Abstract
BACKGROUND
Desmoid tumors (also referred to as aggressive fibromatosis) are connective tissue neoplasms that can arise in any anatomical location and infiltrate the mesentery, neurovascular structures, and visceral organs. There is no standard of care.
METHODS
In this double-blind, phase 3 trial, we randomly assigned 87 patients with progressive, symptomatic, or recurrent desmoid tumors to receive either sorafenib (400-mg tablet once daily) or matching placebo. Crossover to the sorafenib group was permitted for patients in the placebo group who had disease progression. The primary end point was investigator-assessed progression-free survival; rates of objective response and adverse events were also evaluated.
RESULTS
With a median follow-up of 27.2 months, the 2-year progression-free survival rate was 81% (95% confidence interval [CI], 69 to 96) in the sorafenib group and 36% (95% CI, 22 to 57) in the placebo group (hazard ratio for progression or death, 0.13; 95% CI, 0.05 to 0.31; P<0.001). Before crossover, the objective response rate was 33% (95% CI, 20 to 48) in the sorafenib group and 20% (95% CI, 8 to 38) in the placebo group. The median time to an objective response among patients who had a response was 9.6 months (interquartile range, 6.6 to 16.7) in the sorafenib group and 13.3 months (interquartile range, 11.2 to 31.1) in the placebo group. The objective responses are ongoing. Among patients who received sorafenib, the most frequently reported adverse events were grade 1 or 2 events of rash (73%), fatigue (67%), hypertension (55%), and diarrhea (51%).
CONCLUSIONS
Among patients with progressive, refractory, or symptomatic desmoid tumors, sorafenib significantly prolonged progression-free survival and induced durable responses. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT02066181.)
Desmoid tumors (also called aggressive fibromatosis) are rare, locally aggressive neoplasms that arise from connective tissues.1 The annual incidence of the condition is estimated to be 1000 patients in the United States, and the prevalence may be higher. Desmoid tumors typically affect young adults in their 20s and 30s, but they can occur in children, adolescents, and older adults. Most desmoid tumors are sporadic (>90%) and harbor CTNBB1 mutations; a minority of tumors are associated with germline APC mutations and Gardner’s syndrome.2-4 Common primary sites affected by these tumors include the abdominal wall, mesentery, and neurovascular bundle of the extremities. Desmoid tumors do not metastasize and pose a low risk of death (except in Gardner’s syndrome), but they confer substantial complications. Patients may be asymptomatic or may present with severe pain, swelling, deformity, loss of range of motion, bowel obstruction or perforation, or compromise of vital organs.5 Additional associated complications in young adults include long-term opioid use, social isolation, insomnia, anxiety, depression, and interruption of education and employment.6
Although a number of agents have activity against desmoid tumors, no accepted standard of care exists for systemic treatment of the tumors.7 Beyond a few prospective trials, most relevant clinical data have been derived from case series and retrospective analyses. Interpretation of the data is challenging, given the unpredictable natural history of the condition. Desmoid tumors can show rapid growth followed by periods of stabilization, spontaneous regression, or subsequent growth phases.2 Spontaneous regression is reported in up to 20% of patients.8 An up-front watch-and-wait strategy is increasingly advocated for many patients.9-11 Surgery has been the standard of care for primary treatment, but the risk of local recurrence remains unacceptably high (>40%). Local (radiation therapy) or systemic treatments are usually indicated in patients who have disease-related symptoms or progressive disease. Systemic treatment options include hormonal blockade, cytotoxic chemotherapy, and tyrosine kinase inhibitors; the response rates associated with these treatments vary (0 to 40%).12-19 For example, in small prospective studies, imatinib has been found to have limited activity (6 to 11%), and no predictive biomarkers of benefit were found.20
In a retrospective analysis, sorafenib, an oral multitargeted receptor tyrosine kinase inhibitor, at a starting dose of 400 mg once daily was shown to have acceptable safety and was associated with a response rate of 25%, as evaluated with Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, as well as with improvements in quality of life.21 The retrospective study also highlighted that RECIST may underestimate efficacy and that a better criterion may be magnetic resonance imaging (MRI) T2-weighted signal intensity, an imaging biomarker that signifies a biologic transformation from a cellular tumor to a collagenous scar.22 This hypothesis prompted us to conduct a randomized, double-blind, placebo-controlled, phase 3 trial to evaluate the safety and efficacy of sorafenib in the treatment of desmoid tumors.
METHODS
PATIENTS
We enrolled patients 18 years of age or older with a histologically documented desmoid tumor (aggressive fibromatosis) if they had measurable disease and radiographic progression (of ≥10%) in maximum unidimensional measurement within the previous 6 months, recurrent or primary disease that was deemed inoperable or as requiring extensive surgery, or symptomatic disease. An additional entry criterion was an absence of previous sorafenib exposure; no minimum or maximum number of previous systemic treatments was stipulated. The complete entry and crossover eligibility criteria, including baseline laboratory values, are provided in the protocol, available with the full text of this article at NEJM.org.
TRIAL OVERSIGHT
The trial was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and federal and local policy on bioethics and human biologic specimens. Each participating institution obtained approval from a local or central institutional review board. All the patients signed informed consent forms in accordance with federal and institutional guidelines. The trial was designed by the first author and monitored by the Alliance Data and Safety Monitoring Board for the evaluation of safety and the primary end point.
