Abstract
Enteroendocrine cells were historically classified by a letter code, each linked to a single hormone, deduced to be the only hormone produced by the cell. One type, the L cell, was recognised to store and secrete two products, peptide YY (PYY) and glucagon-related peptides. Many other exceptions to the one cell – one hormone classifications have been reported over the last 40 years or so, and yet the one hormone dogma has persisted. In the last 6 years, a plethora of data has appeared that makes the concept unviable. Here we describe the evidence that multiple hormone transcripts and their products reside in single cells and evidence that the hormones are often, but not always, processed into separate storage vesicles. It has become clear that most enteroendocrine cells contain multiple hormones. For example, most secretin cells contain 5-hydroxytryptamine (5-HT), and in mouse many of these also contain cholecystokinin (CCK). Furthermore, CCK cells also commonly store ghrelin, glucose-dependent insulinotropic peptide (GIP), glucagon-like peptide-1 (GLP-1), neurotensin, and PYY. Several hormones, for example secretin and 5-HT, are in separate storage vesicles at a subcellular level. Hormone patterns can differ considerably between species. Another complication is that relative levels of expression vary substantially. This means that data are significantly influenced by the sensitivities of detection techniques. For example, a hormone that can be detected in storage vesicles by super-resolution microscopy may not be above threshold for detection by conventional fluorescence microscopy. New nomenclature for cell clusters with common attributes will need to be devised and old classifications abandoned.
Keywords: gut hormones, enteroendocrine cells, super-resolution microscopy, storage vesicles
Introduction
Investigation of the gastrointestinal system provided the first evidence for the existence of circulating hormones, secretin in 1902 (Bayliss and Starling 1902) and then gastrin in 1905 (Edkins 1906). Earlier than this, a distinctive solitary cell type in the lining of the gastrointestinal tract was revealed by the chromaffin reaction (Heidenhain 1870). These became known as enterochromaffin (EC) cells (Vialli and Erspamer 1933). These cells, and other types of solitary cells, such as those stained by silver salts, were identified as enteroendocrine cells (EECs), the source of gut hormones. In the ensuing years, the chemistries of numerous gastrointestinal hormones were identified, antibodies were raised, and the locations of the cells were determined (Rehfeld 2012). A letter code was developed that classified discrete cell types by the hormone they produced or by ultrastructural features identified with electron microscopy (Table 1). For example, L cells contained ‘large’ secretory vesicles, S cells contained ‘small’ vesicles, and I cells contained ‘intermediate’ vesicles. Gastrin cells were identified as G cells and motilin cells as M cells. With the exception of L cells, which were reported to contain both glucagon-related peptides and PYY (Ali Rachedi et al. 1984; Böttcher et al. 1986), it was thought that each cell type contained a single hormone as indicated in Table 1.
Table 1:
The historical naming of enteroendocrine cells and evidence for multiple rather than single hormone storage. Earlier references showing colocalization, often overlooked, have been included.
| Letter code | Named for | Identifying hormone (historical view) | Examples of colocalization of hormones and/or their gene transcripts (most of recent data is from mouse) |
|---|---|---|---|
| EC (enterochromaffin) | Reaction with chrome salts | 5-HT | Colocalized with CCK, secretin, tachykinins, motilin, ghrelin, GLP-1, and PYY (Pearse et al. 1974; Roth and Gordon 1990; Reynaud et al. 2016; Fothergill et al. 2017; Martins et al. 2017) |
| S | Small vesicles | Secretin | Colocalization with ghrelin, CCK and 5-HT (Lopez et al. 1995; Fothergill et al. 2017) |
| I | Intermediate size vesicles | CCK | Colocalized with proglucagon, PYY, GIP, ghrelin, secretin and neurotensin (Egerod et al. 2012; Sykaras et al. 2014) |
| L | Large vesicles | Proglucagon products (i.e. GLP-1) and PYY | Colocalized with GIP, CCK, secretin, ghrelin, 5-HT and neurotensin (Habib et al. 2012; Glass et al. 2017) |
| ECL (EC-like) | Similarity to EC cells | Histamine | Also contain and secrete pancreastatin (Håkanson et al. 1995) |
| X/A | X for unknown product, A for supposed similarity to pancreatic A cells | Ghrelin (not discovered until 1999) | In the stomach, the majority of ghrelin cells contain colocalized nesfatin-1 (Stengel et al. 2010b). In the small intestine, most express secretin, CCK, proglucagon, and motilin (Wierup et al. 2007; Haber et al. 2017) |
| D | Similarity to pancreatic D cell | Somatostatin | Some also express GIP (Habib et al. 2012) |
| K | Vesicles that differentiate from L cells | GIP | Coexpressed with GLP-1, secretin, CCK, PYY, and somatostatin (Habib et al. 2012) |
| N | Neurotensin content | Neurotensin | Coexpressed with GLP-1 and PYY (Grunddal et al. 2015) |
| G | Gastrin content | Gastrin | Coexpression not investigated adequately |
| M | Motilin content | Motilin | Commonly colocalized with 5-HT and ghrelin (Pearse et al. 1974; Wierup et al. 2007) |
Hidden in some studies from the 1970’s and 80’s were examples that the simple ‘one cell-one (or for L cells, two) hormone’ classification system was not correct. Using markers for EC (5-HT) cells and anti-motilin antisera, it was shown that a proportion of 5-HT cells was motilin immunoreactive (Pearse et al. 1974). By double-labelled immunogold methods, 5-HT immunoreactivity was observed in cells with round PYY-containing vesicles and weak PYY immunoreactivity was found in cells with irregular 5-HT-containing vesicles in human rectal mucosa (Lukinius et al. 1986). By immunohistochemistry, about a third of human secretin cells in the upper small intestine contained 5-HT immunoreactivity (Usellini et al. 1990). In mouse duodenum, this overlap was reported to be as high as 80% of secretin cells (Roth and Gordon 1990). Despite these early findings, the ‘one cell-one hormone’ theory has persisted in text books and top tier journals (Coate et al. 2014).
The present review outlines the evidence that a majority of EECs contain several hormones and that the one cell-one hormone concept is untenable, as other authors have also concluded (Helander and Fändriks 2012; Drucker 2015; Gribble and Reimann 2016). We also review evidence that, at a subcellular level, hormones are commonly concentrated in separate vesicles.
