Abstract
Roughly half the carbon that crop plants fix by photosynthesis is subsequently lost by respiration. Nonessential respiratory activity leading to unnecessary CO2 release is unlikely to have been minimized by natural selection or crop breeding, and cutting this large loss could complement and reinforce the currently dominant yield-enhancement strategy of increasing carbon fixation. Until now, however, respiratory carbon losses have generally been overlooked by metabolic engineers and synthetic biologists because specific target genes have been elusive. We argue that recent advances are at last pinpointing individual enzyme and transporter genes that can be engineered to (1) slow unnecessary protein turnover, (2) replace, relocate, or reschedule metabolic activities, (3) suppress futile cycles, and (4) make ion transport more efficient, all of which can reduce respiratory costs. We identify a set of engineering strategies to reduce respiratory carbon loss that are now feasible and model how implementing these strategies singly or in tandem could lead to substantial gains in crop productivity.
INTRODUCTION
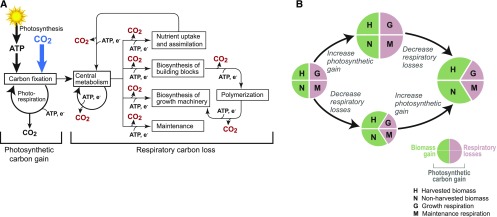
Plant respiration or carbon loss (CO2 efflux) has always received far less attention than photosynthetic carbon gain (CO2 assimilation) in relation to crop yields (Bonner, 1962; Loomis and Williams, 1963; Ainsworth and Ort, 2010; Foyer et al., 2017). One reason is the sheer complexity of the metabolic processes underlying respiratory CO2 loss; these processes involve numerous pathways and are driven and regulated by a vast distributed network of metabolic demands associated with nearly all facets of crop physiology and growth (Figure 1A). Fortunately, it is now possible to pinpoint specific molecular targets to engineer more efficient respiratory processes and therefore minimize CO2 loss. Here, we argue that reducing carbon loss has become as realistic an engineering strategy to raise crop yields as the long-established strategy of increasing carbon gain.
Figure 1.
Plant Respiration and Its Relation to Biomass Yield.
(A) The plurality of diverse metabolic processes that underlie respiratory CO2 losses (red) contrasted with the unitary process of photosynthetic carbon gain (blue).
(B) The main current strategy to enhance crop productivity through modification of central metabolism is to increase net photosynthetic carbon gain. A now-viable alternative is to decrease respiratory carbon loss. These two strategies can be pursued in parallel and then combined.
As context, we first relate respiratory carbon losses to the entire crop carbon budget and describe how respiration can be conceptually divided into “growth” and “maintenance” components. Next, we state the key premises underlying the aim of cutting carbon loss. We then outline a set of strategies that have the potential to cut carbon losses, along with the assumptions and evidence they rest on, and analyze how these strategies can now be implemented. Last, we estimate the increases in crop biomass production that could be obtained by implementing these strategies.
A Primer on Respiratory Carbon Losses
Between 30 and 60% of the carbon assimilated during photosynthesis (net of photorespiration) is subsequently lost by respiration throughout the diel cycle in annual and perennial crops (Gifford et al., 1984; Amthor, 1989, 2000; Cannell and Thornley, 2000). The scale of respiratory losses becomes even more striking when compared with the 15 to 25% of photosynthetically assimilated carbon that is harvested from most crops (Gifford et al., 1984; Unkovich et al., 2010), i.e., two to four times as much carbon can be lost to respiration as goes to harvested yield (Figure 1B).
In plants, respiration rate is mainly directly controlled by consumption of ATP and the resulting supply of ADP (Plaxton and Podestá, 2006). Thus, a slowing or elimination of any nonessential ATP use might immediately curtail respiratory consumption of carbohydrates and CO2 loss. In turn, the spared carbohydrates might become available for other uses, most notably as substrate for additional crop growth.
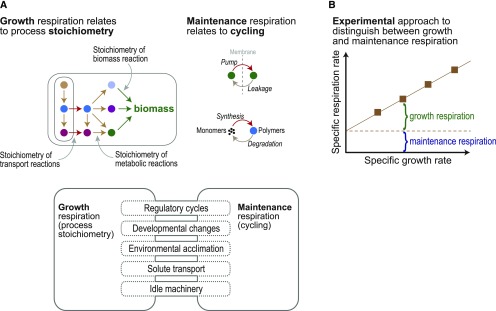
Respiration can be conceptually separated into two components (Amthor, 2000): (1) “growth respiration” that provides the carbon skeletons (e.g., phosphoenolpyruvate produced by glycolysis that serves as precursor to myriad biosynthetic pathways), ATP, and reducing power to drive growth, including the synthesis of cellular components and ion pumping, and hence relates to process stoichiometry (Figure 2A); and (2) “maintenance respiration” that fuels ongoing activities, such as macromolecule turnover and countering ion leakage, and hence relates to cyclic processes that are not directly related to new biomass accumulation (Figure 2A) but are needed to counter accumulation of entropy. These components account for roughly equal carbon losses (Amthor, 1989), each over time consuming ∼20 to 30% of photosynthetically fixed carbon (Figure 1B). A simple way to think of (and experimentally estimate) growth and maintenance respiration is to relate respiration rate to growth rate. Respiration rate increases with growth rate, as higher growth rate requires proportionally higher production rates of precursors, ATP, and reducing power due to stoichiometric demand. The maintenance component on the other hand is not directly linked to growth rate but instead is related to plant size.
Figure 2.
Growth and Maintenance Respiration.
(A) The conceptual split between respiration that drives the stoichiometric synthetic and transport processes required for growth and respiration that fuels cyclic maintenance processes. The bottom part of the figure shows processes that can to some degree be assigned to growth or maintenance.
(B) Experimental measurement of growth and maintenance components of respiration. This relationship is derived by dividing both sides of Equation 1 by biomass amount, giving specific (i.e., per unit dry mass) rates of respiration and growth as follows: R/W = g(dW/dt)/W + m. Respiration rates are measured at various growth rates; the specific respiration rate at zero growth (derived by extrapolation) is taken as the maintenance respiration coefficient m. The maintenance coefficient can be assumed, as a first approximation, not to depend on growth rate. The slope of the relationship is an estimate of the growth respiration coefficient g. This relationship, and Equation 1, is referred to as a two-component coupled respiration model (Hunt and Loomis, 1979) because respiration rate R is coupled to growth rate dW/dt through g.
Mathematically, this relationship between respiratory rate (R, mol CO2 time−1), growth rate (dW/dt, mol C time–1), and biomass amount (W, mol C) can be expressed as follows
| (1) |
where g is the growth respiration coefficient (mol CO2 respired per mol C added to new biomass “structures”) and m is the maintenance respiration coefficient (mol CO2 respired per mol C in biomass per unit time, or simply time−1; Hunt and Loomis, 1979). Here, dW/dt is the rate of conversion of substrate carbon into new “structural” components of biomass (proteins, membranes, and structural carbohydrates, including “storage” compounds in the harvested part) rather than the simple accumulation of dry mass, which would include temporary “storage,” such as starch and sugars in leaves (Amthor, 2000). If R in Equation 1 is a daily rate, so too is the growth rate dW/dt. The value of g varies with biomass composition because converting sugars to proteins, for instance, demands more ATP than converting sugars to cellulose (Penning de Vries et al., 1974; Amthor, 2010). The maintenance coefficient m (typically 0.01 to 0.02 d−1) also depends on biomass composition—enzymes, for instance, need more maintenance than cellulose or lignin—and thus on growth stage because composition changes during development. The maintenance coefficient rises with temperature and in response to environmental stresses (Penning de Vries, 1975; Amthor, 1989, 2000). Growth respiration is the dominant component in fast-growing young plants; maintenance respiration is more important in older plants. If Equation 1 is divided by biomass amount W, a relationship between specific respiration rate and specific growth rate is obtained (Figure 2B), and this relationship can be used to experimentally separate the two respiratory components.
When quantitative relationships between whole-plant photosynthetic rate (P, mol CO2 time−1), growth rate, and respiration rate are stable during periods of interest (i.e., day-to-day changes in the size of temporary storage pools of nonstructural carbohydrates are small), R is approximated by P − dW/dt. In that case, all three rates can be expressed on a daily (24-h) basis and Equation 1 can be rearranged to give (see Supplemental Appendix 1)
| (2) |
where the term P − mW is the photosynthate available for growth, i.e., the amount remaining after maintenance respiration is satisfied. We use Equation 2 to relate our proposed engineered changes in m or g to potential changes in growth (see below and Supplemental Appendix 2).
The growth/maintenance split is not strictly binary because some processes transgress the boundary. Such processes include (1) regulatory metabolite cycles that balance metabolism and growth processes; (2) reconfiguration of metabolic networks by degrading one set of proteins and synthesizing another as development proceeds or environments change; (3) solute transport that supports both growth and maintenance, e.g., source-sink phloem transport and solute uptake to generate turgor pressure; and (4) upkeep of ribosomes and other growth machinery when they are idle (i.e., not in use), causing failure to capitalize fully on the investment involved (Ishihara et al., 2017; Figure 2A).
In the light of the above concepts and numbers, we can revisit Figure 1B to see how strategies to reduce respiration relate to, and can complement, strategies now being pursued to increase photosynthesis (Ainsworth and Ort, 2010; Ort et al., 2015; Erb and Zarzycki, 2016; Schuler et al., 2016; Foyer et al., 2017). In essence, carbon-gain strategies aim to grow the pie in Figure 1B, leaving the relative sizes of the slices for growth respiration, maintenance respiration, and harvested yield the same. Carbon-loss strategies, on the other hand, do not directly change the size of the pie but aim to shrink the slices for growth or maintenance respiration so that more carbon stays in biomass, including harvested parts. A key point is that the carbon gain and carbon loss strategies can—and we argue should—be used together because they reinforce each other (Figure 1B). A larger pie and smaller slices taken by respiration leads to more biomass than either strategy alone.
There are further advantages to engineering lower respiratory carbon loss. First, respiration may take an even larger slice of the carbon pie in the future as global temperatures rise because maintenance respiration rate increases continuously with temperature (Huntingford et al., 2017), reaching maximal values at over 50°C (O’sullivan et al., 2017), whereas the light-saturated photosynthesis rate of C3 crops generally does not increase, and may decrease, when temperatures exceed 20 to 30°C (Porter and Semenov, 2005; Sage and Kubien, 2007). Second, reducing unnecessary carbon loss is predicted to enhance yield in any environment and so should benefit both low- and high-input agriculture. Third, from an operational standpoint, the multifaceted nature of respiration creates a host of potentially stackable targets for engineering interventions.
Engineering Lower Carbon Loss Rests on Reasonable Premises
Respiratory processes are so basic to plant life that the assumption that they can be improved needs critical examination. We argue that the project to engineer lower respiratory carbon loss rests on the two premises below and that both are warranted.
Crop Productivity Can Be Carbon Limited
Crop productivity can be restricted by the net carbon supply (photosynthesis minus respiration) rather than by the capacity to convert assimilated carbon to biomass. The long-running debate on this issue (“source” versus “sink” limitation) has largely converged around the conclusion that productivity is indeed often limited or colimited by carbon supply (Körner, 2015; Ort et al., 2015; Sonnewald and Fernie, 2018). The yield increases in long-term CO2-enrichment experiments (Ainsworth and Long, 2005) support this conclusion, as do the negative correlations sometimes observed between yield and respiration rate (Jacoby et al., 2016). In any case, note that cutting respiratory carbon loss is not alone in resting on the carbon-limitation premise; it is shared with projects to increase photosynthesis or decrease photorespiration.
