infection, potentially leading to an increase in discrimination and stigmatization of outgroup members (Schaller and Park 2011). Experiencing disgust toward obese people is one example of a false-positive error attribution (Park et al. 2007). Since obesity emerged only recently in Homo sapiens, scholars propose that symptoms of obesity, such as fluid build-up, trigger cognitive mechanisms associated with pathogen avoidance, leading to an error-prone, by-product, disgust response (Lieberman et al. 2012). Thus, although the innate drive to avoid infection can be effective in preventing infection among vulnerable populations, it becomes problematic when it is transformed into stigmatization and discrimination, such as with the case of people living with HIV/AIDS (Schaller and Park 2011). Under the guise of life history theory, when individuals are faced with trade-offs in reproduction at the expense of immune function, they might be quick to pass judgment on others regardless of disease status in order to protect current and future offspring. Multiple individuals undergoing similar environmental threats can lead to rapid dissemination of harmful beliefs that exacerbate the problems associated with HIV stigma. Thus, one contribution of this approach is that it highlights the stressors surrounding the stigmatizer: when the stigmatizer experiences heightened stress in the early and/or current environment, this might contribute to the formation or continuation of discriminatory beliefs, thus serving as an added barrier to treatment initiatives.
Social-Ecological Models
Knowledge about the transmission of HIV remained significant across all models for both stigma and fear. This finding replicates previous research in other populations that have found strong links between inaccurate knowledge about transmission and a greater willingness to stigmatize and avoid people with AIDS (Herek and Capitanio 1994, 1997; Price and Hsu 1992). In the current study, even though the majority of participants generally had correct knowledge of the transmission of HIV, two questions raise concern over local beliefs about transmission: 39.4% believed that HIV could be transmitted from being coughed or sneezed on by a person who has HIV and 51.0% believed that mosquitoes or other insects could transmit the virus by biting a person. These findings indicate that more educational programs are needed in this community to provide accurate information about the transmission of HIV. Interestingly, nearly all women in this study had correct knowledge about HIV transmission when the questions focused on infant health, with much lower rates of knowledge in reference to unprotected sex and transmission via syringes. The emphasis on reproductive knowledge in pregnancy makes sense in light of cultural transmission research on pregnancy, whereby women acquire relevant health information from close relatives as means to protect themselves and their fetuses from harm (Henrich and Henrich 2010; Placek et al. 2017).
Findings from the social-ecological model support prior research that investigates the link between stigma, disclosure, and perceived reactions from interpersonal relations. Derlega (2002) found that those who report higher HIV stigma are less willing to disclose their status to family and friends. Similarly, results of a meta-analysis of stigma, social support, and disclosure of HIV status are consistent with our finding that mothers with high levels of perceived stigma demonstrate less willingness to disclose HIV status to interpersonal relations (Smith et al. 2008). Disclosure is an important aspect of eliciting support among people living with HIV, though those experiencing stigma and rejection are less confident in their ability to seek help (Brashers et al. 2004).
One surprising finding in our study was the positive association between HIV fear and willingness to disclose to community/institutional relations. This relationship could be interpreted as those who fear the consequences of HIV might be more willing to seek support and treatment from community stakeholders. A study conducted in Tanzania, for example, found that although religious beliefs predicted higher negative attitudes toward HIV, the majority of participants reported they would disclose their status to religious leaders because they believed prayer could cure HIV (Zou et al. 2009). Our disclosure measure included other community and institutional leaders, but perhaps our participants who adhere closely to social norms also fear the effects of HIV and therefore perceive these stakeholders to be important figures in the treatment process.