Roles of GI hormones
Collectively, gastrointestinal hormones have three major roles: to control the ingestion of food; to control the digestion of the food; and to control metabolic functions (Table 2)
Table 2:
The main functions of gastrointestinal hormones
| Major function | Examples of relevant hormonal influences | Hormones |
|---|---|---|
| Ingestion | Appetite, satiety, rejection of toxic or harmful elements in food | CCK, GLP-1, PYY (satiety); ghrelin (appetite); 5-HT (nausea) |
| Digestion | Secretion of digestive agents (acid, hormones), motility, induction of transporters | Gastrin, histamine, CCK (secretion of digestive agents); motilin, 5-HT (motility), GLP-1 (transporter induction), somatostatin, secretin |
| Metabolism | Glucose uptake and storage, growth hormone release, lipid mobilisation, bone metabolism | GIP, GLP-1, ghrelin, 5-HT |
Ingestion
Enteric hormones are critical in regulating the intake of food. Ghrelin, which reaches peak plasma levels just prior to meals, stimulates the sensation of hunger and is involved in meal initiation (Cummings et al. 2001). Ingested nutrients stimulate the release of several satiety hormones that promote meal cessation, including CCK (Lieverse et al. 1994), GLP-1 (Gutzwiller et al., 1999), PYY (Batterham et al. 2002) and oxyntomodulin (Bagger et al. 2015). Some of these hormones may also promote postprandial satiety, meaning they delay the onset of the next meal. Nausea also reduces food intake. For instance, release of 5-HT can induce nausea and vomiting in response to toxins (Andrews et al. 1990) including toxic substances used for chemotherapy. To manage ingestion of chemotherapy agents, the effects of 5-HT are prevented by 5-HT3 receptor (5-HT3R) antagonists (Carmichael et al. 1989; Wilder-Smith et al. 1993). Interestingly, the orexigenic hormone, ghrelin, suppresses nausea (Sanger and Furness 2016).
Digestion
Several gut hormones are involved in controlling the breakdown of foods by gastric acid, enzymatic degradation, propulsion of contents through the GI tract and removal of waste products (Gribble et al. 2018). Gastrin promotes histamine-stimulated acid secretion from parietal cells in the stomach, whereas secretin and 5-HT help neutralise gastric acid in the duodenum by stimulating bicarbonate release. CCK is secreted in response to breakdown products of fats and proteins and promotes the release of digestive enzymes from the pancreas and bile from the gall bladder, thus assisting in the digestive process. Furthermore, CCK, along with GLP-1, oxyntomodulin, PYY, secretin, 5-HT, somatostatin, and neurotensin, delays gastric emptying, providing more time to adequately process food that has already entered the intestine. Transit throughout the rest of the intestine can be influenced by the actions of neurotensin, motilin, and possibly by 5-HT. Several hormones influence ion transport and, consequentially, fluid movement across the epithelial lining. For example, PYY promotes fluid retention in the colon whereas 5-HT promotes fluid secretion and, when released in large quantities, can induce diarrhoea. In accordance with its effects on motility and secretion, 5-HT has been implicated in irritable bowel syndrome (IBS) with constipation or with diarrhoea (Mawe and Hoffman 2013).
Metabolism
Intestinal hormones convey signals that regulate the metabolism and storage of nutrients. GLP-1, GIP and oxyntomodulin act as incretins, meaning they promote glucose-dependent insulin secretion, protecting the body from the postprandial spike in blood glucose (Schjoldager et al., 1998; Baggio and Drucker 2007). Blood glucose levels can also be influenced by CCK, which lowers glucose production (Cheung et al. 2009), and 5-HT which promotes liver gluconeogenesis (Sumara et al. 2012). 5-HT also influences fat metabolism by promoting lipolysis (Sumara et al. 2012). Similarly, GLP-1 promotes thermogenesis in brown adipose tissue (Krieger et al., 2018), GIP promotes the uptake of triacylglyceride in adipose tissue (Asmar et al. 2017), and PYY inhibits lipolysis (Castan et al. 1993). It is possible that oxyntomodulin, which acts at the glucagon receptor, may influence amino acid metabolism as signalling at the glucagon receptor promotes ureagenesis (Galsgaard et al. 2018). The calcium sensing receptor (CaSR) is associated with several types of EEC, and some of their hormones influence bone metabolism. GIP inhibits bone resorption (Christensen et al. 2018), whereas 5-HT (Yadav et al. 2008) and PYY (Wong et al. 2012) promote bone recycling. Ghrelin has broad metabolic roles (Collden et al, 2017). It is a potent stimulant of growth hormone release, increases fat deposition, and increases circulating glucose levels (Mani and Zigman 2017).
Other roles
A number of other roles of GI hormones have been identified, for example GLP-2 stimulates gut mucosal growth and repair (Burrin et al. 2007), EEC are the source of 5-HT for platelet stores (Mawe and Hoffman 2013) and ghrelin has anti-depressant and anxiolytic effects (Mani and Zigman 2017). Several gut hormones influence the intestinal immune system (Worthington et al. 2018).
Revised narrative of EECs: plurichemical hormone expression
Gene expression
In the past decade, increased interest in the role of EECs in health and disease has precipitated a wave of studies on the expression of GI hormones. A common approach to characterize EECs has been to analyse gene expression of FACS-purified EEC using qPCR, microarray, or RNA sequencing. For example, Egerod et al. (2012) sorted cells from mice expressing eGFP under the control of the CCK promoter, and found gene transcripts for CCK, secretin, GIP, PYY, proglucagon, neurotensin, and ghrelin in the CCK cells (Table 1). By single cell qPCR they revealed that 44% of Cck-positive eGFP cells co-expressed at least one of Sct, Ghrl, or Gip. Most of these overlaps were confirmed by liquid chromatography-mass spectrometry (LC/MS) and/or immunohistochemistry in mouse and human, an exception being an absence of ghrelin peptide (Egerod et al. 2012). Interestingly, the authors did not find somatostatin gene transcripts in CCK-eGFP cells, and in a later study in which gastric somatostatin-RFP reporter cells were characterised they found gene transcripts for PYY and amylin but no other hormone (Egerod et al. 2015). Using the same CCK reporter mice, Sykaras et al. (2014) confirmed the coexpression patterns reported by (Egerod et al. 2012). However, in contrast to Egerod et al. (2012) they observed ghrelin peptide in CCK-eGFP cells using LC/MS and immunohistochemistry, and in human duodenal CCK cells using double labelled immunohistochemistry. Egerod et al. (2012) also ablated cells expressing the proglucagon gene, which reduced GLP-1, PYY, CCK, NTS, GIP, and secretin cell numbers, again demonstrating a close relationship between these hormones. In a similar study using microarrays and qPCR, cells expressing the fluorescent Venus reporter under the control of the glucagon promoter (GLU-Venus) or under the GIP promoter (GIP-Venus) contained transcripts for similar combinations of coexpressed hormones (Habib et al. 2012). Differences from the GLU-Venus cells include the presence of somatostatin and the absence of ghrelin and neurotensin transcripts in GIP-Venus cells. Single cell RNA sequencing of GLU-Venus cells of the first 10 cm of small intestine revealed three clusters, one with especially high levels of Gcg and Pyy, another high in Tph1, and another high in Gip (Glass et al. 2017). Cck, Ghrl, and Sct were especially enriched in the first two clusters.
