Abstract
Objective:
We previously postulated that 2-deoxyglucose (2-DG) activates multiple pro-survival pathways through IGF1R to negate its inhibitory effect on glycolysis. Here, we evaluated whether IGF1R inhibitor synergizes with 2-DG to impede the growth of non-small cell lung cancer (NSCLC).
Materials and methods:
The activation of IGF1R signaling was assessed by the phosphorylation of IGF1R and its downstream target AKT using immunoblot. Drug dose response and combination index analyses were carried out according to the method of Chou and Talalay. Flow cytometry was used to evaluate cell cycle progression. Apoptosis was monitored by caspase-3/PARP cleavages or Annexin V staining. A subcutaneous xenograft model was used to assess this combination in vivo.
Results:
2-DG induces the phosphorylation of IGF1R in its kinase domain, which can be abolished by the IGF1R inhibitor BMS-754807. Furthermore, the combination of 2-DG and BMS-754807 synergistically inhibited the survival of several non-small cell lung cancer (NSCLC) cell lines both in vitro and in vivo. The mechanistic basis of this synergy was cell line-dependent, and LKB1-inactivated EKVX cells underwent apoptosis following treatment with a subtoxic dose of 2-DG and BMS-754807. For these cells, the restoration of LKB1 kinase activity suppressed apoptosis induced by this combination but enhanced G1 arrest. In H460 cells, the addition of 2-DG did not enhance the low level of apoptosis induced by BMS-754807. However, treatment with 0.75 μM of BMS-754807 resulted in the accumulation of H460 cells with 8n-DNA content without affecting cell density increases. Hence, H460 cells may escape BMS-754807-induced G2/M cell cycle arrest through polyploidy. The inclusion of 2-DG blocked formation of the 8n-DNA cell population and restored G2/M phase cell cycle arrest.
Conclusion:
The combination of 2-DG and IGF1R inhibitor BMS-754807 may be used to suppress the proliferation of NSCLC tumors through different mechanisms.
Keywords: cancer therapy, energy metabolism, insulin-like growth factor receptor 1 (IGF1R), lung cancer, tumor suppressor gene
1. Introduction
Non-small cell lung cancer (NSCLC) cells usually have a high rate of aerobic glycolysis. We have previously evaluated the vulnerability of NSCLC cells to 2-deoxyglucose (2-DG), a commonly used glycolytic inhibitor (1–3). This reagent inhibits glycolysis, depletes intracellular ATP level, and suppresses cell growth in general (4–7). At a concentration over 20 mM in vitro, 2-DG has been shown to induce poly ADP ribose polymerase (PARP) cleavage in LKB1-inactivated cervical cancer and NSCLC cells (8,9). Nevertheless, implementation of 2-DG as a therapeutic agent has several obstacles. First, even though 2-DG has been tested in human clinical trials, the highest 2-DG plasma concentration detected was 5.5 mM. Therefore, other reagents may be needed to potentiate 2-DG’s effects in LKB1-inactivated cells at physiologically achievable concentrations. Second, 2-DG has other biological effects in addition to the inhibition of glycolysis (7,10). For example, we previously discovered that 2-DG activates multiple pro-survival signaling pathways through an IGF1R (insulin-like growth factor receptor)-dependent mechanism, which offsets its growth inhibitory effect (1,2). While a similar observation was made in a study of leukemia cells (11), 2-DG failed to induce IGF1R phosphorylation in several breast or cervical cancer cell lines (12). Because of these contradictory findings, we evaluated whether 2-DG is capable of inducing IGF1R phosphorylation in NSCLC cell lines, and more importantly, whether BMS-754807, a clinical grade IGF1R inhibitor, can block 2-DG mediated IGFR activation.
BMS-754807 was developed by Bristol-Myers Squibb as an IGF1R inhibitor in 1999. It co-crystalizes with the kinase domain of IGF1R, and it occupies both the gatekeeper region of the kinase and the sugar pocket (13). In vitro inhibition assays using recombinant proteins indicated IC50 values of BMS-754807 against IGF1R and insulin receptor of 1.8 and 1.7 nM, respectively, and it is also capable of inhibiting IGF1R phosphorylation in vivo (14). This compound is orally available and has no significant ion channel activity, hepatotoxicity or genotoxicity (13). It has been evaluated previously in two lung cancer cell lines for its combined effect with either platinum or gefitinib (15,16). Here, we will determine whether BMS-754807 can be used to sensitize NSCLC cells to 2-DG both in vitro and in vivo.
2. Materials and methods
2.1. Reagents and antibodies
2-DG and mouse polyclonal anti-actin antibody were purchased from Sigma-Aldrich (St. Louis, MO). BMS-754807 was obtained from Bristol-Myers Squibb through an MTA. Antibodies against AKT, phospho-AKT (p-AKTSer473), IGFR, phospho-IGFR (Tyr1135/1136), phospho-ACC, caspase-3, PARP, and MDR1 (cat#13978) were purchased from Cell Signaling Technology, Inc. (Beverly, MA). The MDR1 antibody recognizes both human and mouse MDR1. Mouse monoclonal anti-LKB1 antibody was purchased from Abcam. Anti-actin antibody was purchased from Sigma.
2.2. Cell culture
NSCLC cell lines (H460 and H157) were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA) and were propagated according to the conditions recommended by ATCC. EKVX cells were obtained from NIH. Lkb1-null MEF cells were a gift from Dr. Ronald DePinho’s laboratory. The identities of all cell lines used in this study were validated by genotyping service at Emory University.
2.3. Immunoblot analysis
The procedures for preparation of whole cell protein lysates and for Western blotting were performed as described previously (17). The same blots were used for probing with phospho-specific antibodies and antibodies against total protein. Actin was used as a loading control. The Western analyses presented in this study were carried out at least twice, and representative images were shown in the figures. For quantitation analysis, the density of phospho-IGF1R, total IGF1R, pAKT, and total AKT was determined using the NIH ImageJ package (http://rsbweb.nih.gov/ij/). Phosphorylation ratio was calculated using the following formula:
2.4. SRB assay
2000–3000 cells were seeded in 96-well plates, treated with the indicated chemicals for 48 or 72 hrs and subjected to SRB assay as described previously (1). Reactions were carried out in quadruplicate, and error bars represent one standard deviation. Dose response and combination index analyses were carried out according to the method of Chou and Talalay with CompuSyn software (18).
