Abstract
We evaluated the effects of the non-psychoactive cannabinoid cannabidiol (CBD) on the inflammatory response and recovery of function following spinal cord injury (SCI). Female C57Bl/6 mice were exposed to spinal cord contusion injury (T9-10) and received vehicle or CBD (1.5 mg/kg IP) injections for 10 weeks following injury. The effect of SCI and CBD treatment on inflammation was assessed via microarray, qRT-PCR and flow cytometry. Locomotor and bladder function and changes in thermal and mechanical hind paw sensitivity were also evaluated. There was a significant decrease in pro-inflammatory cytokines and chemokines associated with T-cell differentiation and invasion in the SCI-CBD group as well as a decrease in T cell invasion into the injured cord. A higher percentage of SCI mice in the vehicle-treated group (SCI-VEH) went on to develop moderate to severe (0 – 65.9% baseline thermal threshold) thermal sensitivity as compared with CBD-treated (SCI-CBD) mice. CBD did not affect recovery of locomotor or bladder function following SCI. Taken together, CBD treatment attenuated the development of thermal sensitivity following spinal cord injury and this effect may be related to protection against pathological T-cell invasion.
Keywords: Spinal cord injury, Neuropathic pain, Cannabidiol, Inflammation, T cell
Introduction
Damage to the spinal cord following trauma can lead to motor deficits, changes in sensory sensitivity, and autonomic dysfunction. These deficits result not only from primary insult but also a cascade of reactive changes known as secondary injury. Inflammatory responses such as chemokine and cytokine secretion and immune cell infiltration and/or activation contribute significantly to the development of spinal cord injury as well as to spinal cord injury-associated neuropathic pain (SCI-NP)[1-3]. For example, the incidence of neuropathic pain after spinal cord injury is estimated up to 70% and significantly impacts the patient’s life quality[4, 5]. Inflammatory responses, including the generation of free radicals, proinflammatory cytokines, and chemokines, white blood cell invasion, and activation of resident inflammatory cells, are major components of secondary injury. CXCL-1 and CCL-2 are proinflammatory and can function as chemoattractants for neutrophils and monocytes, respectively, while CXCL9 and CXCL11 may function as Tcell attractants. Recently IL-17-producing T cells have been recognized as contributing to the exacerbation of central nervous system (CNS) injury. Toll-like receptor 2 (TLR2) and TLR4 have also been proposed to function in the regulation of proinflammatory cytokines following spinal cord injury[6]. Interference with the pathophysiological changes that occur during secondary injury offers the opportunity to inhibit the progression of damage that usually develops.
Currently therapeutic options available for treatment of spinal cord injury are extremely limited. The current mainstay of medical therapy for acute injury is high-dose methylprednisolone. However, there is considerable debate as to whether the adverse effects of high-dose steroids outweigh the potential benefits of their use [7-9]. Several of the chemical constituents of Cannabis sativa (aka marijuana) have been shown to possess potent anti-inflammatory and neuroprotective properties, and Cannabis has now been legalized in for medical use in twenty-nine states, with alleviation of symptoms relating to autoimmune and chronic pain disorders as top indications. The phytocannabinoids delta9-tetrahydracannabinol (THC) and cannabidiol (CBD) are the two most abundant and well-studied active ingredients in Cannabis. CBD as a monotherapy is currently in dozens of clinical trials for a wide range of indications from schizophrenia to Crohn’s disease but has yet to be tested in clinical trials for the treatment of spinal cord injury or chronic inflammatory or neuropathic pain. CBD was shown to improve locomotor function in a rat model of cryogenic spinal cord injury[10] and we have recently reported that CBD prevents the development of sensory sensitivity in a mouse model of chemotherapy-induced neuropathic pain. Others have shown anti-neuropathic effects of CBD in a rodent model of diabetic neuropathy and chronic nerve constriction [11-14].
CBD enjoys a rich poly-pharmacology with actions on several substrates within the body relevant to inflammation and pain transmission. While its binding affinity for cannabinoid CB1 and CB2 receptors is negligible, it acts as a direct agonist for serotonin 5-HT1A receptors as well as TRPV1 and glycine channels and is an indirect agonist at adenosine receptors[15-18]. These receptor-mediated actions and possible non-receptor mediated effects can attenuate several pro-inflammatory cellular events such as generation of reactive oxygen and nitrogen species, chemokine and cytokine release, microglial and astrocytic activation, and T cell proliferation[19, 20]. Taken together, these data suggest that CBD will be effective for the treatment of secondary insults following spinal cord injury such as those that are involved in the development of SCI-NP. We hypothesized that treatment with CBD would attenuate sensory alterations and decrease inflammation in the spinal cord of injured mice. To test this hypothesis, we used a spinal cord contusion mouse model to induce injury and treated mice with vehicle or CBD (1.5 mg/kg, IP) intermittently for 10 weeks. Preliminary results from our laboratory showed that across a wide range of doses (1.5 – 20.0 mg/kg) and time courses (intermittent versus daily), the present dosing regimen showed optimal results. The effects of CBD treatment on a range of molecular markers of and inflammation as well as motor and bladder recovery, sensory sensitivity, and were determined. qRT-PCR and microarray analyses were conducted at 48 post-injury and flow cytometry analyses were conducted 2 weeks post-injury based on our previous observations with cannabinoid CB2 agonist effects on inflammation following spinal cord injury[21]. Motor, bladder, and sensory function were measured up to 10 weeks post injury.
Materials and Methods
Animal model of spinal cord injury.
Subjects.
Seven to 9 week old female C57BL/6 mice weighing 16–21 g (Taconic, Hudson, NY) were used. All procedures, interventions, and animal care were done in accordance with the protocol approved by the Temple University Institutional Animal Care and Use Committee, following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were housed for 1 week prior to surgical intervention for acclimation and observation. A light/dark cycle of 12 h was maintained, and the mice were allowed free access to food and water including hydrogel at all times.
Experimental Timeline:
Separate experiments were carried out to determine effects of SCI and CBD on 1) expression of inflammatory markers 48 hours following sham or contusion injury, 2) microglial and T cell numbers in the spinal cord 2 weeks following sham or contusion injury, and 3) assessment of locomotor, bladder, and sensory function for 10 weeks following SCI or sham surgery. These timepoints were chosen based on a large amount of pilot data in our laboratory measuring peak times for changes in cytokine expression, spinal microglial activation and T cell infiltration, recovery of bladder and motor function, and development of sensory sensitivity following SCI in our mouse model.
Surgical procedures.