Most studies using FACS-selected cells are limited by the efficacy and specificity of the fluorescently tagged reporter gene. To overcome this problem, single-cell RNA sequencing can be applied to entire populations of epithelial cells. Recently, Haber et al. (2017) performed single-cell RNA sequencing on a large population of epithelial cells from mouse small intestine. They identified 8 clusters of mature EEC, all of which expressed the gene for secretin. Two clusters expressed Tph1, which encodes the enzyme responsible for synthesising the 5-HT in EEC. Five clusters also expressed Cck along with either Nts, Gip, Gip plus Ghrl, Gcg plus Ghrl, or Gcg plus Pyy. Yet another cluster expressed Sec, Ghrl, Gip, and Sst. In a recent study, an antibody-based method was used to label lysed 5-HT cells for FACS (Lund et al. 2018), after FACS-purification of cells from a chromogranin A reporter failed to effectively isolate 5-HT cells (Engelstoft et al. 2015). 5-HT purified cells from the upper small intestine were enriched for secretin, tachykinin, and CCK gene transcripts, whereas colonic 5-HT cells were enriched for tachykinin but not secretin and CCK. They also demonstrated by immunohistochemistry that tachykinin peptides (substance P and neurokinin A) were expressed in 5-HT cells, confirming earlier observations (Roth and Gordon 1990).
Expression of hormones
Despite the phenomenal amount of data obtained from RNA sequencing experiments, interpretations depend on gene transcripts being translated into functional products. Immunohistochemistry studies, that remain critical to confirm the co-expression of hormones at the peptide/amine level, especially in human tissues, can confirm hormone colocalization (Fig. 1). In earlier studies, immunohistochemistry revealed some colocalizations in mice, notably colocalization of 5-HT, secretin, and tachykinins (Roth and Gordon 1990) and of GLP-1, CCK, and secretin (Aiken et al. 1994). Interestingly, this group also identified CCK cells in mouse proximal colon, where it is not classically thought to be expressed, and observed colocalization with GLP-1, PYY, and neurotensin (Roth et al. 1992). Expression of CCK in mouse colon and overlaps with PYY were recently confirmed (Fakhry et al. 2017). Early examples of colocalizations in human can also be found, for example, in the anal canal almost all GLP-1 cells contained PYY, many contained 5-HT, and none co-expressed somatostatin (Hörsch et al. 1994), and it has long been known that proglucagon peptides are frequently costored with PYY.
Fig 1.
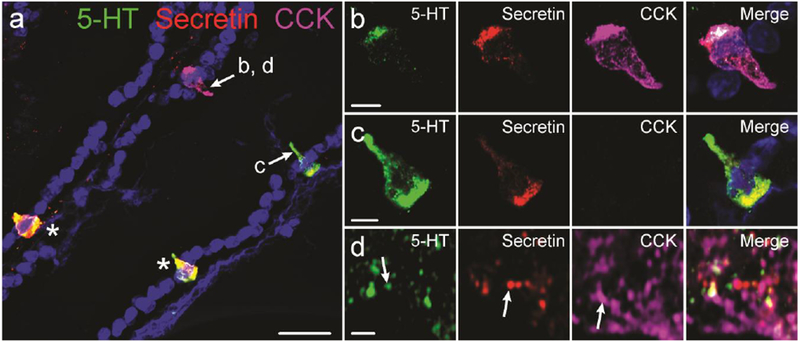
Immunohistochemical colocalization of intestinal hormones. a: 5-HT, secretin, and CCK immunolabelling in mouse duodenum. Cells indicated with arrows are shown in parts b, c, and d. Cells indicated by asterisks are labelled for 5-HT and secretin. Example of a cell containing 5-HT, secretin, and CCK (b), and a cell containing 5-HT and secretin but not CCK (c). Super-resolution image of 5-HT, secretin, and CCK vesicles within an enteroendocrine cell (d). Examples of vesicles in which only one hormone was detected are indicated by arrows. Scale bars: a: 20μm; b, c: 5μm; d: 1μm.
A complex pattern of overlap between GLP-1, PYY, and GIP has been found in mouse, pig and human intestine (Mortensen et al. 2003; Cho et al. 2015). In pigs, the majority of GLP-1 cells contained GIP in the upper small intestine, whereas cells in the lower small intestine predominantly contained PYY (Mortensen et al. 2003). In human upper small intestine, around a third of proglucagon cells contained GIP and only 15% contained PYY, whereas over half the proglucagon cells in the colon contained PYY (Theodorakis et al. 2006; Fothergill et al. 2018). Similar proportions were observed in the mouse intestine (Fothergill et al. 2018), although a study using a GIP-tdRFP mouse line, which tagged around 60% of GIP cells, found that only 5% of GIP-tdRFP cells were immunoreactive for GLP-1 (Svendsen et al. 2016), and similarly low levels of overlap were observed by immunohistochemistry in mouse small intestine (Svendsen et al. 2015). Substantial overlap also occurs between GLP-1 and combinations of neurotensin, CCK, ghrelin, and/or 5-HT in mouse small intestine (Grunddal et al. 2015; Svendsen et al. 2015; Fothergill et al. 2017). Contrary to the hypothesis that EEC begin life by expressing multiple hormones and eventually differentiate to produce only one, Grunddal et al. (2015) found that GLP-1, PYY, and neurotensin are more frequently colocalised in villus tips, suggesting that expression might expand during maturation.