2.5. Colony formation assay
EKVX and Lkb1-null MEF cells were seeded at 4500 cells per well in 12-well plates. H460 cells were seeded at 100 cells per well in 6-well plates. Cells were allowed to attach overnight and then treated with 2-DG, BMS-754807 or their combination for 6 or 14 days, and medium was replaced every 4 days. At the end of this assay, cells were stained with 0.2% crystal violet in 10% unbuffered formalin for 20min. Apoptosis analysis- Apoptosis was measured using the Annexin V-PE Apoptosis Detection kit (BD Pharmingen, San Jose, CA) followed by flow cytometry as previously described (2).
2.6. Cell cycle analysis
Cell cycle analysis was carried out as previously described (19). Briefly, cells were seeded in 6-well plates and treated with the indicated chemicals for the indicated times. Cells were stained with PI/RNASE staining kit (BD Biosciences, San Jose, CA) and analyzed by FACS analysis (BD Biosciences). A total of 10,000 gated cells were acquired for each analysis. Results were analyzed using FlowJo version 7 software (FlowJo. LLC, Ashland, OR).
2.7. Retrovirus infection
pBabe retrovirus encoding empty vector, wild-type LKB1 or dominant-negative LKB1 (K78M) was generated as previously described (19). EKVX and Lkb1-null MEF cells were infected with virus and then selected in puromycin to generate stable cell lines. pBabe vectors were purchased from Addgene.
2.8. Mouse xenograft studies
Human xenograft studies were approved and conducted according to Emory University Institutional Animal Care and Use Committee (IACUC) guidelines. 5–6 week old female athymic nude mice (18–20g) were purchased from Harlan Laboratories. Exponentially growing H460 cells were trypsinized, washed twice with PBS and diluted to 5 × 106 cells per 100 μL PBS. The cell suspension was injected subcutaneously into the right flank of mice with a 1ml-syringe with 26½-gauge needle. Mice were randomly allocated into 4 groups (vehicle control, 2-DG, BMS-754807, and 2-DG+BMS-754807), 7 mice per group. Treatment began when tumors grew to about 50 mm3 (on the 6th day). BMS-754807 was prepared in a mixture of polyethylene glycol 400 (PEG400/water (4:1; vol/vol)) at a concentration of 1.25 mg/ml. 2-DG was dissolved in distilled water at a concentration of 2000 mg/ml. BMS-754807 or 2-DG was administered orally at 0.005 ml/g of body weight. The combination group was administered both agents. The control group was administered PEG400/water. All treatments were administered twice a day for 14 days. Tumor size and weight were measured every other day, and the tumor volume was calculated with the formula [volume = 3.14 × (height × width × width)/6]. Tumors were harvested on the 14th day and weighed. Statistical analysis was performed using SAS Version 9.3. The mixed effects model was mainly used with the tumor volume being log transformed. The fixed effects were group, days, and their interaction. The within-mouse variance covariance structure was chosen to minimize Akaike information criterion (AIC). Based on the fitted model, the tumor growth rate was estimated for each group and compared with the control group. The analysis of tumor weight was carried out by t-test. The p-value was also adjusted by Bonferroni method for multiple comparisons. The underlying statistical assumption was checked accordingly. Xenograft tumor tissues were harvested and analyzed for Ki67 as previously described (pre-diluted from Life Technologies) (20).
3. Results
3.1. BMS-754807 inhibits 2-DG-induced IGF1R phosphorylation
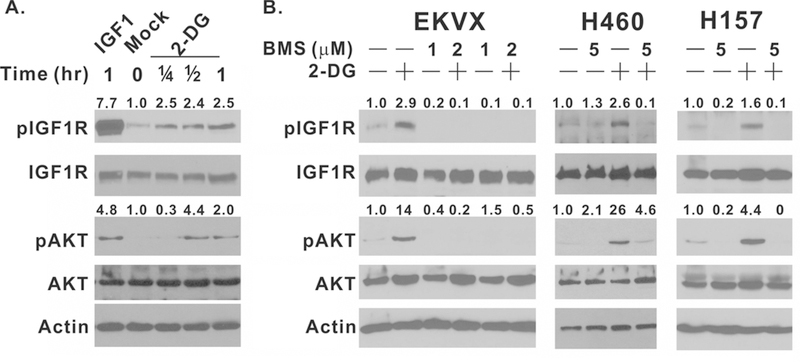
We previously demonstrated that 2-DG treatment activates the pro-survival AKT pathway independently of glycolysis inhibition (1). Furthermore, we used a chemical inhibitor, antibody, and RNAi against IGF1R to demonstrate that 2-DG‒induced IGF1R activation is essential in this process (2). The activation of IGF1R by 2-DG was questioned by Mireuta et al in a study with breast and cervical cancer cell lines, partially because they failed to observe 2-DG‒induced IGF1R phosphorylation at its autophosphorylation site, 1135/1136, in the kinase domain (12). 2-DG‒induced IGF1R phosphorylation at these sites, however, was observed by Estan et al in leukemia cell lines, who also observed a serum-dependent 2-DG induction of AKT phosphorylation as we reported previously (11). To evaluate these contradictory findings in NSCLC cells, we first obtained a panel of phospho-IGF1R antibodies from Cell Signaling. Our analysis indicated that 2-DG‒induced IGF1R phosphorylation can be reliably detected by anti-phospho-Tyr1135/1136 antibody (cat#3024) in EKVX cells within 15 minutes even though the magnitude of IGF1R phosphorylation induced by 25 mM 2-DG was weaker than that by 25 ng/ml IGF1 (Fig. 1A, 7.7-fold vs 2.5-fold). In addition, 2-DG‒induced IGF1R phosphorylation was followed by AKT phosphorylation as predicted by our previous observation (2). 2-DG‒induced AKT phosphorylation was not observed at 15 minutes; consistent with our hypothesis that 2-DG‒induced IGF1R phosphorylation precedes AKT phosphorylation. 2-DG‒induced IGF1R phosphorylation and AKT phosphorylation were also observed in H460 and H157 NSCLC cell lines (Fig. 1B), indicating that this is not a cell line specific phenomenon. Furthermore, BMS-754807, a clinical grade IGF1R inhibitor, completely inhibited 2-DG‒induced IGF1R and AKT phosphorylation in all three cell lines (Fig. 1B). These data support our previous finding that 2-DG is capable of activating AKT signaling through IGF1R in NSCLC cell lines.