The mice were weighed and anesthetized via IP injection of ketamine (100 mg/kg) and xylazine (20 mg/kg). Once under anesthesia, the back hair was clipped and protective eye gel was applied. Body temperature was maintained at 37±0.5°C during the procedure and recovery period with a heating pad and lamp. The ribs were used to localize the T8–T10 laminae. Using a combination of sharp and blunt dissection, the paraspinal musculature was dissected free from the laminae between T8 and T10. The laminectomies were performed at the T8 and T9 levels and adequate length of spinal cord was exposed. The mice were then transferred to the Infinite Horizons (IH) impactor device (PSI Inc., Lexington, KY), where they were suspended via modified Adson forceps clamped to the lateral aspect of the vertebra above and below the level of the laminectomy. The impactor tip was positioned directly above the exposed dura, and then raised to a height of 3 mm. The device was set to deliver a 60-kdyn force to the spinal cord. The actual force, displacement, velocity, and injury time was recorded. The spinal musculature and skin was closed with clips.
Postoperative care and CBD treatment.
After contusion surgery, the mice were placed in a recovery cage under a heating lamp until they were well recovered from anesthesia. All cages were kept on a heating pad on the first postoperative night. The mice were also given subcutaneous injections of fluid (0.9% NSS; 0.5 mL) and antibiotics (enrofloxacin, 2.5 mg/kg) once daily for the first 3 postoperative days. The mice had their bladders emptied twice daily via the Crede maneuver until recovery of autonomic function.
Cannabidiol (CBD) was dissolved with a 1:1:18 ratio of anhydrous ethanol, cremophor, and 0.9% saline. Mice used for behavioral analyses were in study for 10 weeks post-injury. The mice were randomly divided into vehicle-treated (SCI-VEH; n=7), CBD-treated (SCI-CBD; 1.5 mg/kg CBD; n=8), and sham-treated (SHAM; n=10/group) groups. The SCI-VEH and SCI-CBD-treated mice were given injections 1 hr and 24 hr post injury, and on day 3 and then twice a week by intraperitoneal injection (IP) after surgery, until euthanasia. Additional mice were treated as above for RT-PCR analyses, and were euthanized 48 hr post injury (n=6/group). Lastly, a third set of mice were treated as above and used for flow cytometry (n=6/group) and were euthanized 2 weeks post-injury. The investigators were blinded to treatment during all behavioral scoring.
RT2 Profiler PCR array.
Whole spinal cords were harvested 48 hours after surgery. Three spinal cords were pooled together and homogenized. Homogenized spinal cord tissue underwent mRNA extraction and reverse transcription. Mouse cytokines and chemokines RT2 Profiler PCR array (Qiagen, Valencia, CA) was performed to detect gene expression. Briefly, 20 μL of cDNA was diluted with 91 μL of RNase free H2O. Then 102 μL of diluted cDNA was mixed with 1350 μL of 2×SYBR green-containing PCR master mix and 1248 μL of RNase free H2O to prepare 2700 μL of experimental mixture. Twenty five μL of the mixture was loaded in each well of a 96-well plate precoated with primers for different genes. The PCR array was performed using the StepOnePlus Real-Time PCR System (AB Applied Biosystems). The cycling conditions were 15 sec at 95°C, and 1 min at 60°C for 40 cycles, followed by a melting point determination of dissociation curves. Cycle threshold values were determined by automated threshold analysis, and the results were standardized based on 5 housekeeping genes, including glyceraldehyde-3 phosphate dehydrogenase (GAPDH), β2 microglobulin (B2m), glucuronidase beta (Gusβ), heat-shock protein 90 alpha (Hsp90sb1), and β-actin.
Quantitative Real time PCR.
Whole spinal cord tissue was homogenized in Trizol reagent and underwent mRNA extraction, reverse transcription and qRT-PCR to detect gene expression. Genes of interest were selected from those that showed robust changes between vehicle and CBD treated SCI mice from the microarray study described above, in combination with genes that were significantly changed by SCI and CB2 receptor agonist treatment reported in Adhikary et al 2011. The expression of Il23a(p19), IL23R, IFNγ, CXCL-9, CXCL-11, Nos2, TLR4, TNFα, IL-6, IL-10, and Arg1, were detected by SYBR Green-based qRT-PCR as previously described[22]. Real-time PCR was performed using StepOnePlus Real-Time PCR System (AB Applied Biosystems). The following primers were used: Il23a(p19) sense, 5'-TGCTGGATTGCAGAGCAGTAA-3' and antisense, 5'-ATGCAGAGATTCCGAGAGA-3'; ′; IL23R: sense 5′-ACATTGGACTTTTGTCGGGAA-3′ and antisense 5′-AAAATCGGCAACATG-3′; Ifnγ sense, 5’AGCTCATCCGAG and antisense 5’-GCTTCCTGAGGCTGGATACC-3’; CXCL-9: sense 5′-CAAAATTTCATCACGCCCTT and antisense 5′-CCAGACAGCTGTTGTGCATT-3′; CXCL-11: sense 5′-GGGCGCTGTCTTTGCATC and antisense 5′-AAGCTTTCTCGATCTCTGCCAT-3; Nos2 sense, 5′-CGCAGCTGGGCTGTACAA-3′ and antisense, 5′-TGATGTTTGCTTCGGACATCA-3′; Il10 sense, 5′-CCTGGTAGAAGTGATGCCCC-3′ and antisense, 5′-TCCTTGATTTCTGGGCCATG-3′; TLR4 sense 5’GGACCTTACCGGGCAGAAG-3’ and antisense 5’-ACCCCTGGAAAGGAAGGTGT-3’; Arg1 sense, 5′-GGAAGACAGCAGAGGAGGTG-3′ and antisense 5′-TATGGTTACCCTCCCGTTGA-3′; β-actin sense, 5'-TCCACCACCACAGCTGAGAGG-3' and antisense, 5'-CAGCTTCTCTTTGATGTCACG-3'. The expression level of each gene is indicated by the number of cycles needed for the cDNA amplification to reach a threshold. The amount of DNA is calculated from the number of cycles by using standard curves and the results were normalized to β-actin.
Flow cytometry.
Spinal cord injury mice were treated with Vehicle or CBD for 2 weeks. The mice were anesthetized with 20 μl of mix of ketamine HCl and xylazine and perfused through the left cardiac ventricle with 30 ml of HBSS containing 2mM EDTA. The tissue was homogenized by passing through 18-gauge needle repeatedly after spinal cords and spleens were isolated from animals. Spinal cord tissue and spleen was digested in 5 ml HBSS containing 0.5 mg/ml DNAse I and 0.25 mg/ml Liberase for 45 min at 37°C with shaking, followed by blocking solution (10% FCS, 10 mM EDTA in HBSS). The spinal cord tissue was pelleted and resuspended in 10 ml of 30% isotonic Percoll (diluted with 10x HBSS and distilled water), underlaid with 5 ml of 70% isotonic Percoll. Mononuclear cells were isolated from the 30/70 interphase after gradient centrifugation. Cells were washed with RPMI 1640 medium and counted. FACS analysis was performed to detect CD45+ and CD11b+, and CD4+ cells. Splenocytes were prepared by eliminating erythrocytes with red blood cell lysis buffer according to the manufacturer’s protocol (eBioscience). Then splenocytes were counted and underwent FACS analysis to detect CD11b+ cells and CD3+ cells.