Combined Models
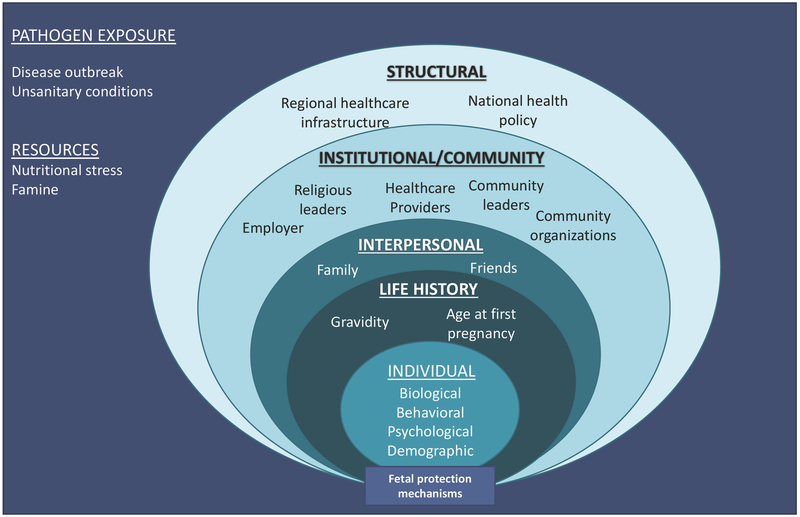
The combined fear model that included significant predictors from the maternal-fetal protection and social-ecological model had the best fit according to AIC. In light of these results, we propose a modified social-ecological model that incorporates maternal reproductive histories, ecological circumstances (access to resources and pathogen risk), and hypothesized maternal-fetal protection mechanisms (Fig. 2). This model is specifically geared toward predicting maternal-fetal protection mechanisms, and the factors that could influence these mechanisms are therefore pregnancy-specific. Furthermore, this model is not intended to be predictive; rather, it can generate new testable hypotheses that draw on the combined perspectives of each model. As the model indicates, environmental exposure to pathogens and resource stress are situated as the backdrop of the social-ecological model. Both exposure to pathogens and resource scarcity can be experienced at the group level, such as disease outbreaks or famine, or at the individual level, such as variation in illness susceptibility and weight. The rest of the model remains the same as the original social-ecological model, with the exception of the individual-level factors, which now include age at first pregnancy and weeks pregnant. We recommend that future research continues to test for other reproductive factors that traditionally predict maternal-fetal protection mechanisms since ecological differences can potentially give rise to variation in these relationships. Lastly, “maternal-fetal protection mechanisms” are nested at the bottom of the model to show how these are ultimately influenced by the preceding factors.
Figure 2.
Combined elements of the social-ecological and modified maternal fetal protection models to explain fetal protection mechanisms, such as HIV stigma and fear.
In addition to using this model to predict behaviors that cause harm to infected others, there are additional ways to incorporate evolutionary theory into the social-ecological model to improve health interventions. First, the bulk of research that utilizes social-ecological theory has demonstrated the efficacy of using education as an intervention tool, and evolutionary medical anthropological research has also shown that health education can reduce stigma and fear (Hewlett and Hewlett 2007). Second, efforts can focus on humans’ innate tendency to cooperate with biological relatives and individuals they are likely to encounter repeatedly (Hamilton 1964; Trivers 1971). Both of these cooperative strategies help increase inclusive fitness. Working within the interpersonal realm of the social-ecological model, public health workers can develop more effective ways to educate and communicate about HIV stigma, treatment, and prevention within family units. Third, humans have evolved to rely on social learning to acquire adaptive information from the local environment. Prestige bias, copying successful and popular individuals, is one major pathway by which information is acquired, and in particular, plays a significant role in norm transmission during pregnancy in traditional societies (Henrich and Henrich 2010; Placek et al. 2017). Within the organizational/institutional and community realms of the social-ecological model, public health interventions should identify the most prestigious individual in terms of the transmission of health knowledge and encourage that individual to transmit updated information. Public health educators and health providers can also use their status to reduce stigma and fear by maintaining a presence in the community, providing updated knowledge, and interacting regularly with people who have HIV/AIDS (Hewlett and Hewlett 2007). Healthcare providers, by virtue of their skills, education, and training, are in a position to provide information that integrates both physical and psychological information that can minimize negative perceptions of HIV-positive individuals (Baidya et al. 2014; D’Souza et al. 2013; Ekstrand et al. 2013; Gopichandran and Chetlapalli 2013). However, this would also require practitioners to gain culturally competent understandings of the various factors influencing stigma across varying levels of influence, and address their own biases about the virus as well (Ekstrand et al. 2013; Mahendra et al. 2007).
Limitations
This study is not without limitations. First, our sample size dropped by more than half by the inclusion of all study measures in the analyses. This is particularly concerning because the goals of the wider Kisalaya project were to provide antenatal care to women, test for HIV, and provide educational counseling on prenatal health. One could speculate that the individuals who withdrew from the study or chose not to answer the questions regarding HIV could represent a portion of the population with high levels of stigma and fear of HIV, or have inaccurate knowledge about the transmission of HIV. Although we cannot be confident in drawing this conclusion, this limitation in the study reflects a larger limitation in PMTCT interventions where women are hesitant to receive antenatal care regarding HIV due to stigma in their families and communities (Turan and Nyblade 2013).