Crop Carbon Losses Have Not Been Fully Optimized for Net Biomass Accumulation by Natural or Artificial Selection
There are several reasons to think this. First, plant metabolism is effectively a “frozen accident” (Shi et al., 2005) in that plants are endowed with just a small subset of the known metabolic pathways and cannot naturally acquire more efficient alternatives from other organisms (Erb et al., 2017). Second, agricultural environments typically differ from natural ones in nutrient and water availability, plant spacing, shading, and pest, pathogen, and weed pressures. The differences have become massive in the past 50 to 100 years, especially in the industrialized world, owing to use of fertilizers, mechanization, and agrochemicals (Khush, 2001; Erisman et al., 2008). Although scientific breeding has successfully adapted crop morphology and phenology to exploit the new “luxury” conditions (Khush, 2001) it has almost surely not fully optimized the metabolic traits that drive carbon loss (Loomis and Amthor, 1999), because these have practically never been directly selected for (Amthor, 2000). More generally, natural selection as well as artificial selection have probably been at least as much for metabolic expediency—the imperative to execute survival-critical processes under all conditions (“systems failure is not an option”)—as for efficient use of energy and carbon (Charles-Edwards, 1975; Barratt et al., 2009).
GENERAL STRATEGIES TO CUT CARBON LOSS AND EVIDENCE THAT THEY WORK
Metabolically based strategies to cut carbon losses and evidence for their plausibility have been discussed for decades. We outline four such long-standing strategies and evidence supporting them, to preface our arguments that they can now be translated into specific engineering targets.
Reduce Unnecessary Turnover of Proteins and Membranes
The energy costs of degrading and resynthesizing proteins and membranes have long been recognized as major drivers of maintenance respiration (Penning de Vries, 1975), making protein and membrane turnover theoretically ripe for reduction (Jacoby et al., 2016). The negative correlation between rates of growth and protein turnover among Arabidopsis (Arabidopsis thaliana) accessions (Ishihara et al., 2017) and similar correlations in animals (Hawkins, 1991) fit with the view that slowing protein turnover conserves carbon.
Replace, Relocate, or Reschedule Metabolic Activities
Replacing energetically inefficient metabolic routes or reactions with efficient ones from other organisms has often been proposed, e.g., the pathway from chorismate to the ubiquinone precursor 4-hydroxybenzoate (Siebert et al., 1996). Plants use a multienzyme, energy-dependent route, whereas Escherichia coli uses just one enzyme (chorismate pyruvate-lyase) that needs no energy input. The chorismate pyruvate-lyase shortcut has been installed in plants (Siebert et al., 1996; Viitanen et al., 2004), albeit not specifically to save energy and carbon. Shifting pathways from elsewhere into leaves and chloroplasts is also often advocated, on the grounds that “surplus” ATP and NAD(P)H are available in illuminated leaves and chloroplasts (Heber, 1976; Krömer et al., 1988; Amthor, 2010; Gardeström and Igamberdiev, 2016). An example is moving nitrate reduction from roots to leaves (Andrews et al., 2004). The same rationale applies to shifting metabolic activities in time instead of space, i.e., running them only during daylight to exploit the surplus energy available.
Suppress Futile Cycles
A futile cycle occurs when enzymes catalyze fluxes in opposing directions (i.e., simultaneously convert metabolite A into B, and B into A) with concomitant loss of energy, usually ATP (Katz and Rognstad, 1978). Suppressing futile cycles in central metabolism is a well-established option to increase efficiency of carbon and energy use (Cairns and Gallagher, 2004; Barratt et al., 2009). It has experimental support, although mainly in the negative sense that increasing cycling raises respiratory losses and diverts carbon away from biomass gain (Morandini and Salamini, 2003).
Reduce Ion Transport Costs
Maintaining ion homeostasis in the face of efflux (“pump and leak”) is a large item in the maintenance energy budget, possibly around half the total (Penning de Vries, 1975; Amthor, 2000), and selecting for low solute efflux from plant tissue is an effective procedure in plant breeding (Whitlow et al., 1992). The context of such breeding, however, has generally been resistance to acute, near-lethal environmental stresses rather than the reduction of ongoing ion efflux from unstressed or moderately stressed tissues (Demidchik et al., 2014).
SPECIFIC CANDIDATE STRATEGIES
Here, we propose specific targets to put the above strategies into practice using metabolic engineering and synthetic biology tools. We also identify a few promising targets that need more research.
Reduce Protein Turnover
The main drivers of protein turnover are (1) the need for flexibility in the face of environmental or developmental change and (2) the need to remove and replace damaged proteins (Hawkins, 1991; Nelson and Millar, 2015). The first driver is still too poorly understood to design rational interventions in crop plants, but the second is now addressable preemptively by mitigating damage to particular proteins. The following are such “upstream” solutions.
Replace the THI4 Thiazole Synthase Enzyme
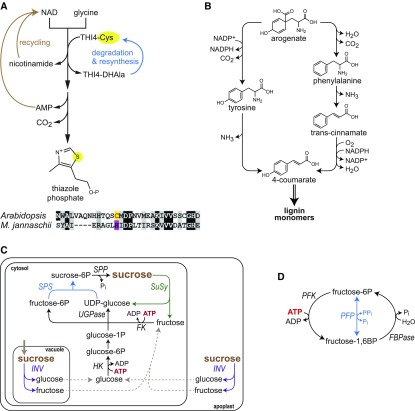
Plant THI4 is a special and extreme instance of protein damage—a suicide enzyme that catalyzes its reaction only once and must then be degraded and resynthesized (Chatterjee et al., 2011). THI4 produces the thiazole precursor of thiamin (vitamin B1) and is suicidal because the sulfur atom for the thiazole ring is obtained by destroying a Cys residue in the THI4 active site (Chatterjee et al., 2011); THI4 is thus a substrate in the reaction, not a catalyst (Figure 3A). The demand for thiamin synthesis is high because thiamin (in its cofactor form, thiamin diphosphate) is short lived (Hanson et al., 2018). The rate of THI4 turnover is therefore also high, in fact higher than all other protein turnover rates measured for barley (Hordeum vulgare) and Arabidopsis (Nelson et al., 2014; Li et al., 2017a). The ATP used in THI4 turnover is estimated to account for 2 to 10% of total maintenance respiration so that replacing THI4 with an efficient non-suicidal (i.e., truly catalytic) enzyme could significantly raise biomass yield (Hanson et al., 2018). Efficient catalytic forms of THI4 that use sulfide as sulfur donor exist in microbes (Figure 3A; Eser et al., 2016) and are candidates for rational engineering or directed evolution to adapt them to function in plants (Hanson et al., 2018). As all plants require thiamin and appear to have suicidal THI4s (Hanson et al., 2018), the strategy of replacing the native THI4 with a non-suicidal one is in principle applicable to any crop.
Figure 3.
Reducing THI4 Turnover, Reconfiguring Lignin Synthesis, and Suppressing Futile Cycles.
(A) Plants synthesize the thiazole phosphate precursor of thiamin from NAD and Gly via a suicidal THI4 enzyme that takes the sulfur atom needed for the thiazole ring from an active-site Cys residue. This converts the Cys to dehydroalanine (DHAla), which inactivates THI4 (Chatterjee et al., 2011). Plant THI4 proteins must consequently be completely degraded and resynthesized after mediating a single reaction. Certain prokaryotes such as Methanococcus jannaschii have THI4s with a His residue in place of the active-site Cys (shown in sequence alignment with Arabidopsis THI4) that use free sulfide as sulfur donor (Eser et al., 2016). These prokaryotic THI4s are true catalysts, i.e., they perform multiple reaction cycles and so make thiazole synthesis far less energetically expensive than when a suicidal THI4 is used.
(B) Alternative plant pathways for conversion of arogenate to 4-coumarate, a precursor to lignin. The reaction set involving Phe, which oxidizes one NADPH per 4-coumarate formed, is found in all plants, but the alternative pathway via Tyr, which reduces one NADP per 4-coumarate formed, is found predominantly in monocots. The overall efficiency of lignin biosynthesis via Tyr is expected to be significantly greater than that of the more common pathway via Phe because of a net reduction in NADPH use.
(C) Futile cycling between Suc synthesis and degradation, highlighting ATP-consuming processes. Suc is synthesized via Suc phosphate synthase (SPS, blue) and sucrose 6-phosphate phosphatase (SPP) and degraded and metabolised either via sucrose synthase (SuSy, green), UDP Glc pyrophosphorylase (UGPase), and fructokinase (FK) or via invertase (INV, purple), hexokinase (HK), and FK. Suc synthesis from two hexose phosphates requires one UTP per Suc, with one PPi being salvaged (not shown). Suc degradation via SuSy requires one PPi per Suc (UTP is internally recycled; not shown). Suc degradation via INV requires two ATP per Suc, plus additional energy for active transport steps (not shown). In the futile cycles, net energy loss is one ATP per Suc in the cycle with SuSY, UGPase and INV, and two ATP per Suc in the cycle with INV, HK, and FK. It is assumed that FK utilizes UTP and/or that the uridine and adenine nucleotide systems are equilibrated via nucleoside diphosphate dikinase (Geigenberger et al., 1993). For simplicity, transport steps needed when INV is located in the vacuole or apoplast are omitted. The rate of cycling can be measured by analyzing labeling patterns after supplying isotopically labeled Suc, Fru, and Glc.
(D) Futile cycling between fructose-6P and fructose-1,6BP. This cycle can occur due to simultaneous activity of two of these three enzymes: ATP-phosphofructokinase (PFK), fructosebisphosphatase (FBPase), and pyrophosphate fructose 6-phosphate phosphotransferase (PFP). PFP (blue) catalyzes a readily reversible reaction. Net energy loss per cycle per unit fructose-6P is one ATP for the cycle between PFK and FBPase, the difference in free energy between one ATP and one PPi for the cycle between PFK and PFP, and one PPi for the cycle between PPi and FBPase. Cycling between fructose-6P and fructose-1,6BP can be measured as randomization of 14C or 13C label between the carbon-1 and carbon-6 positions.
Harden Proteins against Glycation Damage
Glycation—the modification of protein side chains by a cascade of reactions with sugars and other carbonyl compounds—causes major protein damage (Soboleva et al., 2017). The early reactions in the cascade are chemically or enzymatically reversible (Van Schaftingen et al., 2012; Richarme et al., 2015), but later reactions are not and destine the protein to proteolysis (Chondrogianni et al., 2014). Because glycation is site specific and proteomics can identify the particular Lys, Arg, or Cys residues involved (Soboleva et al., 2017), it is feasible to target vulnerable, functionally noncritical amino acids for replacement. Proof of concept comes from the stabilization of industrial enzymes (Gebert et al., 2015) and monoclonal antibodies (Miller et al., 2011) against glycation damage by removing Lys residues. A specific target in plants is a Lys in transketolase 2 (K706 in the Arabidopsis enzyme) that is a glycation hot spot (Bilova et al., 2017). The replacement of this Lys by a nonglycatable residue in some species appears not to affect enzyme function. Mining plant glycated proteome data sets (Bilova et al., 2017) could potentially identify many other targets.
