Abstract
Background
Worldwide, reduction in under-five mortality has not sufficiently included neonates, who represent 45% of deaths in children of age under five years. The least progress has been observed in resource-limited settings.
Methods
This mixed methods study conducted at a Cambodian non-governmental paediatric hospital described the key priorities of the ongoing neonatal service. Routinely collected data from the hospital and microbiology databases included the number of admissions, discharges and deaths and the number of cases of bacteraemias (2011–2016). Semi-structured interviews with the management staff explored the essential features of the service.
Results
There were 2127 neonatal admissions and 247 deaths. The incidence of facility-based neonatal mortality decreased by 81%. Bacteraemic healthcare-associated infections decreased by 68%. A dedicated area for neonatal care was perceived as crucial, allowing better infection control and delivery of staff training.
Conclusions
In this hospital, the neonatal service prioritized basic measures, particularly, having a dedicated neonatal area. Facility-based mortality and bacteraemic healthcare-associated infections decreased.
Keywords: neonatal care, service implementation, neonatal mortality, developing country, Cambodia, NICU
INTRODUCTION
Globally, the proportion of deaths that occur in the neonatal period (first four weeks of life) is increasing [1]. In 2015, there were 2.7 million neonatal deaths, one million of which occurred on the first day of life [2, 3]. Under-five mortality has decreased worldwide, but this has not included neonates to an equal degree [4]. Neonates constitute 45% of deaths in children younger than five years, which is projected to rise to 52% by 2030 [2]. Low and lower-middle income countries (LMICs) have made the least progress towards reducing neonatal mortality [2].
A pervasive perceived barrier to providing neonatal care, particularly in LMICs, is the misconception that it is difficult and expensive [5]. However, simple interventions can be effective. A special care baby unit (SCBU) established at the Maela Camp for Displaced Persons on the Thailand–Myanmar border resulted in halving the neonatal mortality rate within four years [5].
This study describes a neonatal service in a resource-limited setting in Cambodia. Despite its upgrade to a lower-middle income country in 2015, the majority of the Cambodian population live in either poverty or near poverty [6]. The vast majority live in rural areas [7], where the risk of neonatal death is twice that of urban areas [8]. The median neonatal mortality rate is 15 per 1000 live births [3].
This study aims to describe the key components of the neonatal service (as identified by neonatal staff) and to determine the trends in the incidence of facility-based neonatal mortality and morbidity.
METHODS
Setting
Angkor Hospital for Children (AHC), a non-governmental organization-funded hospital in Siem Reap, Cambodia, provides free paediatric healthcare. In 2016, AHC saw 127 900 outpatients and 5596 inpatients [9]. Neonates are brought directly by their caregiver or referred from other facilities. There is no affiliated obstetric unit.
The neonatal unit opened in 2013 as an SCBU. Subsequently a neonatal intensive care unit (NICU) opened. The two adjacent departments form the 12-bed neonatal unit. The neonatal service includes the care and facilities provided to neonates at the neonatal unit and other inpatient departments. The most common reasons for neonatal admissions in 2016 were neonatal sepsis, prematurity and birth asphyxia.
Study design and data management
This mixed methods study evaluated the ongoing neonatal service, and it involved routinely collected quantitative data from the hospital database on all admitted neonates from 1 January 2011 to 31 December 2016 (before and after the neonatal unit was established). Anonymized data collected included dates of birth, admission and discharge/death, ward and gender. The microbiology database was interrogated for neonatal positive blood cultures (excluding those deemed to be contaminated with skin flora). Isolates from samples taken >48 h from admission were categorized as healthcare-associated infections (HCAIs) [10–12].
Routinely collected data were obtained on the cost, equipment and training for the neonatal service.
Temporal trends in the number of admissions, deaths, bacteraemias (particularly HCAIs) and length of stay were determined. A Poisson regression model was fit to the facility-based mortality data to obtain incidence rate ratios, with time as the covariate of interest. The number of neonates admitted to the intensive care unit was used as a proxy for the severity of illness. Data were analysed using the R statistical software package (R Foundation for Statistical Computing, Vienna, Austria).