Figure 1.
BMS-754807 inhibits 2-DG–induced IGF1R phosphorylation. (A) EKVX cells were treated with 25 ng/ml recombinant IGF1 for 1 hr or with 25 mM 2-DG for 0, 15, 30 or 60 min. (B) EKVX cells were treated with 5mM 2-DG, 1 μM BMS, 2μM BMS only, or their combination for 2 hr. H460 and H157 cells were treated with 25 mM 2-DG, 5 μM BMS-754807, or their combination for 2 hr. Whole cell lysates were harvested and subjected to immunoblot with indicated antibodies. pIGF1R and pAKT fold induction was quantitated by ImageJ as described in methods. Each experiment was carried out at least twice, and representative images are shown.
3.2. BMS-754807 synergizes with 2-DG to promote apoptosis in EKVX cells
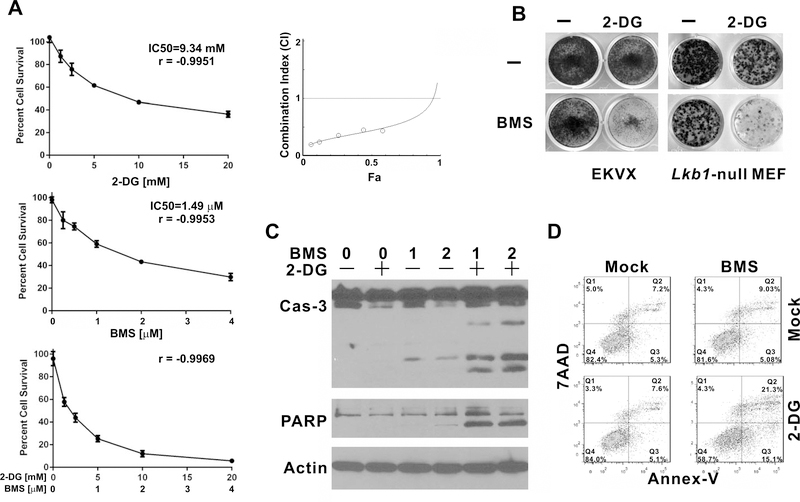
As 2-DG‒induced IGF1R activation may promote cell survival through various downstream signaling pathways including AKT, we next tested whether BMS-754807 can be used to potentiate the effect of 2-DG in NSCLC cells. Combination index (CI) analysis indicated that treatment with these two reagents resulted in CI values less than 1 for all tested concentrations EKVX cell line (Fig. 2A). Synergy between 2-DG and BMS-754807 was also observed in colony formation assays (Fig. 2B). We previously observed that the combination of sub-toxic doses of 2-DG and a compound called IGF1R inhibitor I promotes apoptosis (2). Here, we found that BMS-754807 alone slightly induced caspase-3 or PARP cleavage, and this was significantly enhanced by the combination of 2-DG and BMS-754807 in EKVX cells (Fig. 2C). This synergy in promoting apoptosis was validated by Annexin V/7AAD flow cytometry analysis (Fig. 2D).
Figure 2.
BMS-754807 synergizes with 2-DG to inhibit the proliferation of EKVX and Lkb1-null MEF cells. (A) 2000 EKVX cells were seeded per well in 96-well plates and treated with the indicated concentrations of 2-DG, BMS-754807, or their combination for 72 hrs in quadruplicate. Cells were fixed and analyzed by SRB assay. Dose response and Combination Index analyses were carried out by CompuSyn. Error bars represent one standard deviation. (B) EKVX and Lkb1-null MEF cells were seeded 4500/well in 12-well plates. EKVX cells were treated with 2.5 mM 2-DG, 1μM BMS-754807, or their combination for 6 days. Lkb1-null MEF cells were treated with 1 mM 2-DG, 1μM BMS-754807, or their combination for 14 days. Cells were stained with crystal violet. (C) EKVX cells were treated were treated with 5 mM 2-DG, 1 μM or 2 μM BMS-754807 alone, or their combination for 48 hr. Whole cell lysates were harvested for immunoblot. (D) EKVX cells were treated with 5 mM 2-DG, 2 μM BMS-754807, or their combination for 36 hr. Cells were trypsinized and harvested for apoptosis assay.
3.3. Restoration of kinase-active LKB1 suppressed apoptosis induced by the combination treatment in EKVX and Lkb1-null MEF cells
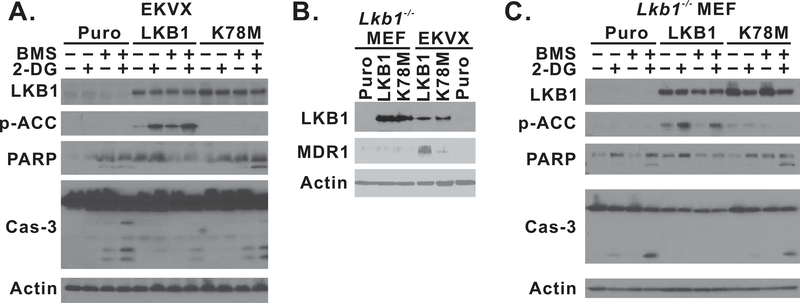
2-DG inhibits the glycolytic pathway to suppress intracellular ATP generation, which can be detected by the LKB1/AMPK metabolic checkpoint (1), and activated LKB1 may protect cells against agents that induce energetic stress (21,22). The EKVX cell line was recently found to contain a Ser to Phe missense mutation at codon 216 in the kinase domain of LKB1, which confers loss of its kinase activity (23). We next determined whether the restoration of LKB1/AMPK signaling is capable of protecting cells against apoptosis induced by the combination of 2-DG and BMS-754807. EKVX cancer cells were transformed with lentiviral constructs containing wild-type (WT), K78M mutant LKB1 gene, or empty vector, and the introduction of wild-type slightly attenuated the proliferation of EKVX cells (supplemental Fig. 1). The phosphorylation of acetyl-CoA carboxylase (ACC) at serine 79 was used as a surrogate marker for the activation of LKB1/AMPK signaling (19,24). 2-DG treatment induced the phosphorylation of ACC only in cells expressing WT-LKB1 protein but not in K78M mutant-expressing cells (Fig. 3A), indicating that LKB1/AMPK signaling was only re-established in EKVX cells with WT-LKB1 expression. BMS-754807 treatment slightly enhanced 2-DG–induced phosphorylation of ACC in WT-LKB1 expressing cells but not in other cells. The re-establishment of wild-type LKB1 expression significantly attenuated caspase-3 and PARP cleavage in EKVX cells (Fig. 3A). The suppression of apoptosis was also validated by Annexin-V/7AAD flow cytometry analysis (supplemental Fig. 2a).