This trial was funded by the National Cancer Institute and was conducted by the Alliance Clinical Trials in Oncology Group and the National Clinical Trials Network (Table S1 in the Supplementary Appendix, available at NEJM.org). Sorafenib was provided by the National Cancer Institute through a research collaboration with Bayer Pharmaceuticals.
All the data were collected, subjected to quality-assurance measures, and analyzed by the Alliance Statistics and Data Center. Archival tumor tissue for central pathological review, biopsy specimens (optional), and MRI scans were de-identified with regard to patient health information and, after completion of quality-assurance measures, were sent for central pathological review and correlative studies. The authors attest to the accuracy and completeness of the data and for the adherence of the trial to the protocol. The first draft of the manuscript was written by the principal investigators (the first and second authors); all the authors reviewed the manuscript. No one who is not an author contributed to the writing of the manuscript.
TRIAL DESIGN AND TREATMENT
In this investigator-initiated, double-blind, placebo-controlled, phase 3 trial, patients were randomly assigned (in a 2:1 ratio) to receive either sorafenib (at a starting dose of 400 mg once daily) or placebo. Desmoid tumors were imaged by means of computed tomography (CT) or MRI at baseline and every 8 weeks. Efficacy was assessed at local institutions with the use of RECIST, version 1.1.23 Administration of sorafenib or placebo continued until disease progression, unacceptable side effects, or withdrawal of consent. At disease progression, the patients were told whether they had been receiving sorafenib or placebo, and those who had been receiving placebo were eligible to cross over to the sorafenib group if they still met the trial entry criteria. Dose interruptions (of up to 28 days) and one dose reduction (to 200 mg once daily) were permitted and described in the trial protocol.
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, as determined by the treating physicians in accordance with RECIST, version 1.1. This end point was defined as the time from randomization to progressive disease (radiographic, clinical, or both) or death, and data were censored at the most recent disease assessment. A modification of the traditional intention-to-treat principle was used for the analysis of the primary end point, in which patients with an incorrect histologic diagnosis were excluded. The secondary end points were toxic effects, the rate of radiographic response, and overall survival. Ineligible patients who received a trial agent were included in the assessment of toxic effects, in which the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03, were used.
At enrollment, patients were given the option of consenting to undergo tumor biopsies and surveys with patient-reported outcome questionnaires at baseline and while taking the trial regimen. Exploratory end points included assessment of pain with the use of the Brief Pain Inventory and assessment of 11 side effects with the patient-reported outcomes version of the CTCAE (PRO-CTCAE, version 1.0) before crossover. Exploratory imaging end points included a comparison of RECIST measurements with total tumor volume and MRI T2-weighted signal intensity in patients.
STATISTICAL ANALYSIS
We calculated that a sample of 75 patients, each with 12 months of follow-up, would provide 90% power at a one-sided significance level of 0.025 (with the use of a stratified log-rank test) to detect a median progression-free survival that was 9 months longer with sorafenib than with placebo (with an expected median progression-free survival of 6 months among patients receiving placebo) and a hazard ratio of 0.4 for progression or death in the sorefenib group relative to the placebo group. Enrollment was estimated at 4 patients per month, for an anticipated duration of 21 months to complete enrollment. The final analysis was to occur at the time that 52 patients had had disease progression or had died. Sorafenib was to be declared as superior with regard to progression-free survival if the onesided P value associated with the stratified log-rank test statistic was less than 0.025. A preplanned, nonbinding futility analysis was performed when 24 (45%) of the 52 required events had been observed.
Kaplan–Meier methods and Cox proportionalhazards modeling were used to estimate the distributions of time-to-event variables and hazard ratios (including confidence intervals), respectively, accounting for stratification factors.24,25 Summary statistics, frequency tables, and parametric and nonparametric statistical tests were used, as applicable. The maximum PRO-CTCAE score for each item during the intervention with accounting for baseline PRO-CTCAE score was tabulated for each trial group, and the difference between the groups in the proportion of patients with a score of at least 1 and, separately, with a score of at least 3 was computed with exact 95% confidence intervals.26 All P values and confidence intervals are two-sided and unadjusted for multiplicity. All the observed data were included in the analysis without imputation for missing data. All the analyses were performed with the use of SAS software, version 9.4 (SAS Institute). The data-lock date was January 31, 2018.
