Abstract
The study of clot retraction in vitro has been adopted as a simple and reproducible approach to assess platelet function. Plasma clots should retract away from the sides of a glass tube within a few hours allowing the rapid characterization of outside-in signalling through platelet integrin αIIbβ3 within individuals or in response to test compounds. In this chapter we describe the role of platelets in fibrin clot retraction and provide a detailed description of the methods used to assess this process.
Keywords: Clot retraction, platelets, integrin αIIbβ3, outside-in signaling
1. Introduction
The conversion of fibrinogen to fibrin at sites of injury result in the formation of threads of fibrin which form a clot trapping platelets and red blood cells to form a plug at the site of injury, and in the arterial circulation consolidating the developing platelet thrombus. Clot retraction is a process driven by outside-in signaling by platelet integrin αIIbβ3 that results in the contraction of the fibrin mesh. The contraction of the fibrin clot, results in the blood clot becoming smaller and excess fluid is extruded. This draws the edges of damaged tissue together and forms a mechanically stable clot.
The study of the retraction of plasma clots formed in vitro has been adopted as a simple and reproducible approach to characterise outside-in signalling through platelet integrin αIIbβ3.
Clot formation, stabilization and retraction
Exposure of sub-endothelial collagen upon vessel injury activates platelets (1-3) and also initiates coagulation resulting in the production of thrombin, a potent platelet agonist (4). Platelet activation results in spreading, the secretion of granules containing pro-thrombotic factors, and the affinity of the fibrinogen receptor, integrin αIIbβ3 for its ligand fibrinogen is increased (5). These ‘inside out’ signals result in platelet aggregation via bivalent binding to fibrinogen. As a consequence of fibrinogen binding, the cytoplasmic tail of β3 integrin subunit becomes phosphorylated triggering an ‘outside-in’ signal that provide a second wave of activatory signaling that is necessary for irreversible platelet thrombus formation. Such ‘outside-in’ signaling through integrin αIIbβ3 plays a key role in clot retraction (6).
The role of platelets in fibrin clot formation is long established. At sites of injury the activation of the tissue factor- (extrinsic) and contact- (intrinsic) dependent coagnulation pathways leads to the activation of factor X, and through subsequent processing of prothrombin to thrombin, lead to the conversion of plasma fibrinogen to fibrin (Figure 1). Activated platelets provide phospholipids released in the form of microparticles that act along with calcium as cofactors for the actions of factor X. The exposure of amino phospholipids such as phosphatidylserine on the surface of activated platelets provides a surface for the assembly of the prothrombinase complex, and thereby thrombin may be produced in the vicinity of a platelet thrombus as it forms, ensuring the incorporation fibrin for thrombus stabilization. Through activation of protease activated receptors on the platelet surface, thrombin also acts as a powerful platelet agonist.
Figure 1. The regulation of fibrin clot formation.