Individual semi-structured interviews were conducted to explore the key priorities for establishing the neonatal service. The purposively sampled participants were medical and nursing managers involved in the implementation and running of the neonatal service. Verbal consent was obtained before participation. The interviews were conducted in English by author WGS; participants were fluent in English. Responses were written and confirmed with the participant. Thematic content analysis using an inductive approach was adopted. Concurrent data collection and analysis allowed iteration. Emerging themes were discussed and agreed upon by the study team.
Ethics
Ethical approval was received from the AHC Institutional Review Board (AHC-IRB 050/17). This study was conducted according to the principles of the Declaration of Helsinki.
RESULTS
Admissions
There were 2127 neonatal admissions from 2011 to 2016. The median age at admission increased from six days (2011) to ten days (2016).
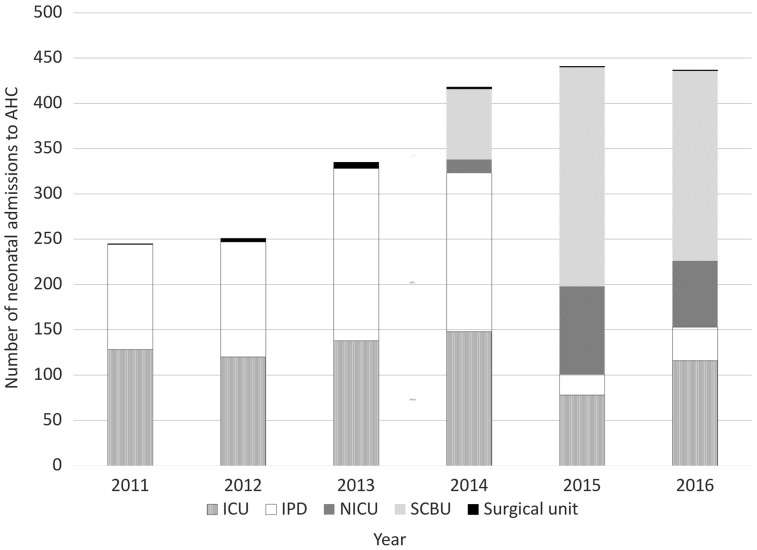
Once the neonatal unit opened, the majority of neonates were admitted to it, with the remainder admitted to the inpatient medical department (IPD) and paediatric intensive care unit (PICU) (Fig. 1). Although the neonatal unit opened in 2013, the hospital database only recorded neonatal unit admissions from 2014.
Fig. 1.
Number of neonatal admissions to AHC per department, by year.
The number of admissions increased annually, with an overall 78% increase from 2011 to 2016.
Facility-based mortality
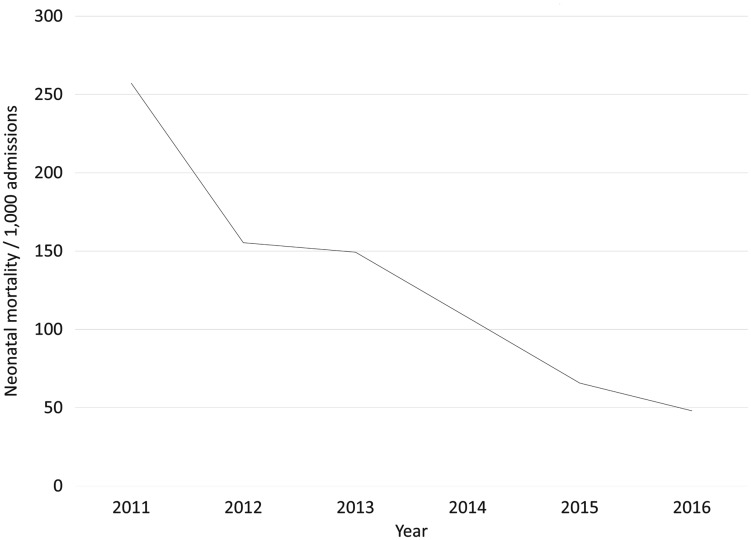
There were 247 neonatal deaths from 2011 to 2016. The incidence of facility-based neonatal mortality decreased by 81%, from 257 of 1000 admissions in 2011 to 48 of 1000 admissions in 2016 (Fig. 2).
Fig. 2.
Incidence of facility-based neonatal mortality per 1000 admissions, by year.