RESULTS
PATIENTS, ENROLLMENT, AND TREATMENT
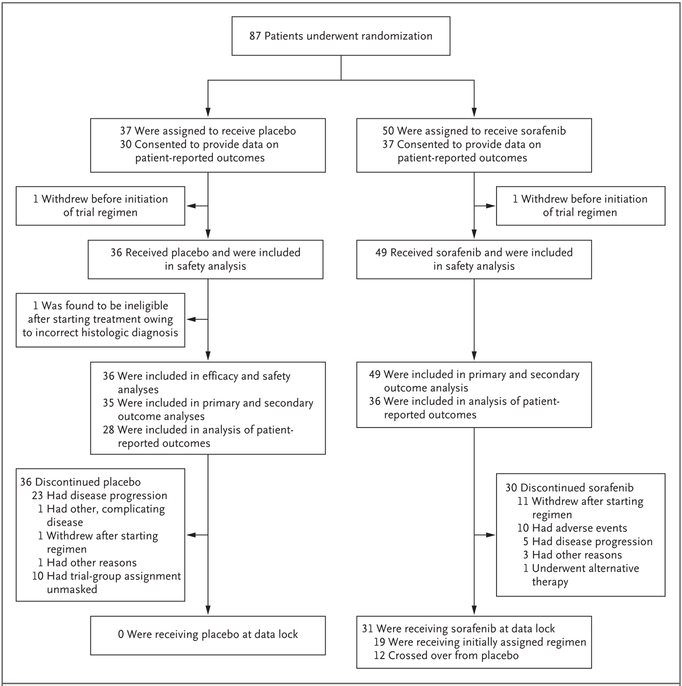
From March 21, 2014, to January 6, 2016, a total of 87 patients were enrolled across 24 sites; 50 patients were randomly assigned to the sorafenib group and 37 to the placebo group (Fig. 1). A systems computer algorithm error was detected after 70 patients (38 in the sorafenib group and 32 in the placebo group) had been enrolled. The randomization ratio was approximately 1.6 to 1.7:1 (sorafenib:placebo) instead of the prespecified 2:1 ratio. This error was shared with the data and safety monitoring board, institutional review boards, treating physicians, and patients (in October 2015), with correction for the remainder of enrollment.
Figure 1.
Randomization and Follow-up among the Patients in the Trial.
The characteristics of the patients at baseline were well balanced between the two trial groups (Table 1).27 A larger percentage of female than male patients were enrolled (69%), and the median age of the patients was 37 years (interquartile range, 28 to 50), findings consistent with the natural history of desmoid tumors; 80% of the patients reported their race as white. The median dose of sorafenib that was administered across the entire trial was 400 mg daily. Dose interruptions occurred in 65% of the patients in the sorafenib group (32 of 49) and 34% of the patients in the placebo group (12 of 35), and dose reductions due to toxic effects occurred in 31% (15 of 49) and 11% (4 of 35), respectively. At data cutoff, 19 patients (39%) who had initially been assigned to the sorafenib group continued to take the drug. At the time of the interim analysis, the data and safety monitoring board also requested an efficacy analysis, and subsequently the trial was halted and unblinded.
Table 1.
Demographic and Clinical Characteristics of the Patients at Randomization.*
| Characteristic | Placebo (N = 37) |
Sorafenib (N = 50) |
|---|---|---|
| Median age (range) — yr | 37 (21–67) | 37 (18–72) |
| Female sex — no. (%) | 26 (70) | 34 (68) |
| ECOG performance-status score — no. (%)† | ||
| 0 | 22 (59) | 35 (70) |
| 1 | 15 (41) | 15 (30) |
| Median sum of target lesions at randomization (range) — cm | 7.6 (2.6–26.5) | 8.4 (1.2–19.3) |
| BPI worst pain score at randomization — no. (%)‡§ | ||
| 0–2 | 14 (38) | 17 (34) |
| 3–6 | 14 (38) | 21 (42) |
| 7–10 | 9 (24) | 12 (24) |
| Intraabdominal disease — no. (%)‡ | 16 (43) | 16 (32) |
| Primary tumor site — no. (%) | ||
| Abdominal | 16 (43) | 14 (28) |
| Extraabdominal | 18 (49) | 32 (64) |
| Both abdominal and extraabdominal | 3 (8) | 4 (8) |
| Previous radiation therapy — no. (%) | 3 (8) | 6 (12) |
| Previous systemic therapy — no. (%) | 15 (41) | 18 (36) |
| Previous surgical resection — no. (%) | 18 (49) | 23 (46) |
| Disease status — no./total no. (%) | ||
| Newly diagnosed | 19/37 (51) | 26/48 (54) |
| Recurrent | 18/37 (49) | 22/48 (46) |
| Trial inclusion criteria — no. (%)¶ | ||
| Disease determined to be unresectable or to require surgery with unacceptably high associated morbidity | 28 (76) | 44 (88) |
| Progression detected by radiographic imaging within 6 months before randomization | 16 (43) | 19 (38) |
| Symptomatic disease with BPI worst pain score ≥3 and consideration of pain narcotic introduction or escalation∥ | 11 (30) | 16 (32) |
The intention-to-treat population included all patients who underwent randomization with the exception of those who were identified after randomization as not having a desmoid tumor and those who did not initiate the trial regimen and did not undergo further follow-up. Randomization was based on a dynamic allocation algorithm developed and implemented by the Alliance Statistics and Data Center. An error in the assignment of the trial regimen was detected and rectified after 70 patients (38 in the sorafenib group and 32 in the placebo group) had been enrolled. The program deriving the assignments of trial regimens incorrectly recognized a patient’s crossover regimen as the initial assigned regimen when balancing for new enrollments. Randomization was stratified according to anatomical location and level of pain at the time of randomization, assessed with the use of the worst pain item of the Brief Pain Inventory (BPI) completed by the patient within 28 days before randomization. There were no significant differences (P<0.05) between the groups in any of the characteristics at the time of randomization. Percentages may not total 100 because of rounding.
Eastern Cooperative Oncology Group (ECOG) performance-status scores are assessed on a 5-point scale, with higher scores indicating greater disability; a score of 5 indicates death.
The characteristic was a stratification factor at randomization.