Figure 3.
The restoration of LKB1/AMPK signaling suppresses apoptosis in EKVX and Lkb1-null MEF cells. (A) EKVX-puro, LKB1, or K78M isogenic cells were seeded 3 × 105/well in 6-well plates. Cells were treated with 1μM BMS-754807, 5mM 2-DG, or their combination for 24 hr and then whole cell lysates were harvested for immunoblot. (B) Immunoblot analysis of MDR1 expression in isogenic Lkb1-null MEF and EKVX cells using an anti-MDR1 antibody that recognizes both human and mouse MDR1 (Cell Signaling, cat#13978). (C) Lkb1-null MEF-puro, -LKB1, or K78M isogenic cells were seeded 3 × 105/well in 6-well plates. Cells were treated with 1μM BMS-754807, 5mM 2-DG, or their combination for 24 hr and then whole cell lysates were harvested for immunoblot. Each experiment was carried out at least twice, and representative data are shown.
The interpretation of this data, however, was complicated by a recent discovery of the elevated expression of MDR1 expression in EKVX cells after the restoration of wild-type LKB1 (23). To address this, we chose to use Lkb1-null MEF cells, which have a genetically defined background. 2-DG synergized with BMS-754807 in a colony formation assay in Lkb1-null MEF cells (Fig. 2B). We also generated isogenic MEF cell lines with vector, WT, or K78M-mutant LKB1 expression, and the combination of 2-DG and BMS-754807 promoted caspase-3 and PARP cleavage in the vector-treated control cells (Fig 3C). The re-expression of active LKB1 in this cell line was confirmed by reactivation of ACC phosphorylation after 2-DG treatment (Fig. 3C), but there was no elevation of MDR1 expression as determined by an antibody that recognizes both human and mouse MDR1 (Fig. 3B). In these MEF isogenic cells, re-establishment of LKB1/AMPK signaling was also capable of suppressing caspase-3 and PARP cleavage induced by the combination of 2-DG and BMS-754807 (Fig. 3C). A similar observation was made by Annexin-V/7AAD flow cytometry analysis (supplemental Fig. 2b). Therefore, the restoration of LKB1 kinase activity is sufficient to rescue LKB1-inactivated cells from apoptosis induced by the combination of 2-DG and BMS-754807 in EKVX and Lkb1-null MEF cells.
We also evaluated whether the restoration of wild-type LKB1 abolished the combined effect of 2-DG and BMS-754807 in EKVX isogenic cell lines. Our data indicated that the effects of the 2-DG and BMS-754807 combination remained synergistic at high concentrations (10 mM/2 μM and 20 μM/4 uM) and were additive at lower concentrations (supplemental Fig. 3a). Cell cycle analysis revealed this combination resulted in the predominant accumulation of a G1 arrested cell population (supplemental Fig. 3b), which is consistent with the role of LKB1 in promoting G1 cell cycle arrest (25–27). Therefore, this combination may also work in some LKB1-wild type NSCLC cells through the promotion of G1 cell cycle arrest.
3.4. 2-DG suppressed 8n-DNA cell population induced by BMS-754807 treatment in H460 cells
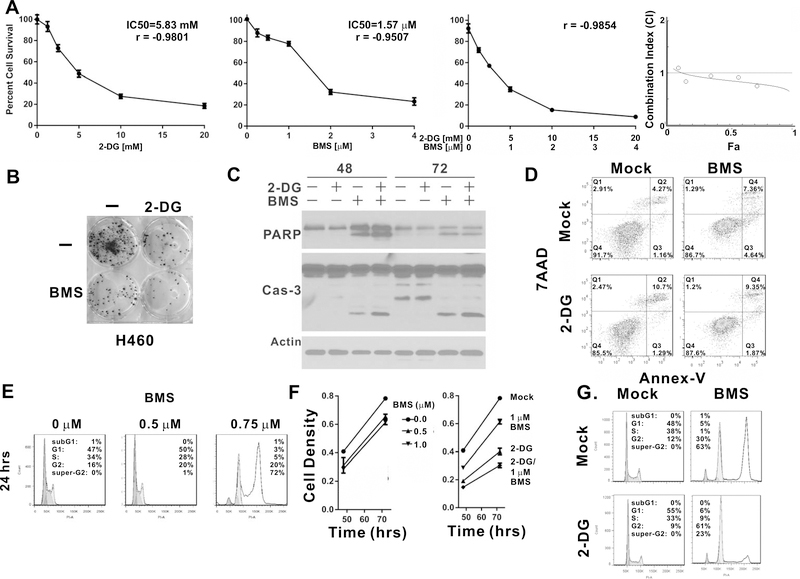
We also evaluated the combination of 2-DG and BMS-754807 in other NSCLC cells, such as H460. CI analysis indicated that 2-DG marginally synergized with BMS-754807, except for the highest concentration combination tested (Fig. 4A). Colony formation assay revealed a similar combinatorial effect (Fig. 4B), but it was weaker than that observed in EKVX or Lkb1-null MEF cells (Fig. 2B).
Figure 4.