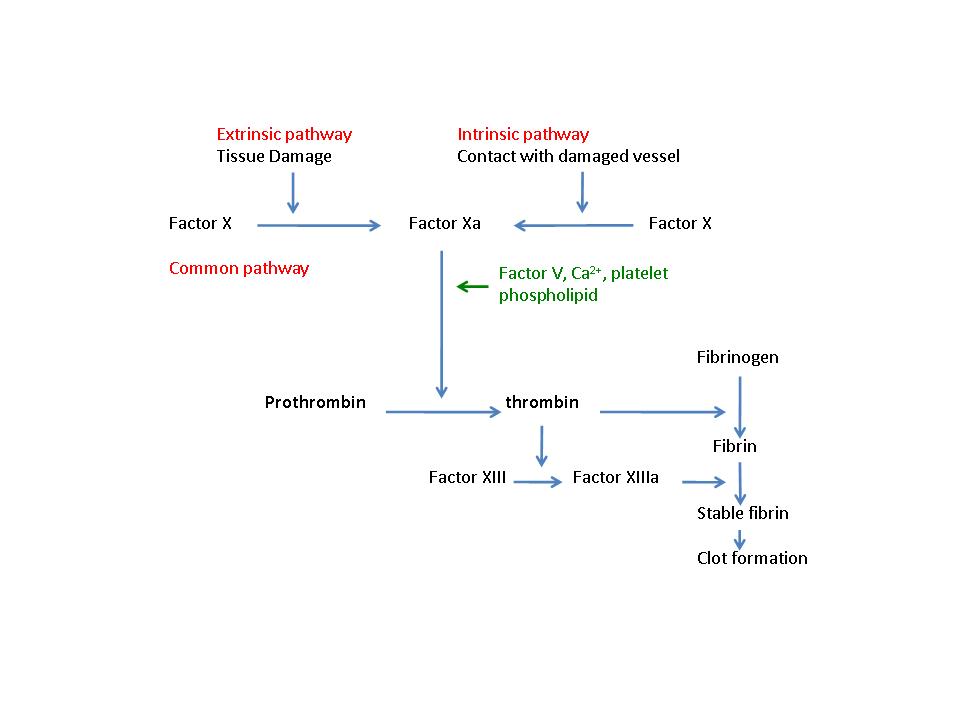
Diagram of the final stages of the coagulation cascade where the intrinsic and extrinsic pathway meet and combine in the final common pathway, that leads to the conversion of fibrinogen to fibrin.
Platelets generate force to contract the fibrin matrix and draw the edges of the wound together. The retraction is driven by the interaction between the fibrin outside the cells and the actin - myosin cytoskeleton of the platelets, which is mediated by integrin αIIbβ3. Myosin binding occurs upon phosphorylation of the β3 subunit of the integrin (7) and the ANK domain-containing Bcl-3 co-localises with the cytoskeleton, which have been shown, along with Src-family kinases to play key regulatory roles in this process (8, 9, 10). Phospholipase Cγ2 (PLCγ2) is also implicated, and following fibrinogen - integrin αIIbβ3 binding, becomes tyrosine phosphorylated by a Src family kinase-dependent mechanism (10). Evidence suggests that tyrosine kinases regulate the attachment of the cytoskeleton to integrin αIIbβ3 and are essential for the transition of cellular contractile forces to the fibrin polymers (11). The process of retraction is energy demanding and the metabolite sensing kinase adenosine monophosphate (AMP)-activated protein kinase α2 (AMPK α2) affects the phosphorylation of the Src-family kinase Fyn, which in turn affects integrin αIIbβ3 signalling and thus clot retraction (12).
Clot retraction as an assay of integrin αIIbβ3 outside-in signalling
The ability of platelets to drive clot retraction has become a surrogate measure of outside in signalling following engagement of the integrin αIIbβ3 that may be readily measure with little required equipment. In this chapter we describe a simple in vitro technique that can be used assess platelet function in human or mouse blood.
2. Materials
Modified Tyrodes-Hepes buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCL, 12mM NaHCO3, 20 mM HEPES, 5 mM glucose, 1 mM MgCL2, pH 7.3)
4% (w/v) sodium citrate (see Note 1)
Thrombin (Sigma, Poole, UK), make a stock of 20 units ml−1 in Tyrodes-Hepes buffer.
Syringes, sealed glass pipettes (see Note 2), reusable tack adhesive (such as Blu-Tack), Glass tubes (10mm diameter tubes with a total capacity of 3ml for mouse clot retraction assays, and 10mm or 12 mm diameter tubes for assays with human blood) and test tube rack
Camera and microbalance
3. Method
In this section we describe the measurement of clot retraction in vitro using platelet rich plasma (PRP), and note considerations for performing and designing experiments.
3.1 Preparation of human blood
3.2 Preparation of mouse blood
Mouse blood (1ml) is drawn into a syringe containing 100μl 4% (w/v) sodium citrate as an anticoagulant. PRP is prepared from whole blood by centrifugation at 200g for 8 min at room temperature in the presence of prostacyclin (0.1μg ml−1).
3.3 Clot retraction assay
Rest the platelets in a warm water bath (30°C) for 30 min.
Count platelet numbers and adjust if necessary using platelet poor plasma (PPP) to ensure consistency (see Note 5).