The BPI worst pain question was “Please rate your pain by circling the one number that best describes your pain at its WORST in the last 24 hours: 0 (no pain)–10 (pain as bad as you can imagine).”
Patients had to meet at least one of these three criteria to be eligible for participation in the trial.
Consideration of pain narcotic introduction or escalation was defined as an inability to control pain with nonsteroidal antiinflammatory drugs and consideration of the addition of narcotics or a more than 30% increase in the current use of narcotics or the addition of a new opioid narcotic.
EFFICACY
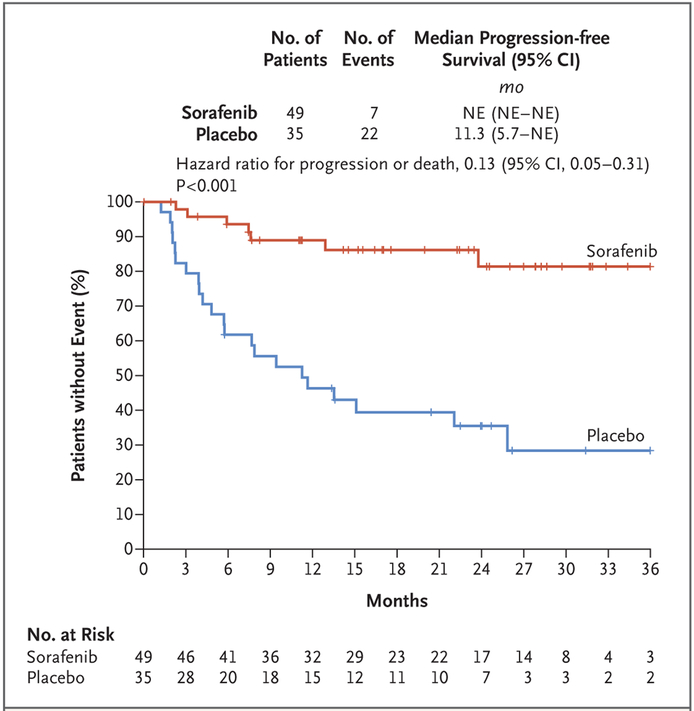
Of the 87 patients who underwent randomization, 84 (97%) were included in the analysis of primary and secondary end points, with a median follow-up of 27.2 months (interquartile range, 22.0 to 31.7) among the 83 surviving patients. Although the median progression-free survival has not yet been reached, the estimates of the progression-free survival rates at 1 year were 89% (95% confidence interval [CI], 80 to 99) in the sorafenib group and 46% (95% CI, 32 to 67) in the placebo group, and the estimates at 2 years were 81% (95% CI, 69 to 96) and 36% (95% CI, 22 to 57), respectively. The results for progression-free survival favored sorafenib, with an 87% lower risk of progression or death in the sorafenib group than in the placebo group (hazard ratio for disease progression or death, 0.13; 95% CI, 0.05 to 0.31; P<0.001) (Fig. 2). Overall, 33% of the patients in the trial (28 of 84) had disease progression: 12% of the patients (6 of 49) in the sorafenib group and 63% of the patients (22 of 35) in the placebo group. Clinical deterioration in the absence of radiographic evidence was the sole indicator of progression in 11 of the 28 patients with progression (39%; 9 patients in the placebo group and 2 in the sorafenib group).
Figure 2. Kaplan–Meier Estimates of the Duration of Progression-free Survival at the Time of the Last Assessment.
Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, were used by the investigators to identify disease progression. Data from patients who did not have progression or who had died were censored and marked by a tick. NE denotes not estimable.
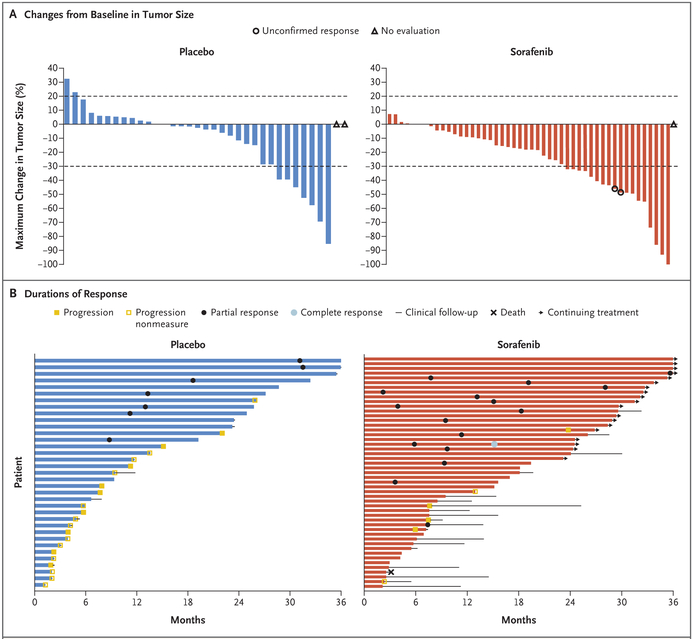
The overall rate of objective response was 33% (95% CI, 20 to 48) in the sorafenib group (16 patients [1 with a complete response and 15 with partial responses] of 49) and 20% (95% CI, 8 to 37) in the placebo group (7 patients [all of whom had a partial response] of 35) (Fig. 3A, and Fig. S1 in the Supplementary Appendix). The mean best percentage change in the sum of the target lesions (RECIST) was −26% (range, −100 to 7) in the sorafenib group and −12% (range, −85 to 32) in the placebo group. The median time to a RECIST-defined response among patients who had a response was 9.6 months (interquartile range, 6.6 to 16.7) in the sorafenib group and 13.3 months (interquartile range, 11.2 to 31.1) in the placebo group (Fig. 3B). The earliest RECIST-defined partial response occurred at 2.2 months in sorafenib group and at 8.8 months in the placebo group.