Reagents.
APC-conjugated anti-mouse CD45, PE-conjugated anti-mouse CD11b, and FITC-conjugated anti-mouse CD3 were purchased from BD PharMingen (San Diego, CA). Trizol reagent was purchased from Invitrogen Corporation (Carlsbad, CA). Percoll was purchased from GE Healthcare (Uppsala, Sweden). Red blood cell lysis buffer was purchased from eBioscience (San Diego, CA).
Motor function evaluation.
The mice were evaluated for motor function recovery using the 9-point Basso Mouse Scale (BMS), an open-field assessment of locomotion. Each mouse was evaluated on postoperative days 1, 3, 7 days and once weekly thereafter for 10 weeks.
Autonomic function evaluation.
All mice had autonomic impairment with urine retention following SCI. To relieve their bladders and to assess autonomic function recovery, urine was expressed twice daily via suprapubic pressure (Credé maneuver), and urine mass was determined. The mice were considered to have recovered autonomic function once the total urine mass expressed was less than 500 mg/day for 3 consecutive days.
Thermal sensitivity test.
Thermal sensitivity of the right hind paw was assessed using a standard rodent thermal algesia meter (Hargreaves Apparatus, 37370, Ugo Basile). Mice were habituated to the compartments for 15 minutes. The infrared source (intensity 25) was then placed under the glass floor and aimed at the center plantar surface of the right hind paw. When the mouse withdrew the hind paw the latency time is automatically recorded. Each mouse was tested 3 times with an inter-test interval of 3 minutes, and average withdrawal latency is determined. If there is no observed response in 22.3 second, the trial was ended automatically to avoid tissue damage.The plantar test in right hind paw in mice was performed before surgery and in 4, 6, and 10 week after surgery. The latency to respond for each mouse after injury was compared with individual baseline before surgery to obtain percent baseline response levels. Based on the distribution of thermal sensitivity scores in the vehicle-treated injured mice, response levels were also divided into 2 categories: mild, or thermal sensitivity scores that represented a ≥66 - 100% baseline threshold response, and moderate to severe, or thermal sensitivity scores that represent a 0 – 65.9% baseline threshold response. Based on our preliminary and present experimental groups, our vehicle-treated SCI mice divide roughly in half into these two categories.
Mechanical allodynia test.
Mechanical sensitivity of the right and left hind paws was measured using standard von Frey filaments (Exacta Touch Test). A baseline measure for each paw was taken prior to inducing the spinal cord injury. Mice were placed in confined chambers on top of an elevated metal grid (Bioseb In Vivo Research Instruments) and acclimated to the environment for 30 minutes prior to testing. Two weeks post spinal cord injury mechanical allodynia testing was conducted once a week for 8 weeks. The up down method described in Chapman et al 1994 was used with filaments ranging from 0.16 – 2.0 grams of force[23]. Pressure was applied to the right hind paw with enough force to create a “C” shape and this pressure was applied for 6 seconds for each filament used. The threshold to respond for each hind paw after injury was compared with individual baseline data before surgery to obtain percent baseline response levels. In addition, because several mice showed both decreased and increased mechanical sensitivity time and between left and right paw measurements, percent baseline changes for each mouse at each time point were calculated for their more sensitive paw measurement and for their less sensitive paw measurement.
Statistics analysis.
Results are summarized as mean ± SEM. Comparisons between two groups (flow cytometry) were done using Student t test whereas comparisons among multiple groups were performed using mixed-effects linear regression or mixed-effects logistic regression/generalized estimating equation (GEE) analysis with Tukey-Kramer multiple comparison post-test adjustments to take the longitudinal correlation in the data into account. Treatment, time, and their interaction terms were included in the regression models whenever appropriate. P-values less than 0.05 were considered statistically significant. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used for all the data analyses.
Results
Impact results.
For the SCI-VEH group, the average kDyn of force measured at time of impact was 63.57 ± 1.90 and the average displacement was 375 ± 29.43 microns. For the SCI-CBD-treated group, the average kDyn of force measured at time of impact was 62.38 ± 1.83 and the average displacement was 363.38 ± 20.54 microns. Therefore the injuries experienced by both groups were well-matched in force, displacement, and deviation from the mean.
SCI-CBD significantly attenuated increased cytokine expression in the injured spinal cord.
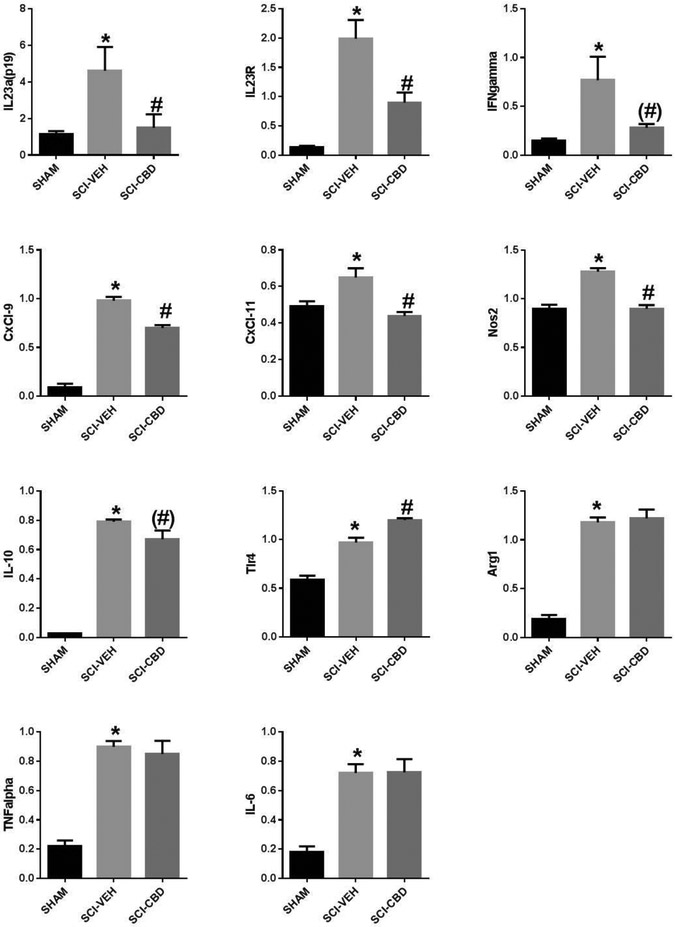
PCR array analysis of spinal cords harvested 48 hr post-injury in vehicle- and CBD-treated mice (Table 1) was performed to measure changes in gene expression of 84 key genes mediating the inflammatory response. CBD treatment was associated with the down-regulation of several chemokines and interleukins, including Ccl11, Cxcl22, Cxcl9, Xcl1, IL12b, and IL17a, and was associated with increased expression of Ccl1, Ccl12, Ccl20, Ccl24, and IL9 (Table 1). Some gene expression changes were confirmed by RT-PCR and other targets of interest were included (Figure 1). One-way ANOVA with Tukey’s multiple comparison post-test adjustments showed that SCI-VEH significantly increased expression of IL-23 (p=0.03) and its receptor (p<0.0001), INFγ (p=0.02), CxCl-9 (p<0.0001), CxCl-11 (p=0.01), iNOS (p<0.0001), TLR-4 (p<0.0001), IL-10 (p<0.0001), Arg1 (p<0.0001), TNFα (p<0.0001), and IL-6 (p<0.0001)as compared with SHAM (significance indicated by * on figures). SCI-CBD significantly decreased the expression of IL-23 (p=0.05) and its receptor (p=0.007), CXCL-9 (p<0.0001), CXCL-11 (p=0.001), and iNOS (p<0.0001), and significantly increased the expression of TRL4 (p=0.001) as compared with SCI-VEH mice (significance indicated by # on figures). CBD treatment did not statistically significantly change INFγ (p = 0.07, Arg-1, IL10 (p =0.06) or IL6 expression compared to SCI-VEH.