Replace, Relocate, or Reschedule Metabolic Activities
Replace the Phe Route to Lignin
Replacing an inefficient biosynthetic pathway with a more efficient one can potentially reduce growth respiration. Conversion of arogenate to 4-coumarate during lignin biosynthesis is one such case. Although otherwise diverse, all pathways to lignin include a short reaction sequence mediating this conversion (Figure 3B; Whetten and Sederoff, 1995; Amthor, 2003). The more common three-reaction sequence via Phe, found in all plants, involves a phenylalanine ammonia-lyase (PAL; Whetten and Sederoff, 1995). This reaction sequence oxidizes one NADPH molecule per molecule of 4-coumarate formed. An alternative two-reaction sequence via Tyr, found mainly in monocots, involves a tyrosine ammonia-lyase (TAL) and reduces one NADP molecule for each 4-coumarate molecule formed. The TAL pathway thus confers an advantage over the PAL pathway of 2 NADPH per 4-coumarate produced. TAL activity in plants is associated with a bifunctional PAL (Louie et al., 2006; Barros et al., 2016); a single amino acid change can introduce TAL activity into a monofunctional PAL (Maeda, 2016). Additionally, TAL enzymes with almost no PAL activity are available from microbes (Jendresen et al., 2015). PAL activity could therefore be supplemented or even replaced by engineering TAL activity. As monocots with TAL activity still apparently synthesize over half their lignin via Phe (Barros et al., 2016), TAL activity engineering might benefit monocots as well as eudicots. The latter possibility rests on the assumption that monocots have not naturally exploited all possibilities for replacing PAL with TAL activity.
With all else equal, synthesis of lignin via the Tyr route instead of the Phe route could substantially reduce growth respiration for the lignin fraction of biomass (Amthor, 2003). In turn, the “production value” (PV) for lignin (g lignin produced per g of sugar substrate consumed) could increase by 5 to 7%, depending on the composition of the lignin (Supplemental Figure 1). Target crops for an altered lignin pathway include hybrid Populus and Eucalyptus (>25% lignin; Whetten and Sederoff, 1995; Novaes et al., 2010), cotton (Gossypium spp.) (>15% lignin; Fukushima et al., 2015: He et al., 2017), and sugarcane (Saccharum spp.) (up to 15% lignin; Masarin et al., 2011). Also, because 4-coumarate is precursor to many crop phenolic compounds such as flavones, flavonols, stilbenes, and anthocyanins, these too could be synthesized more efficiently following replacement (or augmentation) of PAL with TAL activity.
Relocate Nitrate Assimilation from Root to Shoot
Nitrate assimilation occurs to different extents in the roots and leaves of different species and is a major energy demand (Scheurwater et al., 2002; Andrews et al., 2004). Moving all or most nitrate assimilation to the leaf during the day would exploit the excess energy present in the chloroplast in high light and would be more efficient overall, as it would reduce the amount of Suc respired in the root to provide the required energy and carbon skeletons for root nitrate assimilation (Andrews et al., 2004; Shaw and Cheung, 2018). The efficiency calculation includes the additional energy involved in moving nitrate from root to shoot and returning amino acids to the root (Shaw and Cheung, 2018). Temperate cereals such as wheat (Triticum spp.) and barley are targets for such relocation because they assimilate an appreciable amount of nitrate in roots, especially at the relatively low nitrate concentrations in agricultural soils (Andrews, 1986). The simplest engineering strategy is to knock down expression of root nitrate reductase (Andrews et al., 2004), but suppressing nitrite reductase as well could add benefit by eliminating the energy demand of synthesizing this large protein and its iron-sulfur and siroheme prosthetic groups. To ensure that leaves can meet the nitrogen demands of the whole plant, including the root, it may be necessary also to increase the capacities for leaf nitrogen assimilation and for transport of amino acids via the phloem (Yadav et al., 2015; Tegeder and Masclaux-Daubresse, 2018). This strategy would be even more advantageous if the extra cost of nitrate transport to the shoot is offset by improvement in nitrate acquisition and reduction of the futile cycling associated with nitrate transport as noted below.
Reschedule Biosynthetic Processes from Night to Day
Another way to exploit the potential daytime energy excess is to increase the proportion of biosynthesis that happens during the day. The synthesis rates of major biomass components such as protein and cellulose are known to be far lower at night than during the day (Ishihara et al., 2015; Verbančič et al., 2018) and export of Suc from source leaves to the rest of the plant is also slower at night (Brauner et al., 2018). But there is probably scope to further synchronize biosynthesis with the daylight phase. This would better use the energy available during the day and be more efficient overall by saving the energy normally used in the synthesis and breakdown of starch (equivalent to ∼5% of the energy content of the sugar substrate for starch synthesis; Amthor, 2010) that is required to fuel nocturnal biosynthesis. Hence, diel flux-balance analysis models of leaf metabolism optimized for light-use efficiency predict no nocturnal biosynthesis at all unless this is enforced by a constraint (Cheung et al., 2014). Complete minimization of nocturnal activity would, however, likely reduce fitness and carry costs associated with leaving ribosomes and other biosynthetic machinery idle at night (Figure 2A). These costs would include the energetic costs of synthesizing ribosomes as well as the inefficient use of resources invested in them, such as phosphate, of which a considerable proportion is allocated to ribosomes (Raven, 2013). Restricting the biosynthesis of cellular constituents to the daytime would require temporal separation from growth by cell expansion, which is often slower in the light than at night (Poiré et al., 2010; Apelt et al., 2017), presumably reflecting the impact of transpiration on leaf water potential even in well-watered plants. This separation actually occurs, with protein and cell wall biosynthesis being faster in the light (see above). It is possible that more severe changes of leaf water potential in droughted plants might restrict protein and cell wall synthesis in the light (Kawaguchi et al., 2003). Nevertheless, the tradeoffs in crops among energy efficiency, nocturnal growth, and fitness may not be optimal for current agricultural environments, leaving opportunities for tuning. A specific opportunity is to restrict the rate of transitory carbohydrate accumulation during the day. Crops having a strong diurnal pattern of leaf starch accumulation, such as soybean (Glycine max; Leidreiter et al., 1995), would be good targets, with daytime starch accumulation suppressed by leaf-specific knockdown of ADP-Glc pyrophosphorylase.
Another strategy to harness a daytime excess of NAD(P)H and ATP is to increase their export from the chloroplast to support biosynthetic and maintenance processes in the cytosol. NAD(P)H and ATP are exported from the chloroplast indirectly by various metabolite shuttles (Gardeström and Igamberdiev, 2016) that may lack sufficient capacity to support optimal cytosolic energy requirements. It is therefore rational to increase the capacity of existing shuttles or to install direct transport systems for ATP and NAD(P)H in the chloroplast envelope. For example, promoter editing could be used to alter the expression of the Nucleotide Transporter 1 (N TT1) gene encoding the plastid inner envelope nucleotide transporter that is normally only expressed in non-green tissues (Versaw and Garcia, 2017). NTT1 mediates stoichiometric exchange of ATP with ADP + Pi (Trentmann et al., 2008) and, if present in the chloroplast inner envelope, would allow export of excess chloroplastic ATP to the cytosol. However, it might also make the chloroplast a sink for ATP in low-light conditions, to the detriment of ATP/ADP balance in the cytosol. If so, the NTT1 protein would need further engineering to restrict its activity to conditions of chloroplastic energy excess.
Reschedule Expression of the Mitochondrial Alternative Oxidase
The plant respiratory electron transport chain contains various non-phosphorylating bypasses that significantly lower the ATP synthesis per unit of carbon oxidized, most notably the alternative oxidase (AOX; Millar et al., 2011). During the light in photosynthetic tissues of C3 plants, the mitochondria predominantly oxidize Gly and run a modifiedtricarboxylic acid cycle that does not operate cyclically but rather generates intermediates as carbon skeletons for cell biosynthesis (Sweetlove et al., 2010; Tcherkez et al., 2012). Under these conditions, nonphosphorylating bypasses enable the dissipation of excess reducing power generated during photosynthesis (Raghavendra and Padmasree, 2003; Zhang et al., 2017). However, during the night in shoot tissues, these bypasses continue to operate, lowering the ATP synthesis per unit of carbon oxidized (Robinson et al., 1995; Cheah et al., 2014) even though a conventional tricarboxylic acid cycle occurs and ATP biosynthesis is the dominant function of mitochondria. AOX proteins do not have a rapid turnover rate and remain present and active throughout the night in leaves (Lee et al., 2010). The AOX pathway reduces mitochondrial ATP synthesis per unit of carbon oxidized to 33% of the normal cytochrome oxidase pathway (Millar et al., 2011). In vivo measures of AOX operation at night range from 10 to 50% of total respiratory rate (Robinson et al., 1995; Cheah et al., 2014).
Plants have multiple AOX isoforms, typically one that is constitutive and others that are stress inducible (Millar et al., 2011). A solution to the inefficiency that these enzymes present, without compromising stress tolerance, is to replace the constitutive AOX with a synthetic version tailored to have a higher turnover rate and a light-specific promoter to lower the alternative pathway rate at night and raise it again during the day. If this could be engineered perfectly, for the same respiratory ATP synthesis rate, a 5 to 30% of respiratory carbon loss in the dark could be prevented. A crop of choice is soybean, which has an AOX pathway flux in the dark of up to 50% of respiration rate (Robinson et al., 1995; Ribas-Carbo et al., 2005; Cheah et al., 2014). Previous attempts to simply remove AOX from soybean led to a yield penalty (Chai et al., 2012), indicating the importance of a more subtle rescheduling to remove AOX function in the dark. Importantly, as AOX is a low-abundance protein in mitochondria and represents <0.01% of total leaf protein, the ATP efficiency gained by reducing its activity at night would be hundreds of times higher than the loss of ATP required to resynthesize AOX during each day.
Suppress Futile Cycles
Several futile cycles in central metabolism run at high rates (Plaxton, 1996) and could waste a substantial fraction of the ATP produced in respiration (Alonso et al., 2005). Examples include simultaneous synthesis and degradation of starch in leaves in the light (Fernandez et al., 2017), simultaneous synthesis and degradation of Suc (Figure 3C; Geigenberger and Stitt, 1991, 1993; Alonso et al., 2005), and cycling between Fru 6-phosphate (F6P) and Fru1,6-bisphosphate (F16BP; Figure 3D; Keeling et al., 1988; Hatzfeld and Stitt, 1990; Hill and ap Rees, 1994). If a futile cycle reflects only inefficient regulation, suppressing it will decrease respiratory costs without doing collateral damage. Futile cycles may, however, have physiological functions in enabling sensitive metabolic regulation (Samoilov et al., 2005), dissipating energy in stress conditions (Adolfsen and Brynildsen, 2015) or, for F6P-F16BP cycles, regulating the cytosolic pyrophosphate (PPi) pool and hence tonoplast H+-pyrophosphatase activity (Stitt, 1998). The pros and cons of cycle suppression must therefore be carefully assessed for futility versus utility, as follows.
Suc Synthesis/Degradation
A cycle of Suc synthesis and degradation occurs when the enzymes of Suc synthesis (Suc phosphate synthase, Suc phosphate phosphatase) operate concurrently with Suc degradation via (1) Suc synthase and fructokinase or (2) invertase, hexokinase, and fructokinase (Figure 3C). Scenario 2 is more expensive, especially if it involves an invertase located in the vacuole or apoplast and active transport of sugars across the tonoplast or plasma membrane. A Suc synthesis/degradation cycle occurs in source leaves of many species, where it probably serves to regulate the net rate of Suc synthesis and photosynthate allocation to starch (Huber, 1989; Kingston‐Smith et al., 1999; Stitt et al., 2010; Mengin et al., 2017). Suc synthesis/degradation cycles also occur during Suc import in growing tissues (Hargreaves and ap Rees, 1988; Geigenberger and Stitt, 1991, 1993; Nguyen-Quoc and Foyer, 2001; Alonso et al., 2005; Ruan et al., 2010). The costly cycles involving invertase may often be important in driving Suc import or cell expansion (Nguyen-Quoc and Foyer, 2001; Ruan et al., 2010). Cycles involving Suc synthase may sometimes be futile: all major Suc synthase genes can be deleted in Arabidopsis without impacting growth except under low oxygen (Barratt et al., 2009). However, the cycle is not always dispensable: reducing total Suc synthase activity by 75% or more decreases starch and storage protein accumulation in potato (Solanum tuberosum) tubers (Zrenner et al., 1995), and deleting individual Suc synthases in maize (Zea mays) impairs grain development (Chourey et al., 1998) and root growth (Alonso et al., 2005).