The incidence of facility-based neonatal mortality decreased by 27% each year (Poisson regression model: incidence rate ratio, 0.73; 95% confidence interval, 0.67–0.79; p < 0.001). Adjusting for intensive care admissions (as a proxy for severity), the incidence rate ratio was 0.69 (95% confidence interval, 0.55–0.86; p = 0.001).
Healthcare-associated infections
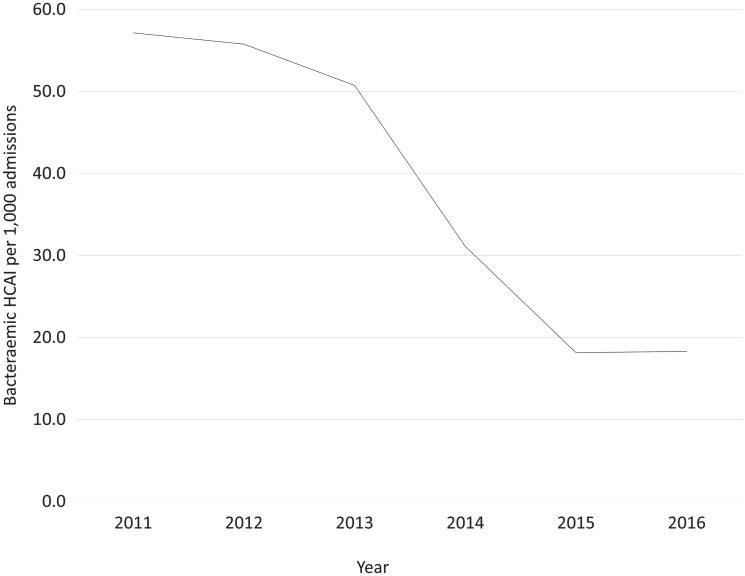
There were 74 blood culture positive HCAI episodes in neonates from 2011 to 2016. The rate of bacteraemic HCAI declined by 68%, from 57 of 1000 admissions to 18 of 1000 admissions during that period (Fig. 3).
Fig. 3.
Number of bacteraemic HCAIs per 1000 admissions, by year.
The steepest decline for bacteraemic HCAI was from 2013 to 2014.
Key priorities of the neonatal service
Six senior management staff members participated in interviews, lasting 15–20 min each. Table 1 shows their characteristics.
Table 1.
Characteristics of semi-structured interview participants
| Characteristic | Number of participants n = 6 (% of n) |
|---|---|
| Gender | |
| Male | 5 (83) |
| Female | 1 (17) |
| Department | |
| Medical | 3 (50) |
| Nursing | 3 (50) |
| Years at AHC (median, range) | 17 (14–19) |
The following four key themes emerged from the data: dedicated area, training, infection prevention and control and attitudes.
Dedicated area
In 2012 an area of the IPD was designated as the “neonatal corner”, to begin cohorting neonates. The neonatal unit opened in 2013; its facilities are shown in Table 2. No new staff were hired for the neonatal service.
Table 2.
Staffing and equipment provided in the neonatal unit
| Neonatal unit staff | SCBU equipment | NICU equipment |
|---|---|---|
| Nurses | General | General |
| 1 unit manager | Bag and mask (two) | Capillary bilirubin meter |
| 4 team leaders | Capillary bilirubin meter | Computer |
| 15 staff nurses | Computer | Digital scale |
| 2–4 student nurses | Digital scale | Emergency trolley |
| Doctors | Emergency trolley | Infant warmer |
| 1 head of neonatology | Handheld pulse oximeter | I-Stat machine |
| 1 senior neonatologist | Infant warmer | Micro haematocrit centrifuge |
| 2 middle-grade neonatologists (≥3 years of clinical training) | Micro haematocrit centrifuge | Nebulizer |
| Nebulizer | Ophthalmoscope | |
| 4 paediatric junior doctors | Ophthalmoscope | Procedure trolley |
| 2–4 paediatric interns | Procedure trolley | Refrigerator |
| Refrigerator | Ultrasonography machine | |
| Water cooler | Water cooler | |
| Per bed | Per bed | |
| Baby respiration apnoea monitor | Baby cot | |
| Basinet baby with trolley | Baby respiration apnoea monitor | |
| LCD phototherapy machine | Bag and mask | |
| Oxygen concentrator | CPAP machine | |
| Stethoscope | Infant incubator | |
| Suction machine | Laryngoscope | |
| Syringe pump | LCD phototherapy machine | |
| Patient monitor, MEC-1200 | ||
| Stethoscope | ||
| Suction machine | ||
| Syringe pump (2) | ||
| Ventilator, NBP840 |
Participants said the leading asset of the neonatal service was the separate unit. This was perceived to be crucial because it allowed better infection prevention and control (IPC), minimizing the risk of HCAI for vulnerable neonates (Table 3, quote 1.1).