Fill glass tubes with 745 μl of warm (30°C) Tyrodes-Hepes buffer and 5 μl RBC (used to colour the clot (see Note 4)). Place all the tubes in the rack on a plain piece of paper or clean bench where it can undisturbed for several hours.
Add 200 μl PRP (this can be reduced to 50μl for mouse platelets) to each tube
At this point any test compounds that may affect clot retraction or platelet function should be added and incubated if required (see Note 6).
Add 50 μl thrombin 20 unit ml−1 (final concentration of 1 unit ml−1) and mix by flicking the tube quickly.
Finally and immediately after adding the thrombin add a sealed glass pipette to the centre of the tube and secure with sticky tac (see Note 7).
Summary of assay setup
| Tyrodes-Hepes buffer | 745 μl |
| PRP | 200 μl |
| Thrombin (20 units /ml stock) | 50 μl |
| RBC | 5 μl |
Amounts can be reduced for the study of mouse platelets or where the volumes of blood samples are limited, using narrow glass tubes (10 mm diameter or less).
| Tyrodes-Hepes buffer | 175 μl |
| PRP | 50 μl |
| Thrombin (20 units /ml stock) | 20 μl |
| RBC | 5 μl |
Assessment of results
Images of the experiment enable the kinetics of clot retraction to be observed, and photographs should be taken at time 0 and then every 30 minutes (or more frequently if desired). Images may also be analysed to provide numerical data, such as apparent thrombus area, using image analysis software.
Results from clot retraction are assessed numerically by the weight of the clot or weight/volume of extruded serum. Typically an experiment is ended after 120 min (although platelet concentration, temperature, and type of experiment will effect this). A point to end the assay should be chosen before all samples reach completion since differences may be less apparent after several hours since all samples may eventually completely contract (some treatments may result in delay in contraction, rather than inhibition of contraction). This will require optimization for particular experimental outcomes. (see Notes 8 & 9)
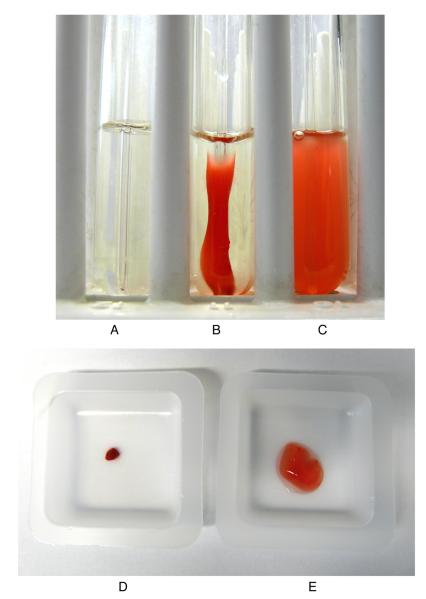
An example of clot retraction using this procedure is shown in Figure 2, where the Src family kinase inhibitor PP2 was used to reduce inhibit clot retraction.
Figure 2. Clot retraction of human PRP.
Photograph was taken after two hours of (A) no thrombin, (B) 1unit ml−1 thrombin (and DMSO solvent control 0.1%), (C) with 1 unit ml−1 thrombin PP2 (25μM) Src family kinase inhibitor (dissolved in DMSO). Clots were weighed after 3 hours (D) thrombin control and (E) thrombin and PP2 Src inhibitor.
Alternative methods
One limitation of the standard method is that it may prove difficult to differentiate between coagulation and platelet defects. Plasma free clot retraction in the presence of purified fibrinogen can be used to investigate the function of integrin αIIbβ3, without the complication of the effects of the coagulation cascade (10, 13, 14).
Abbreviations
- PRP
platelet rich plasma
- RBC
red blood cell
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo-D-3,4-pyrimidine
- PLCγ2
phospholipase Cγ2
- AMPK α2
adenosine monophosphate (AMP)-activated protein kinase α2
4. Notes
Sodium citrate is used as an anticoagulant as this reduces the availability of ionized calcium and magnesium without reducing the pH, which would reduce platelet function.
