Immune checkpoint inhibitors enhance the immune response against tumors but may also trigger immune-related adverse events (IRAEs). Myositis is a rare IRAE. For example, creatine kinase (CK) elevations occurred in just 0.3% of those treated with avelumab, an anti-programmed death-ligand 1 antibody1.
Thymomas are the most common anterior mediastinal masses in adults. Since effective systemic therapies for thymic epithelial tumors are lacking, we included 7 patients with recurrent thymoma and 1 patient with recurrent thymic carcinoma in a phase I trial of avelumab (NCT01772004). Details regarding this trial have been published separately2.
Myasthenia gravis and myositis occur in up to 30% and 5% of thymoma patients, respectively3. Although no patient had a history of autoimmunity or weakness and each had normal baseline CK levels, four patients developed weakness and elevated CK levels, ranging from 762 IU/L to 16,037 IU/L, within 5 weeks of avelumab administration (see Supplementary Text and Supplementary Table 1). CK levels normalized in patients within weeks of stopping avelumab and starting immunosuppressive therapy. Of note, one patient with myositis also had myocarditis and one patient without myositis developed autoimmune enteritis.
We tested for thymoma-associated autoantibodies in sera collected before and after avelumab treatment (Table 1). Four patients had preexisting muscle acetylcholine receptor (mAChR) autoantibodies and each developed CK elevations. No patient without mAChR autoantibodies developed myositis (100% vs. 0%; p=0.029). Myositis and myasthenia have been reported to occur together as an IRAE4. Although we cannot exclude the possibility that our patients could have had both myositis and myasthenia, electrophysiological studies revealed evidence of a neuromuscular junction defect in just one patient. Three patients had both mAChR and striational autoantibodies. Voltage-gated potassium channel autoantibodies were found in two patients and one of them developed myositis; neither patient developed manifestations of potassium-channel autoimmunity. Since approximately 70% of myositis patients have a myositis-specific autoantibody (MSA), we screened pre- and post-avelumab serum samples for 16 MSAs using Autoimmune Inflammatory Myopathies line blots (EUROIMMUN). Although no patient had an MSA on this panel, we cannot exclude the possibility that they may have had an unidentified, potentially pathogenic, autoantibody.
Table 1.
Serum CK levels and thymoma-associated autoantibody levels in thymoma patients before and after avelumab treatment.
| Patient | Serum CK (IU/L) |
Anti-AChR (nmol/L) |
anti-STR (dilutions) |
anti-VGKC (nmol/L) |
anti- GAD65 (nmol/L) |
anti-α3 (nmol/L) |
anti- CRMP5 |
anti- AMPAR |
anti- GABABR |
anti- NMDA |
anti- LGI1 |
anti- Caspr2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 pre-avelumab | 55 | 2.59 | 3840 | 0 | NT | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #1 day 15 post-avelumab | 1792 | 2.36 | 1920 | 0 | 0.02 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #2 pre-avelumab | 86 | 0.21 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #2 day 43 post-avelumab | 1046 | 0.24 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #3 pre-avelumab | 130 | 0.36 | 7680 | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #3 day 15 post-avelumab | 3939 | 0.31 | 7680 | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #4 pre-avelumab | 77 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #4 day 15 post-avelumab | 60 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #5 pre-avelumab | 435 | 0 | Neg | 0.11 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #5 day 15 post-avelumab | 473 | 0 | Neg | 0.15 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #6 pre-avelumab | 91 | 0.73 | 30720 | 0.06 | 0 | 0 | Neg | Neg | Neg | Neg | POS | Neg |
| #6 day 8 post-avelumab | 762 | 0.67 | 61440 | 0.01 | 0 | 0 | Neg | Neg | Neg | Neg | POS | Neg |
| #7 pre-avelumab | 87 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #7 day 15 post-avelumab | 74 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #8 pre-avelumab | 45 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | Neg | Neg |
| #8 day 15 post-avelumab | 28 | 0 | Neg | 0 | 0 | 0 | Neg | Neg | Neg | Neg | NEg | Neg |
CK=creatine kinase; AChR=acetylcholine receptor; STR=striational; VGKC=voltage gated potassium channel; GAD65=glutamic acid decarboxylase 65; α3= ganglionic α3 acetylcholine receptor; CRMP5=collapsing response-mediator protein-5; AMPAR= -amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; GABAR=gamma-aminobutyric acid receptor; NMDA=N-methyl-D-aspartate receptor; LGI1=leucine-rich, glioma-inactivated 1; Casper2=contactin-associated protein-like 2. Note that serum CK levels included in this table are those obtained at the time sera was collected for autoantibody testing and may not reflect peak CK levels for a given patient. Autoantibody testing was performed at the Neuroimmunology Laboratory, Mayo Clinic, Rochester, MN.