Table 1.
Microarray analysis of gene regulation in SCI-VEH- versus SCI-CBD mice 48 hr after spinal cord injury. The genes shown in the table illustrate the expression of 9 genes upregulated by CBD treatment ≥ 4-fold and the expression of 9 genes downregulated by CBD treatment ≥ 4-fold out of 84 key secreted proteins central to the immune response.
| Genes upregulated by CBD treatment |
Fold difference | Genes down- regulated by CBD treatment |
Fold difference |
|---|---|---|---|
| Ccl1 | 13.4064 | Cxcl11 | −9.6237 |
| Ccl12 | 6.2994 | Cxcl22 | −15.7553 |
| Ccl20 | 22.2876 | Cxcl9 | −9.2118 |
| Ccl24 | 10.2979 | Il2 | −5.6824 |
| Cxcl13 | 4.4604 | Ifng | −10.152 |
| Il5 | 6.3942 | Il1b | −6.1986 |
| Il9 | 14.2601 | Il17a | −9.4012 |
| Mstn | 4.3824 | Il12b | −8.4049 |
| Tnfsf10 | 4.6432 | Xcl1 | −29.057 |
Figure 1. SCI-CBD significantly attenuated increased cytokine expression in the injured spinal cord.
RT-PCR 48 hr following injury showed that SCI-VEH mice had increased expression of IL-23, the IL-23 receptor, IFNγ, CxCl-9, CxCl-11, iNOS, TLR-4, IL-10, TNFα, IL-6, and Arg1 as compared with SHAM mice (p < 0.05, indicated by asterisks on figure). SCI-CBD significantly reduced expression of the cytokine IL-23p19 subunit, its receptor IL-23r, the chemokines CXCL-9 and CXCL-11, IFNγ, and iNOS and significantly increased TLR-4 expression compared with the SCI-VEH mice (p < 0.05 as indicated by pound sign on figure). In contrast, there were no significant effects of SCI-CBD versus SCI-VEH differences in IL-10, TNFα, IL-6 and Arg-1 expression. (n=6/group).
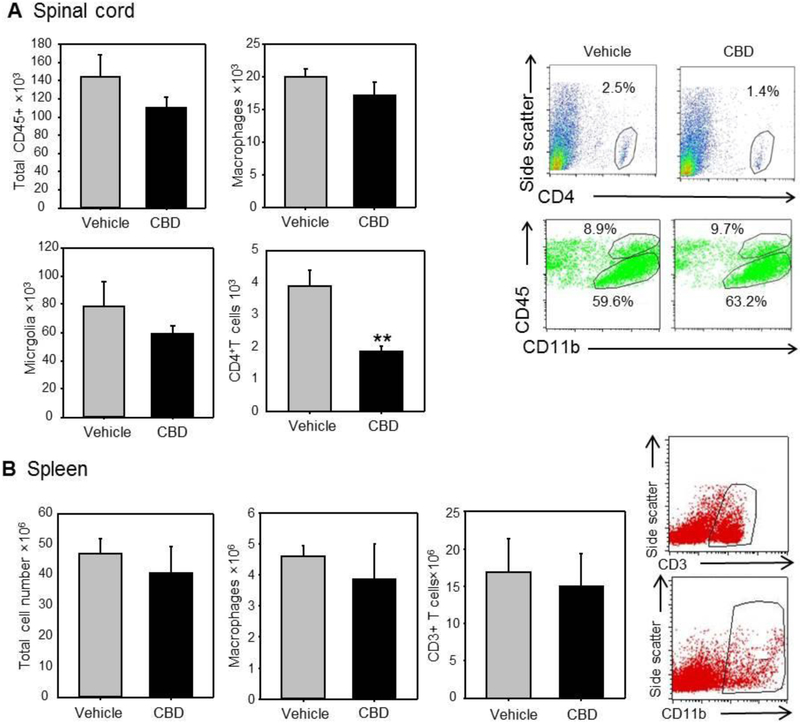
CBD significantly reduced CD4+ T cell numbers but not macrophage or microglia number in the injured spinal cord.
CBD treatment did not significantly decrease macrophage (CD45+high/CD11b+), microglia (CD45+intermediate/CD11b+), or total CD45+ cell populations in the spinal cord compared to vehicle-treated spinal cord-injured mice. CBD treatment did significantly decrease the total number of CD4+ T cells in the injured cord compared with vehicle treatment (Figure 2A). To ensure that the changes in cellular invasion were not the result of a systemic disease, total cell, macrophage, and CD3+ T cell numbers in the spleens of CBD- and vehicle-treated animals were compared. The results showed there was no significant difference in cell population between the two treatment groups (Figure 2B).
Figure 2. SCI-CBD significantly decreased CD4+ T cell population in the injured spinal cord, but does not significantly alter microglial or macrophage populations.