F6P/F16BP Cycling
There is rapid cycling between F6P and F16BP in many respiring tissues (Figure 3D). This cycle usually involves pyrophosphate Fru 6-phosphate phosphotransferase (PFP; Hajirezaei et al., 1993), which operates close to equilibrium in vivo (Weiner et al., 1987; Geigenberger et al., 1993; Stitt, 1998). A futile cycle with wastage of energy will occur when PFP operates together with either ATP-phosphofructokinase or fructosebisphosphatase, although in some circumstances this cycle may deliver PPi to support Suc mobilization via Suc synthase and UDP Glc pyrophosphorylase or energization of the tonoplast (Plaxton, 1996, 2010; Stitt, 1998). Evidence that such cycles are futile is provide by the observation that strongly decreasing PFP expression does not affect growth in several species (Hajirezaei et al., 1993; Nielsen and Stitt, 2001; Duan et al., 2016) and even increases the Suc content in sugarcane (van der Merwe et al., 2010). However, negative phenotypes do occasionally appear: for example, strong reductions in PFP expression inhibited growth under salt and osmotic stress conditions in Arabidopsis seedlings (Lim et al., 2013) and decreased rice (Oryza sativa) grain quality (Duan et al., 2016).
Thus, current evidence indicates that both the Suc synthesis/degradation and F6P/F16BP cycles can be safely suppressed in certain species, tissues, and conditions. Given the high estimated energetic costs of these cycles (see above), potential gains are substantial. To achieve them, enzymes will need to be engineered in a suitably cell-, development-, or condition-specific way. An example of an immediate target would be seed fill in crops like rape (Brassica napus) and soybean, where photosynthesis in the seed minimizes the risk of low oxygen. More generally, research into the impact of partial reductions in expression on performance in different species and conditions would sharpen planning of strategies to optimize tradeoffs between energetic optimization and metabolic flexibility.
Improve Efficiency of Nitrate Acquisition
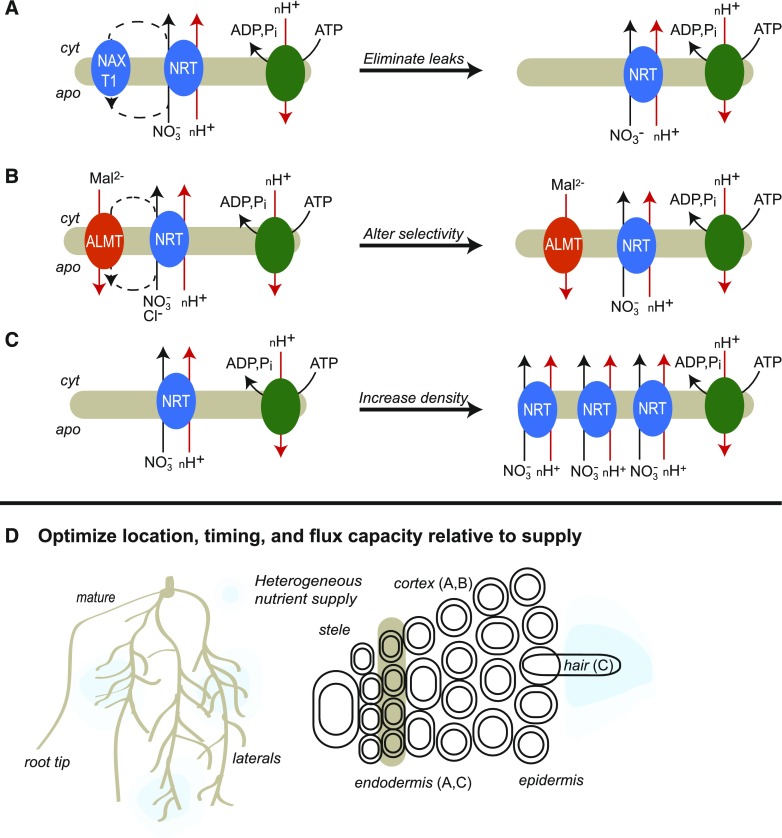
The transporters that mediate nitrate (NO3−) uptake in roots are promising targets because NO3− is the main nitrogen source for most plants, although NO3− versus NH4+ preference varies depending on selection for particular soil types (Dechorgnat et al., 2018). The ATP-driven H+ pumping that generates the electrochemical proton gradient for NO3− uptake (Figure 4) is likely the highest macronutrient acquisition cost (Glass, 2003; Xu et al., 2012).
Figure 4.
Strategies to Reduce the Costs of NO3− Acquisition.
In the schemes above the line, the native state is on the left and the reduced-cost engineered state is on the right. Red arrows are proton fluxes or malate (Mal2−) fluxes as indicated, solid black arrows are NO3− transport fluxes, and dashed black arrows are NO3− leaks. Blue ovals are NO3− transporters, i.e., NRT family members and the NAXT NO3− excretion transporter. The red oval represents aluminum-activated malate transporters (ALMTs). The green oval is the ATP-driven H+ pump that generates the electrochemical proton gradient for NO3− uptake. Cost reduction strategies are as follows.
(A) Identifying and eliminating NO3− leaks, e.g., via the NAXT1 transporter.
(B) Modifying selectivity of NO3− transporters and anion channels. Anion channels such as the ALMTs that can be permeable to NO3− and that would normally allow leakage out of the cytoplasm to the external medium (negative membrane potential and low external NO3−), potentially enable a futile cycle. Altering the selectivity of NRT transporters is possible to reduce competing Cl− transport.
(C) Increasing the density on specific membranes (e.g., in root hair cells) of an optimized NO3− transporter would effectively increase the flux density (Vmax) that may more efficiently capture NO3− when and where it becomes available in soil and possibly linked to high water flows to the root.
(D) Location, timing, and flux capacity are critical for any strategy. For instance, eliminating an efflux transporter from the xylem parenchyma as well as from the root cortex would compromise transport to the shoot. Similarly, expression of an influx transporter in the xylem parenchyma could result in a futile cycle where a net efflux is required to the xylem. Heterogeneous concentrations of NO3− in the soil require control of lateral root foraging matched with transport capacity and demand by the shoot.
Plants have several families of transporters and channels that can transport NO3− (Krapp et al., 2014). TheNitrate Transporter 2s (NRT2s) are high-affinity transporters responsible for NO3− uptake at low concentrations; the nitrate/peptide family (NPF) transporters and dual-affinity NRT2s are low-affinity transporters that work at higher concentrations. Some NPF members can also transport K+ and other substrates or act as nitrate sensors (Krapp et al., 2014; Li et al., 2017c); these and similar moonlighting activities by other transporters (Krapp et al., 2014; Corratgé-Faillie and Lacombe, 2017; Ramesh et al., 2018) are considerations for engineering as they can compromise outcomes. Another engineering consideration is that the expression of engineered NO3− transporters would need to be as precisely spatially and developmentally regulated as that of natural NO3− transporters (Garnett et al., 2013). A further consideration is that NO3− assimilation requires regulation of pH since NO3− reduction produces OH−. This is turn impacts carboxylate production and transport across the tonoplast, with links to K+ transport (Raven, 1985).
Several engineering strategies to reduce the costs of NO3− acquisition can be proposed, with maize (Zea mays) and wheat (Triticum aestivum) as promising target crops for their implementation (Xu et al., 2012).
Identify Transporters That Allow Efflux of Nitrate to the Rhizosphere and Engineer to Reduce or Eliminate This Efflux
A precedent for this strategy comes from Arabidopsis NPF2.7 (Nitrate Excretion Transporter, NAXT1), a plasma membrane transporter that is expressed mainly in root cortex and that can efflux NO3− under acidic conditions (Figure 4A; Segonzac et al., 2007). RNAi knockdown of NAXT1 increased root NO3− level but also decreased shoot NO3− level; this unwanted decrease may have been due to collateral knockdown of other NAXT family members (Segonzac et al., 2007). Similarly, someAluminium-Activated Malate Transporter (ALMT) anion transporters can readily efflux NO3− (Piñeros et al., 2008; Sharma et al., 2016). As residues that affect their substrate selectivity or activity have been identified (Zhang et al., 2013; Ramesh et al., 2015), the selectivity of these transporters could in principle be rationally engineered (Figure 4B). Other transporters can efflux NO3−, e.g., Arabidopsis NPF7.3 (NRT1.5; Lin et al., 2008), NPF6.3 (NRT1.1; Léran et al., 2013), and maize NPF6.4 (Wen et al., 2017) or Cl− e.g., Arabidopsis NPF2.5 (Li et al., 2017b). These transporters are candidates for pre-engineering research to identify the residues that govern NO3− efflux and NO3−/Cl− selectivity.
Overexpress More Efficient Transporters to Increase Their Density in Specific Membranes
“More efficient” in this case means transporters that do not allow efflux under certain conditions have high selectivity for NO3− (e.g., over Cl−) and/or have high Vmax values (Figure 4C). This type of strategy has been applied in rice, where overexpressing an NRT2 transporter increased nitrogen use efficiency, growth, and yield but was also linked to pH regulation (Fan et al., 2016). The overexpression of more efficient transporters could be especially effective in “gatekeeper” cells, which carry much of the transport flux but may be only a small fraction of the cell population (Xu et al., 2018). Note, however, that changing selectivity away from Cl− has some potential to disrupt osmoregulation (Wege et al., 2017).
Optimize Location, Timing, and Flux Capacity of Root NO3− Transporters Relative to NO3− Supply
A target location (Figure 4D) is root hairs and root zones with a high density of water influx, since NO3− is readily mobile in the soil solution and is convected to the root surface during transpiration, and NO3− and water transport are positively correlated (Tyerman et al., 2017). Nitrate flux into roots varies as demand by the plant changes, and the heterogenous nature of soil requires control over lateral root development to forage for the NO3− supply (York et al., 2016). The complex transcriptomic and proteomic mechanisms that match supply with demand in this multivariate system are now sufficiently known to begin designing engineering strategies (Wang et al., 2018).
POTENTIAL BIOMASS GAINS FROM REDUCED RESPIRATORY CARBON LOSS
Biomass gains can potentially come from reducing carbon loss associated with maintenance and/or growth. Maintenance respiration losses can be reduced by lowering the rate of cyclic processes such as protein turnover or by increasing the efficiency of respiratory ATP production (Amthor, 2000). Growth respiration losses can be reduced by increasing biosynthetic efficiency or by increasing the efficiency of respiratory ATP production (Penning de Vries et al., 1974). Below, we quantitatively assess biomass gains that could accrue from implementing the strategies described above.