Figure 3. Tumor Responses and Clinical Outcomes.
Panel A shows waterfall plots of percentage changes from baseline in tumor size as assessed by investigators according to RECIST, version 1.1. Each bar represents one patient. Horizontal dashed lines indicate the changes in tumor size that would represent a partial response (30% decrease) or progressive disease (20% increase). One patient in the sorafenib group had a complete response, defined as total disappearance of tumor. Panel B shows swimmer plots of the duration of response and clinical outcomes among patients during the trial. “Progression nonmeasure” denotes clinical progression without radiographic progression (20% growth). One patient in the sorafenib group died from disease-related intestinal perforation. Duration of response was calculated as the time between the first objective response and disease progression; data from patients with ongoing responses were censored at the most recent disease assessment. Time to response during the time of the blinded trial intervention was calculated from the start of the intervention to the date of the first objective response or to the most recent disease assessment (for patients without a response). Time to progression was the time to disease progression or to the most recent assessment if the patient did not have disease progression.
In the exploratory imaging analysis, 498 MRI scans were obtained from 55 patients. We selected a training set of 11 patients who were treated at a single institution (Memorial Sloan Kettering Cancer Center), and we analyzed 167 MRI scans for changes in tumor dimension (according to RECIST) and compared this value with the changes in total tumor volume and T2-weighted signal intensity. As shown in Figure S2 in the Supplementary Appendix, changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.
SAFETY
A total of 85 patients (49 in the sorafenib group and 36 in the placebo group) were included in the assessment of safety with the use of CTCAE, version 4.0. A summary of the most common adverse events is provided in Table 2. Adverse events led to a significantly higher rate of discontinuation of the trial regimen in the sorafenib group than in the placebo group (20% vs. no patients). The most common reason for dose reduction in the sorafenib group was skin disorders. Grade 3 adverse events that were attributed to the trial regimen by the investigators occurred in 29% of patients in the sorafenib group and 14% of patients in the placebo group. Grade 4 events that were associated with sorafenib included thrombocytopenia (2%) and anemia (2%). One patient in the sorafenib group died from disease-related bowel perforation. A list of the side effects reported by the patients with the use of PRO-CTCAE is provided in Table S4 and Figure S3 in the Supplementary Appendix. The proportions of patients with nausea, diarrhea, rash, and hand–foot syndrome were higher in the sorafenib group than in the placebo group.
Table 2.
Incidence of Adverse Events of Any Cause According to Initially Assigned Trial Regimen.*
| Event | Sorafenib (N = 49) |
Placebo (N = 36) |
||
|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 or 4 | Grade 1 or 2 | Grade 3 or 4 | |
| number of patients (percent) | ||||
| Any adverse event | 26 (53) | 23 (47) | 25 (69) | 9 (25) |
| Events during receipt of trial regimen with incidence ≥10%† | ||||
| Palmar–plantar erythrodysesthesia syndrome | 34 (69) | 1 (2) | 8 (22) | 0 |
| Rash | ||||
| Any rash or skin disorder | 36 (73) | 7 (14) | 15 (42) | 0 |
| Papulopustular | 24 (49) | 6 (12) | 6 (17) | 0 |
| Acneiform | 6 (12) | 0 | 0 | 0 |
| Maculopapular | 7 (14) | 0 | 1 (3) | 0 |
| Skin or subcutaneous tissue disorders —other‡ | 7 (14) | 1 (2) | 5 (14) | 0 |
| Pruritus | 7 (14) | 0 | 0 | 0 |
| Fatigue | 33 (67) | 3 (6) | 22 (61) | 1 (3) |
| Hypertension | 27 (55) | 4 (8) | 14 (39) | 0 |
| Diarrhea | 25 (51) | 0 | 12 (33) | 0 |
| Nausea | 24 (49) | 0 | 14 (39) | 1 (3) |
| Myalgia | 18 (37) | 1 (2) | 12 (33) | 0 |
| Alopecia | 18 (37) | 0 | 3 (8) | 0 |
| Arthralgia | 17 (35) | 1 (2) | 9 (25) | 0 |
| Abdominal pain | 15 (31) | 1 (2) | 9 (25) | 4 (11) |
| Anorexia | 15 (31) | 0 | 9 (25) | 0 |
| Constipation | 11 (22) | 0 | 4 (11) | 0 |
| Oral mucositis | 11 (22) | 0 | 6 (17) | 0 |
| Vomiting | 10 (20) | 1 (2) | 6 (17) | 2 (6) |
| Anemia | 8 (16) | 1 (2) | 2 (6) | 1 (3) |
| Increase in alanine aminotransferase level | 7 (14) | 0 | 4 (11) | 0 |
| Decrease in platelet count | 6 (12) | 2 (4) | 1 (3) | 0 |
| Hyperglycemia | 6 (12) | 1 (2) | 3 (8) | 0 |
| Peripheral sensory neuropathy | 6 (12) | 0 | 1 (3) | 0 |
| Increase in aspartate aminotransferase level | 5 (10) | 1 (2) | 3 (8) | 0 |
| Increase in blood bilirubin level | 5 (10) | 0 | 3 (8) | 1 (3) |
| Decrease in neutrophil count | 5 (10) | 0 | 2 (6) | 0 |
| Dry skin | 5 (10) | 0 | 1 (3) | 0 |
| Headache | 4 (8) | 0 | 6 (17) | 0 |
| Decrease in white-cell count | 3 (6) | 0 | 6 (17) | 0 |
| Musculoskeletal connective-tissue disorders — other§ | 3 (6) | 0 | 4 (11) | 0 |
Events that occurred while the patient was taking the initially assigned trial regimen (before crossover) are shown. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The events reported reflect the maximum severity in each category for a given patient during the treatment period; multiple occurrences of the same event in a single patient were counted once, at the highest grade at which it occurred. All 85 patients were included in the assessment of safety.