Furthermore, future applications of this combined approach should consider structural factors impacting stigmatization, such as discriminatory policies or healthcare infrastructure. Although we did not expect a significant difference in structural determinants of stigma between the villages we sampled in Mysore district, individual perceptions of the structural factors that influence the factors that enable or prevent individuals from testing for HIV or receiving treatment could have shed light on appropriate steps to take in future prevention efforts.
Conclusion
This study is among the first to empirically compare the social-ecological model and the evolutionary model of maternal-fetal protection in predicting HIV stigma and fear. Numerous studies in the public health literature view stigma as a mediating factor of health behavior and disease (Corrigan 2004; Hatzenbuehler et al. 2013; Puhl and Latner 2007; Sayles et al. 2009). However, internalized stigma itself has never been studied as an outcome in the social-ecological model, which is concerning given the corrosive effect stigma can have on health (Hatzenbuehler et al. 2013). Using the constructs of the social-ecological model, we identified individual, interpersonal, and community/institutional facilitators of stigma among pregnant women. Our findings suggest that perceived social support (or discrimination), anticipated reactions of community members, friends and family, as well as women’s reproductive life histories and nutritional stress are significant factors that influence HIV-related stigma and fear. This study went further by developing a combined predictive model that will hopefully set the stage for future research focused on hypothesized strategies pregnant women use to protect themselves and their offspring. Understanding these factors that shape HIV stigma and fear is crucial because treatment providers can learn to reframe their communication techniques in a way that is more culturally sensitive and considers the long-standing resource and immunological stressors women face. Our hope, therefore, is that the combined understanding of social-ecological predictors and humans’ evolved psychology will improve public health efforts to reduce HIV-related stigma and fear among vulnerable populations.
Supplementary Material
Acknowledgments
The authors would like to thank study participants, research assistants, and PHRII staff for their assistance in the study design and data collection process. Thank you to James Jones, Edward Hagen, and Marsha Quinlan for comments and suggestions on earlier iterations of this manuscript. The Kisalaya mobile clinic project was funded by the Elizabeth Glaser Pediatric AIDS Foundation International Leadership Award to Purnima Madhivanan. Caitlyn Placek was supported by the Global Health Equity Scholars Training Grant from Fogarty International Center at National Institutes of Health (R25 TW009338). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Biography
Caitlyn Placek, PhD, is a biocultural medical anthropologist and assistant professor of biological anthropology at Ball State University. She specializes in evolutionary approaches to maternal health and diet, religion, and substance use.
Holly Nishimura, MPH, is a PhD student at the Bloomberg School of Public Health at Johns Hopkins University. Her research interests focus on sexual and reproductive health in LMICs.
Dionne Stephens, PhD, is a psychologist at Florida International University who examines sociohistorical factors that shape minority populations’ sexual scripting and sexual health processes, with emphasis on gender and ethnic/racial identity development.
Natalie Hudanick is an undergraduate student in anthropology at Ball State University. Her interests include public health and women’s health.
Purnima Madhivanan, PhD, is the founder of PHRII and an associate professor and director of the Epidemiology PhD program at Florida International University. She has been working in the field of public health for more than a decade, particularly in the realm of maternal health and HIV/AIDS.