Maintenance
To translate reductions in maintenance respiration rate into gains in crop biomass accumulation, we compared the growth rate Equation 2 with and without a reduction in maintenance respiration coefficient. The comparison is summarized by the following (see Supplemental Appendix 2):
| (3) |
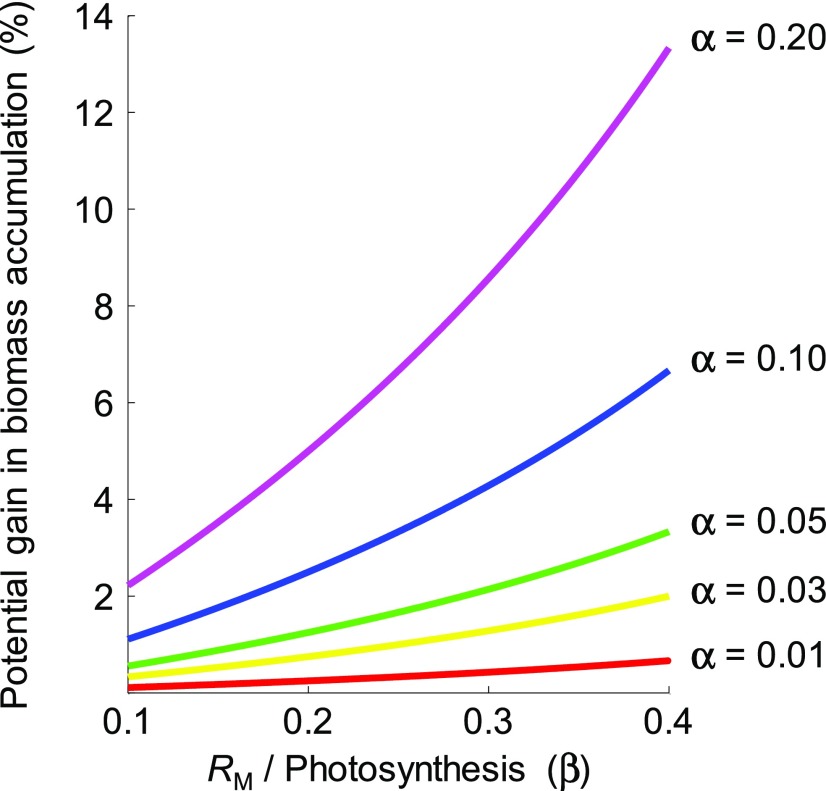
where dW*/dt is growth rate resulting from a reduced maintenance respiration coefficient, dW/dt is growth rate with “normal” maintenance respiration coefficient, α is the fraction (0 to 1) of whole-plant maintenance respiration that engineering eliminates, and β is the average whole-plant ratio of maintenance respiration to photosynthesis mW / P over a period of interest. Based on references cited above (see “a primer on respiratory carbon losses”) we take 0.23 as the central tendency of β. For this value of β, potential percentage gains in biomass accumulation are nearly one third the percentage reductions in the maintenance respiration coefficient m (Figure 5).
Figure 5.
Potential Biomass Gains Accruing from Reduced Maintenance Respiration.
A simple integrative model to quantify potential effects of a fractional reduction in the maintenance respiration coefficient (m) on the potential proportional gain in whole-plant growth written as αβ/(1 − β), where α is the fraction of m that is “engineered away” and β is the whole-plant mW:P (maintenance respiration [mW = RM]/photosynthesis) ratio. Potential biomass gains are plotted for discrete values of α (different lines) over the range 0.10 to 0.40 for β. Over a growing season, we expect β to be in the range 0.20 to 0.25 for non-stressed crops.
Our target values of α, described below, are used to quantify potential biomass gains for a range of strategies to reduce maintenance respiration (Table 1).
Table 1. Potential Biomass Gains Resulting from Elimination of a Portion of Maintenance Respiration.
| Mechanism to Reduce Maintenance Respiration | Range of Potential Reduction in Maintenance Respiration (%)a | Reduction of Maintenance Respiration Deemed Feasible (α)(%)b | Potential Biomass Gain for β = 0.23 (%)b |
|---|---|---|---|
| Eliminate THI4 turnover | 2 to10c | 4 | 1.2 |
| Reduce protein glycationd | 1 to 7 | 4 | 1.2 |
| Reschedule expression of AOX, including in nonphotosynthetic tissuese | 5 to 30 | 15 | 4.5 |
| Suppress futile cyclesf | 5 to 40 | 15 | 4.5 |
| Reduce intracellular membrane leaks | 5 to 30 | ?g | ?g |
The upper end of the ranges sums to more than 100% because of uncertainty about individual processes. There is also uncertainty about how the ranges vary between species and environments.
See text for definitions of α and β. Supplemental Appendix 2 outlines the method of calculating potential biomass gain based on α and β. We take 0.23 as the central tendency for β in crops.
Based on Hanson et al. (2018).
Based on the following assumptions, which we deem reasonable but subject to significant uncertainty: glycation amenable to reduction is associated with constitutive proteins; integrated over a growing season and throughout the plant 30 to 50% of proteins turned over are constitutive; 40 to 60% of constitutive protein degradation is associated with glycation; 20 to 40% of constitutive-protein glycation could be eliminated by engineering; and 30 to 55% of maintenance respiration supports protein turnover. We take midpoint values as an estimate of a feasible reduction in maintenance respiration.
Based on the following assumptions: 5 to 40% of respiratory ATP production in maintenance conditions supports futile cycles; for some crops 15% of maintenance respiration could be eliminated by stopping unnecessary cycles (e.g., if 30% of maintenance respiration is used to provide ATP consumed in futile cycles, and half the activity of those cycles is removed, 15% of maintenance respiration could be eliminated).
Plasmalemma and tonoplast leaks may constitute half of maintenance costs (Penning de Vries, 1975; Amthor, 2010), in which case suppressing leaks could yield savings in respiration comparable to, or even exceeding, those from rescheduling AOX expression and suppressing futile cycles. More research is necessary, however, before firmer quantitative estimates for potential reductions in those leaks, and the metabolic engineering targets that might be associated with reducing those leaks, will be possible. As a result, we presently do not provide an estimate of a feasible respiratory savings, but merely note that it could be large.
Because of the high turnover rate and relatively high abundance of the THI4 protein, crop biomass accumulation might be increased up to 4% by essentially eliminating THI4 turnover (Hanson et al., 2018); we suggest a feasible target of ∼1 to 2% (Table 1). Potential biomass gains associated with reduced protein glycation cannot yet be predicted confidently, but we derive a value of ∼1 to 2% (Table 1).
Potential biomass gains from reducing AOX activity depend on the proportion of mitochondrial electron transport accounted for by AOX, and other factors set out in Supplemental Appendix 3. Measured values for this proportion (10% to 50%) indicate a possible biomass increase of up to ∼9% (see figure in Supplemental Appendix 3), with a target of 4% to 5% considered feasible in some crops (Table 1). A modest additional biomass increase due to higher efficiency of ATP formation for growth processes is also expected (Penning de Vries et al., 1974). Similarly, if maintenance respiration is reduced by 15% by eliminating unnecessary (futile) cycling, biomass gains of 4 to 5% could be achieved (Table 1).
We did not include a strategy to reduce respiratory costs of active transmembrane transport to counteract membrane “leaks” (other than those associated with NO3− acquisition) because we lack specific knowledge, inter alia, of leak rates and molecular targets to reduce them. Nevertheless, we note that order-of-magnitude calculations indicate that plasmalemma and tonoplast leaks may constitute up to half of maintenance costs in whole plants (Penning de Vries, 1975; Amthor, 2010), representing potential savings in respiration comparable to those possible through rescheduling AOX expression and suppressing futile cycles. Advancing research on leaks across apoplastic barriers in roots (e.g., Pfister et al., 2014) might allow both assessments of their quantitative significance to crop carbon budgets and, if the costs are high, possibilities for eliminating leaks through genetic engineering.
Growth
For the Tyr shortcut to lignin (Figure 3B), an increase in the PV of the lignin fraction of biomass translates into less Suc substrate being used to generate each unit of lignin. The Suc that would have been used for the same amount of lignin biosynthesis may become available for additional biomass growth. For a range of crop-specific biomass lignin concentrations, a PV increase of 6% for the lignin fraction of biomass could translate into 0.5 to 2.5% increases in whole-plant PV (Table 2), which is equivalent to the percentage gain in biomass accumulation.
Table 2. Potential Effect of Increased Lignin Production Value on Whole-Plant PV Brought about by Replacing PAL Activity with TAL Activity in Support of Lignin Biosynthesis.
| Biomass Constituent | Low-Lignin Crop | Medium-Lignin Crop | High-Lignin Crop | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction (g/g) | PV Native | PV Altered | Gain in PV (%) | Fraction (g/g) | PV Native | PV Altered | Gain in PV (%) | Fraction (g/g) | PV Native | PV Altered | Gain in PV (%) | |
| Lignin | 0.05 | 0.415 | 0.440 | 6.02 | 0.15 | 0.415 | 0.440 | 6.02 | 0.30 | 0.415 | 0.440 | 6.02 |
| Non-lignin | 0.95 | 0.720 | 0.720 | 0.00 | 0.85 | 0.720 | 0.720 | 0.00 | 0.70 | 0.720 | 0.720 | 00.0 |
| Whole plant | 1.00 | 0.705 | 0.706 | 0.48 | 1.00 | 0.674 | 0.678 | 1.35 | 1.00 | 0.629 | 0.636 | 2.48 |
Production value (PV) is g biomass grown from 1 g Suc substrate used for carbon skeletons and energy used in biosynthesis (Penning de Vries et al., 1974). PV (native) is for lignin biosynthesis involving PAL; PV (improved) is for lignin biosynthesis involving TAL (see Figure 3C). PV of the nonlignin biomass fraction is the weighted average of all nonlignin biomass components. Whole-plant PV is amount of total biomass grown from 1 g Suc plus inorganic N, S, and minerals. Column labeled “gain” is percentage increase in PV due to transformation of lignin biosynthetic pathway(s) from those using PAL to those using TAL. PV for the nonlignin fraction of biomass is illustrative only (not intended to reflect any specific crop). Low-, medium-, and high-lignin crops might be represented by many cereal and oilseed crops, by cotton and sugarcane, and by tree plantation crops, respectively.
Acquisition and assimilation of N is an important requirement for crop growth, and respiration associated with NO3− uptake (separate from that associated with NO3− assimilation) can be a significant fraction of root respiration (Bouma et al., 1996; Kurimoto et al., 2004). If leaks of NO3− back out of the root could be reduced, additional growth would be possible because substrate that would normally be respired to support reuptake of NO3− could instead be used for growth. However, the growth gain is likely to be quite modest. Our assessment indicates that except for the extreme case of complete elimination of a 25% leak fraction in a crop with high N:C ratio and extensive AOX activity, the biomass gains achievable by reducing NO3− leakage from roots are < 1% (Supplemental Appendix 4).
Biomass Gains Can Potentially Be Partitioned to Harvested Organs
Percentage gains in harvested yield could in principle exceed percentage gains in total crop biomass. This would occur if biomass gains (i.e., the “extra” carbohydrate and downstream biomass made available through maintenance savings) were partitioned preferentially to harvested organs. For example, for a harvest index of 0.5 and assuming that “extra” carbohydrate saved before grain filling is stored in a form (e.g., stem reserves) that can be mobilized to grain and/or that “extra” carbohydrate saved during grain filling is preferentially partitioned to grain, a 3% increase in total biomass could translate into as much as a 6% increase in grain yield.