Several peptide hormones are costored with the amine 5-HT. For example, in human colon, 5-HT cells frequently contained PYY and somatostatin (Martins et al. 2017). Over half of 5-HT cells in mouse duodenum contained secretin, and smaller proportions contained CCK, ghrelin, and GLP-1 (Fothergill et al. 2017). When assessing vesicle abundance at a super-resolution level, 5-HT and chromogranin A were frequently present in peptide storing EEC in relatively small numbers that may not be detected by cell-level analysis (Fothergill et al. 2017), which may explain why other studies have observed minimal overlap between 5-HT and other hormones (Aiken et al. 1994). Although chromogranin A is frequently used as a pan-enteroendocrine cell marker (Mazzawi and El-Salhy 2016), it is more closely associated with amine-storing cells, i.e. 5-HT and histamine (Engelstoft et al. 2015). Furthermore, antibodies raised against different regions of chromogranin A can also differentially label amine or peptide storing EECs (Portela–Gomes and Stridsberg 2002).
Species and regional differences in EEC populations
Although there are many similarities across species, there are also examples of marked regional and species differences in hormone expression and coexpression. One radical species difference is that motilin is expressed in humans, dogs and cats but not mice, rats or guinea-pigs (Smith et al. 1981; Sanger et al. 2011). PYY is equally abundant in the porcine duodenum and distal colon, whereas it is not present in mouse duodenum, although it is abundant in mouse colon (Mortensen et al. 2003; Cho et al. 2015; Wewer Albrechtsen et al. 2016). GIP is predominantly expressed in the upper small intestine across species. In rat and pig, neurotensin is most abundant in the ileum and upper small intestine, whereas in mice it is equally expressed in the proximal colon as in the ileum (Wewer Albrechtsen et al. 2016). CCK, which is generally assumed to be expressed in the small intestine only and is absent from human large intestine (Rehfeld 1978; Maton et al. 1984; Martins et al. 2017), is abundant in mouse cecum and proximal colon and is in lower amounts in the distal colon (Fakhry et al. 2017). Furthermore, while CCK was commonly colocalized with 5-HT in the small intestine, these hormones were never colocalized in the colon (Fakhry et al. 2017). In another example of differences between species, 5-HT is expressed in all secretin cells in cow, cat, and guinea-pig, in 70% of secretin cells in human, but very rarely in pig secretin cells (Cetin 1990).
Given the heterogeneity of EEC, it may be expected that functional differences exist between EEC subpopulations. Indeed, 5-HT cells in the duodenum exhibit a remarkably distinct repertoire of nutrient receptors compared to 5-HT cells in the colon (Martin et al. 2017). Another study by this group compared functional responses in duodenal and colonic mouse 5-HT cells, and found that duodenal cells secreted more 5-HT in response to glucose than to fructose or sucrose, and that the opposite was true of colonic 5-HT cells (Martin et al. 2017). Intraluminal glucose stimulated GLP-1 secretion from mouse upper small intestine but not from the colon (Moriya et al. 2009). A subpopulation of 5-HT cells express the mechanosensitive channel, piezo2 (Alcaino et al. 2018), whereas others do not express this channel. Thus, some EC cells, but not all, release 5-HT in response to mechanical stimulation (Alcaino et al. 2018). Single cell RNA sequencing of GLU-Venus cells also revealed differences in receptor profiles, for example, a cluster containing high levels of Tph1 was relatively enriched for CaSR, TLR5, MC4R, and SSTR2 transcripts, but not GPR119 (Glass et al. 2017). Free fatty acid receptor 2 was relatively enriched in the clusters high in Pyy and Tph1 compared to the cluster high in Gip.
Storage of hormones at a subcellular level
The recent development of super-resolution microscopy has advanced studies of the subcellular storage of hormones of EEC because it has extended resolution to below 50 nm and allowed storage vesicles, that are generally 100 - 400 nm in diameter, to be visualised in multicolour fluorescence (Stengel et al. 2010a; Cho et al. 2014; Grunddal et al. 2015; Fothergill et al. 2017)(Fig 2). Prior to this, analysis of subcellular storage required electron microscope immunocytochemistry.
Fig 2.
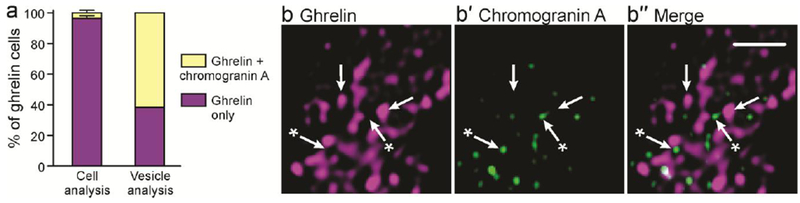
a: Contrast of the quantitative colocalisation of ghrelin and chromogranin A. Using cell level analysis, only ghrelin was detected in most cells, whereas a super-resolution vesicle analysis revealed chromogranin A in over 50% of ghrelin immunoreactive cells. Subcellular stores of ghrelin (b) and small numbers of chromogranin A vesicles (b′) are observed using super-resolution microscopy. Arrows indicate vesicles that contain ghrelin, and arrows with an asterisk indicate vesicles that contain chromogranin A. Scale bar: 1μm.
There are some instances in which hormones are clearly segregated, with some vesicles having strong immunoreactivity for one hormone, and other vesicles having immunoreactivity for another hormone, with no detectable second hormone. In other cases, two hormones appear to be in the same storage vesicle. An example of segregated storage is of ghrelin and nesfatin in the gastric X/A cells (Stengel et al. 2009). Neurotensin in EEC of human and mouse ileum is in separate stores to GLP-1 and PYY, but GLP-1 and PYY were only sometimes observed in separate stores (Grunddal et al. 2015). Furthermore, electron microscopy demonstrated that in cells coexpressing proglucagon product and PYY in human ileum, nearly all granules were labelled with GLP-1 and glicentin antisera, whereas a minority of vesicles contained PYY immunoreactivity (Eissele et al. 1992). In the colon, GLP-1 and PYY are generally in the same vesicles (Billing et al. 2018; Fothergill et al. 2018). In fact, Billing et al (2018) found that GLP-1, PYY and INSL5 were generally costored in the same vesicles in murine colonic EECs. This is consistent with an electron microscope study showing that PYY and proglucagon-derived peptides are costored within secretory vesicles in cat colon and human rectum (Böttcher et al. 1986). An electron microscope study of rabbit colon cells suggested that around 15% of vesicles contained only PYY, and that the relative abundance of PYY and proglucagon-derived peptide immunoreactivity varies substantially between vesicles (Nilsson et al. 1991).