Spinal cords were harvested 2 weeks following SCI-VEH or SCI-CBD treatment. SCI-CBD did not significantly decrease total CD45+ population (Figure 5A top left), microglia (bottom left), and macrophage (top right) population, but did significantly decrease CD4+ T cell population (bottom right). There were no significant differences in total spleen cell, T cell, or macrophage numbers in the spleens of CBD-treated versus vehicle-treated animals (Figure 5B) (n=6/group; tissues from three mice were pooled for each run, and the results from the two runs were averaged).
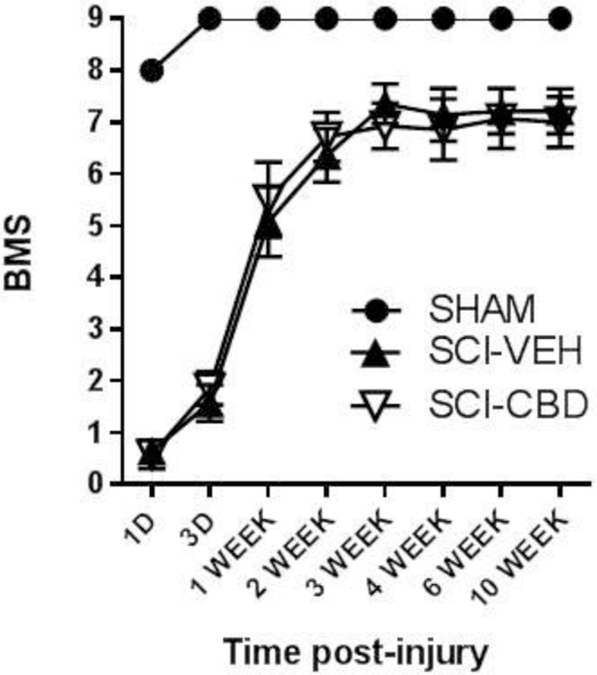
CBD did not significantly improve motor or bladder function following spinal cord injury.
There was no statistically significant effect of SCI-CBD treatment on BMS score when compared to the SCI-VEH group [F(1,13)<1.0, n.s.]. There was an overall main effect of time [F(7, 91) = 136.5, p <0.0001], and no significant interaction [F(7, 91) = 1.1, n.s.] (Figure 3). CBD treatment did not increase the number of mice who recovered bladder function by 4 weeks post-spinal cord injury (70.4% for SCI-VEH, 68.8% for SCI-CBD group).
Figure 3. SCI-CBD did not significantly affect motor function following spinal cord injury (Basso mouse scale; BMS).
Locomotor recovery was assessed using the BMS. BMS scores for mice in each treatment group at each time point were averaged (±SEM). Mixed-effects linear regression showed no statistically significant effect of SCI-CBD as compared with SCI-VEH.
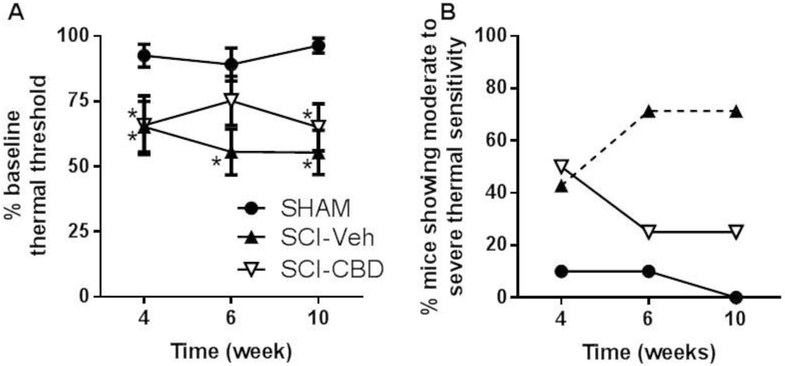
CBD treatment leads to less severe thermal sensitivity after spinal cord injury.
Following injury, median percent thermal threshold scores compared to baseline were approximately 50% by week 10 post-injury in SCI-VEH mice and approximately 70% in SCI-CBD mice. Mixed-effects linear regression showed a significant main effect of treatment [F(2, 22) = 20.75, p<0.0001], but no significant main effect of time [F(2, 44) < 1, n.s.] and no significant interaction between treatment and time [F(4, 44) = 1.17, n.s.]. Tukey’s multiple comparison post-tests showed that at Week 4, both SCI-VEH and SCI-CBD mice were significantly more sensitive compared with SHAM mice, at Week 6 only SCI-VEH mice were significantly different from SHAM mice, and at Week 10 both SCI-VEH and SCI-CBD mice showed significantly more sensitivity compared with SHAM mice (Figure 4A). As described in the methods section, because we observe a range of the severity of thermal sensitivity following SCI, we also analyzed the data by generating two sensitivity categories (“mild” = ≥66 - 100% baseline thermal threshold response and “moderate to severe” = 0 – 65.9% baseline thermal threshold response) and calculating the percentage of animals which fell into each category by treatment group and time point. Approximately 70% of SCI-VEH mice went on to develop moderate to severe thermal sensitivity that progressed in severity between 4 to 10 weeks post-impact (similar to what has been reported previously by Nesic et al 2005, while only 25% of SCI-CBD mice developed moderate to severe thermal sensitivity (Figure 4B) [24]. GEE/mixed-effects logistic regression analysis showed a significant effect of treatment (p=0.01) but no significant effect of time (p=0.85). There were too few observations to analyze the data for a significant interaction. Post-test analysis showed that vehicle treated mice had a significantly higher proportion of “moderate to severe” than the sham injured mice across time (p<0.01). SCI-CBD treated mice were not significantly different from SHAM mice across time.
Figure 4. CBD treatment attenuates the development of thermal sensitivity in the hind paw following spinal cord injury.
Mice received sham or T9-10 contusion injury and treated with vehicle (SCI-VEH) or 1.5 mg/kg CBD (SCI-CBD) 1h pre and 24h post injury and twice weekly thereafter for 10 weeks. Thermal sensitivity of the right hindpaw was measured using the plantar test. (A) The percent baseline latency scores for each treatment group (SHAM, n=10; SCI-VEH, n=7; SCI-CBD, n=8) at each time point (4, 6, and 10 weeks post-injury) were averaged (±SEM). Mixed-effects linear regression revealed a significant treatment effect; * represents adjusted p<0.05 as compared to sham control using Tukey-Kramer’s multiple comparison method. (B) The percentage of mice in each treatment group which displayed moderate to severe changes in thermal sensitivity (thermal sensitivity scores that represent a 0 – 65.9% baseline response) is depicted. Mixed-effects logistic regression analysis showed a significant overall treatment effect, revealing a statistically significant difference between SHAM and SCI-VEH groups (p<0.05, indicated by dotted line).