References
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta T-A, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J (2012). A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLOS One, 7(6), e36466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidya M, Gopichandran V, and Kosalram K (2014). Patient-physician trust among adults of rural Tamil Nadu: A community-based survey. Journal of Postgraduate Medicine, 60(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, and O’Connor TG (2011). Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosomatic Medicine, 73(8), 656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers DE, Neidig JL, and Goldsmith DJ (2004). Social support and the management of uncertainty for people living with HIV or AIDS. Health Communication, 16(3), 305–331. [DOI] [PubMed] [Google Scholar]
- Brown L, Macintyre K, and Trujillo L (2003). Interventions to reduce HIV/AIDS stigma: what have we learned? AIDS Education and Prevention, 15(1), 49–69. [DOI] [PubMed] [Google Scholar]
- Cameron AC, and Trivedi PK (2005). Microeconometrics: Methods and applications. Cambridge: Cambridge University Press. [Google Scholar]
- Census of India. (2011). Census of India: census data online. Retrieved from http://censusindia.gov.in/2011-common/censusdataonline.html?drpQuick=anddrpQuickSelect=andq=jenukuruba
- Chisholm JS, Quinlivan JA, Petersen RW, and Coall DA (2005). Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Human Nature, 16(3), 233–265. [DOI] [PubMed] [Google Scholar]
- Corrigan P (2004). How stigma interferes with mental health care. American Psychologist, 59(7), 614–625. [DOI] [PubMed] [Google Scholar]
- Cumberland P, Shulman CE, Maple C, A P, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT (2007). Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. The Journal of Infectious Diseases 196(4), 550–557. [DOI] [PubMed] [Google Scholar]
- Derlega VJ, Winstead BA, Greene K, Serovich J, and Elwood WN (2002). Perceived HIV-related stigma and HIV disclosure to relationship partners after finding out about the seropositive diagnosis. Journal of Health Psychology, 7(4), 415–432. [DOI] [PubMed] [Google Scholar]
- Drake A, Wagner A, Richardson B, and John-Stewart G (2014). Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: a systematic review and meta-analysis. PLoS Medicine, 11 Retrieved from http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS, Karkada SN, Somayaji G, and Venkatesaperumal R (2013). Women’s well-being and reproductive health in Indian mining community: need for empowerment. Reproductive Health, 10, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand ML, Ramakrishna J, Bharat S, and Heylen E (2013). Prevalence and drivers of HIV stigma among health providers in urban India: implications for interventions. Journal of the International AIDS Society, 16(3, Suppl 2), 18717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler DMT (2002). Reproductive Immunosuppression and Diet: An evolutionary perspective on pregnancy sickness and meat consumption. Current Anthropology, 43(1) 19–61. [DOI] [PubMed] [Google Scholar]
- Fessler DMT, Eng SJ, and Navarrete CD (2005). Elevated disgust sensitivity in the first trimester of pregnancy. Evolution and Human Behavior, 26(4), 344–351. [Google Scholar]
- Flaxman SM, and Sherman PW (2000). Morning sickness: a mechanism for protecting mother and embryo. Quarterly Review of Biology, 75(2), 113–148. [DOI] [PubMed] [Google Scholar]
- Georgiev AV, Kuzawa CW, and McDade TW (2016). Early developmental exposures shape trade-offs between acquired and innate immunity in humans. Evolution, Medicine, and Public Health 2016(1), 256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs CM, Wendt A, Peters S, and Hogue CJ (2012). The impact of early age at first childbirth on maternal and infant health. Paediatric and Perinatal Epidemiology, 26(s1), 259–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go VF, Sethulakshmi CJ, Bentley ME, Sivaram S, Srikrishnan AK, Solomon S, and Celentano DD (2003). When HIV-prevention messages and gender norms clash: the impact of domestic violence on women’s HIV risk in slums of Chennai, India. AIDS and Behavior, 7(3), 263–272. [DOI] [PubMed] [Google Scholar]
- Goffman E (1963). Stigma; notes on the management of spoiled identity. New York: Simon & Schuster. [Google Scholar]