2-DG suppresses 8N-DNA and BMS-754807 inhibits the proliferation of H460 cells in vitro. (A) 3000 H460 cells were seeded per well in 96-well plates and treated with the indicated concentrations of 2-DG, BMS-754807 or their combination for 72 hrs in quadruplicate. Cells were fixed and analyzed by SRB assay. Combination Index analyses were carried out by CompuSyn. Error bars represent one standard deviation. (B) H460 cells were seeded 100/well in 6-well plates. Cells were treated with 2mM 2-DG, 2μM BMS-754807, or their combination for 14 days. Cells were stained with crystal violet for colony formation analysis. (C) H460 cells were treated with 1 mM 2-DG, 2.5 μM BMS-754807 alone, or their combination for 48 or 72 hrs. Whole cell lysates were harvested for immunoblot with the indicated antibodies. (D) H460 cells were treated with 5 mM 2-DG, 4 μM BMS-754807, or their combination for 36 hr. Cells were trypsinized and harvested for Annexin-V/7AAD flow cytometry analysis. (E) H460 cells were treated with 0, 0.5, and 0.75 μM BMS-754807 for 24 hrs followed by cell cycle analysis. (F) Left panel: 3000 H460 cells were seeded per well in 96-well plates and treated with the indicated concentration of BMS-754807 for 48 or 72 hrs in quadruplicate. Cell density was analyzed by SRB assay. Right panel: 3000 H460 cells were seeded per well in 96-well plates and treated with 5 mM 2-DG, 1 μM BMS-754807 or their combination for 48 or 72 hrs in quadruplicate. Cell density was analyzed by SRB assay. Errors bar represent one standard deviation. (G) H460 cells were treated with 2 mM 2-DG, 4 μM BMS-754807, or their combination for 36 hrs followed by cell cycle analysis.
Treatment with 2.5 μM of BMS-754807 alone was sufficient to induce caspase-3 and PARP cleavage in H460 cells, but the inclusion of a sub-toxic level of 2-DG (1 mM) did not significantly enhance caspase-3 or PARP cleavage (Fig. 4C). Annexin V/7AAD flow cytometry analysis revealed that treatment with 5 mM 2-DG alone or 4μM BMS-754807 alone induced approximately 12% Annexin V positive cells at 36 hrs, but the total number of Annexin V-positive cells was only 10% with the combination treatment (Fig. 4D). Therefore, the combinatorial effect of 2-DG and BMS-754807 treatment on H460 cells was not due to an increase in apoptotic cells.
A previous study indicated that treatment of NSCLC cells with 1 μM BMS-754807 resulted in significant accumulation of G2/M phase cells and 4% of cells became polyploid with 8n DNA content (16). We observed a similar pattern in EKVX cells; BMS-754807 induced the accumulation of G2/M phase cells in a dose dependent manner and also resulted in the suppression of cell proliferation (supplemental Fig. 4). In H460 cells, treatment with 0.75 μM of BMS-754807 also induced the accumulation of G2/M phase cells, but the majority of cells appeared to have 8n-DNA content at 24 hrs after treatment (Fig. 4E). The extensive accumulation of cells with 8n-DNA content was also observed following treatment with 2 and 4 μM (data not shown) but not with 0.5 μM BMS-754807 (Fig. 4E). This phenomenon was novel, and the underlying mechanism was not known. More importantly, the increase in cell density was not attenuated by treatment with up to 1 μM BMS-754807 between 48 and 72 hrs, based on SRB assay (Fig. 4F, left panel), indicating that the formation of 8n DNA did not reduce the increase in cell density. The addition of 5 mM 2-DG to this treatment, however, suppressed the increase in cell density (Fig. 4F, right panel). Interestingly, the combination treatment attenuated the accumulation of cells with 8n-DNA content and restored G2/M cell cycle arrest even though 2-DG treatment alone did not cause G2/M cell cycle arrest (Fig. 4G). Therefore, the combinatorial effect of 2-DG and BMS-754807 in H460 cells is due to the re-establishment of G2/M phase cell cycle arrest but not the potentiation of apoptosis.
3.5. The combination of BMS-754807 and 2-DG suppresses the growth of H460 xenografts in vivo
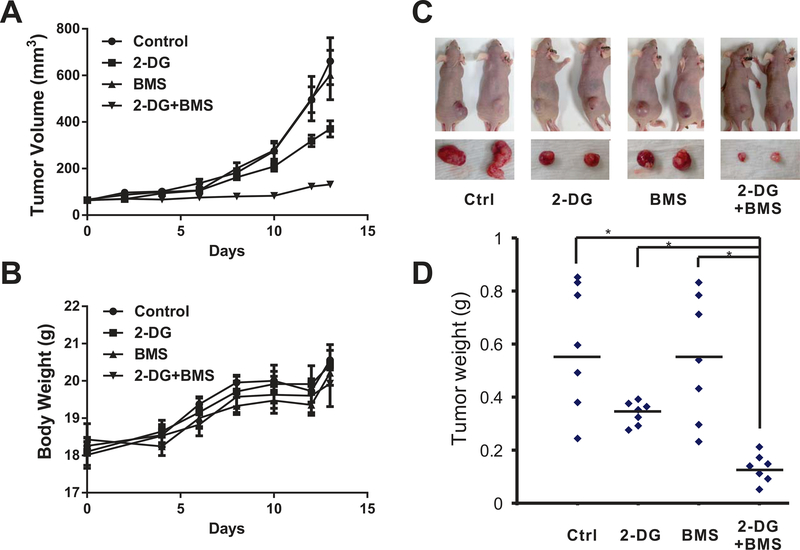
We next tested whether this combination had similar effects in vivo. EKVX and Lkb1-null MEF cells did not consistently form xenografts in our hands, so we focused our analysis on xenografts derived from H460 cells. When the xenografts reached an average size of 50 mm3, mice were treated with BMS-754807 alone, 2-DG alone, or the combination for 14 days (n=7 in each group). As shown in Table 1 and Figure 5A, the control group had the fastest tumor growth over time (growth rate = 0.171 log-mm3/day). While BMS-754807 treatment alone did not significantly suppress tumor growth (growth rate = 0.161 log-mm3/day) compared to the control group (p = 0.999), 2-DG alone was capable of slowing down tumor growth (growth rate = 0.135 log-mm3/day) with marginal significance (p = 0.135). In the mice treated with both 2-DG and BMS-754807, we observed a significantly slower and suppressed tumor growth (growth rate = 0.053 log-mm3/day) in the first two weeks compared to the control (p < 0.001). None of these treatment methods resulted in significant changes in animal body weight (Fig. 5B and supplemental Table 1). There was a significant decrease in tumor weight in the group receiving the combination treatment (Fig. 5C and D). H460 xenografts contained extensive necrosis, and we were unable to assess apoptosis through TUNEL analysis. We evaluated Ki-67 levels and saw a slight decrease in the combined treatment group but not at a statistically significant level (supplemental Fig 5). In combination, these data indicate that BMS-754807 also synergized with 2-DG in the suppression of tumor growth in vivo.