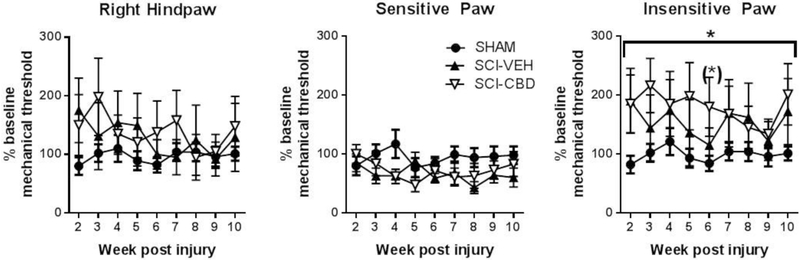
The T9-10 SCI contusion mouse model was not significantly associated with increased mechanical sensitivity. Unlike what was observed for thermal sensitivity, spinal cord injured mice did not develop significant sensitivity to touch (Figure 5A, data for right paw shown for consistency with thermal sensitivity data). In fact, although a weak trend toward reduced sensitivity in the SCI-VEH and SCI-CBD groups compared with the SHAM group was observed during the first half of the 10 week study, mixed-effects linear regression analysis revealed no statistically significant main effect of treatment [F(2,22) = 0.98, p=0.39], and no statistically significant main effect of time [F(8,175) = 1.03, ns], and no statistically significant interaction between treatment and time [F(16,175) =1.17, ns]. As mentioned in the Methods and depicted in Figure 5A, the data were variable, reflecting both increases and decreases in mechanical sensitivity, sometimes in the same mouse at the same time point between the right and left paws. Therefore, we also analyzed the more sensitive and less sensitive values obtained by mice at each time point Figure 5B). When the more sensitive paw values were averaged from each animal at each time point, it was observed that SCI-VEH and SCI-CBD mice seemed to display higher sensitivity to touch than SHAM mice. Mixed-effects linear regression analysis revealed a marginally significant main effect of treatment [F(2,22) = 2.42, p=0.11], and no statistically significant main effect of time [F(8,175) = 1.05, ns], and no statistically significant interaction between treatment and time [F(16,175) =1.16, ns]. Tukey-Kramer’s post-tests showed (marginally) significant differences between CBD and sham-injured groups at week 4 (p=0.08) and between vehicle and sham-injured groups at week 4 (p=0.06), week 6 (p=0.03), and week 8 (p=0.02). When the less sensitive paw values were averaged, it was observed that SCI-VEH and SCI-CBD mice displayed less sensitivity to touch than SHAM mice. Mixed-effects linear regression analysis revealed a statistically significant main effect of treatment [F(2,22) = 3.70, p=0.04], and no statistically significant main effect of time [F(8,175) = 1.32, ns], and no statistically significant interaction between treatment and time [F(16,175) = 0.78, ns]. Tukey-Kramer’s post-tests showed marginally significant differences between SCI-CBD and SHAM groups at week 6 (p=0.09), and marginally significant differences overall among the three groups at week 2 (p=0.03), week 5 (p=0.07), and week 6 (p=0.07).
Figure 5. The T9-10 SCI contusion mouse model was not significantly associated with increased mechanical sensitivity.
Mechanical sensitivity of the right and left hind paw was measured using the Von Frey test. The percent baseline sensitivity scores from the right paw for each treatment group at each time point (4, 6, and 10 weeks post-injury) were averaged (±SEM). For the right hindpaw, which was measured for the thermal sensitivity studies, mixed-effects linear regression revealed no overall significant effect of treatment, time or their interaction (Figure 2A). The more sensitive percent baseline scores for each mouse (left vs right paw) were also averaged and statistically compared between groups and across time (Figure 2B), and mixed-effects linear regression revealed a marginally significant overall treatment effect. The less sensitive percent baseline scores for each mouse (left vs right paw) were also averaged and statistically compared between groups and across time (Figure 2C), and mixed-effects linear regression revealed a significant overall treatment effect (p < 0.04 indicated by bracket and asterisk) and a marginally significant difference between SHAM and SCI-CBD on Week 6 (p = 0.09 indicated by asterisk above data point) with multiple comparison post-test adjustments.
Discussion
In the present study we demonstrated that following spinal cord contusion injury in mice, CBD treatment was associated with a decrease in several pro-inflammatory cytokines and chemokines associated with secondary injury following damage to the spinal cord and with the development of neuropathic pain. CBD also significantly attenuated the presence of CD4+ T cells, but not CD45+ macrophages or microglia, within the damaged spinal cord. These neuroimmune outcomes were associated with a decrease in thermal sensitivity in these mice. Results also showed that changes in mechanical sensitivity in this mouse model of thoracic contusion injury were variable even between the left and right hind paw of an individual mouse, with increases and decreases in sensitivity observed. There was a trend toward statistical significance for the effect of SCI, regardless of vehicle or CBD treatment, to increase sensitivity, while there was a significant effect of SCI on insensitivity with a trend toward SCI-CBD producing the most mechanical insensitivity. CBD treatment also did not improve motor or bladder function.
As neuroinflammation is associated with SCI and SCI-NP, we determined the effects of CBD treatment on immune markers in SCI mice 48 hr post injury. Microarray analysis (Table 1) showed that CBD downregulated or upregulate by several fold, a multitude of key chemoattractant factors and cytokines. qRT-PCR results similarly showed that CBD decreased IL-23 and its IL23 receptor and reduced expression of CXCL-9 and CXCL-11, IFNγ and iNOS. No significant reductions in TRL4 receptor, Arg-1, and IL-10 expression were observed. Taken together, these results suggest that CBD may attenuate neuroinflammation by suppressing the expression of molecules involved in immune cell communication, activation, migration and cross-talk with neuronal populations.
We and others have demonstrated that resident microglia can be activated chronically after spinal cord injury in both experimental animals and patients [21, 25-27]. Moreover, there is a growing literature implicating spinal microglial activation in the development of neuropathic pain by enhancing the hyperactivity of spinal nociceptive neurons and promoting central sensitization [28-31]. In addition, others have reported in alternate models of neuropathic pain that CBD’s anti-neuropathic effects co-occur with a reduction in microglial activation [13, 14, 32]. Some changes in cytokine and chemokine expression following CBD treatment observed in the present study are indicative of alterations in macrophage/microglial activation, such as Il1β, IL12β, and IL23α. However, expression of other common markers of microglial activity remained unchanged with CBD treatment, including the TRL4 receptor, Arg-1, TNFα, IL6, and IL10 expression. This observation is further substantiated by the flow cytometry results showing no significant decrease in microglial and macrophage populations within the injured spinal cord in CBD-treated vs vehicle-treated mice. However it must be noted that while microglial number was not reduced by CBD treatment, this does not preclude a possible effect of CBD on the functionality of these cells following injury.