Events that had an incidence of 10% or higher in either trial group are shown. One patient in the sorafenib group died from disease-related bowel perforation (not shown in this table) that was judged by the investigators not to have been related to the drug; no other grade 5 events occurred.
Events in this category included callus, swelling, plantar wart, hidradenitis supportiva, and pain.
Events in this category included pain and cramping.
CROSSOVER
In the placebo group, 27 patients met the eligibility criteria for open-label sorafenib treatment (20 at disease progression and 7 when the data and safety monitoring board released results), and 12 patients continue to take sorafenib; however, the data remain immature. The toxic effects among the patients receiving open-label sorafenib were similar to those among the patients who were initially randomly assigned to receive sorafenib and are listed in Table S7 in the Supplementary Appendix.
DISCUSSION
This randomized trial provides data on the efficacy of sorafenib in patients with progressive or symptomatic desmoid tumors. Other agents that are used to treat these tumors include anthracyclines (e.g., pegylated liposomal doxorubicin), vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors.
For a locally infiltrative tumor, the prevention of further worsening or compromise of vital structures is a clinically meaningful end point. In that context, among patients with progressive, symptomatic, or recurrent desmoid tumors, the rate of progression-free survival with sorafenib at 1 year was 89%. Patients treated with sorafenib had an 87% lower risk of disease progression or death than those who received placebo. To balance the efficacy of the drug against the long-term drug-related toxic effects, we chose a starting dose of sorafenib (one 400-mg tablet daily) that was lower than the dose used in other types of cancer and permitted dose interruptions and reductions.5 The modest toxicity of sorafenib was confirmed in both clinicianreported and patient-reported assessments of adverse events. Consistent with previous literature, the rates of adverse events that were based on clinician reporting were substantially lower than those that were based on patient reporting.28 Many of these differences were due to the ability to detect more lower-grade mild-to-moderate side effects with the use of the patient-reported PRO-CTCAE. Accordingly, we surmised that the high rate of withdrawal from the trial due to adverse events (20%) suggests that even greater dose flexibility may be necessary to balance toxicity and benefit.
This trial highlights the importance of randomization in the conduct of clinical trials. Spontaneous regression was once considered to be anecdotal and rare (occurring in <5% of patients), but more recent retrospective, nonrandomized studies have shown higher rates of spontaneous remission.8,9 Our prospective trial, in which desmoid tumors in patients who were taking placebo were evaluated, provides evidence in support of an initial period of observation in patients with newly diagnosed desmoid tumors, given that 20% of the patients in the placebo group had disease regression. In this trial, late responses were observed in the sorafenib group, and response rates may increase with further data maturation.
A final important clinical issue to note regards the feasibility and challenges of conducting clinical trials in very rare cancers. Rare cancers are defined as those with an incidence of less than 15 cases per 100,000 persons per year. Although individually uncommon, rare cancers account for 25% of all cancers and are associated with poor survival.29,30 The main challenges in the design and execution of this phase 3 trial were the incidence of the cancer (0.3 cases per 100,000 persons per year), the lack of consensus on the standard of care, the lack of predictive biomarkers for the selection of patients, and the lack of validated, desmoid-specific patient-reported outcome measures. The unreliability of historical data on treatment and natural history (e.g., the rate of spontaneous regression) was an additional design challenge. All potential trial designs (e.g., frequentist or Bayesian) should be considered on the basis of not only their statistical properties but also their feasibility with regard to late events or logistic support for realtime data entry. The trial conducted was an international collaboration among U.S. and Canadian National Cancer Institutes, cooperative research groups, patient advocacy groups, and physician outreach groups, an endeavor that facilitated the enrollment of 87 patients in 17 months.29,31
A limitation of this trial is that it was not designed to directly compare the primary or secondary end points with meaningful improvements in pain palliation, functionality, or quality of life. The use of pain-palliation questionnaires was optional, and limited results were available. In our exploratory analysis, we were unable to use the Brief Pain Inventory to discern any difference between the groups (data not shown), contrary to previous reports. Symptoms that affect patients with desmoid tumors are wide-ranging, and since this trial was conducted, a prospective, desmoid tumor–specific, patient-reported outcome tool has been developed for future trials.6 Beyond the traditional end points that are used in clinical trials, incorporating an evaluation of the patient experience is critical.32
The ability to use RECIST-defined responses to correlate with treatment effect and survival among patients with solid tumors is debated. Data from our exploratory analysis suggested that there is anatomical and mathematical discordance among assessments that are based on unidimensional measurement (RECIST), tumor volume, and T2-weighted signal intensity; therefore, RECIST — the current regulatory metric — may underestimate treatment effects. This phenomenon is observed in other sarcomas, such as tenosynovial giant-cell tumors and gastrointestinal stromal tumors, in which tumor volume and density are better predictors of treatment effect than RECIST measurements.33,34 Similarly, data have suggested that tumor volume or MRI T2-weighted signal intensity — namely, a shift from a cellular mass to a collagenous scar — may be additional imaging biomarkers that can potentially be used to assess treatment effects on desmoid tumors.22,35 The appropriate duration of sorafenib treatment and its cost and benefit relative to those of existing therapies remain unknown. Finally, the mechanism of action of sorafenib in desmoid tumors36 is not known. Investigations into changes in gene expression and protein phosphorylation of receptor tyrosine kinases (e.g., platelet-derived growth factor receptor, fibroblast growth factor receptor, and transforming growth factor beta receptor) and the Wnt signaling pathway are ongoing in the 25 sets of paired biopsy specimens we obtained.