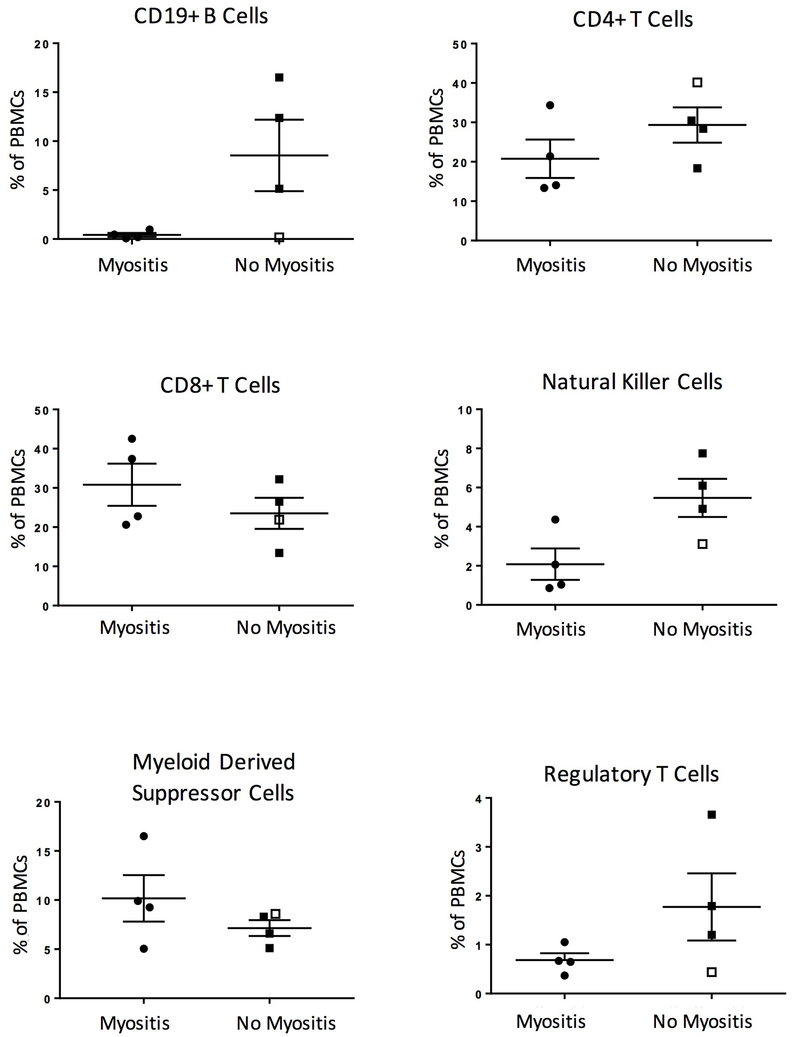
Flow cytometry performed on peripheral blood mononuclear cells collected prior to avelumab therapy revealed that patients who developed myositis had low B cell frequencies (Figure 1 and Supplementary Tables 2 and 3). A single patient without myositis, but who developed enteritis, also had low B cell levels. Taken together, thymoma patients who developed myositis or enteritis had lower B cell frequencies (0.19%, 0.12-0.73%; median, interquartile range) than thymoma patients who did not (12.37%, 5.14-16.5%), those with non-thymic malignancies (8.3%, 2.4-11.7%), or healthy controls (16.3%, 11.9-17.65%).
Figure 1.
Immune cell subsets in thymoma patients prior to treatment with avelumab. Flow cytometry was performed on peripheral blood mononuclear cells prior to avelumab treatment. Cell types were defined as follows: regulatory T cells are CD4+ CD25+ FoxP3+ CD127−, natural killer cells are CD56+ CD3−, and myeloid derived suppressor cells are CD11b+ HLA-DRlow/− CD33+. The median and interquartile ranges are indicated by long and short horizontal bars, respectively. The patient without myositis who developed autoimmune enteritis is noted with an open square.
These observations suggest that testing for mAChR autoantibodies and/or B cell levels may identify thymoma patients most at risk for developing myositis with avelumab. Since mAChR autoantibodies cause myasthenia but not myositis or elevated CK levels, and because mAChR autoantibody levels did not increase with myositis, we conclude that they are most likely a marker of preexisting autoimmunity rather than the direct cause of muscle damage. B cell lymphopenia, which occurs in half of thymoma patients5, has not been described in myositis. Interestingly, a recent study reported that declining B cells preceded the development of IRAEs in melanoma patients following combination CTLA4 and PD1 checkpoint blockade; however, unlike the patients described here, these patients had normal B cell levels prior to checkpoint blockade6. It remains unclear why declining B cell levels would be associated with IRAEs, including myositis.
Additional studies are needed to confirm these findings and to determine whether preexisting autoantibodies or immune cell subset dysregulation predicts which non-thymic tumor patients are at increased risk for IRAEs.
Supplementary Material
Acknowledgments
Funding sources: This work was financially supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases and National Cancer Institute of the National Institutes of Health. LCR is funded in part by the Donald B. and Dorothy L. Stabler Foundation.
REFERENCES
- 1.Kelly K, Infante JR, Taylor MH, et al. Safety profile of avelumab in patients with advanced solid tumors: A pooled analysis of data from the phase 1 JAVELIN solid tumor and phase 2 JAVELIN Merkel 200 clinical trials. Cancer 2018. doi: 10.1002/cncr.31293 [published Online First: 2018/02/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heery CR, O'Sullivan-Coyne G, Madan RA, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol 2017;18(5):587–98. doi: 10.1016/S1470-2045(17)30239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard C, Frih H, Pasquet F, et al. Thymoma associated with autoimmune diseases: 85 cases and literature review. Autoimmun Rev 2016;15(1):82–92. doi: 10.1016/j.autrev.2015.09.005 [published Online First: 2015/09/27] [DOI] [PubMed] [Google Scholar]
- 4.Chen JH, Lee KY, Hu CJ, et al. Coexisting myasthenia gravis, myositis, and polyneuropathy induced by ipilimumab and nivolumab in a patient with non-small-cell lung cancer: A case report and literature review. Medicine (Baltimore) 2017;96(50):e9262. doi: 10.1097/MD.0000000000009262 [published Online First: 2018/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montella L, Masci AM, Merkabaoui G, et al. B-cell lymphopenia and hypogammaglobulinemia in thymoma patients. Ann Hematol 2003;82(6):343–7. doi: 10.1007/s00277-003-0635-z [published Online First: 2003/04/26] [DOI] [PubMed] [Google Scholar]
- 6.Das R, Bar N, Ferreira M, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 2018;128(2):715–20. doi: 10.1172/JCI96798 [published Online First: 2018/01/09] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.