In a recent study, co-storage of a wider range of hormones, 5-HT, chromogranin A, secretin, CCK, ghrelin, and GLP-1 has been investigated in mouse duodenum (Fothergill et al. 2017). Examples of separate storage could be demonstrated for all hormones investigated. Similarly, Sykaras (2014) found substantial separation of CCK and ghrelin, and of PYY and GIP in vesicle stores. Furthermore, GIP and oxyntomodulin vesicle stores were frequently segregated in human jejunum cells (Fothergill et al. 2018). Examples of hormones that appear predominantly co-stored in vesicles includes ghrelin and motilin, which are frequently co-expressed at a cell level in human small intestine (Wierup et al. 2007), and neurokinin A and 5-HT in mouse intestine (Lund et al. 2018), in addition to PYY and GLP-1, that are discussed above.
Most EEC hormones are stored in electron dense secretory vesicles, and a homogenous distribution of immunoreactivity for costored hormones has been demonstrated for GLP-1 and PYY by electron microscopy (Eissele et al. 1992). However, secretin and chromogranin A immunoreactivity was topologically segregated in vesicles, with secretin present in the electron dense core and chromogranin A present in the electron lucent halo (Usellini et al. 1990). This sub-vesicular difference in localisation could result in a difference in location by fluorescence microscopy that is interpreted as storage in separate vesicles. The co-storage of hormones may have functional significance, as costored hormones are presumably co-released, whereas it is theoretically possible that hormones stored in separate vesicles could be differentially released.
Relative amounts of stored hormones, the threshold problem
The strengths of immunoreactivity, amounts of hormone, and expression levels of hormone-producing genes differ considerably between cells, an example being expression of Cck varying over 1000 fold between Cck cells of the mouse duodenum (Egerod et al. 2012; Habib et al. 2012; Fothergill et al. 2017). Thus, both gene and hormone expression determinations are subject to false negatives based on thresholds for detection. However, very low levels of hormones in an EEC may not be functionally significant. In the mouse duodenum, gene expression analysis showed Ghrl was expressed at about 25% of the Cck gene copy number in FACS-separated Cck cells, and at the single cell level 13% of Cck cells had detectable Ghrl transcripts (Egerod et al. 2012). But by immunohistochemistry, no ghrelin immunoreactivity was found in CCK cells. In this same study, 37% of Cck cells expressed detectable Sct (Egerod et al. 2012), whereas by immunohistochemistry, secretin was present in 73% of CCK cells (Fothergill et al. 2017).
Examination of EEC at a resolution sufficient to investigate colocalization at a cell level may not detect hormones that are expressed in low amounts, but can be detected by super-resolution microscopy (Fig 3). In a study of colocalization of chromogranin A and ghrelin, conventional fluorescence microscopy revealed chromogranin A in only 3.5% of ghrelin cells, whereas super-resolution microscopy revealed that most ghrelin cells contained at least some chromogranin A vesicles (Fothergill et al. 2017) (Fig. 2). Moving between super-resolution microscopy and electron microscopy potentially presents the same type of problem (Fig 3). To detect a signal by fluorescence, even by super-resolution microscopy, requires photons from multiple fluorescence events, whereas the binding of a single antibody molecule linked to a gold particle can be detected by electron microscopy. Thus, a vesicle with detectable hormone by electron microscopy may not have detectable hormone by immunohistochemistry. At the vesicle level, as at the cell level, ratios of costored hormones may differ. By double immunogold staining, ratios of PYY to proglucagon-derived peptides in storage vesicle were spread over a 40-fold range (Nilsson et al. 1991).
Fig 3.
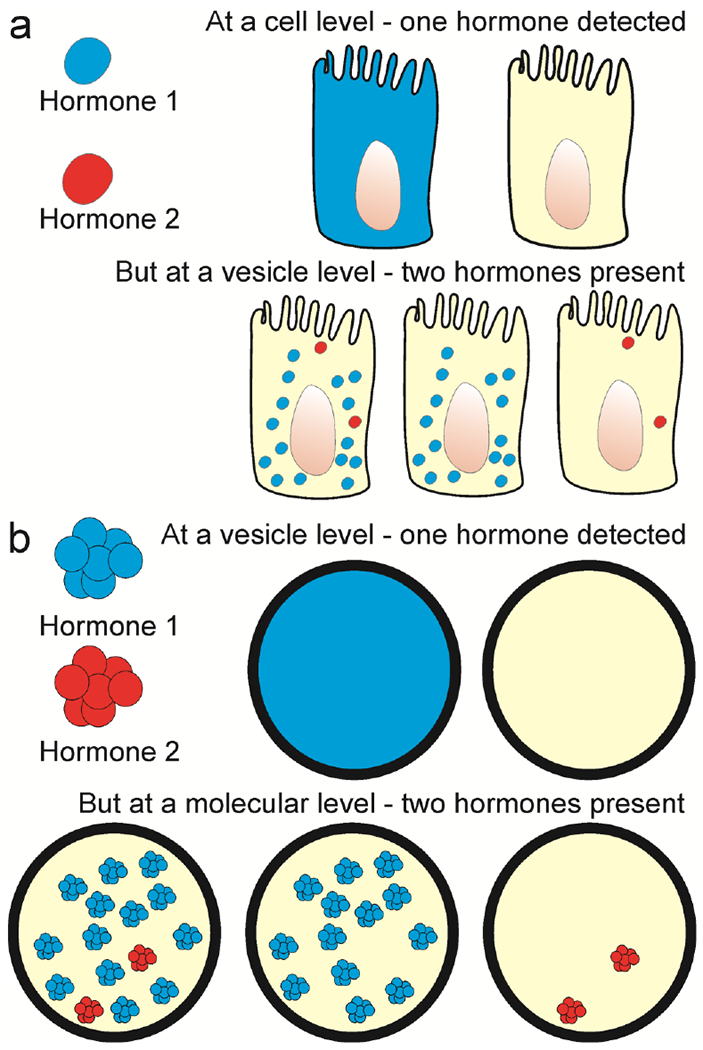
Differences in sensitivities of detection at cellular, subcellular and molecular levels. A: At a cell level, a hormone present in low abundance, in this example Hormone 2 (red), is not detectable above background at a cell level, but can be found in storage vesicles by super-resolution microscopy. B: At a storage vesicle level, hormone molecules that are present might not provide a signal that is seen by super-resolution fluorescence, but could be revealed by other methods, such as immunogold electron microscopy.