Glass pipettes can easily be sealed by holding the tip over a Bunsen flame for a few seconds.
Do not add ACD during platelet preparation, since this will lower pH and chelate ions preventing clot retraction from occurring.
Red blood cells are used to colour the clot for easy visualization. Use the RBCs that have been pelleted in the preparation of PRP as these are more concentrated than whole blood and will provide more defined colour.
When comparing the effect of a compound on an individual each donor provides a full set of data. However when comparing different individuals the platelet number is likely to be variable. To keep platelet levels standardised samples can be diluted with platelet poor plasma. With human work this will be more easily available as blood volumes can be larger, however, with mouse work when comparing test and control animals this may involve taking blood from another animal (test animal for test samples and control for control samples) in order to prepare some PPP. For this type of work it is worth preparing samples from several animals at once to allow pairing up similar platelet concentrations and use of PPP can be shared. PPP may be prepared by centrifugation of PRP (1400g for 10 minutes at room temperature) to remove platelets, and can be stored at −20°C for future use).
When testing compounds that may influence clot retraction it is important to incorporate a vehicle control.
Developing clots may collapse if disturbed. The sticky tac is used to ensure that the glass pipette does not move.
Setting up experiments in duplicate will allow clots to be weighed to be taken at a point where differences are pronounced while keeping another set to examine if the same end point is eventually reached.
Temperature can affect the speed of clot retraction. If the laboratory temperature is variable, assays should be conducted within an incubator at 20°C.
References
- (1).Farndale RW, Sixma JJ, Barnes MJ, de Groot PG. The role of collagen in thrombosis and hemostasis. J Thromb Haemost. 2004;2:561–73. doi: 10.1111/j.1538-7836.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- (2).Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–12. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- (3).Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–9. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- (4).Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–64. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- (5).Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–15. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- (6).Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–11. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- (7).Shattil SJ, Kashiwagi H, Pampori N. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–57. [PubMed] [Google Scholar]
- (8).Weyrich AS, Denis MM, Schwertz H, Tolley ND, Foulks J, Spencer E, Kraiss LW, Albertine KH, McIntyre TM, Zimmerman GA. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109:1975–83. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Berndt MC, Andrews RK. Full clot retraction: running on mTOR. Blood. 2007;109:1791–1792. [Google Scholar]
- (10).Suzuki-Inoue K, Hughes CE, Inoue O, Kaneko M, Cuyun-Lira O, Takafuta T, Watson SP, Ozaki Y. Involvement of Src kinases and PLCgamma2 in clot retraction. Thromb Res. 2007;120:251–8. doi: 10.1016/j.thromres.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Schoenwaelder SM, Jackson SP, Yuan Y, Teasdale MS, Salem HH, Mitchell CA. Tyrosine kinases regulate the cytoskeletal attachment of integrin alpha IIb beta 3 (platelet glycoprotein IIb/IIIa) and the cellular retraction of fibrin polymers. J Biol Chem. 1994;269:32479–87. [PubMed] [Google Scholar]
- (12).Randriamboavonjy V, Isaak J, Fromel T, Viollet B, Fisslthaler B, Preissner KT, Fleming I. AMPK alpha2 subunit is involved in platelet signaling, clot retraction, and thrombus stability. Blood. 116:2134–40. doi: 10.1182/blood-2010-04-279612. [DOI] [PubMed] [Google Scholar]
- (13).Osdoit S, Rosa JP. Fibrin clot retraction by human platelets correlates with alpha(IIb)beta(3) integrin-dependent protein tyrosine dephosphorylation. J Biol Chem. 2001;276:6703–10. doi: 10.1074/jbc.M008945200. [DOI] [PubMed] [Google Scholar]
- (14).Seiffert D, Pedicord DL, Kieras CJ, He B, Stern AM, Billheimer JT. Regulation of clot retraction by glycoprotein IIb/IIIa antagonists. Thromb Res. 2002;108:181–9. doi: 10.1016/s0049-3848(02)00395-x. [DOI] [PubMed] [Google Scholar]