- Gopichandran V, and Chetlapalli SK (2013). Factors influencing trust in doctors: a community segmentation strategy for quality improvement in healthcare. BMJ Open, 3(12), e004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, Nalugoda F, Kiddugavu M, Sewankambo N, Quinn TC, Reynolds SJ, Wawer MJ (2005). Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study. The Lancet, 366(9492), 1182–1188. [DOI] [PubMed] [Google Scholar]
- Hamilton WD (1964). The genetical evolution of social behaviour. II. Journal of Theoretical Biology, 7(1), 17–52. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Phelan JC, and Link BG (2013). Stigma as a fundamental cause of population health inequalities. American Journal of Public Health, 103(5), 813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, and Henrich N (2010). The evolution of cultural adaptations: Fijian food taboos protect against dangerous marine toxins. Proceedings of the Royal Society of London B: Biological Sciences, 277(1701), 3715–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herek GM, and Capitanio JP (1994). Conspiracies, contagion, and compassion: trust and public reactions to AIDS. AIDS Education and Prevention, 6(4), 365–375. [PubMed] [Google Scholar]
- Herek GM, and Capitanio JP (1997). AIDS stigma and contact with persons with AIDS: effects of direct and vicarious contact. Journal of Applied Social Psychology, 27(1), 1–36. [Google Scholar]
- Herek GM, Capitanio JP, and Widaman KF (2002). HIV-related stigma and knowledge in the United States: prevalence and trends 1991–1999. American Journal of Public Health, 92(3), 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett BS, and Hewlett BL (2007). Ebola, culture and politics: The anthropology of an emerging disease. Cengage Learning. [Google Scholar]
- Hook EB (1978). Dietary cravings and aversions during pregnancy. The American Journal of Clinical Nutrition, 31(8), 1355–1362. [DOI] [PubMed] [Google Scholar]
- Jones BC, Hahn AC, Fisher CI, Wang H, Kandrik M, Lee AJ, Tybur JM and DeBruine LM (2018). Hormonal correlates of pathogen disgust: testing the compensatory prophylaxis hypothesis. Evolution and Human Behavior, 39(2), 166–169. [Google Scholar]
- Kerrigan D, Moreno L, Rosario S, Gomez B, Jerez H, Barrington C, Weiss E, Sweat M (2006). Environmental–structural interventions to reduce HIV/STI risk among female sex workers in the Dominican Republic. American Journal of Public Health, 96(1), 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N, Krupp K, Ravi K, Gowda S, Jaykrishna P, Leonardson-Placek C, Siddhaiah A, Bristow C, Arun A, Klausner J, Madhivanan P (2017). Implementing and sustaining a mobile medical clinic for prenatal care and sexually transmitted infection prevention in rural Mysore, India. BMC Infectious Diseases, 17, 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Read JS, and Jamieson DJ (2014). Pregnancy and infection. New England Journal of Medicine, 370(23), 2211–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, and Choudhry VP (2010). Iron deficiency and infection. The Indian Journal of Pediatrics, 77(7), 789–793. [DOI] [PubMed] [Google Scholar]
- Kurzban R, and Leary MR (2001). Evolutionary origins of stigmatization: the functions of social exclusion. Psychological Bulletin, 127(2), 187–208. [DOI] [PubMed] [Google Scholar]
- Larios SE, Lozada R, Strathdee SA, Semple SJ, Roesch S, Staines H, Orozovich P, Fraga M, Amaro H, de la Torre A, Magis-Rodriguez C, Patterson TL (2009). An exploration of contextual factors that influence HIV risk in female sex workers in Mexico: the social ecological model applied to HIV risk behaviors. AIDS Care, 21(10), 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman D, Tybur J, and Latner J (2012). Disgust sensitivity, obesity stigma, and gender: contamination psychology predicts weight bias for women, not men. Obesity 20(9), 1803–1814. [DOI] [PubMed] [Google Scholar]
- Mahendra VS, Gilborn L, Bharat S, Mudoi R, Gupta I, George B, Samson L, Daly C, Pulerwitz J (2007). Understanding and measuring AIDS-related settings: A developing country perspective. SAHARA-J: Journal of Social Aspects of HIV/AIDS, 4(2), 616–625. Retrieved from https://www.ajol.info/index.php/saharaj/article/view/30125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeroy KR, Bibeau D, Steckler A, and Glanz K (1988). An ecological perspective on health promotion programs. Health Education Quarterly, 15(4), 351–377. [DOI] [PubMed] [Google Scholar]
- Mikolić A (2016). Disgust and facial expression recognition across the menstrual cycle. Human Ethology Bulletin, 1, 31–60. [Google Scholar]
- Miller EM (2010). Maternal hemoglobin depletion in a settled Northern Kenyan pastoral population. American Journal of Human Biology, 22(6), 768–774. 10.1002/ajhb.21078 [DOI] [PubMed] [Google Scholar]