Table 1.
Estimated growth rate by experiment groups.
| Groups | Tumor growth Rate (log-mm3/day) | p-value | Adjust p-value* | p-value |
|---|---|---|---|---|
| Control | 0.171 | ref | ref | - |
| BMS-754807 | 0.161 | 0.582 | 0.999 | - |
| 2-DG | 0.135 | 0.045 | 0.135 | ref |
| Combination | 0.053 | <0.001 | <0.001 | <0.001 |
Comparison is made relative to control group.
The adjusted p-value is by Bonferroni method.
Figure 5.
The combination of BMS-754807 and 2-DG suppresses the growth of LKB1-inactivated cells in vivo. Female athymic nude mice were implanted with 5 × 106 H460 cells in 100 μL PBS on the right flank. Tumors were allowed to grow to about 50mm3 (on the 6th day), then mice were allocated into 4 groups and administered vehicle control, 2-DG, BMS-754807, or the combination of 2-DG and BMS-754807. Mice were treated twice daily for 14 days. Body weight and tumor size were measured every other day, and the tumor volume was calculated with the formula [volume = 3.14 × (height × width × width)/6]. (A) Tumor volumes determined by caliper measurement. Error bars represent one S.E.M. within group. (B) Animal body weight measurements. Error bars represent one SEM. (C) Mice and xenografts in different groups. (D) Tumors were weighed upon harvest. * indicates p < 0.05.
4. Discussion
Lung cancer cells usually display a high rate of glycolysis, and we have studied the growth suppressing effects of 2-DG because it is a known inhibitor of glycolysis that depletes intracellular ATP production (1,28,29). The safety of 2-DG in patients has been evaluated in several clinical trials (30), but 2-DG does not have anti-tumor activity as a single agent. Our previous mechanistic study indicated that 2-DG also activates multiple pro-survival pathways through IGF1R which is independent of its inhibition of glycolysis (1,2). Our finding, however, was questioned by a subsequent study which failed to identify 2-DG–induced IGF1R phosphorylation in several breast and cervical cancer cell lines (12). 2-DG–induced IGF1R phosphorylation, however, was observed in a myeloid leukemia study (11). These conflicting findings suggest that 2-DG–induced IGF1R phosphorylation may be tissue type-specific, and led us to re-evaluate 2-DG–induced IGF1R phosphorylation in NSCLC cancer cells. Our analysis indicated that 2-DG induces IGF1R phosphorylation at Tyr1135/1136 in its kinase domain, which is necessary for its kinase activation. Furthermore, pre-treatment with BMS-754807 was sufficient to abolish 2-DG–induced IGF1R phosphorylation. Therefore, these data are consistent with our previous finding that 2-DG is capable of activating IGF1R signaling in NSCLC cells. The mechanism underlying 2-DG–induced IGF1R activation is still unclear. We previously postulated that 2-DG interferes with the interaction between IGF1/IGF1BP3, which is unlikely to be the case given the experimental evidence provided by Mireuta et al (12). Therefore, it will be of interest to determine in the future how 2-DG activates IGF1R in cell lines of lung cancer and myeloid leukemia origins.
The activation of IGF1R signaling by 2-DG to offset its growth inhibitory effect is also supported by our cell proliferation studies which revealed a synergistic interaction between 2-DG and BMS-754807. In EKVX and Lkb1-null MEF cells, the combination of 5 mM 2-DG and 1μM BMS-754807 promotes apoptosis. 2-DG–induced apoptosis was previously reported in LKB1-inactivated cells at 45 mM (8), but the highest 2-DG plasma concentration detected in patients was approximately 5.5 mM (30), which was not sufficient to induce apoptosis. Our data indicate that the inclusion of IGF1R inhibitor enables 2-DG to induce apoptosis at a physiologically relevant concentration.
For Lkb1-null MEF and EKVX cells that undergo apoptosis with the combination treatment, the re-establishment of LKB1/AMPK signaling is sufficient to alleviate apoptosis. Interestingly, the combination of 2-DG and BMS-754807 remains additive or synergistic in EKVX-LKB1 wild type cells because the presence of functional LKB1 promotes G1 cell cycle arrest. The mechanism underlying LKB1-mediated G1 arrest has been known for over a decade (25–27), and our findings indicate that the combination of 2-DG and BMS-754807 may also be effective in some LKB1-wild type NSCLCs through this well-established mechanism. However, our finding does not mean that the 2-DG/BMS combination is effective in all lung cancer cells. A challenge in any drug screening with human NSCLC cell lines is the extensive genetic heterogeneity even in lines that that share common driver mutations. We carried out combination index analysis in other NSCLC cancer cell lines. Even though we observed synergistic interaction between 2-DG and BMS in some LKB1-wild type cell lines, such as NCI-H520 and NCI-H226, we also found that the 2-DG/BMS combination is antagonistic in some LKB1-wild-type cell lines, such as NCI-1792 and NCI-1703 (data not shown). These data suggest that LKB1 status alone cannot be used to predict NSCLC’s response to the combination of 2-DG and BMS
A surprising discovery of this study is that treatment with 0.75 μM of BMS-754807 was sufficient to induce 8n DNA content in the majority of H460 cells. This is in contrast to EKVX cells or H292 tumors treated with BMS-754807 where most cells are arrested in G2/M phase (16). The inhibition of IGF1R function has previously been shown to enhance radiation-induced polyploidy in DU145 prostate cancer cells (31), and BMS-754807 was able to induce polyploidy with 8n-DNA content in 4% of the cell population (16). Because H460 cells have wild-type p53, BMS-754807 induced polyploidy formation is unlikely to be related to p53 inactivation. In addition, the EKVX cell contains p53 inactivation mutations, and BMS-754807 failed to induce a polyploid cell population in the EKVX background. Therefore, this response is not related to p53 loss. A recent study indicated that polyploidy formation in doxorubicin-treated cancer cells can promote the escape from senescence (32). Hence, it is possible that the formation of 8n-DNA facilitates the escape of H460 cells from G2/M phase cell cycle checkpoint. The inclusion of 2-DG re-established the G2/M checkpoint in H460 cells and attenuated cell proliferation in vitro. We also evaluated this phenomenon in the H460 xenograft model, using a moderate dose of 2-DG and BMS-754807, and this combination remained effective in vivo.