CLOSING POINTS
We end with five take-home points. First, it is hard to overstate the significance of respiratory carbon loss to yield improvement. Respiration is as central to plant productivity as photosynthesis; together, they power all plant metabolism and—indirectly—nearly all non-plant metabolism on Earth. The processes driving respiratory carbon loss consequently merit as much attention from metabolic engineering and synthetic biology as photosynthesis and high-value secondary product biosynthesis. The potential payoffs from cutting carbon losses are simply too large to ignore. Second, the specific proteins, pathways, and processes that we identify as candidates for engineering are just initial targets of opportunity in what is becoming a target-rich arena as knowledge of respiratory drivers and the technology to modify them keep advancing. As this happens, the many substrates, enzymes, transporters, and effectors involved in respiration become a plus instead of a minus because they offer multiple options to reduce carbon loss in various contexts. Third, respiratory loss reduction candidates are present in all plants and very likely genetically independent—making them applicable to many species and potentially stackable. Fourth, as noted throughout this article, because plants are highly differentiated multicellular organisms, not just E. coli writ large, it matters greatly where, when, and how much engineered genes are expressed. At present, almost all engineering of plant gene expression uses natural regulatory parts, leaving engineered genes open to unwanted crosstalk from endogenous control systems in the host. Fortunately, the fast-growing field of synthetic regulatory circuits in plants (Boehm et al., 2017; Kassaw et al., 2018) can provide orthogonal systems to modulate gene expression; these may be very useful in re-engineering genes that drive carbon loss. Finally, we acknowledge that smart breeding approaches that integrate multiple processes and focus on phenotypic outputs like growth rate and yield provide an alternative to directed engineering of metabolic subprocesses, and that directed approaches might fail if the various subprocesses interact in a nonlinear and hence difficult-to-predict manner on emergent agronomic traits. However, breeding depends on the available genetic diversity in the breeding population and this, as argued earlier, may be limited or poorly suited for yield maximization in the agricultural context. In this context, directed engineering provides a complementary approach to smart breeding, with potential synergies if it highlights candidate genes and polymorphisms, provides strategies to create missing diversity for highlighted traits related to respiratory loss, or provides knowledge that supports modeling of emergent traits. We recognize there is likely a deep tradeoff between minimization of respiration and optimization of energy use, on the one hand, and robustness and yield stability in a fluctuating environment on the other hand. Even so, the directed approaches proposed here would still provide a much needed quantitative analysis of this tradeoff to learn which apparently wasteful components are in fact important and which can be decreased or suppressed to the benefit of crop productivity.
Supplemental Data
Supplemental Figure 1. Theoretical PVs of lignin synthesized from Suc (g lignin g−1 Suc substrate) via Phe (blue bars) or via Tyr (orange bars).
Supplemental Appendix 1. Derivation of Equation 2.
Supplemental Appendix 2. Relating a reduction in the maintenance respiration coefficient to potential growth increase.
Supplemental Appendix 3. Translating reduced AOX activity into potential crop biomass gain.
Supplemental Appendix 4. Translating an engineered reduction in NO3− leakage from roots into potential crop biomass gain.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
The authors’ work was supported by NSF award IOS-1444202 to A.D.H., by Australian Research Council funding (CE140100008) to A.H.M. and S.D.T., by ERA-CAPS (BO 1482/18-1 | FE 552/33-1 | RE 1351/2-1 | SW 122/2-1) to L.J.S., by Max Planck Society funding to A.B.-E. and M.S., and by a grant from the Bill and Melinda Gates Foundation (CASS, OP1113365) to M.S. We thank Dr. Jaya Joshi for help in drawing figures.
AUTHOR CONTRIBUTIONS
All authors contributed to the manuscript; A.D.H. integrated the contributions.
Footnotes
Articles can be viewed without a subscription.
References
- Adolfsen K.J., Brynildsen M.P. (2015). Futile cycling increases sensitivity toward oxidative stress in Escherichia coli. Metab. Eng. 29: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth E.A., Long S.P. (2005). What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 165: 351–371. [DOI] [PubMed] [Google Scholar]
- Ainsworth E.A., Ort D.R. (2010). How do we improve crop production in a warming world? Plant Physiol. 154: 526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A.P., Vigeolas H., Raymond P., Rolin D., Dieuaide-Noubhani M. (2005). A new substrate cycle in plants. Evidence for a high glucose-phosphate-to-glucose turnover from in vivo steady-state and pulse-labeling experiments with [13C]glucose and [14C]glucose. Plant Physiol. 138: 2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor J.S. (1989). Respiration and Crop Productivity. (New York: Springer-Verlag; ). [Google Scholar]
- Amthor J.S. (2000). The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Ann. Bot. 86: 1–20. [Google Scholar]
- Amthor J.S. (2003). Efficiency of lignin biosynthesis: A quantitative analysis. Ann. Bot. 91: 673–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor J.S. (2010). From sunlight to phytomass: On the potential efficiency of converting solar radiation to phyto-energy. New Phytol. 188: 939–959. [DOI] [PubMed] [Google Scholar]
- Andrews M. (1986). The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ. 9: 511–519. [Google Scholar]
- Andrews M., Lea P.J., Raven J.A., Lindsey K. (2004). Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Ann. Appl. Biol. 145: 25–40. [Google Scholar]
- Apelt F., Breuer D., Olas J.J., Annunziata M.G., Flis A., Nikoloski Z., Kragler F., Stitt M. (2017). Circadian, carbon, and light control of expansion growth and leaf movement. Plant Physiol. 174: 1949–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt D.H., Derbyshire P., Findlay K., Pike M., Wellner N., Lunn J., Feil R., Simpson C., Maule A.J., Smith A.M. (2009). Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 106: 13124–13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros J., Serrani-Yarce J.C., Chen F., Baxter D., Venables B.J., Dixon R.A. (2016). Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants 2: 16050. [DOI] [PubMed] [Google Scholar]
- Bilova T., et al. (2017). Global proteomic analysis of advanced glycation end products in the Arabidopsis proteome provides evidence for age-related glycation hot spots. J. Biol. Chem. 292: 15758–15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm C.R., Pollak B., Purswani N., Patron N., Haseloff J. (2017). Synthetic botany. Cold Spring Harb. Perspect. Biol. 9: a023887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. (1962). The upper limit of crop yield: This classical problem may be analyzed as one of the photosynthetic efficiency of plants in arrays. Science 137: 11–15. [DOI] [PubMed] [Google Scholar]
- Bouma T.J., Broekhuysen A.G.M., Veen B.W. (1996). Analysis of root respiration of Solanum tuberosum as related to growth, ion uptake and maintenance of biomass. Plant Physiol. Biochem. 34: 795–806. [Google Scholar]
- Brauner K., Birami B., Brauner H.A., Heyer A.G. (2018). Diurnal periodicity of assimilate transport shapes resource allocation and whole-plant carbon balance. Plant J. 94: 776–789. [DOI] [PubMed] [Google Scholar]
- Cairns A.J., Gallagher J.A. (2004). Absence of turnover and futile cycling of sucrose in leaves of Lolium temulentum L.: Implications for metabolic compartmentation. Planta 219: 836–846. [DOI] [PubMed] [Google Scholar]
- Cannell M.G.R., Thornley J.H.M. (2000). Modelling the components of plant respiration: some guiding principles. Ann. Bot. 85: 45–54. [Google Scholar]
- Chai T.T., Simmonds D., Day D.A., Colmer T.D., Finnegan P.M. (2012). A GmAOX2b antisense gene compromises vegetative growth and seed production in soybean. Planta 236: 199–207. [DOI] [PubMed] [Google Scholar]
- Charles-Edwards D.A. (1975). Efficiency and expediency in plant growth. Ann. Bot. 39: 161–162. [Google Scholar]
- Chatterjee A., Abeydeera N.D., Bale S., Pai P.J., Dorrestein P.C., Russell D.H., Ealick S.E., Begley T.P. (2011). Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase. Nature 478: 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M.H., Millar A.H., Myers R.C., Day D.A., Roth J., Hillier W., Badger M.R. (2014). Online oxygen kinetic isotope effects using membrane inlet mass spectrometry can differentiate between oxidases for mechanistic studies and calculation of their contributions to oxygen consumption in whole tissues. Anal. Chem. 86: 5171–5178. [DOI] [PubMed] [Google Scholar]
- Cheung C.Y., Poolman M.G., Fell D.A., Ratcliffe R.G., Sweetlove L.J. (2014). A diel flux balance model captures interactions between light and dark metabolism during day-night cycles in C3 and crassulacean acid metabolism leaves. Plant Physiol. 165: 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N., Petropoulos I., Grimm S., Georgila K., Catalgol B., Friguet B., Grune T., Gonos E.S. (2014). Protein damage, repair and proteolysis. Mol. Aspects Med. 35: 1–71. [DOI] [PubMed] [Google Scholar]
- Chourey P.S., Taliercio E.W., Carlson S.J., Ruan Y.L. (1998). Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 259: 88–96. [DOI] [PubMed] [Google Scholar]
- Corratgé-Faillie C., Lacombe B. (2017). Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J. Exp. Bot. 68: 3107–3113. [DOI] [PubMed] [Google Scholar]
- Dechorgnat J., Francis K.L., Dhugga K.S., Rafalski J.A., Tyerman S.D., Kaiser B.N. (2018). Root ideotype influences nitrogen transport and assimilation in maize. Front. Plant Sci. 9: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V., Straltsova D., Medvedev S.S., Pozhvanov G.A., Sokolik A., Yurin V. (2014). Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 65: 1259–1270. [DOI] [PubMed] [Google Scholar]
- Duan E., Wang Y., Liu L., Zhu J., Zhong M., Zhang H., Li S., Ding B., Zhang X., Guo X., Jiang L., Wan J. (2016). Pyrophosphate: fructose-6-phosphate 1-phosphotransferase (PFP) regulates carbon metabolism during grain filling in rice. Plant Cell Rep. 35: 1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb T.J., Zarzycki J. (2016). Biochemical and synthetic biology approaches to improve photosynthetic CO2-fixation. Curr. Opin. Chem. Biol. 34: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb T.J., Jones P.R., Bar-Even A. (2017). Synthetic metabolism: Metabolic engineering meets enzyme design. Curr. Opin. Chem. Biol. 37: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisman J.W., Sutton M.A., Galloway J., Klimont Z., Winiwarter W. (2008). How a century of ammonia synthesis changed the world. Nat. Geosci. 1: 636–639. [Google Scholar]
- Eser B.E., Zhang X., Chanani P.K., Begley T.P., Ealick S.E. (2016). From suicide enzyme to catalyst: The iron-dependent sulfide transfer in Methanococcus jannaschii thiamin thiazole biosynthesis. J. Am. Chem. Soc. 138: 3639–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Tang Z., Tan Y., Zhang Y., Luo B., Yang M., Lian X., Shen Q., Miller A.J., Xu G. (2016). Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 113: 7118–7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez O., Ishihara H., George G.M., Mengin V., Flis A., Sumner D., Arrivault S., Feil R., Lunn J.E., Zeeman S.C., Smith A.M., Stitt M. (2017). Leaf starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 174: 2199–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer C.H., Ruban A.V., Nixon P.J. (2017). Photosynthesis solutions to enhance productivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372: 20160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima R.S., Kerley M.S., Ramos M.H., Porter J.H., Kallenbach R.L. (2015). Comparison of acetyl bromide lignin with acid detergent lignin and Klason lignin and correlation with in vitro forage degradability. Anim. Feed Sci. Technol. 201: 25–37. [Google Scholar]
- Gardeström P., Igamberdiev A.U. (2016). The origin of cytosolic ATP in photosynthetic cells. Physiol. Plant. 157: 367–379. [DOI] [PubMed] [Google Scholar]
- Garnett T., et al. (2013). The response of the maize nitrate transport system to nitrogen demand and supply across the lifecycle. New Phytol. 198: 82–94. [DOI] [PubMed] [Google Scholar]
- Gebert M.S., Dalsgaard S., Ortiz-Johnson M., Garske A.L. (2015). Stable enzymes by glycation reduction. US Patent Application No. WO2015073772A1.