- Miller EM (2016). The reproductive ecology of iron in women. American Journal of Physical Anthropology, 159(S61), 172–195. [DOI] [PubMed] [Google Scholar]
- Navarrete CD, Fessler DMT, and Eng SJ (2007). Elevated ethnocentrism in the first trimester of pregnancy. Evolution and Human Behavior, 28(1), 60–65. [Google Scholar]
- Nettle D, Coall DA, and Dickins TE (2011). Early-life conditions and age at first pregnancy in British women. Proceedings of the Royal Society of London B: Biological Sciences, 278(1712), 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgan NG (1997). The beneficial effects of body fat and adipose tissue in humans*. International Journal of Obesity, 21(9), 738–746. [DOI] [PubMed] [Google Scholar]
- Osamor PE, and Grady C (2016). Women’s autonomy in health care decision-making in developing countries: A synthesis of the literature. International Journal of Women’s Health, 8 191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Schaller M, and Crandall CS (2007). Pathogen-avoidance mechanisms and the stigmatization of obese people. Evolution and Human Behavior, 28(6), 410–414. [Google Scholar]
- Parker BJ, Barribeau SM, Laughton AM, Roode J. C. de, and Gerardo NM (2011). Non-immunological defense in an evolutionary framework. Trends in Ecology and Evolution, 26(5), 242–248. [DOI] [PubMed] [Google Scholar]
- Parker R, and Aggleton P (2003). HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Social Science and Medicine, 57(1), 13–24. [DOI] [PubMed] [Google Scholar]
- Placek CD (2017). A test of four evolutionary hypotheses of pregnancy food cravings: evidence for the social bargaining model. Royal Society Open Science, 4(10), 170243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placek CD, and Hagen EH (2013). A test of three hypotheses of pica and amylophagy among pregnant women in Tamil Nadu, India. American Journal of Human Biology, 25(6), 803–813. [DOI] [PubMed] [Google Scholar]
- Placek CD, and Hagen EH (2015). Fetal protection: the roles of social learning and innate food aversions in South India. Human Nature, 26(3), 255–276. [DOI] [PubMed] [Google Scholar]
- Placek CD, and Madhivanan P (2017). Exploring the perceptions of pregnancy loss between two South Indian women: A pilot study. Public Health, 148, 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placek CD, Madhivanan P, and Hagen EH (2017). Innate food aversions and culturally transmitted food taboos in pregnant women in rural southwest India: separate systems to protect the fetus? Evolution and Human Behavior, 38(6), 714–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price V, and Hsu M-L (1992). Public opinion about AIDS policies: the role of misinformation and attitudes toward homosexuals. The Public Opinion Quarterly, 56(1), 29–52. [DOI] [PubMed] [Google Scholar]
- Profet M (1988). The evolution of pregnancy sickness as protection to the embryo against Pleistocene teratogens. Evolutionary Theory, 8(3), 177–190. [Google Scholar]
- Profet M (1995). Pregnancy sickness as adaptation: a deterrent to maternal ingestion of teratogens In (Barkow JH, Cosmides L, and Tooby J, eds.) The adapted mind: Evolutionary psychology and the generation of culture (pp. 327–366). New York: Oxford University Press. [Google Scholar]
- Puhl RM, and Latner JD (2007). Stigma, obesity, and the health of the nation’s children. Psychological Bulletin, 133(4), 557–580. [DOI] [PubMed] [Google Scholar]
- Qiao S, Li X, Zhou Y, Shen Z, Tang Z, and Stanton B (2015). Factors influencing the decision-making of parental HIV disclosure: a socio-ecological approach. AIDS, 29(1). Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4618838/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K, Kwon J-Y, Aldo P, Silasi M, and Mor G (2014). Understanding the complexity of the immune system during pregnancy. American Journal of Reproductive Immunology, 72(2), 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Kapur S, Salhan S, and Mittal A (1999). Chlamydia trachomatis infection in pregnancy: risk factor for an adverse outcome. British Journal of Biomedical Science; London, 56(2), 94–98. [PubMed] [Google Scholar]
- Sandelowski M, Lambe C, and Barroso J (2004). Stigma in HIV-Positive women. Journal of Nursing Scholarship, 36(2), 122–128. [DOI] [PubMed] [Google Scholar]