The utility of this combination in other cancer types, such as breast cancer, is uncertain for two reasons. First, we have previously shown that PI3K/AKT signaling is one of the major downstream targets of 2-DG-induced IGF1R activation (1,2). Because the combined rate of somatic mutation of PI3KCA, PTEN, and AKT is relatively low in lung cancer (e.g. approximately 10~13% in lung adenocarcinoma), 2-DG treatment is likely to induce significant changes in AKT activity to promote cell survival. In contrast, the PIK3CA somatic mutation rate alone in invasive breast carcinoma is ~36%, and the rate of PTEN inactivation mutation is ~9%. As a result, these cell lines are likely to have high baseline AKT activity, and it is unclear whether 2-DG-induced IGF1R phosphorylation can cause sufficient change in AKT phosphorylation to promote cell survival. Second, 2-DG-induced AKT phosphorylation can be significantly attenuated by BMS-754807 in NSCLC. In contrast, Chakraborty et al recently demonstrated that BMS-754807 treatment alone caused little to no suppression of phosphorylated AKT in six commonly used breast cancer cell lines (33). Therefore, it is unclear whether BMS754807 treatment can significantly attenuate AKT phosphorylation in breast cancers.
5. Conclusions
Our work demonstrated that IGF1R signaling can be activated in non-small cell lung cancer by 2-DG, and this activation is suppressed by an IGF1R inhibitor, BMS-754807. Furthermore, we demonstrated that the combination of 2-DG and BMS-754807 inhibits the proliferation of non-small cell lung cancer both in vitro and in vivo. The mechanistic basis of this inhibition was cell line-dependent. In genetically defined Lkb1-null MEF cells and EKVX cells, the combination of subtoxic dose of 2-DG and BMS-754807 induced apoptosis. The restoration of LKB1 kinase activity was sufficient to suppress apoptosis, but this combination is still effective because the presence of LKB1 promotes G1 cell cycle arrest. H460 cells may escape the inhibitory effect of BMS-754807 by forming polyploid cells with 8n DNA content, and the combination with 2-DG restored G2/M phase cell cycle arrest. The ability of this combination to activate various tumor suppressive pathways further supports the notion that the combination of 2-DG and BMS-754807 may be beneficial to some lung cancer patients in the clinic.
Supplementary Material
Acknowledgments
We would like to thank Dr. Anthea Hammond for editing this manuscript.
Funding
Funding was provided by grants to W.Z. (R01-CA203928, R01-CA140571), to A.I.M and W.Z (R01CA194027), to F.R.K (P01 CA116676), and to S.Y.S. (R01-CA160522) from the National Institute of Cancer, China Scholarship Council to F.L., and Anise McDaniel Brock Scholar fund to W.Z. This work was also supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
Abbreviations:
- 2-DG
2-deoxyglucose
- IGF-1R
insulin-like growth factor 1 receptor
- NSCLC
non-small cell lung cancer
- PARP
poly ADP ribose polymerase
References
- 1.Zhong D, Liu X, Schafer-Hales K, Marcus AI, Khuri FR, Sun SY, and Zhou W (2008) 2-Deoxyglucose induces Akt phosphorylation via a mechanism independent of LKB1/AMP-activated protein kinase signaling activation or glycolysis inhibition. Mol Cancer Ther 7, 809–817 [DOI] [PubMed] [Google Scholar]
- 2.Zhong D, Xiong L, Liu T, Liu X, Liu X, Chen J, Sun SY, Khuri FR, Zong Y, Zhou Q, and Zhou W (2009) The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J. Biol. Chem 284, 23225–23233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun L, Liu X, Fu H, Zhou W, and Zhong D (2016) 2-Deoxyglucose Suppresses ERK Phosphorylation in LKB1 and Ras Wild-Type Non-Small Cell Lung Cancer Cells. PLoS One 11, e0168793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karczmar GS, Arbeit JM, Toy BJ, Speder A, and Weiner MW (1992) Selective depletion of tumor ATP by 2-deoxyglucose and insulin, detected by 31P magnetic resonance spectroscopy. Cancer Res. 52, 71–76 [PubMed] [Google Scholar]
- 5.McComb RB, and Yushok WD (1964) Metabolism of Ascites Tumor Cells. Iv. Enzymatic Reactions Involved in Adenosinetriphosphate Degradation Induced by 2-Deoxyglucose. Cancer Res. 24, 198–205 [PubMed] [Google Scholar]
- 6.Sridhar R, Stroude EC, and Inch WR (1979) Cytotoxicity of glucose analogues in V79 multicell spheroids. In Vitro 15, 685–690 [DOI] [PubMed] [Google Scholar]
- 7.Kang HT, and Hwang ES (2006) 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 78, 1392–1399 [DOI] [PubMed] [Google Scholar]
- 8.Nafz J, De-Castro Arce J, Fleig V, Patzelt A, Mazurek S, and Rösl F (2007) Interference with energy metabolism by 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside induces HPV suppression in cervical carcinoma cells and apoptosis in the absence of LKB1. Biochem. J 403, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inge LJ, Coon KD, Smith MA, and Bremner RM (2009) Expression of LKB1 tumor suppressor in non-small cell lung cancer determines sensitivity to 2-deoxyglucose. J. Thorac. Cardiovasc. Surg 137, 580–586 [DOI] [PubMed] [Google Scholar]
- 10.Brown J (1962) Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism 11, 1098–1112 [PubMed] [Google Scholar]
- 11.Estan MC, Calvino E, de Blas E, Boyano-Adanez Mdel C, Mena ML, Gomez-Gomez M, Rial E, and Aller P (2012) 2-Deoxy-D-glucose cooperates with arsenic trioxide to induce apoptosis in leukemia cells: involvement of IGF-1R-regulated Akt/mTOR, MEK/ERK and LKB-1/AMPK signaling pathways. Biochem. Pharmacol 84, 1604–1616 [DOI] [PubMed] [Google Scholar]