Classifying enteroendocrine cells
It is clear that new concepts of EEC taxonomy are emerging and that a nomenclature that recognises this fact is required. These cells can no longer be considered as belonging to types that are defined by a single hormone, and the letter code that has served until recently is no longer useful. Some suggestions have been made. One is that EEC be defined by organ, species, and hormones (Drucker 2015). An example would be a cell in the mouse jejunum that contains GIP and other hormones being given the designation JMGIP+SST+ GCG+PYY− (Drucker 2015). In this example, PYY minus is included to distinguish this type of cell from similar cells that do contain PYY. The difficulty with this nomenclature is that is does not easily cater for the variation between closely related cells. For example, cells isolated because of their expression of CCK, express transcripts for Gcg products, Gip, Nts, Pyy and Sct in variable amounts. Another scheme is to define cells by the now deficient letter codes in combination (Haber et al. 2017). Using this system, cells of the SILA type are typified by expression the S cell hormone gene (Sct), the I cell hormone gene (Cck), the L cell hormone gene (Gcg), and the A cell hormone gene (Ghrl). This will become confusing if the letter code is abandoned.
It is feasible to use cluster analysis to define cell groupings. For example, analysis of expression profiles of EEC draws the cells into definable clusters, within which there is cell to cell variation (Glass et al. 2017; Haber et al. 2017). A new classification could include features of clusters that go beyond hormone expression, to include, for example receptors and transcription factors.
Conclusions
Studies of EECs in the last 7 years have revealed a complexity that was only hinted at by earlier studies. It is now clear that most EECs express multiple hormones and that, at a sub-cellular level, hormones are often, but not always, concentrated in separate storage organelles. Moreover, patterns of colocalization differ between species and regions. The classification of EEC is complicated by hormones being expressed at widely different levels, meaning that individual cells may be classified differently depending on the sensitivity of detection and the ability to distinguish signal from background. In the future it is likely that EECs will be defined by clusters of attributes, not by a single hormone marker.
Acknowledgements
This work was supported by NIH (SPARC) grant ID # 1OT2OD023847 (TW Powley) to JBF and an Australian Government Research Training Program Scholarship to LJF.
References
- Aiken KD, Kisslinger JA, Roth KA (1994) Immunohistochemical studies indicate multiple enteroendocrine cell differentiation pathways in the mouse proximal small intestine. Dev Dyn 201:63–70 [DOI] [PubMed] [Google Scholar]
- Alcaino C, Knutson KR, Treichel AJ, Yildiz G, Strege PR, Linden DR, Li JH, Leiter AB, Szurszewski JH, Farrugia G, Beyder A (2018) A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci USA 10.1073/pnas.1804938115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali Rachedi A, Varndell IM, Adrian TE, Gapp DA, Van Noorden S, Bloom SR, Polak JM (1984) Peptide YY immunoreactivity is co-stored with glucagon related immunoreactants in endocrine cells of the gut and pancreas. Histochemistry 80:487–491 [DOI] [PubMed] [Google Scholar]
- Andrews PLR, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L (1990) The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 68:325–345 [DOI] [PubMed] [Google Scholar]
- Asmar M, Asmar A, Simonsen L, Gasbjerg LS, Sparre-Ulrich AH, Rosenkilde M, Hartmann B, Dela F, Holst JJ, Bülow J (2017) The gluco- and liporegulatory and the vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes db 170480. [DOI] [PubMed] [Google Scholar]
- Bagger JI, Holst JJ, Hartmann B, Andersen B, Knop FK, Vilsbøll T (2015) Effect of oxyntomodulin, glucagon, GLP-1, and combined glucagon +GLP-1 infusion on food intake, appetite, and resting energy expenditure. J Clin Endocrinol Metab 100:4541–4552 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, et al. (2002) Gut hormone PYY3-36 physiologically inhibits food intake. Nature 418:650. [DOI] [PubMed] [Google Scholar]
- Bayliss WM, Starling EH (1902) The mechanism of pancreatic secretion. J Physiol (Lond) 28:325–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing LJ, Smith CA, Larraufie P, et al. (2018) Co-storage and release of insulin-like peptide-5, glucagon-like peptide-1 and peptideYY from murine and human colonic enteroendocrine cells. Molec Metab 10.1016/j.molmet.2018.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher G, Alumets J, Håkanson R, Sundler F (1986) Co-existence of glicentin and peptide YY in colorectal L-cells in cat and man. An electron microscopic study. Regul Pept 13:283–291 [DOI] [PubMed] [Google Scholar]
- Burrin DG, Stoll B, Guan X, Cui L, Chang X, Hadsell D (2007) GLP-2 rapidly activates divergent intracellular signaling pathways involved in intestinal cell survival and proliferation in neonatal piglets. Am J Physiol Endocrinol Metab 292:E281–E291 [DOI] [PubMed] [Google Scholar]
- Carmichael J, Cantwell BMJ, Edwards CM, Zussman BD, Thompson S, Rapeport WG, Harris AL (1989) A pharmacokinetic study of granisetron (BRL 43694A), a selective 5-HT3 receptor antagonist: correlation with anti-emetic response. Cancer Chemother Pharmacol 24:45–49 [DOI] [PubMed] [Google Scholar]
- Castan I, Valet P, Larrouy D, Voisin T, Remaury A, Daviaud D, Laburthe M, Lafontan M (1993) Distribution of PYY receptors in human fat cells: an antilipolytic system alongside the α2-adrenegic system. Am J Physiol Endocrinol Metab 265:E74–E80 [DOI] [PubMed] [Google Scholar]
- Cetin Y (1990) Secretin-cells of the mammalian intestine contain serotonin. Histochemistry 93:601–606 [DOI] [PubMed] [Google Scholar]
- Cheung GWC, Kokorovic A, Lam CKL, Chari M, Lam TKT (2009) Intestinal cholecystokinin controls glucose production through a neuronal network. Cell Metabolism 10:99–109 [DOI] [PubMed] [Google Scholar]
- Cho H-J, Kosari S, Hunne B, Callaghan B, Rivera LR, Bravo DM, Furness JB (2015) Differences in hormone localisation patterns of K and L type enteroendocrine cells in the mouse and pig small intestine and colon. Cell Tissue Res 359:693–698 [DOI] [PubMed] [Google Scholar]
- Cho H-J, Robinson ES, Rivera LR, McMillan PJ, Testro A, Nikfarjam M, Bravo DM, Furness JB (2014) Glucagon-like peptide 1 and peptide YY are in separate storage organelles in enteroendocrine cells. Cell Tissue Res 357:63–69 [DOI] [PubMed] [Google Scholar]