Table 3.
Quotes from the semi-structured interviews, by theme
| Theme | Quote number | Quote | Participant |
|---|---|---|---|
| Dedicated area | 1.1 | “So neonates are very new and very weak, so it is easy for them to get an infection from the other older patients. I think it is better to separate them.” | IPD, Nursing |
| 1.2 | “I think that there are now better outcomes for neonates. Particularly when we talk about infection control, it is very important. Another side is that we can provide certain features, like keeping the area warm, the room is not too light, sound is limited in here, procedures should be done at the same time … Another thing that is different here is the equipment, the ventilator machine and equipment and supplies allow better outcomes for the small baby also.” | PICU, Nursing | |
| 1.3 | “I think there (neonatal unit) a nurse has three or four patients, but here (IPD) they have four to six. So the care provided to the neonate out there is better because they can attend to the patient more closely. As you know the care needs of neonates are more than paediatric patients.” | IPD, Nursing | |
| 1.4 | “Since we opened up the neonatal unit mortality has dropped … our care is better. Before we could not help a baby weighing less than one kilogram to survive. Now we can help, and those babies can survive.” | Neonatal unit, Medical | |
| Training | 2.1 | “Some nurses have gone to train in Thailand for four months and after that they come back to work in the neonatal unit. Also we still receive training from volunteers, for example neonatologists from Singapore, nurses from America, England.” | Neonatal unit, Nursing |
| 2.2 | “We now have a neonatal team so they have continuing medical education on neonates only, so they gain their specific knowledge and are more confident.” | PICU, Medical | |
| Attitudes | 3.1 | “And before according to our knowledge and skills we thought it’s the same caring for a big child and a small tiny baby. But actually right after we split into two units, we realised that the small baby has special needs to take care of them.” | PICU, Nursing |
| 3.2 | “Now, we try to focus on specific areas. Even now we sometimes have neonates here (PICU), but we have neonatal specialists and when we have neonates we try to coordinate with the specialty doctors and nurses to come to discuss.” | PICU, Nursing | |
| 3.3 | “Yeah, all the neonatal staff are very happy because they work in the neonatal unit, not in general paediatrics. They can focus on nursing care only for neonates. So they are happy and they feel happy to work. They feel it is easy to make patients much better because the quality of care is much better, and infection control is much better and the equipment is much better. And basic competencies and knowledge are much better than before. Yeah, it’s very good, very good now it’s separate.” | Neonatal unit, Nursing |
Participants mentioned that a dedicated area also allowed regulation of the environment and convenient location of specific equipment (Table 3, quote 1.2).
Participants felt that better quality and more attentive care was delivered at the neonatal unit owing to optimal staff:patient ratios (Table 3, quote 1.3).
All participants perceived better patient outcomes owing to the neonatal service (Table 3, quote 1.4).
Training
Neonatal training was provided by the local staff and volunteers (Table 3, quote 2.1). A senior neonatologist received training in Singapore in 2004–05 and subsequently taught neonatal care at AHC. The paediatric junior doctor neonatology teaching hours increased from 16 h per rotation (2003) to 42 h per rotation (2016).
Participants reported that creating a neonatal unit enabled the neonatal team to better consolidate their experiential learning, and it allowed specific training to be delivered more easily (Table 3, quote 2.2).
Infection prevention and control
IPC roles were established during the study period, incorporating the eight key principles of the WHO [13]. The multidisciplinary IPC committee comprised medical and nursing directors, department leads, clinical microbiologists and one infection control nurse (meeting the WHO recommendation for one full-time trained IPC staff member per 100 beds) [13]. IPC activities included monthly committee meetings, regular training and audits (e.g. hand hygiene).