In conclusion, in this trial, therapy with sorafenib appeared to be effective in slowing disease progression in patients with desmoid tumors.
Supplementary Material
Acknowledgments
Supported by grants from the National Cancer Institute, National Institutes of Health (U10CA180821, U10CA180882, and U24CA196171 to the Alliance for Clinical Trials in Oncology; U10CA180833, U10CA180838, U10CA180857, U10CA180858, UG1CA189850, UG1CA189957, U10CA180820 [to the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network], U10CA180868 [to NRG], U10CA180888, and U10CA180826 [to the Southwest Oncology Group]), Bayer Pharmaceuticals, a grant from Memorial Sloan Kettering Cancer Center (P30 CA008748), a grant from the American Society of Clinical Oncology (Career Development Award, to Dr. Gounder), the Desmoid Tumor Research Foundation (to Dr. Gounder), and an Orphan Products Clinical Trials Grant from the Food and Drug Administration (R01 FD005105, to Dr. Gounder).
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families and caregivers for participating in this trial; Jeanne Whitting and Marlene Portnoy of the Desmoid Tumor Research Foundation and patient advocacy group; Alex Zoroufy, Tiffany Winfield, Brenda Ginos, and Vera Suman, all at the Alliance Statistics and Data Center, Mayo Clinic, Rochester, MN, for additional support with statistical analyses; Krista Garbacz, research coordinator at Alliance Protocol Office, Chicago; David Poon, Alliance Imaging Core Laboratory, Ohio State University, Columbus; Yujia Wen, Alliance Biorepository, Ohio State University, Columbus; and the Translational Research Operations and Alliance (A091105) investigators and site personnel: Mark Agulnik (Northwestern University, Chicago), John Carlson (Froedtert and the Medical College of Wisconsin, Milwaukee), Scott Cole (Oklahoma Cancer Specialists and Research Institute, Tulsa), Rangaswamy Govindarajan (University of Arkansas for Medical Sciences, Little Rock), Khawaja S. Jahangir and Grzegorz Obara (Nevada Cancer Research Foundation NCORP, Las Vegas), Janelle Meyer (Loyola University Medical Center, Maywood, IL), Jennifer Wright (University of Utah–Huntsman Cancer Institute, Salt Lake City), Andrew Pippas (John B. Amos Cancer Center, Columbus, GA), Thierry Alcindor (McGill University, Montreal), and Kashfia Haque, Lee Schenider, Mark Dickson, Joseph Erinjeri, and Mercedes Condy (Memorial Sloan Kettering Cancer Center, New York).