- Christensen MB, Lund A, Calanna S, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK (2018) Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab 103:288–294 [DOI] [PubMed] [Google Scholar]
- Coate KC, Kliewer SA, Mangelsdorf DJ (2014) Snapshot: Hormones of the gastrointestinal tract. Cell 159:1478–1479 [DOI] [PubMed] [Google Scholar]
- Collden G, Tschop MH, Muller TD, 2017. Therapeutic potential of targeting the ghrelin pathway. Int J Mol Sci 18, 798–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001) A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50:1714–1719 [DOI] [PubMed] [Google Scholar]
- Drucker DJ (2015) Evolving concepts and translational relevance of enteroendocrine cell biology. J Clin Endocrinol Metab 101:778–786 [DOI] [PubMed] [Google Scholar]
- Edkins JS (1906) The chemical mechanism of gastric secretion. J Physiol (Lond) 34:133–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Grunddal KV, et al. (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153:5782–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod KL, Engelstoft MS, Lund ML, Grunddal KV, Zhao M, Barir-Jensen D, Nygaard EB, Petersen N, Holst JJ, Schwartz TW (2015) Transcriptional and functional characterization of the G protein-coupled receptor repertoire of gastric somatostatin cells. Endocrinology 156, 3909–3923 [DOI] [PubMed] [Google Scholar]
- Eissele R, Göke R, Willemer S, Harthus H-P, Vermeer H, Arnold R, Göke B (1992) Glucagon-like peptide-I cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22:283–291 [DOI] [PubMed] [Google Scholar]
- Engelstoft MJ, Lund ML, Grunddal KV, Egerod KL, Osborne-Lawrence S, Poulsen SS, Zigman JM, Schwartz TW (2015) Research resource: a chromogranin A reporter for serotonin and histamine secreting enteroendocrine cells. Mol Endocrinol 29:1658–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhry J, Wang J, Martins P, Fothergill LJ, Hunne B, Prieur P, Shulkes A, Rehfeld JF, Callaghan B, Furness JB (2017) Distribution and characterisation of CCK containing enteroendocrine cells of the mouse small and large intestine. Cell Tissue Res 369:245–253 [DOI] [PubMed] [Google Scholar]
- Fothergill LJ, Callaghan B, Hunne B, Bravo DM, Furness JB (2017) Costorage of enteroendocrine hormones evaluated at the cell and subcellular levels in male mice. Endocrinology 158:2113–2123 [DOI] [PubMed] [Google Scholar]
- Fothergill LJ, Ringuet MT, Sioras E, Hunne B, Fazio Coles TE, Martins P, Furness JB (2018) Cellular and sub-cellular localisation of oxyntomodulin-like immunoreactivity in enteroendocrine cells of human, mouse, pig, and rat. Cell Tissue Res in press [DOI] [PubMed] [Google Scholar]
- Galsgaard KD, Winther-Sørensen M, Ørskov C, et al. (2018) Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol. Endocrinol Metab 314:E93–E103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass LL, Calero-Nieto FJ, Jawaid W, Larraufie P, Kay RG, Göttgens B, Reimann F, Gribble FM (2017) Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab 6:1296–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Reimann F (2016) Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu Rev Physiol 78:277–299 [DOI] [PubMed] [Google Scholar]
- Gribble FM, Reimann F, Roberts GP, 2018. Gastrointestinal Hormones In: Physiology of the Gastrointestinal Tract (Sixth Edition), ed. Said HM. Elsevier, pp. 31–70. [Google Scholar]
- Grunddal KV, Ratner CF, Svendsen B, et al. (2015) Neurotensin is co-expressed, co-released and acts together with GLP-1 and PYY in enteroendocrine control of metabolism. Endocrinology 157:176–194 [DOI] [PubMed] [Google Scholar]
- Gutzwiller J, et al. , 1999. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber AL, Biton M, Rogel N, et al. (2017) A single-cell survey of the small intestinal epithelium. Nature 551:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153:3054–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson R, Ding X-Q, Norlén P, Chen D (1995) Circulating pancreastatin is a marker for the enterochromaffin-like cells of the rat stomach. Gastroenterology 108:1445–1452 [DOI] [PubMed] [Google Scholar]
- Heidenhain R (1870) Untersuchungen über den Bau der Labdrüsen. Arch Mikroskop Anat 6:368–406 [Google Scholar]
- Helander HF, Fändriks L (2012) The enteroendocrine “letter cells” – time for a new nomenclature? Scand J Gastroenterol 47:3–12 [DOI] [PubMed] [Google Scholar]
- Hörsch D, Fink T, Göke B, Arnold R, Büchler M, Weihe E (1994) Distribution and chemical phenotypes of neuroendocrine cells in the human anal canal. Regul Pept 54:527–542 [DOI] [PubMed] [Google Scholar]
- Krieger J-P, et al. , 2018. Glucagon-like peptide-1 regulates brown adipose tissue thermogenesis via the gut-brain axis in rats. Am J Physiol 315: R708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieverse RJ, Jansen JBMJ, Masclee AAM, Rovati LC, Lamers CBHW (1994) Effect of a low dose of intraduodenal fat on satiety in humans: studies using the type A cholecystokinin receptor antagonist loxiglumide. Gut 35:501–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MJ, Upchurch BH, Rindi G, Leiter AB (1995) Studies in transgenic mice reveal potential relationships between secretin-producing cells and other endocrine cell types. J Biol Chem 270:885–891 [DOI] [PubMed] [Google Scholar]
- Lukinius AIC, Ericsson JLE, Lundqvist MK, Wilander EMO (1986) Ultrastructural localization of serotonin and polypeptide YY (PYY) in endocrine cells of the human rectum. J Histochem Cytochem 34:719–726 [DOI] [PubMed] [Google Scholar]
- Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW (2018) Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. MolMetab 11, 70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Zigman JM (2017) Ghrelin as a survival hormone. Trends Endocrinol. Metab 28:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Lumsden AL, Young RL, Jessup CF, Spencer NJ, Keating DJ (2017) The nutrient-sensing repertoires of mouse enterochromaffin cells differ between duodenum and colon. Neurogastroenterol Motil 29, el3046. [DOI] [PubMed] [Google Scholar]
- Martins P, Fakhry J, Chaves de Oliveira E, Hunne B, Fothergill LJ, Ringuet M, d’Ávila Reis D, Rehfeld JF, Callaghan B, Furness JB (2017) Analysis of enteroendocrine cell populations in the human colon. Cell Tissue Res 367:361–368 [DOI] [PubMed] [Google Scholar]