Formal prospective HCAI surveillance commenced in 2015, facilitated by the clinical microbiologists. Locally appropriate case definitions were based on WHO, Centers for Disease Control and Prevention and UK guidelines [10–12]. Monthly reports were circulated to senior management.
A dedicated area for neonatal care was considered an essential part of good IPC practice (Table 3, quotes 1.2, 1.3).
Attitudes
Participants said that separating neonates changed perceptions, underscoring the difference between neonatal and general paediatric patients (Table 3, quote 3.1).
After the neonatal unit was established, the expertise within the neonatal team was increasingly recognized and sought for neonates admitted to other wards (Table 3, quote 3.2).
Participants reported good teamwork with neonatal staff gathered together. A positive working environment, coupled with observing better patient outcomes, fostered greater confidence and a sense of pride in their work within the neonatal team (Table 3, quote 3.3).
Cost of the neonatal service
All costs are reported in US dollars. Developing the neonatal unit cost $63 692. A further $261 947 of equipment was purchased for the neonatal service from 2013 to 2016.
The annual operational cost of the neonatal service has remained largely constant since its establishment; in 2016, this was $354 552. On the basis of a total of 4447 patient-days in 2016, the cost of care per neonate per day was $79.73.
DISCUSSION
Greater efforts are needed to reduce neonatal mortality, especially in LMICs [2]. This study described the key priorities of a neonatal service in Cambodia, with substantial decreases in facility-based neonatal mortality and morbidity observed.
In six years, the incidence of facility-based neonatal mortality decreased by 81%. The increased median age at admission could indicate that admitted neonates were less sick. However, even after adjusting for the severity of illness, a 31% annual decrease in neonatal deaths was observed.
The rate of bacteraemic HCAIs decreased by 68%. The steepest decline (2013 to 2014) corresponded with the opening of the SCBU. A major perceived benefit of the neonatal unit was better IPC, by cohorting neonates away from general paediatric inpatients.
Laxminarayan et al. [14] found that HCAI prevalence in developing country NICUs is nine times that in the USA. Increasingly births in LMICs occur at healthcare facilities, placing more neonates at risk of HCAI. Too often healthcare providers in LMICs do not know how to implement good IPC [15]. In this low-resource setting, IPC measures were deployed and a reduction in HCAI was seen.
Neonatal care can be considered too costly or difficult to deliver in resource-limited settings [16–18]. In this study, relatively simple measures were perceived to result in better outcomes. A similar study in Uganda described two stages to developing neonatal care, with the same essential priorities found—a dedicated space, neonatal staff training and good IPC [19].
In this study the essential feature of the neonatal service was perceived to be the dedicated area for neonatal care. This finding is mirrored by various guidelines as important for improving neonatal outcomes [20, 21]. The dedicated area facilitated the other key features of training, good IPC, teamwork and promoting a strong identity as a specialist team. The importance of training and involving international expertise when developing NICU facilities in LMICs has also been shown previously [15–18].
Limitations of this study include that it was conducted at a single non-governmental site. This allowed the in-depth multi-departmental exploration of the neonatal service presented. Conclusions from this study could be generalizable to a facility with inpatient paediatric care and teaching infrastructures, without the need for extensive additional resources. Existing staff were allocated to the neonatal service. Existing space was appropriated for the neonatal unit. Training was provided through the existing teaching programme.
The population of neonates admitted may be self-selected owing to caregivers’ ability to bring the baby to AHC. However, because AHC does not restrict access to its services by any means (other than age less than 16 years), neonates from various geographical locations and socioeconomic backgrounds could present. These factors would have an equal effect throughout the study period and would therefore not be expected to impact the results.
This study relied on routinely collected quantitative data; it was outside the scope to collect purposive prospective quantitative data. The purposively collected qualitative data may have been biased owing to the small sample size; however, all relevant managers were included. The methodology described in this study could be used by other facilities to evaluate similar ongoing programmes.
CONCLUSIONS
A neonatal service was implemented in a resource-limited setting in Cambodia. Basic measures were found to be fundamental, particularly creating a dedicated area and delivering training. These simple measures were associated with a decrease in neonatal mortality and morbidity.