- Saute F, Menendez C, Mayor A, Aponte J, Gomez‐Olive X, Dgedge M, and Alonso P (2002). Malaria in pregnancy in rural Mozambique: the role of parity, submicroscopic and multiple Plasmodium falciparum infections. Tropical Medicine and International Health, 7(1) 19–28. [DOI] [PubMed] [Google Scholar]
- Sayles JN, Wong MD, Kinsler JJ, Martins D, and Cunningham WE (2009). The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. Journal of General Internal Medicine, 24(10), 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M, and Park JH (2011). The behavioral immune system (and why it matters). Current Directions in Psychological Science 20(2), 99–103. [Google Scholar]
- Senarath U, and Gunawardena Nalika Sepali. (2009). Women’s autonomy in decision making for health care in South Asia. Asia Pacific Journal of Public Health, 21(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Banks B, Jonas D, Miles MS, and Smith GC (2011). HIV interventions to reduce HIV/AIDS stigma: a systematic review. AIDS and Behavior, 15(6), 1075–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman CE, Marshall T, Dorman EK, Bulmer JN, Cutts F, Peshu N, and Marsh K (2001). Malaria in pregnancy: adverse effects on haemoglobin levels and birthweight in primigravidae and multigravidae. Tropical Medicine and International Health, 6(10), 770–778. [DOI] [PubMed] [Google Scholar]
- Silverman JG, Decker MR, Saggurti N, Balaiah D, and Raj A (2008). Intimate partner violence and HIV infection among married Indian women. Journal of the American Medical Association, 300(6), 703–710. [DOI] [PubMed] [Google Scholar]
- Smith R, Rossetto K, and Peterson B (2008). A meta-analysis of disclosure of one’s HIV-positive status, stigma and social support. AIDS Care 20(10). Retrieved from https://www.tandfonline.com/doi/abs/10.1080/09540120801926977 [DOI] [PubMed] [Google Scholar]
- Stangl AL, Lloyd JK, Brady LM, Holland CE, and Baral S (2013). A systematic review of interventions to reduce HIV-related stigma and discrimination from 2002 to 2013: how far have we come? Journal of the International AIDS Society, 16(3). 10.7448/ias.16.3.18734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee RW (2003). Pregnancy, nutrition and parasitic diseases. The Journal of Nutrition, 133(5), 1661S–1667S. [DOI] [PubMed] [Google Scholar]
- Stephenson R (2007). Human immunodeficiency virus and domestic violence: the sleeping giants of Indian health? Indian Journal of Medical Sciences, 61(5). Retrieved from http://go.galegroup.com/ps/i.do?p=AONEandsw=wandissn=00195359andv=2.1andit=randid=GALE%7CA163159785andsid=googleScholarandlinkaccess=abs [PubMed] [Google Scholar]
- Steward WT, Herek GM, Ramakrishna J, Bharat S, Chandy S, Wrubel J, and Ekstrand ML (2008). HIV-related stigma: adapting a theoretical framework for use in India. Social Science and Medicine (1982), 67(8), 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokols D (1996). Translating social ecological theory into guidelines for community health promotion. American Journal of Health Promotion, 10(4), 282–298. [DOI] [PubMed] [Google Scholar]
- Taha T, Dallabetta G, Hoover D, Chiphangwi J, Mtimavalye L, Liomba G, Newton I Kumwenda NI, Miotti P (1998). Trends of HIV‐1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi: AIDS, 12(2) 197–203. [DOI] [PubMed] [Google Scholar]
- Timmers AD, Bossio JA, and Chivers ML (2018). Disgust, sexual cues, and the prophylaxis hypothesis. Evolutionary Psychological Science, 4(2), 179–190. [Google Scholar]
- Trivers RL (1971). The evolution of reciprocal altruism. Quarterly Review of Biology, 46(1), 35–57. [Google Scholar]
- Turan JM, and Nyblade L (2013). HIV-related stigma as a barrier to achievement of global PMTCT and maternal health goals: a review of the evidence. AIDS and Behavior, 17(7), 2528–2539. [DOI] [PubMed] [Google Scholar]
- UNAIDS. (2003). UNAIDS fact sheet on stigma and discrimination. Retrieved from http://data.unaids.org/publications/fact-sheets03/fs_stigma_discrimination_en.pdf
- Van Hollen C (2007). Navigating HIV, pregnancy, and childbearing in South India: pragmatics and constraints in women’s decision making. Medical Anthropology, 26(1), 7–52. [DOI] [PubMed] [Google Scholar]
- Young AG, and Pike IL (2012). A biocultural framework for examining maternal cravings and aversions among pastoral women in East Africa. Ecology of Food and Nutrition, 51(5), 444–462. [DOI] [PubMed] [Google Scholar]
- Zou J, Yamanaka Y, John M, Watt M, Ostermann J, and Thielman N (2009). Religion and HIV in Tanzania: influence of religious beliefs on HIV stigma, disclosure, and treatment attitudes. BMC Public Health, 9, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.