A major function of activated microglial and macrophages following injury is the recruitment of lymphocytes such as T cells. Indeed in contrast to the microglia and macrophage results, flow cytometry revealed a significant reduction in CD4+ T cells following treatment with CBD. In agreement, a majority of mRNA expression changes observed with CBD in this study implicate alterations in T cell activation and migration, including Xcl-1, Ifn-γ, Ccl-9, Ccl11, CcL-20, Ccl-22, lL-17, IL-12β, IL-23 and its receptor. In addition to microglia and macrophages, T cells have also been strongly implicated in the secondary inflammatory response following spinal cord injury [33-35]. More recently T cells have been suggested to play an essential role in SCI-NP, although the underlying mechanisms remained understudied [36-38]. A caveat to our results regarding this is that we measured T cell infiltration into the spinal cord at 2 weeks following injury, as this is when we have observed peak T cell numbers in the injured cord, while SCI-NP tends to development later than this 2 week time point. Future studies are required to determine the specific role of T cell infiltration on subsequent development of SCI-NP. Perhaps most relevant to the present results and the preclinical modeling of SCI in general is the reported differential role that microglia versus lymphocytes may play in the modulation of neuropathic pain in male and female models. Of course this is especially important to consider given the heavy reliance on female rodent models for SCI studies. Taken together, these data suggest that CBD may either decrease the release of T-cell chemo-attractant molecules from cells such as microglia, monocytes, and dendritic cells, or act directly on T cells to inhibit activation and/or migration. Again, while we did not see a significant effect of CBD treatment on microglial and macrophage number, we cannot rule out that CBD alters the phenotype of these cells to regulate their functionality. However, there is mounting evidence from elegant cell culture studies that CBD application can directly alter T cell gene expression and phenotype [20, 39].
In our spinal cord contusion mouse model, we observed a range in the degree of thermal sensitivity developed across vehicle-treated SCI subjects, and analysis of mean % baseline thermal threshold scores showed this variability, with a trend toward CBD attenuating the development of thermal sensitivity following SCI. Based on this wide distribution, we also analyzed our data by dividing subjects into 2 categories: mild, or thermal threshold scores that represented a ≥66 - 100% baseline response, and moderate to severe, or thermal threshold scores that represent a 0 – 65.9% baseline response. As seen in Figure 1B, the percentage of mice that developed moderate to severe thermal sensitivity is similar between vehicle- and CBD-treated SCI at week 4. However, at weeks 6 and 10, approximately 70% of vehicle-treated mice show moderate to severe sensitivity, while only 20% of CBD-treated mice do.
In contrast to effects on thermal sensitivity, we found little evidence of the development of below-level mechanical sensitivity in our model. Specifically, when compared to sham-injured mice, mice exposed to SCI showed an increase in mechanical threshold indicative of a loss of tactile sensation, with a trend toward CBD producing the most mechanical insensitivity. Mechanical allodynia is a common observation in rats following contusion injury of the spinal cord, but a review of literature shows a more complex range of outcomes in mouse models. Even among studies that focus on contusion injury of the thoracic T9-12, resultant effects on tactile sensitivity vary across reports. For example, Hoschouer et al 2009 reported no changes in mechanical sensitivity following contusion injury to the thoracic region, while Chen et al 2012 reported tactile allodynia in their model[40, 41]. Moreover, insensitivity has been reported at early time points and hypersensitivity at later time points, and hypersensitivity to thin filaments but hyposensitivity to thicker filaments[42, 43]. Taken together the presence and direction of tactile changes following spinal contusion injury in mice is variable across laboratories and likely relates to the level and nature of damage to the dorsal column pathway transmitting ascending mechanical touch information.
While CBD treatment was associated with less severe thermal sensitivity, this was not accompanied by improvements in motor and bladder function recovery that we have previously reported with treatment of the cannabinoid CB2 receptor agonist O-1966 [21]. These results emphasize the very complex pathophysiological mechanisms underlying SCI and SCI-NP. Clearly, both motor and sensory pathways are damaged in this model and our data suggest that treatment with CBD preferentially protects from dysregulation of nociceptive systems over attenuation of/ repair to damaged touch or motor pathways.
In conclusion, our data suggest that treatment with CBD can mitigate the development of thermal sensitivity following spinal cord injury, and that these effects may in part due to a suppression of release of cytokines, chemokines, and other signaling molecules involved in T cell activation and recruitment into the spinal cord. In addition, these effects seem largely separate from improvements in motor and bladder function, suggesting that mechanisms involved in the development of neuropathic pain following spinal cord injury are not identical to those that underlie deficits in motor and autonomic function. Further research is necessary in vivo to determine the initial site(s) of action for CBD necessary to attenuate the development of neuropathic pain following spinal cord injury.
Highlights.
The non-psychoactive cannabinoid cannabidiol (CBD) significantly decreased pro-inflammatory cytokines and chemokines associated with T-cell differentiation and invasion in mice following spinal cord injury (SCI).
CBD also decreased T cell invasion into the injured cord.
A higher percentage of SCI mice in the vehicle-treated group went on to develop moderate to severe thermal sensitivity as compared with CBD-treated mice.
These anti-inflammatory effects of CBD were not associated with improved recovery of locomotor or bladder function following SCI.
Acknowledgements:
This work was funded by Department of Defense Award W81XWH-14-1-0389 (SJW) and supported by P30 DA013429 (EMU)
Abbreviations
- BMS
Basso Mouse Scale
- CBD
Cannabidiol
- SCI
spinal cord injury
- SCI-NP
spinal cord injury neuropathic pain
- TLR
Toll like receptor
- THC
delta9-tetrahydracannabinol
Footnotes
Author Disclosure Statement: No competing financial interests exist for any of the manuscript authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hongbo Li, Center for Substance Abuse Research, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, hongbo.li@temple.edu, ph 215-707-6112, fax 215-707-5307.
Weimin Kong, Microbiology and Immunology Department, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, kongw@mail.med.upenn.edu, ph 215-707-6112, fax 215-246-4090.
Christina R. Chambers, Center for Substance Abuse Research, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, christinachambers@yahoo.com, ph 215-707-6112, fax 215-707-5307.
Daohai Yu, Department of Clinical Sciences, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, dyu@temple.edu, ph 215-707-6525, fax 215-707-3160.
Doina Ganea, Microbiology and Immunology Department, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, doina.ganea@temple.edu, ph 215-707-9921, 215-707-7788.
Ronald F. Tuma, Center for Substance Abuse Research, Department of Physiology, Lewis Katz School of Medicine, Temple University, Philadelphia PA 19140, tumarf@temple.edu, ph 215-707-5485, 215-707-5307.