ACKNOWLEDGEMENTS
The authors would like to thank the staff and volunteers who have given their time to AHC over the years, and Dr Mavuto Mukaka for assistance with statistical analysis.
FUNDING
This work was internally funded.
REFERENCES
- 1.World Health Organisation. Accountability for maternal, newborn and child survival: the 2013 Update. Geneva: WHO, 2013.
- 2. You D, Hug L, Ejdemyr S, et al. Levels & trends in child mortality estimates developed by the UN inter-agency group for child mortality estimation. Report 2015. United Nations Inter-agency Group for Child Mortality Estimation, 2015.
- 3.UNICEF. UNICEF data: monitoring the situation of children and women. https://data.unicef.org/topic/child-survival/neonatal-mortality/ (3 August 2017, date last accessed).
- 4. Wardlaw T, You D, Hug L, et al. UNICEF Report: enormous progress in child survival but greater focus on newborns urgently needed. Reprod Health 2014;11:82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner C, Carrara V, Aye Mya Thein N, et al. Neonatal intensive care in a Karen refugee camp: a 4 year descriptive study. PLoS One 2013;8:e72721.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The World Bank. The World Bank Cambodia overview. http://www.worldbank.org/en/country/cambodia/overview#12016 (18 January 2017, date last accessed).
- 7.World Health Organisation and Ministry of Health Cambodia. Health service delivery profile Cambodia, 2012. http://www.wpro.who.int/health_services/service_delivery_profile_cambodia.pdf (18 January 2017, date last accessed).
- 8. Hong R, Ahn PY, Wieringa F, et al. The unfinished health agenda: neonatal mortality in Cambodia. PLoS One 2017;12:e0173763.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angkor Hospital for Children. Angkor Hospital for Children annual report, 2016. http://angkorhospital.org/wp-content/uploads/newsletters/2016-annual-report.pdf (3 August 2017, date last accessed).
- 10.CDC/NHSN. Surveillance definitions for specific types of infections, 2014. http://www.socinorte.com/wp-content/uploads/2014/06/17pscNosInfDef_current.pdf (29 September 2017, date last accessed).
- 11.English national point prevalence survey on healthcare-associated infections and antimicrobial use, 2011. 2011. http://webarchive.nationalarchives.gov.uk/20140714095446/http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317134304594 (29 September 2017, date last accessed).
- 12.Prevention of Hospital-Acquired Infections A Practical Guide. 2nd edn, 2002. http://www.who.int/csr/resources/publications/whocdscsreph200212.pdf (29 September 2017, date last accessed).
- 13.Guidelines on core components of infection prevention and control programmes at the national and acute health care facility level, 2016. http://www.who.int/gpsc/ipc-components/en/. (29 September 2017, date last accessed). [PubMed]
- 14. Laxminarayan R, Duse A, Wattal C, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis 2013;13:1057–98. [DOI] [PubMed] [Google Scholar]
- 15. Zaidi AK, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet 2005;365:1175–88. [DOI] [PubMed] [Google Scholar]
- 16. Ho JJ, Chang AS.. Changes in the process of care and outcome over a 10-year period in a neonatal nursery in a developing country. J Trop Pediatr 2007;53:232–7. [DOI] [PubMed] [Google Scholar]
- 17. Umran RM, Al-Jammali A.. Neonatal outcomes in a level II regional neonatal intensive care unit. Pediatr Int 2017;59:557–63. [DOI] [PubMed] [Google Scholar]
- 18. Martinez AM, Khu DT, Boo NY, et al. Barriers to neonatal care in developing countries: parents' and providers' perceptions. J Paediatr Child Health 2012;48:852–8. [DOI] [PubMed] [Google Scholar]
- 19. Burgoine K, Ikiror J, Akol S, et al. Staged implementation of a two-tiered hospital-based neonatal care package in a resource-limited setting in Eastern Uganda. BMJ Glob Health 2018;3:e000586.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White RD. Recommended standards for the newborn ICU. J Perinatol 2007;27:S4–S19. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health. Health Building Note 09-03: neonatal units, 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/147879/HBN_09-03_Final.pdf (29 September 2017, date last accessed).