- Maton PN, Selden AC, Chadwick VS (1984) Differential distribution of molecular forms of cholecystokinin in human and porcine small intestinal mucosa. Regul. Pept 8:9–19 [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM (2013) Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10:473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzawi T, El-Salhy M (2016) Changes in small intestinal chromogranin A-immunoreactive cell densities in patients with irritable bowel syndrome after receiving dietary guidance. Int J Molec Med 37:1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya R, Shirakura T, Ito J, Mashiko S, Seo T (2009) Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297:E1358–E1365 [DOI] [PubMed] [Google Scholar]
- Mortensen K, Christensen LL, Holst JJ, Orskov C (2003) GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 114:189–196 [DOI] [PubMed] [Google Scholar]
- Nilsson O, Bilchik AJ, Goldenring JR, Ballantyne GH, Adrian TE, Modlin IM (1991) Distribution and immunocytochemical colocalization of peptide YY and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology 129:139–148 [DOI] [PubMed] [Google Scholar]
- Pearse AGE, Polak JM, Bloom SR, Adams C, Dryburgh JR, Brown JC (1974) Enterochromaffin cells of the mammalian small intestine as the source of motilin. Virchows Arch B 16:111–120 [DOI] [PubMed] [Google Scholar]
- Portela–Gomes GM, Stridsberg M (2002) Chromogranin A in the human gastrointestinal tract: an immunocytochemical study with region-specific antibodies. J Histochem Cytochem 50:1487–1492 [DOI] [PubMed] [Google Scholar]
- Rehfeld JF (1978) Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog. J Biol Chem 253:4022–4030 [PubMed] [Google Scholar]
- Rehfeld JF (2012) Beginnings: A reflection on the history of gastrointestinal endocrinology. Regul Pept 177: S1–S5 [DOI] [PubMed] [Google Scholar]
- Reynaud Y, Fakhry J, Fothergill L, Callaghan B, Ringuet MT, Hunne B, Bravo DM, Furness JB (2016) The chemical coding of 5-hydroxytryptamine containing enteroendocrine cells in the mouse gastrointestinal tract. Cell Tissue Res 364:489–497 [DOI] [PubMed] [Google Scholar]
- Roth KA, Gordon JI (1990) Spatial differentiation of the intestinal epithelium: Analysis of enteroendocrine cells containing immunoreactive serotonin, secretin, and substance P in normal and transgenic mice. Proc Natl Acad Sci USA 87:6408–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, Kim S, Gordon JI (1992) Immunocytochemical studies suggest two pathways for enteroendocrine cell differentiation in the colon. Am J Physiol 263:G174–G180 [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Furness JB (2016) Ghrelin and motilin receptors as drug targets for gastrointestinal disorders. Nat Rev Gastroenterol Hepatol 19:38–48 [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Holbrook JD, Andrews PLR (2011) The translational value of rodent gastrointestinal functions: a cautionary tale. Trends Pharmacol Sci 32:402–409 [DOI] [PubMed] [Google Scholar]
- Schjoldager B, et al. , 1988. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Europ J Clinical Invest 18: 499–503. [DOI] [PubMed] [Google Scholar]
- Smith PH, Davis BJ, Seino Y, Yanaihara N (1981) Localization of motilin-containing cells in the intestinal tract of mammals: a further comparison using region-specific motilin antisera. Gen Comp Endocrinol 44:288–291 [DOI] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Taché Y (2010a) Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: Role as regulators of food intake and body weight. Peptides 31:357–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Wang L, Taché Y, Sachs G, Lambrecht NWG (2010b) Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem Biophys Res Commun 392:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, Tache Y, Scachs G, Lambrecht NWG (2009) Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology 150:232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara G, Sumara O, Kim JK, Karsenty G (2012) Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metabolism 16:588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen B, Pais R, Engelstoft MS, et al. (2016) GLP1- and GIP-producing cells rarely overlap and differ by bombesin receptor-2 expression and responsiveness. J Endocrinol 228:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen B, Pedersen J, Albrechtsen NJW, Hartmann B, Toräng S, Rehfeld JF, Poulsen SS, Holst JJ (2015) An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 156:847–857 [DOI] [PubMed] [Google Scholar]
- Sykaras AG, Demenis C, Cheng L, Pisitkun T, Mclaughlin JT, Fenton RA, Smith CP (2014) Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155:3339–3351 [DOI] [PubMed] [Google Scholar]
- Theodorakis MJ, Carlson O, Michopoulos S, Doyle ME, Juhaszova M, Petraki K, Egan JM (2006) Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290:E550–E559 [DOI] [PubMed] [Google Scholar]
- Usellini L, Finzi G, Riva C, Capella C, Mochizuki T, Yanaihara C, Yanaihara N, Solcia E (1990) Ultrastructural identification of human secretin cells by the immunogold technique. Their costorage of chromogranin A and serotonin. Histochemistry 94:113–120 [DOI] [PubMed] [Google Scholar]
- Vialli M, Erspamer V (1933) Celluli enterocromaffini e cellule basigranulose acidofile nei vertebrati. Z Zellforsch Mikroskop Anat 19:743–773 [Google Scholar]
- Wewer Albrechtsen NJ, Kuhre RE, Toräng S, Holst JJ (2016) The intestinal distribution pattern of appetite and glucose regulatory peptides in mice, rats and pigs. BMC Research Notes 9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierup N, Björkqvist M, Weström B, Pierzynowski S, Sundler F, Sjölund K (2007) Ghrelin and motilin are cosecreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab 92:3573–3581 [DOI] [PubMed] [Google Scholar]
- Wilder-Smith OHG, Borgeat A, Chappuis P, Fathi M, Forni M (1993) Urinary serotonin metabolite excretion during cisplatin chemotherapy. Cancer 72:2239–2241 [DOI] [PubMed] [Google Scholar]
- Wong IPL, Driessler F, Khor EC, Shi Y-C, Hörmer B, Nguyen AD, Enriquez RE, Eisman JA, Sainsbury A, Herzog H, Baldock PA (2012) Peptide YY regulates bone remodeling in mice: a link between gut and skeletal biology. PLoS One 7:e40038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington JJ, Reimann F, Gribble FM (2018) Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity. Mucosal Immunology 11:3. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Ryu J-H, Suda N, et al. (2008) Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837 [DOI] [PMC free article] [PubMed] [Google Scholar]