- Geigenberger P., Stitt M. (1991). A “futile” cycle of sucrose synthesis and degradation is involved in regulating partitioning between sucrose, starch and respiration in cotyledons of germinating Ricinus communis L. seedlings when phloem transport is inhibited. Planta 185: 81–90. [DOI] [PubMed] [Google Scholar]
- Geigenberger P., Stitt M. (1993). Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 189: 329–339. [DOI] [PubMed] [Google Scholar]
- Geigenberger P., Langenberger S., Wilke I., Heineke D., Heldt H.W., Stitt M. (1993). Sucrose is metabolised by sucrose synthase and glycolysis within the phloem complex of Ricinus communis L. seedlings. Planta 190: 446–453. [Google Scholar]
- Gifford R.M., Thorne J.H., Hitz W.D., Giaquinta R.T. (1984). Crop productivity and photoassimilate partitioning. Science 225: 801–808. [DOI] [PubMed] [Google Scholar]
- Glass A.D.M. (2003). Nitrogen use efficiency of crop plants: Physiological constraints upon nitrogen absorption. CRC Crit. Rev. Plant Sci. 22: 453–470. [Google Scholar]
- Hajirezaei M., Sonnewald U., Viola R., Carlisle S., Dennis D., Stitt M. (1993). Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192: 16–30. [Google Scholar]
- Hanson A.D., Amthor J.S., Sun J., Niehaus T.D., Gregory J.F. III, Bruner S.D., Ding Y. (2018). Redesigning thiamin synthesis: Prospects and potential payoffs. Plant Sci. 273: 92–99. [DOI] [PubMed] [Google Scholar]
- Hargreaves J.A., ap Rees T. (1988). Turnover of starch and sucrose in roots of Pisum sativum. Phytochemistry 27: 1627–1629. [Google Scholar]
- Hatzfeld W.-D., Stitt M. (1990). A study of the rate of recycling of triose phosphates in heterotrophic Chenopodium rubrum cells, potato tubers, and maize endosperm. Planta 180: 198–204. [DOI] [PubMed] [Google Scholar]
- Hawkins A.J.S. (1991). Protein turnover: A functional appraisal. Funct. Ecol. 5: 222–233. [Google Scholar]
- He Z., Zhang H., Tewolde H., Shankle M. (2017). Chemical characterization of cotton plant parts for multiple uses. Agric. Environ. Lett. 2: 110044. [Google Scholar]
- Heber U. (1976). Energy coupling in chloroplasts. J. Bioenerg. Biomembr. 8: 157–172. [DOI] [PubMed] [Google Scholar]
- Hill S.A., ap Rees T. (1994). Fluxes of carbohydrate metabolism in ripening bananas. Planta 192: 52–60. [Google Scholar]
- Huber S.C. (1989). Biochemical mechanism for regulation of sucrose accumulation in leaves during photosynthesis. Plant Physiol. 91: 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt W.F., Loomis R.S. (1979). Respiration modelling and hypothesis testing with a dynamic model of sugar beet growth. Ann. Bot. 44: 5–17. [Google Scholar]
- Huntingford C., et al. (2017). Implications of improved representations of plant respiration in a changing climate. Nat. Commun. 8: 1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Obata T., Sulpice R., Fernie A.R., Stitt M. (2015). Quantifying protein synthesis and degradation in Arabidopsis by dynamic 13CO2 labeling and analysis of enrichment in individual amino acids in their free pools and in protein. Plant Physiol. 168: 74–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H., Moraes T.A., Pyl E.T., Schulze W.X., Obata T., Scheffel A., Fernie A.R., Sulpice R., Stitt M. (2017). Growth rate correlates negatively with protein turnover in Arabidopsis accessions. Plant J. 91: 416–429. [DOI] [PubMed] [Google Scholar]
- Jacoby R.P., Millar A.H., Taylor N.L. (2016). Opportunities for wheat proteomics to discover the biomarkers for respiration-dependent biomass production, stress tolerance and cytoplasmic male sterility. J. Proteomics 143: 36–44. [DOI] [PubMed] [Google Scholar]
- Jendresen C.B., Stahlhut S.G., Li M., Gaspar P., Siedler S., Förster J., Maury J., Borodina I., Nielsen A.T. (2015). Highly active and specific Tyr ammonia-lyases from diverse origins enable enhanced production of aromatic compounds in bacteria and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 81: 4458–4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassaw T.K., Donayre-Torres A.J., Antunes M.S., Morey K.J., Medford J.I. (2018). Engineering synthetic regulatory circuits in plants. Plant Sci. 273: 13–22. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. (1978). Futile cycling in glucose metabolism. Trends Biochem. Sci. 3: 171–174. [Google Scholar]
- Kawaguchi R., Williams A.J., Bray E.A., Bailey-Serres J. (2003). Water-deficit-induced translational control in Nicotiana tabacum. Plant Cell Environ. 26: 221–229. [Google Scholar]
- Keeling P.L., Wood J.R., Tyson R.H., Bridges I.G. (1988). Starch biosynthesis in developing wheat grain: evidence against the direct involvement of triose phosphates in the metabolic pathway. Plant Physiol. 87: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khush G.S. (2001). Green revolution: the way forward. Nat. Rev. Genet. 2: 815–822. [DOI] [PubMed] [Google Scholar]
- Kingston‐Smith A.H., Walker R.P., Pollock C.J. (1999). Invertase in leaves: Conundrum or control point? J. Exp. Bot. 50: 735–743. [Google Scholar]
- Körner C. (2015). Paradigm shift in plant growth control. Curr. Opin. Plant Biol. 25: 107–114. [DOI] [PubMed] [Google Scholar]
- Krapp A., David L.C., Chardin C., Girin T., Marmagne A., Leprince A.S., Chaillou S., Ferrario-Méry S., Meyer C., Daniel-Vedele F. (2014). Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 65: 789–798. [DOI] [PubMed] [Google Scholar]
- Krömer S., Stitt M., Heldt H.W. (1988). Mitochondrial oxidative phosphorylation participating in photosynthetic metabolism of a leaf cell. FEBS Lett. 226: 352–356. [Google Scholar]
- Kurimoto K., Day D.A., Lambers H., Noguchi K. (2004). Effect of respiratory homeostasis on plant growth in cultivars of wheat and rice. Plant Cell Environ. 27: 853–862. [DOI] [PubMed] [Google Scholar]
- Lee C.P., Eubel H., Millar A.H. (2010). Diurnal changes in mitochondrial function reveal daily optimization of light and dark respiratory metabolism in Arabidopsis. Mol. Cell. Proteomics 9: 2125–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidreiter K., Heineke D., Heldt H.W., Müller-Röber B., Sonnewald U., Willmitzer L. (1995). Leaf-specific antisense inhibition of starch biosynthesis in transgenic potato plants leads to an increase in photoassimilate export from source leaves during the light period. Plant Cell Physiol. 36: 615–624. [Google Scholar]
- Léran S., Muños S., Brachet C., Tillard P., Gojon A., Lacombe B. (2013). Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot nitrate translocation. Mol. Plant 6: 1984–1987. [DOI] [PubMed] [Google Scholar]
- Li B., Qiu J., Jayakannan M., Xu B., Li Y., Mayo G.M., Tester M., Gilliham M., Roy S.J. (2017b). AtNPF2.5 Modulates chloride (Cl−) efflux from roots of Arabidopsis thaliana. Front. Plant Sci. 7: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Yu M., Du X.Q., Wang Z.F., Wu W.H., Quintero F.J., Jin X.H., Li H.D., Wang Y. (2017c). NRT1.5/NPF7.3 Functions as a proton-coupled H+/K+ antiporter for K+ loading into the xylem in Arabidopsis. Plant Cell 29: 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Nelson C.J., Trösch J., Castleden I., Huang S., Millar A.H. (2017a). Protein degradation rate in Arabidopsis thaliana leaf growth and development. Plant Cell 29: 207–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H., Cho M.H., Bhoo S.H., Hahn T.R. (2013). Pyrophosphate: fructose-6-phosphate 1-phosphotransferase is involved in the tolerance of Arabidopsis seedlings to salt and osmotic stresses. In Vitro Cell. Dev. Biol. Plant 50: 84–91. [Google Scholar]
- Lin S.H., Kuo H.F., Canivenc G., Lin C.S., Lepetit M., Hsu P.K., Tillard P., Lin H.L., Wang Y.Y., Tsai C.B., Gojon A., Tsay Y.F. (2008). Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20: 2514–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis R.S., Amthor J.S. (1999). Yield potential, plant assimilatory capacity, and metabolic efficiencies. Crop Sci. 39: 1584–1596. [Google Scholar]
- Loomis R.S., Williams W.A. (1963). Maximum crop productivity: An estimate. Crop Sci. 3: 67–72. [Google Scholar]
- Louie G.V., Bowman M.E., Moffitt M.C., Baiga T.J., Moore B.S., Noel J.P. (2006). Structural determinants and modulation of substrate specificity in Phe-Tyr ammonia-lyases. Chem. Biol. 13: 1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H.A. (2016). Lignin biosynthesis: Tyrosine shortcut in grasses. Nat. Plants 2: 16080. [DOI] [PubMed] [Google Scholar]
- Masarin F., Gurpilhares D.B., Baffa D.C., Barbosa M.H., Carvalho W., Ferraz A., Milagres A.M. (2011). Chemical composition and enzymatic digestibility of sugarcane clones selected for varied lignin content. Biotechnol. Biofuels 4: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengin V., Pyl E.T., Alexandre Moraes T., Sulpice R., Krohn N., Encke B., Stitt M. (2017). Photosynthate partitioning to starch in Arabidopsis thaliana is insensitive to light intensity but sensitive to photoperiod due to a restriction on growth in the light in short photoperiods. Plant Cell Environ. 40: 2608–2627. [DOI] [PubMed] [Google Scholar]
- Millar A.H., Whelan J., Soole K.L., Day D.A. (2011). Organization and regulation of mitochondrial respiration in plants. Annu. Rev. Plant Biol. 62: 79–104. [DOI] [PubMed] [Google Scholar]
- Miller A.K., Hambly D.M., Kerwin B.A., Treuheit M.J., Gadgil H.S. (2011). Characterization of site-specific glycation during process development of a human therapeutic monoclonal antibody. J. Pharm. Sci. 100: 2543–2550. [DOI] [PubMed] [Google Scholar]
- Morandini P., Salamini F. (2003). Plant biotechnology and breeding: Allied for years to come. Trends Plant Sci. 8: 70–75. [DOI] [PubMed] [Google Scholar]
- Nelson C.J., Millar A.H. (2015). Protein turnover in plant biology. Nat. Plants 1: 15017. [DOI] [PubMed] [Google Scholar]
- Nelson C.J., Alexova R., Jacoby R.P., Millar A.H. (2014). Proteins with high turnover rate in barley leaves estimated by proteome analysis combined with in planta isotope labeling. Plant Physiol. 166: 91–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Quoc B., Foyer C.H. (2001). A role for ‘futile cycles’ involving invertase and sucrose synthase in sucrose metabolism of tomato fruit. J. Exp. Bot. 52: 881–889. [DOI] [PubMed] [Google Scholar]
- Nielsen T.H., Stitt M. (2001). Tobacco transformants with strongly decreased expression of pyrophosphate:fructose-6-phosphate expression in the base of their young growing leaves contain much higher levels of fructose-2,6-bisphosphate but no major changes in fluxes. Planta 214: 106–116. [DOI] [PubMed] [Google Scholar]
- Novaes E., Kirst M., Chiang V., Winter-Sederoff H., Sederoff R. (2010). Lignin and biomass: A negative correlation for wood formation and lignin content in trees. Plant Physiol. 154: 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’sullivan O.S., et al. (2017). Thermal limits of leaf metabolism across biomes. Glob. Change Biol. 23: 209–223. [DOI] [PubMed] [Google Scholar]
- Ort D.R., et al. (2015). Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 112: 8529–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning de Vries F.W.T. (1975). The cost of maintenance processes in plant cells. Ann. Bot. 39: 77–92. [Google Scholar]