Sara Jane Ward, Center for Substance Abuse Research, Department of Pharmacology, Lewis Katz School of Medicine, Temple University, Philadelphia PA, saraward@temple.edu, ph 215-707-5485, 215-707-5307.
References
- 1.White FA, Bhangoo SK, and Miller RJ, Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov, 2005. 4(10): p. 834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popovich PG, Wei P, and Stokes BT, Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol, 1997. 377(3): p. 443–64. [DOI] [PubMed] [Google Scholar]
- 3.Gal P, et al. , Chemokines as possible targets in modulation of the secondary damage after acute spinal cord injury: a review. Cell Mol Neurobiol, 2009. 29(6-7): p. 1025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yezierski RP, et al. , Excitotoxic spinal cord injury: behavioral and morphological characteristics of a central pain model. Pain, 1998. 75(1): p. 141–55. [DOI] [PubMed] [Google Scholar]
- 5.Siddall PJ, et al. , A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain, 2003. 103(3): p. 249–57. [DOI] [PubMed] [Google Scholar]
- 6.Kigerl KA, et al. , Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem, 2007. 102(1): p. 37–50. [DOI] [PubMed] [Google Scholar]
- 7.Coleman WP, et al. , A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord, 2000. 13(3): p. 185–99. [DOI] [PubMed] [Google Scholar]
- 8.Hurlbert RJ, Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg, 2000. 93(1 Suppl): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 9.Qian T, Campagnolo D, and Kirshblum S, High-dose methylprednisolone may do more harm for spinal cord injury. Med Hypotheses, 2000. 55(5): p. 452–3. [DOI] [PubMed] [Google Scholar]
- 10.Kwiatkoski M, Guimaraes FS, and Del-Bel E, Cannabidiol-treated rats exhibited higher motor score after cryogenic spinal cord injury. Neurotox Res, 2012. 21(3): p. 271–80. [DOI] [PubMed] [Google Scholar]
- 11.Ward SJ, et al. , Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female C57Bl6 mice. Anesth Analg, 2011. 113(4): p. 947–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward SJ, et al. , Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol, 2014. 171(3): p. 636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toth CC, et al. , Cannabinoid-mediated modulation of neuropathic pain and microglial accumulation in a model of murine type I diabetic peripheral neuropathic pain. Mol Pain, 2010. 6: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa B, et al. , The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol, 2007. 556(1-3): p. 75–83. [DOI] [PubMed] [Google Scholar]
- 15.Russo EB, et al. , Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res, 2005. 30(8): p. 1037–43. [DOI] [PubMed] [Google Scholar]
- 16.Bisogno T, et al. , Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol, 2001. 134(4): p. 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrens J, et al. , The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology, 2009. 83(4): p. 217–22. [DOI] [PubMed] [Google Scholar]
- 18.Carrier EJ, Auchampach JA, and Hillard CJ, Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A, 2006. 103(20): p. 7895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booz GW, Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic Biol Med, 2011. 51(5): p. 1054–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozela E, et al. , Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J Neuroinflammation, 2016. 13(1): p. 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adhikary S, et al. , Modulation of inflammatory responses by a cannabinoid-2-selective agonist after spinal cord injury. J Neurotrauma, 2011. 28(12): p. 2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kong W, Yen JH, and Ganea D, Docosahexaenoic acid prevents dendritic cell maturation, inhibits antigen-specific Th1/Th17 differentiation and suppresses experimental autoimmune encephalomyelitis. Brain, behavior, and immunity, 2011. 25(5): p. 872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaplan SR, et al. , Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods, 1994. 53(1): p. 55–63. [DOI] [PubMed] [Google Scholar]
- 24.Nesic O, et al. , Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem, 2005. 95(4): p. 998–1014. [DOI] [PubMed] [Google Scholar]
- 25.Chang HT, Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal Cord, 2007. 45(2): p. 174–82. [DOI] [PubMed] [Google Scholar]
- 26.Sroga JM, et al. , Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol, 2003. 462(2): p. 223–40. [DOI] [PubMed] [Google Scholar]
- 27.Zai LJ and Wrathall JR, Cell proliferation and replacement following contusive spinal cord injury. Glia, 2005. 50(3): p. 247–57. [DOI] [PubMed] [Google Scholar]
- 28.Tsuda M and Inoue K, Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology, 2016. 104: p. 76–81. [DOI] [PubMed] [Google Scholar]
- 29.Detloff MR, et al. , Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol, 2008. 212(2): p. 337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hains BC and Waxman SG, Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci, 2006. 26(16): p. 4308–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang D, Couture R, and Hong Y, Activated microglia in the spinal cord underlies diabetic neuropathic pain. Eur J Pharmacol, 2014. 728: p. 59–66. [DOI] [PubMed] [Google Scholar]
- 32.Costa B, et al. , Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch Pharmacol, 2004. 369(3): p. 294–9. [DOI] [PubMed] [Google Scholar]
- 33.Jones TB, et al. , Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci, 2004. 24(15): p. 3752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu HZ, et al. , Effects of autoimmunity on recovery of function in adult rats following spinal cord injury. Brain Behav Immun, 2008. 22(8): p. 1217–30. [DOI] [PubMed] [Google Scholar]
- 35.Hauben E, et al. , Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci, 2000. 20(17): p. 6421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potas JR, et al. , Augmented locomotor recovery after spinal cord injury in the athymic nude rat. J Neurotrauma, 2006. 23(5): p. 660–73. [DOI] [PubMed] [Google Scholar]
- 37.Moalem G, Xu K, and Yu L, T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience, 2004. 129(3): p. 767–77. [DOI] [PubMed] [Google Scholar]
- 38.Costigan M, et al. , T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci, 2009. 29(46): p. 14415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozela E, et al. , Cannabinoids decrease the th17 inflammatory autoimmune phenotype. J Neuroimmune Pharmacol, 2013. 8(5): p. 1265–76. [DOI] [PubMed] [Google Scholar]
- 40.Hoschouer EL, Yin FQ, and Jakeman LB, L1 cell adhesion molecule is essential for the maintenance of hyperalgesia after spinal cord injury. Exp Neurol, 2009. 216(1): p. 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen MJ, et al. , Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia, 2012. 60(11): p. 1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murakami T, et al. , Anti-interleukin-6 receptor antibody reduces neuropathic pain following spinal cord injury in mice. Exp Ther Med, 2013. 6(5): p. 1194–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nees TA, et al. , Early-onset treadmill training reduces mechanical allodynia and modulates calcitonin gene-related peptide fiber density in lamina III/IV in a mouse model of spinal cord contusion injury. Pain, 2016. 157(3): p. 687–97. [DOI] [PubMed] [Google Scholar]