- 12.Mireuta M, Hancock MA, and Pollak M (2011) Binding between insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 is not influenced by glucose or 2-deoxy-D-glucose. J. Biol. Chem. 286, 16567–16573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wittman MD, Carboni JM, Yang Z, Lee FY, Antman M, Attar R, Balimane P, Chang C, Chen C, Discenza L, Frennesson D, Gottardis MM, Greer A, Hurlburt W, Johnson W, Langley DR, Li A, Li J, Liu P, Mastalerz H, Mathur A, Menard K, Patel K, Sack J, Sang X, Saulnier M, Smith D, Stefanski K, Trainor G, Velaparthi U, Zhang G, Zimmermann K, and Vyas DM (2009) Discovery of a 2,4-disubstituted pyrrolo[1,2-f][1,2,4]triazine inhibitor (BMS-754807) of insulin-like growth factor receptor (IGF-1R) kinase in clinical development. J. Med. Chem 52, 7360–7363 [DOI] [PubMed] [Google Scholar]
- 14.Carboni JM, Wittman M, Yang Z, Lee F, Greer A, Hurlburt W, Hillerman S, Cao C, Cantor GH, Dell-John J, Chen C, Discenza L, Menard K, Li A, Trainor G, Vyas D, Kramer R, Attar RM, and Gottardis MM (2009) BMS-754807, a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther 8, 3341–3349 [DOI] [PubMed] [Google Scholar]
- 15.Franks SE, Jones RA, Briah R, Murray P, and Moorehead RA (2016) BMS-754807 is cytotoxic to non-small cell lung cancer cells and enhances the effects of platinum chemotherapeutics in the human lung cancer cell line A549. BMC Res. Notes 9, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SJ, Kim EJ, Lee HJ, Kim SY, Oh SJ, Ryu JS, Moon DH, Ahn JH, and Kim SW (2013) A pilot study for the early assessment of the effects of BMS-754807 plus gefitinib in an H292 tumor model by [(18)F]fluorothymidine-positron emission tomography. Invest. New Drugs 31, 506–515 [DOI] [PubMed] [Google Scholar]
- 17.Zhong D, Liu X, Khuri FR, Sun S, Vertino PM, and Zhou W (2008) LKB1 is Necessary for Akt-mediated Phosphorylation of Pro-apoptotic Proteins. Cancer Res. 68, 7270–7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou TC, and Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul 22, 27–55 [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Jin R, Liu X, Huang H, Wilkinson SC, Zhong D, Khuri FR, Fu H, Marcus A, He Y, and Zhou W (2015) LKB1 promotes cell survival by modulating TIF-IA-mediated pre-ribosomal RNA synthesis under uridine downregulated conditions. Oncotarget [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Nannapaneni S, Wang D, Liu F, Wang X, Jin R, Liu X, Rahman MA, Peng X, Qian G, Chen ZG, Wong KK, Khuri FR, Zhou W, and Shin DM (2017) Phenformin enhances the therapeutic effect of selumetinib in KRAS-mutant non-small cell lung cancer irrespective of LKB1 status. Oncotarget 8, 59008–59022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao RX, and Xu ZX (2014) Targeting the LKB1 tumor suppressor. Curr. Drug Targets 15, 32–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou W, Zhang J, and Marcus AI (2014) LKB1 Tumor Suppressor: Therapeutic Opportunities Knock when LKB1 Is Inactivated. Genes & Diseases 1, 64–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mao K, Liu F, Liu X, Khuri FR, Marcus AI, Li M, and Zhou W (2015) Re-expression of LKB1 in LKB1-mutant EKVX cells leads to resistance to paclitaxel through the up-regulation of MDR1 expression. Lung Cancer 88, 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shackelford DB, Abt E, Gerken L, Vasquez DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS, and Shaw RJ (2013) LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell 23, 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie X, Wang Z, and Chen Y (2007) Association of LKB1 with a WD-repeat protein WDR6 is implicated in cell growth arrest and p27(Kip1) induction. Mol. Cell. Biochem 301, 115–122 [DOI] [PubMed] [Google Scholar]
- 26.Zeng PY, and Berger SL (2006) LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 66, 10701–10708 [DOI] [PubMed] [Google Scholar]
- 27.Tiainen M, Ylikorkala A, and Makela TP (1999) Growth suppression by Lkb1 is mediated by a G(1) cell cycle arrest. Proc. Natl. Acad. Sci. U. S. A 96, 9248–9251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jha B, and Pohlit W (1992) Effect of 2-deoxy-D-glucose on DNA double strand break repair, cell survival and energy metabolism in euoxic Ehrlich ascites tumour cells. Int. J. Radiat. Biol 62, 409–415 [DOI] [PubMed] [Google Scholar]
- 29.Dwarkanath BS, Zolzer F, Chandana S, Bauch T, Adhikari JS, Muller WU, Streffer C, and Jain V (2001) Heterogeneity in 2-deoxy-D-glucose-induced modifications in energetics and radiation responses of human tumor cell lines. Int. J. Radiat. Oncol. Biol. Phys 50, 1051–1061 [DOI] [PubMed] [Google Scholar]
- 30.Mohanti BK, Rath GK, Anantha N, Kannan V, Das BS, Chandramouli BA, Banerjee AK, Das S, Jena A, Ravichandran R, Sahi UP, Kumar R, Kapoor N, Kalia VK, Dwarakanath BS, and Jain V (1996) Improving cancer radiotherapy with 2-deoxy-D-glucose: phase I/II clinical trials on human cerebral gliomas. Int. J. Radiat. Oncol. Biol. Phys 35, 103–111 [DOI] [PubMed] [Google Scholar]
- 31.Chitnis MM, Lodhia KA, Aleksic T, Gao S, Protheroe AS, and Macaulay VM (2014) IGF-1R inhibition enhances radiosensitivity and delays double-strand break repair by both non-homologous end-joining and homologous recombination. Oncogene 33, 5262–5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosieniak G, Sliwinska MA, Alster O, Strzeszewska A, Sunderland P, Piechota M, Was H, and Sikora E (2015) Polyploidy Formation in Doxorubicin-Treated Cancer Cells Can Favor Escape from Senescence. Neoplasia 17, 882–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chakraborty A, Hatzis C, and DiGiovanna MP (2017) Co-targeting the HER and IGF/insulin receptor axis in breast cancer, with triple targeting with endocrine therapy for hormone-sensitive disease. Breast Cancer Res. Treat 163, 37–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.