- Penning de Vries F.W.T., Brunsting A.H.M., van Laar H.H. (1974). Products, requirements and efficiency of biosynthesis: a quantitative approach. J. Theor. Biol. 45: 339–377. [DOI] [PubMed] [Google Scholar]
- Pfister A., et al. (2014). A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3: e03115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros M.A., Cançado G.M.A., Maron L.G., Lyi S.M., Menossi M., Kochian L.V. (2008). Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: The case of ZmALMT1 - an anion-selective transporter. Plant J. 53: 352–367. [DOI] [PubMed] [Google Scholar]
- Plaxton W.C. (1996). The organization and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 185–214. [DOI] [PubMed] [Google Scholar]
- Plaxton W.C. (2010). Metabolic flexibility helps plants to survive stress. In Plant Physiology and Development, 6th ed., Web essay 12.1, Taiz L., Zeiger E., Møller I.M., Murphy A., eds (Oxford: Sinauer Associates; ), http://6e.plantphys.net/essay12.01.html [Google Scholar]
- Plaxton W.C., Podestá F.E. (2006). The functional organization and control of plant respiration. Crit. Rev. Plant Sci. 25: 159–198. [Google Scholar]
- Poiré R., Wiese-Klinkenberg A., Parent B., Mielewczik M., Schurr U., Tardieu F., Walter A. (2010). Diel time-courses of leaf growth in monocot and dicot species: endogenous rhythms and temperature effects. J. Exp. Bot. 61: 1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J.R., Semenov M.A. (2005). Crop responses to climatic variation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360: 2021–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra A.S., Padmasree K. (2003). Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 8: 546–553. [DOI] [PubMed] [Google Scholar]
- Ramesh S.A., et al. (2015). GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 6: 7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh S.A., Kamran M., Sullivan W., Chirkova L., Okamoto M., Degryse F., McLaughlin M., Gilliham M., Tyerman S.D. (2018). Aluminum-activated malate transporters can facilitate GABA transport. Plant Cell 30: 1147–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven J.A. (1985). Regulation of pH and generation of osmolarity in vascular plants: A cost‐benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol. 101: 25–77. [DOI] [PubMed] [Google Scholar]
- Raven J.A. (2013). RNA function and phosphorus use by photosynthetic organisms. Front. Plant Sci. 4: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Carbo M., Taylor N.L., Giles L., Busquets S., Finnegan P.M., Day D.A., Lambers H., Medrano H., Berry J.A., Flexas J. (2005). Effects of water stress on respiration in soybean leaves. Plant Physiol. 139: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richarme G., Mihoub M., Dairou J., Bui L.C., Leger T., Lamouri A. (2015). Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated Cys, Arg, and Lys residues. J. Biol. Chem. 290: 1885–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.A., Ribas-Carbo M., Yakir D., Giles L., Reuveni Y., Berry J.A. (1995). Beyond SHAM and cyanide: opportunities for studying the alternative oxidase in plant respiration using oxygen-isotope discrimination. Aust. J. Plant Physiol. 22: 487–496. [Google Scholar]
- Ruan Y.L., Jin Y., Yang Y.J., Li G.J., Boyer J.S. (2010). Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 3: 942–955. [DOI] [PubMed] [Google Scholar]
- Sage R.F., Kubien D.S. (2007). The temperature response of C(3) and C(4) photosynthesis. Plant Cell Environ. 30: 1086–1106. [DOI] [PubMed] [Google Scholar]
- Samoilov M., Plyasunov S., Arkin A.P. (2005). Stochastic amplification and signaling in enzymatic futile cycles through noise-induced bistability with oscillations. Proc. Natl. Acad. Sci. USA 102: 2310–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurwater I., Koren M., Lambers H., Atkin O.K. (2002). The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. J. Exp. Bot. 53: 1635–1642. [DOI] [PubMed] [Google Scholar]
- Schuler M.L., Mantegazza O., Weber A.P. (2016). Engineering C4 photosynthesis into C3 chassis in the synthetic biology age. Plant J. 87: 51–65. [DOI] [PubMed] [Google Scholar]
- Segonzac C., Boyer J.C., Ipotesi E., Szponarski W., Tillard P., Touraine B., Sommerer N., Rossignol M., Gibrat R. (2007). Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell 19: 3760–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma T., Dreyer I., Kochian L., Piñeros M.A. (2016). The ALMT family of organic acid transporters in plants and their involvement in detoxification and nutrient security. Front. Plant Sci. 7: 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R., Cheung C.Y.M. (2018). A dynamic multi-tissue flux balance model captures carbon and nitrogen metabolism and optimal resource partitioning during Arabidopsis growth. Front. Plant Sci. 9: 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T., Bibby T.S., Jiang L., Irwin A.J., Falkowski P.G. (2005). Protein interactions limit the rate of evolution of photosynthetic genes in cyanobacteria. Mol. Biol. Evol. 22: 2179–2189. [DOI] [PubMed] [Google Scholar]
- Siebert M., Sommer S., Li S.M., Wang Z.X., Severin K., Heide L. (1996). Genetic engineering of plant secondary metabolism. Accumulation of 4-hydroxybenzoate glucosides as a result of the expression of the bacterial ubiC gene in tobacco. Plant Physiol. 112: 811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboleva A., Schmidt R., Vikhnina M., Grishina T., Frolov A. (2017). Maillard proteomics: Opening new pages. Int. J. Mol. Sci. 18: E2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U., Fernie A.R. (2018). Next-generation strategies for understanding and influencing source-sink relations in crop plants. Curr. Opin. Plant Biol. 43: 63–70. [DOI] [PubMed] [Google Scholar]
- Stitt M. (1998). Pyrophosphate as an alternative energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot. Acta 111: 167–175. [Google Scholar]
- Stitt M., Lunn J., Usadel B. (2010). Arabidopsis and primary photosynthetic metabolism—more than the icing on the cake. Plant J. 61: 1067–1091. [DOI] [PubMed] [Google Scholar]
- Sweetlove L.J., Beard K.F., Nunes-Nesi A., Fernie A.R., Ratcliffe R.G. (2010). Not just a circle: Flux modes in the plant TCA cycle. Trends Plant Sci. 15: 462–470. [DOI] [PubMed] [Google Scholar]
- Tcherkez G., Boex-Fontvieille E., Mahé A., Hodges M. (2012). Respiratory carbon fluxes in leaves. Curr. Opin. Plant Biol. 15: 308–314. [DOI] [PubMed] [Google Scholar]
- Tegeder M., Masclaux-Daubresse C. (2018). Source and sink mechanisms of nitrogen transport and use. New Phytol. 217: 35–53. [DOI] [PubMed] [Google Scholar]
- Trentmann O., Jung B., Neuhaus H.E., Haferkamp I. (2008). Nonmitochondrial ATP/ADP transporters accept phosphate as third substrate. J. Biol. Chem. 283: 36486–36493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman S.D., Wignes J.A., Kaiser B.N. (2017). Root hydraulic and aquaporin responses to N availability. In Plant Aquaporins: From Transport to Signaling, Chaumont F. and Tyerman S.D., eds (Cham, Switzerland: Springer International Publishing; ), pp. 207–236. [Google Scholar]
- Unkovich M., Baldock J., Forbes M. (2010). Variability in harvest index of grain crops and potential significance for carbon accounting: examples from Australian agriculture. Adv. Agron. 105: 173–219. [Google Scholar]
- van der Merwe M.J., Groenewald J.H., Stitt M., Kossmann J., Botha F.C. (2010). Downregulation of pyrophosphate: D-fructose-6-phosphate 1-phosphotransferase activity in sugarcane culms enhances sucrose accumulation due to elevated hexose-phosphate levels. Planta 231: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Collard F., Wiame E., Veiga-da-Cunha M. (2012). Enzymatic repair of Amadori products. Amino Acids 42: 1143–1150. [DOI] [PubMed] [Google Scholar]
- Verbančič J., Lunn J.E., Stitt M., Persson S. (2018). Carbon supply and the regulation of cell wall synthesis. Mol. Plant 11: 75–94. [DOI] [PubMed] [Google Scholar]
- Versaw W.K., Garcia L.R. (2017). Intracellular transport and compartmentation of phosphate in plants. Curr. Opin. Plant Biol. 39: 25–30. [DOI] [PubMed] [Google Scholar]
- Viitanen P.V., Devine A.L., Khan M.S., Deuel D.L., Van Dyk D.E., Daniell H. (2004). Metabolic engineering of the chloroplast genome using the Echerichia coli ubiC gene reveals that chorismate is a readily abundant plant precursor for p-hydroxybenzoic acid biosynthesis. Plant Physiol. 136: 4048–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Cheng Y.H., Chen K.E., Tsay Y.F. (2018). Nitrate transport, signaling, and use efficiency. Annu. Rev. Plant Biol. 69: 85–122. [DOI] [PubMed] [Google Scholar]
- Wege S., Gilliham M., Henderson S.W. (2017). Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 68: 3057–3069. [DOI] [PubMed] [Google Scholar]
- Weiner H., Stitt M., Heldt H.W. (1987). Subcellular compartmentation of pyrophosphate and alkaline pyrophosphatase in leaves. Biochim. Biophys. Acta Bioenerg 893: 13–21. [Google Scholar]
- Wen Z., Tyerman S.D., Dechorgnat J., Ovchinnikova E., Dhugga K.S., Kaiser B.N. (2017). Maize NPF6 proteins are homologs of Arabidopsis CHL1 that are selective for both nitrate and chloride. Plant Cell 29: 2581–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten R., Sederoff R. (1995). Lignin biosynthesis. Plant Cell 7: 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow T.H., Bassuk N.L., Ranney T.G., Reichert D.L. (1992). An improved method for using electrolyte leakage to assess membrane competence in plant tissues. Plant Physiol. 98: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Waters S., Byrt C.S., Plett D., Tyerman S.D., Tester M., Munns R., Hrmova M., Gilliham M. (2018). Structural variations in wheat HKT1;5 underpin differences in Na+ transport capacity. Cell. Mol. Life Sci. 75: 1133–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Fan X., Miller A.J. (2012). Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 63: 153–182. [DOI] [PubMed] [Google Scholar]
- Yadav U.P., Ayre B.G., Bush D.R. (2015). Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: assessing the potential to improve crop yields and nutritional quality. Front. Plant Sci. 6: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York L.M., Silberbush M., Lynch J.P. (2016). Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architectural phenes. J. Exp. Bot. 67: 3763–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Baetz U., Krügel U., Martinoia E., De Angeli A. (2013). Identification of a probable pore-forming domain in the multimeric vacuolar anion channel AtALMT9. Plant Physiol. 163: 830–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.S., Liu M.J., Scheibe R., Selinski J., Zhang L.T., Yang C., Meng X.L., Gao H.Y. (2017). Contribution of the alternative respiratory pathway to PSII photoprotection in C3 and C4 plants. Mol. Plant 10: 131–142. [DOI] [PubMed] [Google Scholar]
- Zrenner R., Salanoubat M., Willmitzer L., Sonnewald U. (1995). Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 7: 97–107. [DOI] [PubMed] [Google Scholar]