Contributor Information
Mrinal M. Gounder, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Michelle R. Mahoney, Alliance Statistics and Data Center, Mayo Clinic, Rochester, MN
Brian A. Van Tine, Washington University School of Medicine, St. Louis
Vinod Ravi, M.D. Anderson Cancer Center, University of Texas, Houston
Steven Attia, Mayo Clinic in Florida, Jacksonville
Hari A. Deshpande, Yale University, New Haven, CT
Abha A. Gupta, University Health Network Princess Margaret Cancer Centre, Toronto
Mohammed M. Milhem, University of Iowa-Holden Comprehensive Cancer Center, Iowa City
Robert M. Conry, University of Alabama at Birmingham Cancer Center, Birmingham
Sujana Movva, Fox Chase Cancer Center, Philadelphia
Michael J. Pishvaian, Georgetown University, Lombardi Comprehensive Cancer Center, Washington, DC
Richard F. Riedel, Duke Cancer Institute, Duke University Medical Center, Durham, North Carolina
Tarek Sabagh, Dayton National Cancer Institute Community Oncology Research Program, Dayton, OH
William D. Tap, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Natally Horvat, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Ethan Basch, Lineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, North Carolina
Lawrence H. Schwartz, Columbia University Vagellos College of Physicians and Surgeons and New York Presbyterian Hospital, New York
Robert G. Maki, Northwell Cancer Institute and Cold Spring Harbor Laboratory, Lake Success, New York
Narasimhan P. Agaram, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Robert A. Lefkowitz, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Yousef Mazaheri, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
Rikiya Yamashita, Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center, New York
John J. Wright, National Cancer Institute, Bethesda, MD
Amylou C. Dueck, Alliance Statistics and Data Center, Mayo Clinic, Scottsdale, AZ
Gary K. Schwartz, Columbia University Vagellos College of Physicians and Surgeons and New York Presbyterian Hospital, New York
REFERENCES
- 1.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon, France: IARC Press, 2013. [Google Scholar]
- 2.Brennan MF, Antonescu CR, Alektiar K, Maki RG. Management of soft tissue sarcoma. Cham, Switzerland: Springer, 2016. [Google Scholar]
- 3.Salas S, Chibon F, Noguchi T, et al. Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer 2010;49:560–8. [DOI] [PubMed] [Google Scholar]
- 4.Alman BA, Li C, Pajerski ME, Diaz-Cano S, Wolfe HJ. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997;151:329–34. [PMC free article] [PubMed] [Google Scholar]
- 5.Gounder MM, Thomas DM, Tap WD. Locally aggressive connective tissue tumors. J Clin Oncol 2018;36:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paty J, Maddux L, Gounder MM. Prospective development of a patient reported outcomes (PRO) tool in desmoid tumors: a novel clinical trial endpoint. J Clin Oncol 2017;35:Suppl:11022. abstract. [Google Scholar]
- 7.Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol 2017; 28:2399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonvalot S, Ternès N, Fiore M, et al. Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 2013;20:4096–102. [DOI] [PubMed] [Google Scholar]
- 9.Colombo C, Miceli R, Le Péchoux C, et al. Sporadic extra abdominal wall desmoid-type fibromatosis: surgical resection can be safely limited to a minority of patients. Eur J Cancer 2015;51:186–92. [DOI] [PubMed] [Google Scholar]
- 10.Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 2009;16:2587–93. [DOI] [PubMed] [Google Scholar]
- 11.Penel N, Le Cesne A, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: a nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 2017;83:125–31. [DOI] [PubMed] [Google Scholar]
- 12.de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010;116: 2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chugh R, Wathen JK, Patel SR, et al. Efficacy of imatinib in aggressive fibromatosis: results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 2010;16:4884–91. [DOI] [PubMed] [Google Scholar]
- 14.Palassini E, Frezza AM, Mariani L, et al. Long-term efficacy of methotrexate plus vinblastine/vinorelbine in a large series of patients affected by desmoid-type fibromatosis. Cancer J 2017;23:86–91. [DOI] [PubMed] [Google Scholar]
- 15.Skapek SX, Ferguson WS, Granowetter L, et al. Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 2007;25:501–6. [DOI] [PubMed] [Google Scholar]
- 16.Skapek SX, Anderson JR, Hill DA, et al. Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer 2013;60:1108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kummar S, O’Sullivan Coyne G, Do KT, et al. Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol 2017;35:1561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiore M, Colombo C, Radaelli S, et al. Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer 2015;51:2800–7. [DOI] [PubMed] [Google Scholar]
- 19.Villalobos VM, Hall F, Jimeno A, et al. Long-term follow-up of desmoid fibromatosis treated with PF-03084014, an oral gamma secretase inhibitor. Ann Surg Oncol 2018;25:768–75. [DOI] [PubMed] [Google Scholar]
- 20.Heinrich MC, McArthur GA, Demetri GD, et al. Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 2006;24:1195–203. [DOI] [PubMed] [Google Scholar]
- 21.Gounder MM, Lefkowitz RA, Keohan ML, et al. Activity of sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 2011;17:4082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundaram M, McGuire MH, Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol 1987; 148:1247–50. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81. [Google Scholar]
- 25.Cox DR. Regression models and lifetables. J R Stat Soc B 1972;34:187–202. [Google Scholar]
- 26.Basch E, Rogak LJ, Dueck AC. Methods for implementing and reporting patient-reported outcome (PRO) measures of symptomatic adverse events in cancer clinical trials. Clin Ther 2016;38:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. [PubMed] [Google Scholar]
- 28.Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol 2017;3:1043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blay JY, Coindre JM, Ducimetière F, Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol 2016;17(2):e62–e69. [DOI] [PubMed] [Google Scholar]
- 30.Boyd N, Dancey JE, Gilks CB, Huntsman DG. Rare cancers: a sea of opportunity. Lancet Oncol 2016;17(2):e52–e61. [DOI] [PubMed] [Google Scholar]
- 31.Ingelfinger JR, Drazen JM. Patient organizations and research on rare diseases. N Engl J Med 2011;364:1670–1. [DOI] [PubMed] [Google Scholar]
- 32.Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol 2018; 19(5):e267–e274. [DOI] [PubMed] [Google Scholar]
- 33.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 2015;373:428–37. [DOI] [PubMed] [Google Scholar]
- 34.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. [DOI] [PubMed] [Google Scholar]
- 35.Vandevenne JE, De Schepper AM, De Beuckeleer L, et al. New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol 1997;7:1013–9. [DOI] [PubMed] [Google Scholar]
- 36.Gounder MM. Notch inhibition in desmoids: “Sure it works in practice, but does it work in theory?” Cancer 2015;121:3933–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.